Abstract
ATP-sensitive potassium (KATP) channels regulate insulin secretion from pancreatic beta-cells. Gain-of-function mutations in the genes encoding the Kir6.2 and SUR1 subunits of this channel cause neonatal diabetes. We report two novel mutations on the same haplotype (cis), F60Y and V64L, in the slide helix of Kir6.2 in a patient with neonatal diabetes, developmental delay and epilepsy. Functional analysis revealed the F60Y mutation increases the intrinsic channel open probability (Po(0)), thereby indirectly producing a marked decrease in channel inhibition by ATP and an increase in whole-cell KATP currents. When expressed alone, the V64L mutation caused a small reduction in apparent ATP inhibition, by enhancing the ability of MgATP to stimulate channel activity. The V64L mutation also ameliorated the deleterious effects on the F60Y mutation when it was expressed on the same (but not a different) subunit. These data indicate that F60Y is the pathogenic mutation and reveal that interactions between slide helix residues can influence KATP channel gating.
INTRODUCTION
ATP-sensitive potassium (KATP) channels are found in a variety of tissues where they couple the metabolism of the cell to its electrical activity via changes in plasma membrane potential (1–3). KATP channels are inhibited by intracellular ATP and activated by intracellular MgADP (4) and thus their activity is regulated by the balance between the intracellular levels of these nucleotides. In pancreatic beta-cells, plasma glucose concentration regulates insulin secretion via metabolically generated changes in adenine nucleotides: increased metabolism leads to closure of KATP channels, membrane depolarization, activation of voltage-dependent calcium channels and thereby to calcium-induced exocytosis of insulin (3,5).
Activating mutations in the genes KCNJ11 and ABCC8, encoding the KATP channel subunits Kir6.2 and SUR1, respectively, are associated with neonatal diabetes neonatal diabetes (6,7), a rare genetic disease characterized by elevated blood glucose levels within the first 6 months of life. These mutations decrease the ability of ATP to inhibit channel activity and result in abnormally large KATP currents that hyperpolarize the beta-cell and lead to reduced calcium influx and insulin secretion (7–10). The degree of reduction in channel ATP sensitivity is correlated with severity of the disease (3,11). Mutations that cause a small decrease in ATP sensitivity result in either transient or permanent diabetes. Large reductions in ATP produce developmental delay, epilepsy and muscle weakness as well as neonatal diabetes, a condition called DEND syndrome (7). An intermediate decrease in ATP sensitivity leads to intermediate DEND (iDEND) syndrome, characterized by developmental delay, muscle weakness and neonatal diabetes but not epilepsy.
Beta-cell KATP channels are hetero-octameric complexes of four pore-forming Kir6.2 subunits and four regulatory SUR1 subunits (12). ATP inhibits the KATP channel activity by binding to Kir6.2 (13), whereas MgADP activates the channel by interaction with the nucleotide-binding domains of SUR1 (14–17). Sulfonylurea drugs close KATP channels by binding to SUR1, and thereby stimulate insulin secretion (18). They have been used for many years to treat type 2 diabetes and more recently neonatal diabetes (19).
Activating mutations are found in both Kir6.2 and SUR1 subunits: they reduce the ability of ATP to inhibit the channel and in some cases enhance the stimulatory activity of MgADP (3,7,20–22). Some mutations cluster around the ATP-binding site of Kir6.2 and directly reduce ATP binding (6,23,24). Other mutations are found outside the ATP-binding region and reduce the inhibitory effect of ATP on Kir6.2 indirectly by increasing the intrinsic channel open probability and/or disrupting the mechanism by which conformational changes induced by ATP binding lead to channel closure (25,26). Indeed, mutations that cause DEND syndrome are often found in regions distant from the ATP-binding site, such as the gating loop (T293N), slide helix (V59G) or the pore-occluding ‘gate’ regions (I167L, I296L, L164P) that influence the channel gating (20,25,27,28). Mutations in SUR1 can act by increasing Mg-nucleotide activation (29,30) or enhancing the intrinsic channel open probability (22,31).
We report a DEND patient with heterozygous mutations in two residues that lie in the same slide helix of Kir6.2, F60Y and V64L. In functional studies, we found F60Y to be a pathogenic KATP channel mutation, whereas the V64L mutation had only subtle effects on channel ATP sensitivity when expressed alone. When both mutations were expressed on the same subunit (as is found in the patient), V64L ameliorated the effect of the F60Y mutation on the channel ATP sensitivity. This is the first report of neonatal diabetes in a patient with two mutations in Kir6.2.
RESULTS
Patient characteristics and genetics
A male patient diagnosed with diabetes at 4 weeks of age (birth weight 2315 g at 36 weeks’ gestation) was referred for genetic testing at 14 years of age with developmental delay, aggressive behaviour and epilepsy (an average of four generalized clonic tonic seizures per year). Two novel heterozygous KCNJ11 mutations [F60Y (c.179T>A) and V64L (c.190G>C)] were identified. The patient was subsequently able to transfer from insulin (0.8 U/kg/day) to glibenclamide (1.75 mg/kg/day taken in three doses) with an improvement in HbA1c from 8.4% (at referral) to 5.6%. His motor development improved so that he is now able to walk long distances, and his behaviour became less aggressive and more easily managed following drug transfer. His seizures also ceased.
The patient's unaffected mother did not carry either of the mutations, and both DNA from the father and information regarding the paternal family history of diabetes was unavailable. It is therefore not possible to tell whether the mutations arose de novo or if they have been inherited. Allele-specific polymerase chain reaction (PCR) was undertaken and sequencing of the PCR amplicon demonstrated that the two mutations were present on the same chromosome. We sequenced KCNJ11 in >4000 individuals and neither mutation was found in any other person except for this patient.
Effects of F60Y and V64L mutations on KATP channel ATP sensitivity
To determine the molecular mechanism of disease, we first measured the ability of ATP to inhibit KATP channel activity in inside-out membrane patches excised from Xenopus oocytes heterologously expressing wild-type or mutant KATP channels. Because Mg2+ is needed for the interaction of ATP with Kir6.2 but not SUR1, experiments were first carried out in the absence of Mg2+ to examine the effect of the mutations on ATP block at Kir6.2. The effect of the mutations on the stimulation of channel via SUR1 was subsequently studied by measuring ATP concentration–inhibition curves in the presence of Mg2+.
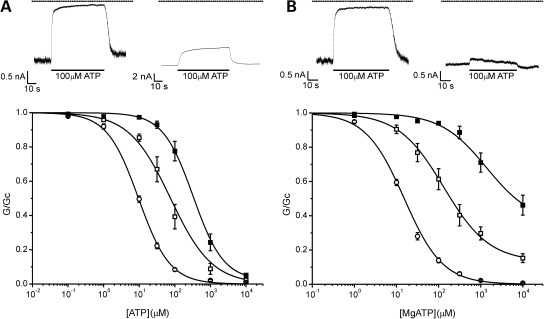
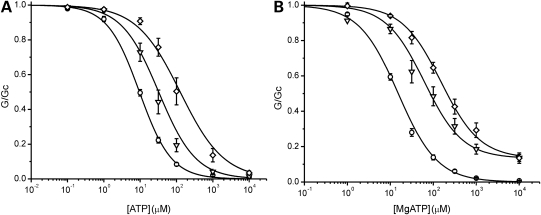
Homomeric (hom) F60Y and heterozygous (het) F60Y channels were significantly less sensitive to inhibition by ATP than wild-type channels (Fig. 1A). The ATP concentration producing half-maximal block (IC50) was 378, 93 and 10 µm for homF60Y, hetF60Y and wild-type channels, respectively (Table 1). These results indicate that the F60Y mutation decreases ATP block at Kir6.2. In contrast, there was little effect of the V64L mutation on channel ATP sensitivity in the absence of Mg2+ (Fig. 2A).
Figure 1.
Effects of the F60Y mutation. ATP sensitivity in the absence (A) and presence (B) of Mg2+. Above: KATP currents recorded at −60 mV in inside-out patches excised from oocytes expressing Kir6.2/SUR1 (wild-type, WT) (left) and homKir6.2-F60Y/SUR1 (homF60Y) (right) channels. The dashed line indicates the zero current level. Below: mean relationship between [ATP] and KATP conductance (G), expressed relative to that in the absence of nucleotide (Gc), for WT (open circles, n = 13), hetF60Y (open squares, n = 7) or homF60Y (filled squares, n = 7) channels in the absence of Mg2+ (A); or for wild-type (open circles, n = 18), hetF60Y (open squares, n = 9) and homF60Y (filled squares, n = 8) channels in the presence of Mg2+ (B). The lines are drawn to Eq. (1), with the following parameters. (A) WT (IC50 = 10 µm, h = 0.99), hetF60Y (IC50 = 76 µm, h = 0.75), homF60Y (IC50 = 328 µm, h = 1.02, a = 0.022). (B) WT (IC50 = 15 µm, h = 0.95), hetF60Y (IC50 = 133 µm, h = 0.81, a = 0.13), homF60Y (IC50 = 1.38 mm, h = 0.8, a = 0.36).
Table 1.
Mean data for wild-type and mutant KATP channels.
| IC50 | IC50 | Fractional current in 3 mm MgATP | Po(0) | |
|---|---|---|---|---|
| Mg2+-free | Mg2+ | Mg2+ | ||
| WT | 10 ± 1 (n = 13) | 16 ± 1 (n = 18) | 1.0 ± 0.1 (n = 18) | 0.29 ± 0.02 (n = 25) |
| homF60Y | 378 ± 82 (n = 7)** | 1781 ± 321 (n = 8)*** | 54 ± 7 (n = 8)*** | 0.86 ± 0.01 (n = 26)*** |
| hetF60Y | 93 ± 27 (n = 7)* | 188 ± 35 (n = 9)** | 21 ± 3 (n = 9)*** | |
| homV64L | 14 ± 1 (n = 7)** | 36 ± 5 (n = 9)** | 5 ± 1 (n = 9)* | 0.41 ± 0.02 (n = 19)*** |
| hetV64L | 13 ± 2 (n = 8)* | 27 ± 4 (n = 8)* | 2 ± 1 (n = 8) | |
| F60Y + V64L | 160 ± 42 (n = 8)** | 189 ± 25 (n = 15)*** | 19 ± 3 (n = 15)*** | |
| homF60Y/V64L | 233 ± 80 (n = 9)* | 316 ± 47 (n = 12)*** | 34 ± 3 (n = 12)*** | 0.79 ± 0.02 (n = 22)*** |
| hetF60Y/V64L | 30 ± 6 (n = 7)* | 97 ± 22 (n = 11)** | 14 ± 2 (n = 11)*** |
Data are mean ± SEM (n = number of patches).
*P ≤ 0.05 against wild-type (Student's t-test).
**P ≤ 0.01 (Student's t-test).
***P ≤ 0.001 (Student's t-test).
Figure 2.
Effects of the V64L mutation. ATP sensitivity in the absence (A) and presence (B) of Mg2+. Above: KATP currents recorded at −60 mV in inside-out patches excised from oocytes expressing Kir6.2/SUR1 (WT) (left) and homKir6.2-V64L/SUR1 (right) channels. The dashed line indicates the zero current level. Below: mean relationship between [ATP] and KATP conductance (G), expressed relative to that in the absence of nucleotide (Gc), for WT (open circles, n = 13), hetV64L (open triangles, n = 8) and homV64L (filled triangles, n = 7) channels in the absence of Mg2+ (A); or for WT (open circles, n = 18), hetV64L (open triangles, n = 8) and homV64L (filled triangles, n = 9) channels in the presence of Mg2+ (B). The lines are drawn to Eq. (1), with the following parameters. (A) hetV64L (IC50 = 13 µm, h = 1.16) and homV64L (IC50 = 14 µm, h = 1.21). (B) hetV64L (IC50 = 24 µm, h = 0.95) and homV64L (IC50 = 32 µm, h = 0.93, a = 0.04). Data and lines for WT are the same as in Figure 1.
In the presence of Mg2+, the ATP sensitivity of both hetF60Y and homF60Y channels was significantly less than that in its absence: IC50 for both homF60Y (1.8 mm) and hetF60Y channels (188 µm) were substantially less than that of wild-type channels (16 µm; Table 1). The fraction of unblocked current at 3 mm MgATP was also increased, being 21 ± 3% (n = 9) for hetF60Y channels and even greater for homF60Y (60% of the current was blocked even at MgATP concentrations as high as 10 mm) (Fig. 1B). As found for other mutations causing neonatal diabetes (20), the increase in the IC50 produced by Mg2+ was greater for homF60Y channels (5-fold) than wild-type channels (1.6-fold) (compare Fig. 1A and B).
Interestingly, in the presence of Mg2+, both homV64L and hetV64L channels were significantly less ATP sensitive than wild-type channels, despite the fact that there was no difference in the absence of the cation (Fig. 2B; Table 1). This argues that the V64L mutation must somehow facilitate the mechanism by which MgATP interaction with SUR1 leads to opening of the KATP channel, and thus it must influence interactions of SUR1 with Kir6.2.
These results indicate that the F60Y mutation is the pathogenic mutation. We next examined if the effect of the mutation is influenced by the presence of the V64L mutation, either on the same or a different subunit.
Effects of F60Y and V64L mutations in the same subunit
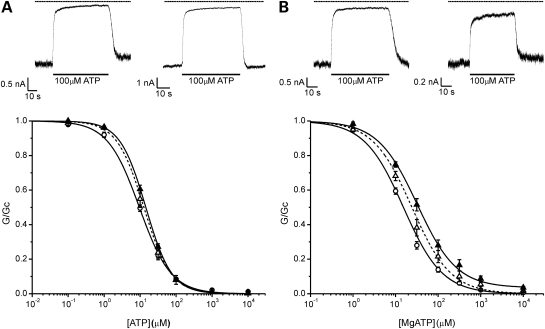
Genetic analysis showed that the F60Y and V64L mutations lie on the same chromosome. Thus, because the patient is heterozygous, they will express both wild-type subunits and subunits containing the double mutation F60Y/V64L. Figure 3 illustrates ATP concentration–inhibition curves for homF60Y/V64L and hetF60Y/V64L channels. In both the absence and presence of Mg2+, homF60Y/V64L channels had a significantly lower IC50 than homF60Y channels, being 233 versus 378 µm in Mg2+-free and 316 versus 1781 µm in Mg2+-containing solutions, for wild-type and homF60Y/V64L channels, respectively (Fig. 3; Table 1). A similar effect was found for hetF60Y/V64L channels: 30 versus 93 µm in Mg2+-free and 97 versus 188 µm in Mg2+-containing solutions (Fig. 3; Table 1). These results suggest that the V64L mutation reduces the deleterious effect of the F60Y mutation when both mutations are on the same subunit, and that it is able to do so in both the absence and the presence of Mg2+.
Figure 3.
Mutations in the same subunit. (A and B) Mean relationship between [ATP] and KATP conductance (G), expressed relative to that in the absence of nucleotide (Gc), for wild-type (WT, open circles, n = 13), homF60Y (filled squares, n = 7) and homF60Y/V64L (filled inverted triangles, n = 9) channels in the absence of Mg2+ (A); or for WT (open circles, n = 18), homF60Y (filled squares, n = 8) and homF60Y/V64L (filled inverted triangles, n = 12) channels in the presence of Mg2+ (B). The lines are drawn to Eq. (1), with the following parameters. (A) homF60Y/V64L (IC50 = 160 µm, h = 0.93, a = 0.08). (B) homF60Y/V64L (IC50 = 197 µm, h = 0.98, a = 0.29). Data and lines for WT and homF60Y are the same as in Figure 1. (C and D) Mean relationships between [ATP] and KATP conductance (G), expressed relative to that in the absence of nucleotide (Gc), for WT (open circles, n = 13), hetF60Y (open squares, n = 7) and hetF60Y/V64L (open inverted triangles, n = 7) channels in the absence of Mg2+ (C); or for wild-type (open circles, n = 18), hetF60Y (open squares, n = 9) and hetF60Y/V64L (open inverted triangles, n = 11) channels in the presence of Mg2+ (D). The lines are drawn to Eq. (1), with the following parameters. (C) hetF60Y/V64L (IC50 = 32 µm, h = 0.85). (D) hetF60Y/V64L (IC50 = 62 µm, h = 0.97, a = 0.13). Data and lines for WT and hetF60Y are the same as in Figure 1.
Effects of F60Y and V64L mutations in different subunits
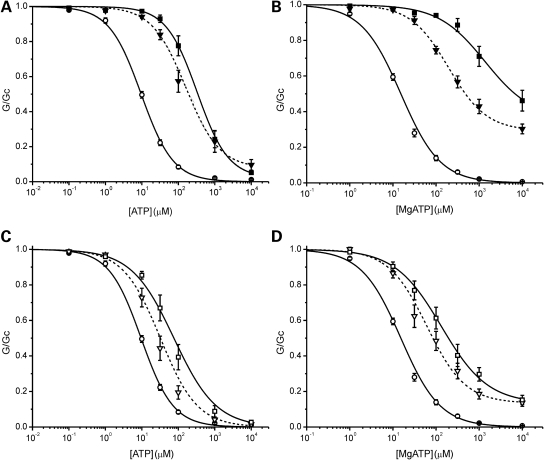
We next examined if the ability of the V64L mutation to ameliorate the effect of the F60Y mutation is also observed when the mutations are on separate subunits. We refer to this combination as F60Y + V64L. It produces a reduction in ATP sensitivity that is not significantly different from that found for hetF60Y channels, in either the absence or the presence of Mg2+ (Fig. 4; Table 1). Thus the F60Y mutation is dominant and the presence of V64L on a different subunit has no modifying effect.
Figure 4.
Mutations in separate subunits. Mean relationship between [ATP] and KATP conductance (G), expressed relative to that in the absence of nucleotide (Gc), for wild-type (WT) (open circles, n = 13), hetF60Y (open squares, n = 7), hetV64L (open triangles, n = 8) and F60Y + V64L (open diamonds, n = 8) channels in the absence of Mg2+ (A); or for WT (open circles, n = 18), hetF60Y (open squares, n = 9), hetV64L (open triangles, n = 8) and F60Y + V64L (open diamonds, n = 15) in the presence of Mg2+ (B). The lines are drawn to Eq. (1), with the following parameters. (A) F60Y + V64L (IC50 = 126 µm, h = 0.72). (B) F60Y + V64L (IC50 = 151 µm, h = 0.96, a = 0.13). Data and lines for WT, hetF60Y and hetV64L are the same as in Figures 1 and 2.
Figure 5 compares the ATP sensitivity of F60Y + V64L and hetF60Y/V64 in the presence and absence of Mg2+. It is clear that the F60Y + V64L combination causes a greater reduction in ATP sensitivity than hetF60Y/V64, reflecting the ability of the V64L mutation to ameliorate that of the F60Y mutation when expressed on the same subunit.
Figure 5.
Comparison of mutations in the same and different subunits. ATP concentration–response relations in the absence (A) and presence (B) of Mg2+ for WT (open circles), hetF60Y/V64L (open inverted triangles) and F60Y + V64L (open diamonds) channels. Same data as in Figures 3 and 4.
F60Y channels have an increased intrinsic open probability
Many neonatal diabetes mutations reduce the ATP sensitivity of the KATP channel by impairing ATP binding and/or transduction, whereas others influence ATP sensitivity indirectly by increasing the intrinsic (ligand-independent) open probability (Po(0)) of the channel (3). Mutations that lie within the slide helix have previously been shown to influence intrinsic gating: for example, V59G favours the open conformation of the channel (25).
We therefore examined the effect of the F60Y mutation on the intrinsic open probability in the absence of nucleotides. We found that Po(0) was markedly increased by the F60Y mutation, being 0.86 ± 0.01 (n = 26) for homF60Y compared with 0.29 ± 0.02 (n = 25) for wild-type channels (Fig. 6). This increase in Po(0) can account for the reduced ATP sensitivity of the F60Y mutant channel (26,32,33). Consistent with the lack of an effect on ATP sensitivity, the V64L mutation had only a small effect on Po(0), whether expressed alone or in combination with the F60Y mutation (Table 1).
Figure 6.
Single-channel currents. Single-channel currents recorded at −60 mV from an inside-out patch expressing WT (above) and homF60Y (below) channels. The dashed line indicates the zero current level.
Effects of F60Y and V64L mutations on whole-cell KATP currents
We next assessed the effects of the mutations on whole-cell KATP currents. Under resting conditions, the intracellular ATP concentration ([ATP]i) of the oocyte is high enough to keep wild-type KATP channels closed (Fig. 7). Lowering of [ATP]i by the metabolic inhibitor sodium azide reduces KATP channel inhibition and results in a substantial current. This current was inhibited by the KATP channel blocker tolbutamide, confirming that it flows through the KATP channel.
Figure 7.
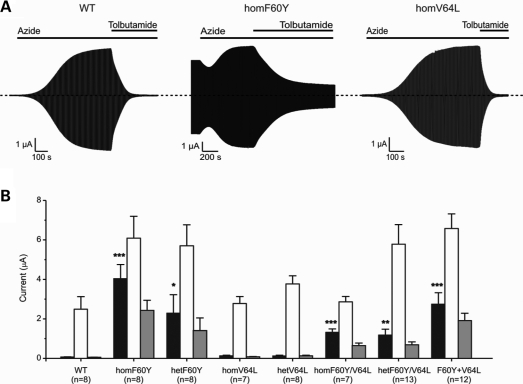
Whole-cell KATP currents. (A) Representative wild-type, homF60Y and homV64L whole-cell currents evoked by repeated voltage steps from −10 to +10 and −30 mV. The bars indicate the application of 3 mm azide or 0.5 mm tolbutamide. As previously noted for other mutations azide causes a small initial block when resting currents are large. (B) Mean steady-state whole-cell KATP currents evoked by a voltage step from −10 to −30 mV before (control, black bars) and after (white bars) application of 3 mm azide, and in the presence of 3 mm azide plus 0.5 mm tolbutamide (grey bars) for wild-type and mutant channels, as indicated. The number of oocytes tested is given below the bars. *P ≤ 0.05 against wild-type, **P ≤ 0.01, ***P ≤ 0.001 (Student's t-test).
Resting currents in oocytes expressing homF60Y channels were substantially higher than for oocytes expressing wild-type channels (Fig. 7), as expected from the lower ATP sensitivity of the mutant channel. Metabolic inhibition further increased homF60Y currents suggesting that the channels are partially blocked at resting [ATP]i and can be activated further by lowering [ATP]i. Resting hetF60Y currents were greater than wild-type but less than those of homF60Y channels: the amplitude of the azide-induced currents was similar for hetF60Y and homF60Y channels. In contrast, hetV64L and homV64L currents resembled those of wild-type channels. As expected from the lower ATP sensitivity, channels containing both the F60Y and V64L mutations also had increased resting currents. Consistent with the ATP concentration–inhibition curves, oocytes expressing channels with F60Y and V64L in the same subunit showed smaller resting currents than oocytes expressing channels containing the F60Y mutation alone (Fig. 7). This is in agreement with the relative extent of block by 3 mm MgATP (Table 1).
Channels containing the F60Y mutation were significantly less sensitive to tolbutamide at concentrations that maximally block wild-type channels, whereas those containing only the V64L mutation were unaffected. Inhibition by 0.5 mm tolbutamide was 99 ± 1% (n = 8) for wild-type channels and 89 ± 1% (n = 13) for hetF60Y/V64L channels (Fig. 7), consistent with the fact that the patient is sensitive to sulfonylureas.
DISCUSSION
The results reported here indicate the F60Y mutation is pathogenic. The V64L mutation partially ameliorates the effects of the F60Y mutation when it is present on the same, but not a different, subunit. Fortuitously, the patient carries these mutations on the same chromosome, and this combination is expected to result in a less severe phenotype than if they had only the F60Y mutation.
Given the severity of the phenotype, and the fact that it can be attributed solely to the F60Y mutation, it seems very likely that the F60Y mutation arose de novo.
Functional implications
The large amplitude of hetF60Y/V64L currents recorded in inside-out patches exposed to physiological levels of MgATP explains the dramatic increase in the resting whole-cell KATP currents. A similar increase in the beta-cell KATP current would be expected to produce beta-cell hyperpolarization and decrease the membrane depolarization, Ca2+ influx and insulin secretion evoked by glucose. Thus the reduced ATP inhibition of hetF60Y/V64L can explain the diabetic phenotype of the patient.
The large whole-cell currents and the extent of reduction in MgATP sensitivity are also consistent with the neurological symptoms of the patient. Previous studies have shown that mutations which cause iDEND or DEND syndrome give rise to heterozygous KATP currents that are much less sensitive to MgATP inhibition than mutations associated with neonatal diabetes alone (3). This is probably because KATP channels are normally shut in many muscle and nerve tissues and open only under metabolic stress (2). Thus, a greater reduction in ATP sensitivity is required to increase the resting KATP current sufficiently to influence electrical activity in these cells.
The increase in heterozygous KATP currents recorded in the presence of MgATP is similar to that found for other neonatal diabetes mutations associated with neurological symptoms and greater than for mutations that cause neonatal diabetes alone (3). For mutations that cause DEND syndrome, between 26 and 40% of the heterozygous current is not blocked by 3 mm MgATP, compared with 12–20% for mutations causing iDEND, 4–10% (in general) for mutations causing neonatal diabetes alone and <1% for wild-type channels (3). The patient described here carries a combination of two mutations on the same subunit (hetF60Y/V64L), which results in an unblocked current in 3 mm MgATP that is 14% of that in the absence of nucleotide, a magnitude similar to that associated with mutations that cause iDEND syndrome. This accounts for the fact the patient has developmental delay. The increase in muscle strength and behavioural problems observed following transfer to sulfonylureas is explained by the block of over-active KATP channels in extra-pancreatic tissues.
The magnitude of the increase in KATP current at 3 mm MgATP is the smallest reported to date to be associated with epilepsy (3). This could indicate that the patient's epilepsy has a different origin or that it results from a different phenotypic background. However, we favour the idea it is a consequence of the F60Y mutation, because the patient's seizures ceased following transfer to sulfonylureas, as reported for at least one other gain-of-function mutation in Kir6.2 (34).
Molecular mechanism
The increased KATP current produced by the F60Y mutation results from a decreased sensitivity to MgATP block, which is due to two mechanisms. The first is a reduced ability of ATP to close the KATP channel, which is mediated via Kir6.2 as it is present in the absence of Mg2+. This can be accounted for by the large increase in intrinsic Po(0). Second, the enhanced reduction in ATP sensitivity in the presence of Mg2+ argues that the stimulatory actions of MgATP, mediated via SUR1, are enhanced by the mutation. This is also found for some other neonatal diabetes mutations (20). Interestingly, although the F60Y mutation results in a 5-fold shift in ATP sensitivity on the addition of Mg2+, this is not the case for the double mutation F60Y/V64L which has a shift of similar magnitude to wild-type channel. This suggests that F60Y influences the mechanism by which MgATP binding/hydrolysis at SUR1 is translated into opening of the Kir6.2 pore, and that the V64L mutation ameliorates the effect of the F60Y mutation.
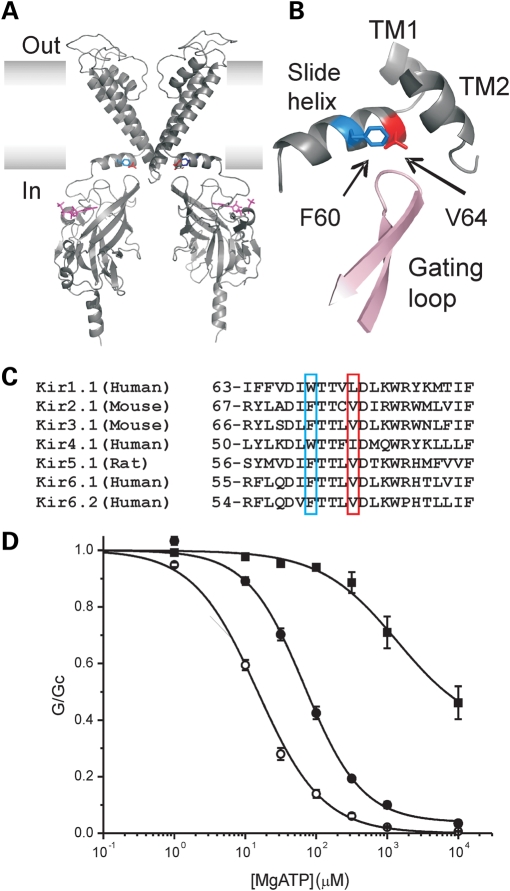
In a molecular model of Kir6.2, residues F60 and V64 lie on the slide helix, one helical turn apart (35) (Fig. 8A). This amphipathic helix lies in the plane of the membrane and is believed to be intimately involved in Kir channel gating (36,37). The phenylalanine at the position corresponding to F60 is well conserved throughout Kir channels, although it is replaced by a tryptophan in some Kir families (Fig. 8B). Valine or another small hydrophobic residue (isoleucine, leucine) is often found at the position corresponding to V64. A key question is how the F60Y mutation causes its deleterious effect and how the V64L mutation is able to ameliorate its effect on the intrinsic channel open probability.
Figure 8.
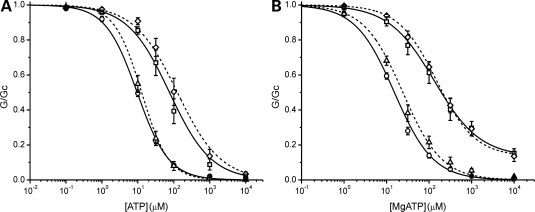
Molecular mechanism. (A) A molecular model of Kir6.2 (35) showing the location of residues F60 (blue) and V64 (red). For clarity only two transmembrane domains and two cytosolic domains are illustrated. ATP is shown in magenta. (B) A close-up of slide helix region showing the location of F60 and V64 and their proximity to the gating loop of adjacent subunit and transmembrane domains 1 and 2 (TM). Pink and grey indicate different subunits. (C) Alignment of the slide helix region for seven Kir channels. Residues corresponding to positions F60 and V64 in Kir6.2 are highlighted. (D) ATP concentration–response relations in the presence of Mg2+ for WT (open circles), homF60W (solid circles) and F60Y (squares) channels. For WT and homF60Y, the data are the same as in Figure 1B. The line for F60W is drawn to Eq. (1) with IC50 = 69 µm, h = 1.07, a = 0.04.
One possibility is that the larger size and polarity of the tyrosine residue might prevent the channel from closing and that this would be somehow counteracted by V64L. We tested this idea by mutating F60 to tryptophan (W), which in some Kir families is expressed at an equivalent position to F60 and is the largest natural amino acid. The F60W mutation reduced the channel sensitivity to inhibition by MgATP (IC50 = 73 ± 5 µm for homF60W, n = 5, Fig. 8C), but not nearly as much as homF60Y (1.8 mm). This suggests that steric repulsion is not the primary cause of the functional effects of the F60Y mutation.
Tyrosine substitution adds a hydroxyl (–OH) group to the aromatic side chain of phenylalanine. A second possibility therefore is that the OH group of tyrosine creates an H bond that stabilizes the open state of the channel, which phenylalanine cannot do. Such hydrogen bonding might occur between F60Y and lipid head groups or other regions of the channel such as the gating loop. The presence of leucine, which is slightly larger than valine, at position 64 might hinder this interaction. This might also explain why the V64L mutation must be on the same subunit to influence the effect of the F60Y mutation.
Clinical implications
Sulfonylureas are an effective therapy for most patients with neonatal diabetes caused by KATP channel mutations, and many have now successfully transferred from insulin to sulfonylureas (19,38). The magnitude of tolbutamide block of mutant KATP channels in functional studies correlates well with the success of the sulfonylurea therapy (3,19). We observed that tolbutamide caused 89% block of hetF60Y/V64L channels. This value lies within the range in which patients respond to sulfonylurea therapy, which explains why our patient could be successfully treated with glibenclamide.
Finally, although the V64L mutation cannot explain the severe phenotype of our patient, it does induce a small reduction in ATP sensitivity. However, this decrease is smaller in magnitude than those produced by other mutations associated with neonatal diabetes, including those that do not cause neurological complications (3). Thus, it seems unlikely (although not impossible) that the V64L mutation alone could cause neonatal diabetes and a different cause of disease should be considered in any patients carrying this mutation, particularly those with a severe phenotype.
MATERIALS AND METHODS
Mutation detection
Genomic DNA was extracted from peripheral leucocytes using standard procedures. The single-coding exon of KCNJ11 was amplified by the PCR (primer sequences are available on request). Unidirectional sequencing was performed using universal M13 primers and a Big Dye Terminator Cycler Sequencing Kit v3.1 (Applied Biosystems, Warrington, UK) according to the manufacturer's instructions. Reactions were analysed on an ABI 3730 capillary sequencer (Applied Biosystems, Warrington, UK) and sequences compared with the reference sequence NM_000525 using Mutation Surveyor v2.61 (SoftGenetics, PA, USA). To determine whether the mutations were in cis or trans, primers were designed to amplify a single copy of the patient's KCNJ11 gene using a primer complementary to the V64L (c.190G>A) mutation (primer sequences available on request). Sequence analysis of the PCR amplicon revealed that the two mutations were on the same chromosome.
Molecular biology and oocyte preparation
Human Kir6.2 (GenBank NM000525; E23 and I337) and rat SUR1 (GenBank L40624) were used. Site-directed mutagenesis, synthesis of capped mRNA and preparation Xenopus laevis oocytes was performed as previously reported (20,25). The oocytes were coinjected with ∼4 ng of SUR1 mRNA and ∼0.8 ng wild-type or mutant Kir6.2 mRNA. To simulate the heterozygous state, SUR1 was coexpressed with a 1:1 mixture of wild-type and mutant Kir6.2, or where indicated F60Y-Kir6.2 plus V64L-Kir6.2 mRNAs. For each batch of oocytes, all mutations were injected to enable direct comparison of their effects. Oocytes were incubated in Barth's solution and studied 1–4 days after injection.
Electrophysiology
Whole-cell currents were recorded using a two-electrode voltage clamp in response to voltage steps of ±20 mV from a holding potential of −10 mV, in a solution containing (in mm): 90 KCl, 1 MgCl2, 1.8 CaCl2, 5 HEPES (pH 7.4 with KOH). Metabolic inhibition was induced by 3 mm Na-azide, and 0.5 mm tolbutamide was used to block KATP channels, as indicated.
The patch-clamp recordings were performed in inside-out patches. The pipette solution contained (mm): 140 KCl, 1.2 MgCl2, 2.6 CaCl2, 10 HEPES (pH 7.4 with KOH). The Mg-free solution contained (mm): 107 KCl, 1 K2SO4, 10 EGTA, 10 HEPES (pH 7.2 with KOH) and K2ATP, as indicated. Mg-containing solution contained (mm): 107 KCl, 11 EGTA, 2 MgCl2, 1 CaCl2, 10 HEPES (pH 7.2 with KOH) and adding MgATP rather than K2ATP. The current was measured at a constant holding voltage of −60 mV. To control for possible rundown or activation by MgATP, Gc was taken as the mean of the conductance in control solution before and after ATP application. ATP concentration–response curves were fit with a modified Hill equation:
| 1 |
where [ATP] is the ATP concentration, IC50 is the ATP concentration at which inhibition is half maximal, h is the slope factor (Hill coefficient) and a represents the fraction of unblocked current at saturating [ATP] (a = 0 except where specified).
Single-channel currents were recorded at −60 mV, filtered at 5 kHz and sampled at 20–50 kHz, and analysed using a combination of Clampfit (Axon Instruments, Foster City, CA, USA) and Origin (OriginLab Corporation, Northampton, MA, USA). The open probability in the absence of ATP (Po(0)) was determined from single- and multi-channel patches as the fraction of time spent in the open state for recordings of ∼0.5 min duration after onset of the rinse of bath solution. Channel activity (NPo) was measured as the mean current (I) divided by the single-channel amplitude (i) for current records of ∼1 min duration. Po(0) was calculated from NPo/N, where N is number of channels in the patch estimated from the maximum number of superimposed events. Alternatively, the Po(0) was estimated by fitting idealized traces to recording using 50% threshold crossing method or by fitting an all-points current amplitude histogram with Gaussian functions. For multichannel patches with high open probability, Po(0) was estimated by dividing the mean current by maximum current.
Data are given as mean ± SEM. Significance was evaluated using Student's t-test.
FUNDING
This work was supported by Wellcome Trust [076436/Z/05/Z and 081188/A/06/Z], the Royal Society, and the European Union [EuroDia-(LSHM-CT-2006-518153)]. S.E.F. is the Sir Graham Wilkins, Peninsula Medical School Research Fellow and F.M.A. is a Royal Society Research Professor.
ACKNOWLEDGEMENTS
The authors would like to thank Annet Damhuis for her technical assistance.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Miki T., Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J. Mol. Cell. Cardiol. 2005;38:917–925. doi: 10.1016/j.yjmcc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Nichols C.G. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft F.M. ATP-sensitive K+ channels and disease: from molecule to malady. Am. J. Physiol. Endocrinol. Metab. 2007;293:E880–E889. doi: 10.1152/ajpendo.00348.2007. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft F.M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog. Biophys. Mol. Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 5.Ashcroft F.M., Harrison D.E., Ashcroft S.J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 6.Gloyn A.L., Pearson E.R., Antcliff J.F., Proks P., Bruining G.J., Slingerland A.S., Howard N., Srinivasan S., Silva J.M., Molnes J., et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 7.Hattersley A.T., Ashcroft F.M. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 8.Koster J.C., Marshall B.A., Ensor N., Corbett J.A., Nichols C.G. Targeted overactivity of beta cell KATP channels induces profound neonatal diabetes. Cell. 2000;100:645–654. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 9.Girard C.A., Wunderlich F.T., Shimomura K., Collins S., Kaizik S., Proks P., Abdulkader F., Clark A., Ball V., Zubcevic L., et al. A mouse model of neonatal diabetes caused by the KATP channel mutation Kir6.2-V59M. J. Clin. Invest. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remedi M.S., Kurata H.T., Scott A., Wunderlich F.T., Rother E., Kleinridders A., Tong A., Brüning J.C., Koster J.C., Nichols C.G. Secondary consequences of beta cell inexcitability: identification and prevention in a murine model of KATP-induced neonatal diabetes mellitus. Cell. Metab. 2009;9:140–151. doi: 10.1016/j.cmet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashcroft F.M. ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shyng S.L., Nichols C.G. Octameric stoichiometry of the KATP channel complex. J. Gen. Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tucker S.J., Gribble F.M., Zhao C., Trapp S., Ashcroft F.M. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 14.Nichols C.G., Shyng S.L., Nestorowicz A., Glaser B., Clement J.P., Gonzalez G., Aguilar-Bryan L., Permutt M.A., Bryan J. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 15.Shyng S.L., Ferrigni T., Nichols C.G. Regulation of KATP channel activity by diazoxide and MgADP—distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J. Gen. Physiol. 1997;110:643–654. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gribble F.M., Tucker S.J., Ashcroft F.M. The essential role of the Walker motifs of SUR1 in KATP channel activation by MgADP and diazoxide. EMBO J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gribble F.M., Tucker S.J., Haug T., Ashcroft F.M. MgATP activates the beta-cell KATP channel by interaction with its SUR1 subunit. Proc. Natl Acad. Sci. USA. 1998;95:7185–7190. doi: 10.1073/pnas.95.12.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gribble F.M., Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 19.Pearson E.R., Flechtner I., Njolstad P.R., Malecki M.T., Flanagan S., Larkin B., Ashcroft F.M., Klimes I., Codner E., Iotova V., et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N. Eng. J. Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 20.Proks P., Girard C., Ashcroft F.M. Functional effects of KCNJ11 mutations causing neonatal diabetes: enhanced activation by MgATP. Hum. Mol. Genet. 2005;14:2717–2726. doi: 10.1093/hmg/ddi305. [DOI] [PubMed] [Google Scholar]
- 21.Babenko A.P., Polak M., Cavé H., Busiah K., Czernichow P., Scharfmann R., Bryan J., Aguilar-Bryan L., Vaxillaire M., Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N. Engl. J. Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 22.Proks P., Girard C., Bævre H., Njolstad P.R., Ashcroft F.M. Functional effects of mutations at F35 in the NH2-terminus of Kir6.2 (KCNJ11), causing neonatal diabetes, and response to sulphonylurea therapy. Diabetes. 2006;55:1731–1737. doi: 10.2337/db05-1420. [DOI] [PubMed] [Google Scholar]
- 23.Shimomura K., Girard C., Proks P., Nazim J., Lippiat J.D., Cerutti F., Lorini R., Ellard S., Hattersley A.T., Barbetti F., et al. Mutations at the same residue (R50) of Kir6.2 (KCNJ11) that cause neonatal diabetes produce different functional effects. Diabetes. 2006;55:1705–1712. doi: 10.2337/db05-1640. [DOI] [PubMed] [Google Scholar]
- 24.Masia R., Koster J.C., Tumini S., Chiarelli F., Colombo C., Nichols C.G., Barbetti F. An ATP-binding mutation (G334D) in KCNJ11 is associated with a sulfonylurea-insensitive form of developmental delay, epilepsy, and neonatal diabetes. Diabetes. 2007;56:328–336. doi: 10.2337/db06-1275. [DOI] [PubMed] [Google Scholar]
- 25.Proks P., Antcliff J.F., Lippiat J., Gloyn A.L., Hattersley A.T., Ashcroft F.M. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc. Natl Acad. Sci. USA. 2004;101:17539–17544. doi: 10.1073/pnas.0404756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proks P., Girard C., Haider S., Gloyn A.L., Hattersley A.T., Sansom M.S., Ashcroft F.M. A novel gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep. 2005;6:470–475. doi: 10.1038/sj.embor.7400393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tammaro P., Flanagan S.E., Zadek B., Srinivasan S., Woodhead H., Hameed S., Klimes I., Hattersley A.T., Ellard S., Ashcroft F.M. A Kir6.2 mutation causing severe functional effects in vitro produces neonatal diabetes without the expected neurological complications. Diabetologia. 2008;51:802–810. doi: 10.1007/s00125-008-0923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimomura K., Flanagan S.E., Zadek B., Lethby M., Zubcevic L., Girard C.A., Petz O., Mannikko R., Kapoor R.R., Hussain K., et al. Adjacent mutations in the gating loop of Kir6.2 produce neonatal diabetes and hyperinsulinism. EMBO Mol. Med. 2009;1:166–177. doi: 10.1002/emmm.200900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Wet H., Rees M.G., Shimomura K., Aittoniemi J., Patch A.M., Flanagan S.E., Ellard S., Hattersley A.T., Sansom M.S., Ashcroft F.M. Increased ATPase activity produced by mutations at arginine-1380 in nucleotide-binding domain 2 of ABCC8 causes neonatal diabetes. Proc. Natl Acad. Sci. USA. 2007;104:18988–18992. doi: 10.1073/pnas.0707428104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babenko A.P. A novel ABCC8 (SUR1)-dependent mechanism of metabolism-excitation uncoupling. J. Biol. Chem. 2008;283:8778–8782. doi: 10.1074/jbc.C700243200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proks P., Shimomura K., Craig T.J., Girard C.A., Ashcroft F.M. Mechanism of action of a sulphonylurea receptor SUR1 mutation (F132L) that causes DEND syndrome. Hum. Mol. Genet. 2007;16:2011–2019. doi: 10.1093/hmg/ddm149. [DOI] [PubMed] [Google Scholar]
- 32.Trapp S., Proks P., Tucker S.J., Ashcroft F.M. Molecular analysis of ATP-sensitive K channel gating and implications for channel inhibition by ATP. J. Gen. Physiol. 1998;112:333–349. doi: 10.1085/jgp.112.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enkvetchakul D., Loussouarn G., Makhina E., Shyng S.L., Nichols C.G. The kinetic and physical basis of KATP channel gating: toward a unified molecular understanding. Biophys. J. 2000;78:2334–2348. doi: 10.1016/S0006-3495(00)76779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimomura K., Hörster F., de Wet H., Flanagan S.E., Ellard S., Hattersley A.T., Wolf N.I., Ashcroft F., Ebinger F. A novel mutation causing DEND syndrome: a treatable channelopathy of pancreas and brain. Neurology. 2007;69:1342–1349. doi: 10.1212/01.wnl.0000268488.51776.53. [DOI] [PubMed] [Google Scholar]
- 35.Antcliff J.F., Haider S., Proks P., Sansom M.S., Ashcroft F.M. Functional analysis of a structural model of the ATP-binding site of the KATP channel Kir6.2 subunit. EMBO J. 2005;24:229–239. doi: 10.1038/sj.emboj.7600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo A., Gulbis J.M., Antcliff J.F., Rahman T., Lowe E.D., Zimmer J., Cuthbertson J., Ashcroft F.M., Ezaki T., Doyle D.A. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 37.Nishida M., Cadene M., Chait B.T., MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 2007;26:4005–4015. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafiq M., Flanagan S.E., Patch A.M., Shields B.M., Ellard S., Hattersley A.T. Neonatal Diabetes International Collaborative Group. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care. 2008;31:204–209. doi: 10.2337/dc07-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]