Abstract
Leber congenital amaurosis (LCA) caused by mutations in Aryl hydrocarbon receptor interacting protein like-1 (Aipl1) is a severe form of childhood blindness. At 4 weeks of age, a mouse model of LCA lacking AIPL1 exhibits complete degeneration of both rod and cone photoreceptors. Rod cell death occurs due to rapid destabilization of rod phosphodiesterase, an enzyme essential for rod survival and function. However, little is understood regarding the role of AIPL1 in cone photoreceptors. Cone degeneration observed in the absence of AIPL1 could be due to an indirect ‘bystander effect’ caused by rod photoreceptor death or a direct role for AIPL1 in cones. To understand the importance of AIPL1 in cone photoreceptor cells, we transgenically expressed hAIPL1 exclusively in the rod photoreceptors of the Aipl1−/− mouse. Transgenic expression of hAIPL1 restored rod morphology and the rod-derived electroretinogram response, but cone photoreceptors were non-functional in the absence of AIPL1. In addition, the cone photoreceptors degenerate, but at a slower rate compared with Aipl1−/− mice. This degeneration is linked to the highly reduced levels of cone PDE6 observed in the hAIPL1 transgenic mice. Our studies demonstrate that AIPL1 is needed for the proper functioning and survival of cone photoreceptors. However, rod photoreceptors also provide support that partially preserves cone photoreceptors from rapid death in the absence of AIPL1.
INTRODUCTION
Leber congenital amaurosis (LCA) is an autosomal recessive disease that causes visual impairment in children. Fifteen different genes have been identified in which mutations result in LCA (1,2). Among these genes, mutations in Aipl1 have been associated with the most severe form of LCA leading to the degeneration of photoreceptor cells (3,4). A mouse model of LCA lacking Aipl1 (Aipl1−/−) exhibited rapid degeneration of both rods and cones and did not produce any light-dependent electrical response, recapitulating the disease phenotype seen in humans (5,6). The lack of rod photoreceptor function in this model has been linked to the destabilization of rod phosphodiesterase (rod PDE6), an essential photoreceptor enzyme (6).
Unlike rod photoreceptors, the role of AIPL1 in cone photoreceptors is unknown. In human retina, AIPL1 is expressed as early as fetal week 11 in cone progenitors, but is undetectable in adult cone photoreceptor cells (7–9). The absence of AIPL1 in adult human cones suggests that AIPL1 may be essential for development, but is not essential for cone cell survival. A hypomorphic mouse model with reduced AIPL1 expression showed rapid rod photoreceptor cell death, but cones seem to be normal up to 11 months (10). This study suggests that AIPL1 is critical for rod photoreceptors, but is dispensable in cone photoreceptors. Further evidence that AIPL1 functions primarily in the rod photoreceptors comes from clinical findings that a heterozygous parent of an LCA patient exhibited a reduction in rod photoreceptor function (3). In addition, an adult LCA patient with a mutation in AIPL1 exhibited predominant rod photoreceptor degeneration with surviving cone cells (11).
Cone cell death observed in AIPL1 deficient mice could be due to the indirect effect of rapid rod photoreceptor cell degeneration. Secondary cone photoreceptor cell death is well characterized in a mouse model of retinitis pigmentosa, rd1/rd1, where cone degeneration is slow and preceded by rapid rod photoreceptor loss that is linked to a defect in the rod phototransduction pathway (12). This model of cone degeneration is recognized as a ‘bystander’ effect, secondary to rod photoreceptor degeneration. The bystander effect is a phenomenon acknowledged across disciplines and can be initiated in varying ways, but in retinal diseases like retinitis pigmentosa, it is typically thought of as the induction of rod or cone photoreceptor cell death by loss of the other photoreceptor cell type. Bystander photoreceptor cell death is believed to result from altered cone metabolism or due to the lack of rod-derived trophic factors (13–15). The observations that AIPL1 may not be important for cone photoreceptors imply that the cone degeneration observed as a result of AIPL1-deficiency is due to the death of the rod photoreceptor cells in this disease.
However, a lack of measurable cone photoreceptor function in patients carrying homozygous mutations in Aipl1 indicates that AIPL1 is necessary for human cones (3). Consistent with this finding, the Aipl1−/− mouse is deficient in photopic electroretinograms (ERGs) at any age tested and undergoes rapid cone photoreceptor degeneration (6). This is in contrast to the results from rd1/rd1 mice where cone photoreceptors are functional during the initial phase of rod photoreceptor degeneration (16). Altogether, these observations argue for a direct and an essential role for AIPL1 in cones.
To test our hypothesis that AIPL1 has a specific role in cones, we developed transgenic mice that express human AIPL1 (hAIPL1) solely in rod photoreceptor cells. The loss of AIPL1 from cone photoreceptors in the presence of viable rods results in the complete loss of the cone electrical response and a reduced rate of cone degeneration. Our results indicate that not only is AIPL1 necessary for the function and survival of cones, but also that rods provide some form of protection to cone photoreceptors that prolongs their survival when AIPL1 is absent.
RESULTS
AIPL1 is expressed in adult cone photoreceptors
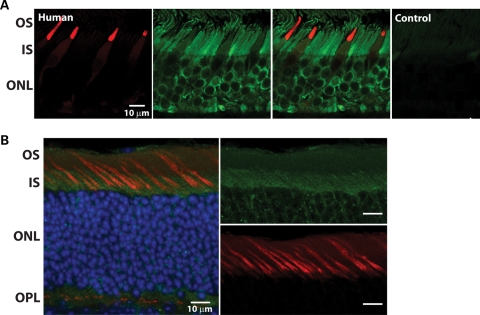
AIPL1 is expressed very early in both rod and cone photoreceptors (7–9). In humans at fetal week 11, AIPL1 can be detected in the pure cone area along with cone transducin (7). Along the edges of the pure cone area, AIPL1 is co-localized in early rods with the transcription factor, NRL (neural retina leucine zipper) (7). While the expression of AIPL1 is maintained in adult rod photoreceptor cells, one study illustrated that AIPL1 was undetectable in adult cone photoreceptor cells (8). The inability to detect AIPL1 in adult cones could be due to epitope masking or to lower sensitivity of the peptide antibody used. To circumvent these issues and to test whether AIPL1 is expressed in adult cone photoreceptors, we used a highly sensitive antibody generated against full-length human AIPL1 protein (4365L) (17). In normal adult human retinal tissue, AIPL1 expression was robust in both rod inner segments and nuclei (green; Fig. 1A). In comparison, AIPL1 immunostaining was weak in the inner segments of cones. This staining co-localized with a known cone marker, cone arrestin (red; Fig. 1A). Staining for both AIPL1 and cone arrestin was lost when the primary antibodies were omitted, confirming the specificity of the antibodies used in this study (control; Fig. 1A). To extend these findings, we verified the presence of AIPL1 in adult (P135) murine cones, using an AIPL1 antibody generated against mAIPL1 protein (10). In the mouse, AIPL1 (green) localizes to the inner segments of cone photoreceptors that are also positive for peanut agglutinin (PNA; red), a known cone marker (Fig. 1B). These results indicate that AIPL1 is present endogenously in adult mouse and human cones, albeit at lower levels in human retina in comparison to rod cells.
Figure 1.
AIPL1 is expressed in adult cone photoreceptors. (A) Immunolocalization of human-specific AIPL1 antibody (green) to the outer nuclear layer (ONL) of human retina. Anti-AIPL1 labeled rods intensely from the synaptic terminals to the proximal outer segment, extending along the axoneme. Cones, identified by mAb 7G6 labeling (red), appeared weakly labeled in the myoid and ellipsoid. Sections with omission of both primary antibodies served as negative controls. OS, outer segment; IS, inner segment. Scale bars, 10 µm. (B) AIPL1 (green) is expressed in both rod and cone photoreceptors of C57/BL6 mouse retina (post-natal day 135). Colocalization (yellow) of mAIPL1 immunoreactivity and peanut agglutinin (red), a cone marker, suggests that AIPL1 is expressed in mouse cones. ONL nuclei are visualized by DAPI staining (blue). OPL, outer plexiform layer. Scale bars, 10 µm.
Transgenic mice express hAIPL1 exclusively in rod photoreceptors
A mouse model of LCA that is deficient in AIPL1 exhibits rapid photoreceptor degeneration and lacks functional photoreceptor cells (5,6). Rod photoreceptor loss of function and degeneration are associated with defects in rod PDE6 stability (18). From our localization studies, it is evident that AIPL1 is expressed in mouse cone photoreceptors (Fig. 1A). However, the importance of AIPL1 in the function and maintenance of cones is not known. To decipher the need for AIPL1 in cone photoreceptor cells, our experimental design was to eliminate AIPL1 from cones in the presence of viable rod photoreceptors. This experimental approach will avoid any ‘bystander’ cone death as rod photoreceptor loss is prevented.
We created a transgenic mouse model that expresses AIPL1 exclusively in rods. The transgene, full-length human Aipl1, was placed under the control of a 2.5 kb Nrl promoter (19). This Nrl promoter has been shown to drive the expression of genes exclusively in rod photoreceptors (19). We used hAipl1 cDNA for transgenic expression because hAIPL1 protein has a unique C-terminal extension resulting in a larger protein (50 versus 37 kDa) allowing for unambiguous distinction of transgenically expressed human protein from native murine AIPL1 (20). The founders derived from injections of the Nrlp-hAipl1 construct were subsequently crossed into Aipl1−/− mice, so that AIPL1 expression in the retina would be controlled solely by the transgene and thus secluded to the rod photoreceptors.
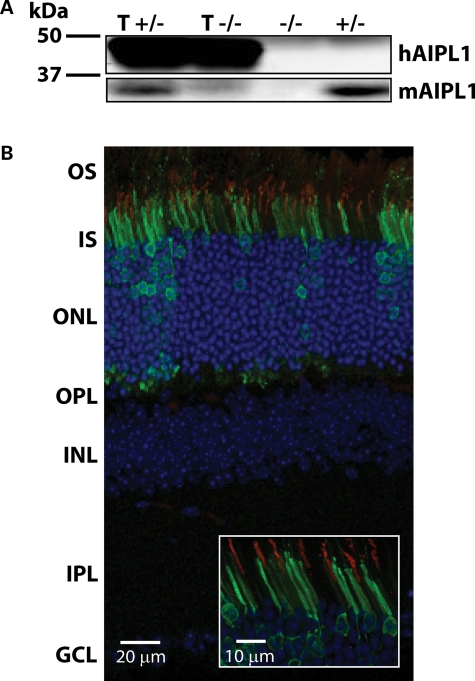
Immunoblotting using a polyclonal antibody (4365L) that recognizes both human and mouse AIPL1 reveals a robust expression of hAIPL1 at post-natal day 8 (Fig. 2A) (17). Immunolocalization of hAIPL1 in adult (P100) retinal tissue from transgenic mice (tg hAipl1; Aipl1+/− or T+/−) shows that hAIPL1 expression continues to adulthood and is highly compartmentalized (Fig. 2B). Similar to native murine AIPL1, expression of the transgene is localized mainly to the inner segment of the photoreceptors and in some photoreceptor nuclei (Fig. 2B) (17). To ensure that AIPL1 was being expressed only in rod photoreceptors, we verified the location of hAIPL1 relative to the α-subunit of cone transducin (Gtα) in adult transgenic mice expressing hAIPL1 (tg hAipl1; Aipl1+/−) (Fig. 2B). It is evident that AIPL1 is localized to the inner segments of cells that are not positive for cone transducin, thus illustrating the exclusion of hAIPL1 from cone photoreceptors (Fig. 2B, inset). Altogether, these results demonstrate the early expression of hAIPL1 exclusively in inner segments and nuclei of rod photoreceptor cells. Our results also prove that the Nrl promoter driven transgene expression is indeed rod-specific.
Figure 2.
The hAipl1 transgene is expressed exclusively in rod photoreceptor cells. (A) Western blot analysis with anti-hAIPL1 antibody indicates that hAIPL1 is expressed early at post-natal day 8 (P8). T+/−, transgenic hAipl1 in Aipl1+/− background; T−/−, transgenic hAipl1 in Aipl1−/− background; +/−, Aipl1+/−; −/−, Aipl1−/−. (B) Immunohistochemistry showing AIPL1 localization with an affinity-purified AIPL1 antibody conjugated with alexa 488 (green). Cone outer segments (OS) were identified using a cone marker, cone transducin α (Gtα, red). The segregation of AIPL1 from Gtα-positive cones indicates that hAIPL1 is expressed exclusively in rod photoreceptors. Section was taken from P100 tg hAipl1; Aipl1+/− mouse. OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Transgenic expression of hAIPL1 rescues rod morphology, restores rod function and stabilizes the rod PDE6 heteromer
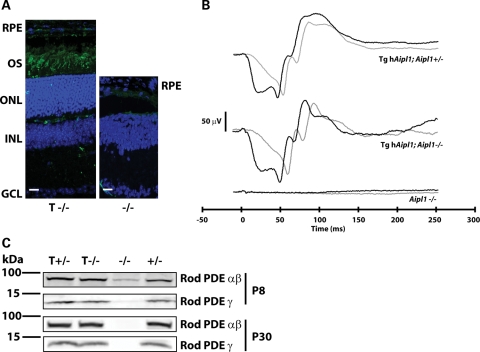
To test whether transgenic expression of hAIPL1 can prevent the rapid retinal degeneration associated with AIPL1 deficiency, we examined the morphology of retina from transgenic mice expressing hAIPL1 in an AIPL1 knockout background (tg hAipl1; Aipl1−/− or T−/−). In retina from adult mice expressing transgenic hAIPL1, we observe 8–10 layers of photoreceptor nuclei (Fig. 3A, left panel, blue). In comparison, no photoreceptor nuclei are observed in retina from adult mice lacking AIPL1 (Fig. 3A, right panel, blue). Immunolocalization of rod opsin, a marker for rod photoreceptors, demonstrates a robust rescue of rod photoreceptor cells and proper formation of photoreceptor outer segments (Fig. 3A, left panel, green).
Figure 3.
Transgene expression of human AIPL1 restores rod morphology and function. (A) Expression of hAIPL1 in rod photoreceptors abrogates the rod cell death observed in Aipl1−/− mice. Rod outer segments (OS) were labeled with anti-rod opsin antibody (4D2, green) and the outer nuclear layer (ONL) was visualized with DAPI (blue). In tg hAipl1; Aipl1−/−, the ONL was recovered to near normal thickness (8–10 nuclear layers) and rod opsin is clearly visible in the ROS (left panel). However, at the same age, degeneration of the ONL is complete and no rod opsin can be detected in the retina of Aipl1−/− mice (right panel). Sections are taken from P100 mice. RPE, retinal pigment epithelium; OS, outer segments; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bars, 20 µm. (B) Full-field dark-adapted ERG recording from P15 mice show complete recovery of rod light response. Tg hAipl1; Aipl1+/− mice exhibit robust rod-derived electrical responses that are reciprocated in tg hAipl1; Aipl1−/− mice. This is in contrast to Aipl1−/− mice which exhibit no light-driven rod photoreceptor response. Black lines represent a light intensity of 2.5 cd s m−2 and gray lines represent a lower light intensity of 0.16 cd s m−2. These recordings are an average of at least three different mice. Littermates were used to compare responses. (C) Recovery of rod PDE6 expression. Using MOE antibody (1:2000), rod PDE6 can be detected in Aipl1+/− (+/−), tg hAipl1; Aipl1+/− (T +/−) and tg hAipl1; Aipl1−/− (T −/−) mice as early as P8. However, rod PDE6 levels are reduced in Aipl1−/− (−/−) mice at P8 and undetectable at P30.
To investigate whether rod photoreceptors expressing hAIPL1 are functional, we performed dark-adapted ERGs on tg hAipl1; Aipl1−/− mice. As a control, we compared the ERG response of tg hAipl1; Aipl1−/− mice to Aipl1+/− mice expressing hAIPL1 in rods (tg hAipl1; Aipl+/− or T+/−), with normal ERG response (Fig. 3B). These mice express both murine and human forms of Aipl1 in rods and control for the presence of the transgene. We also used Aipl1−/− as a control for the absence of an ERG response. As expected, due to extensive photoreceptor degeneration, there is no recordable dark-adapted ERG response in mice lacking AIPL1 (Fig. 3B). However, in tg hAipl1; Aipl1−/− mice, dark-adapted ERGs resemble the normal responses (Fig. 3B). These recordings unequivocally demonstrate that transgenic expression of hAIPL1 rescues the rod ERG response (Fig. 3B).
Rod PDE6 instability is the main cause of defective phototransduction and consequent photoreceptor cell death in mice lacking AIPL1 (6,18). Therefore, we examined whether rod PDE6 expression is recovered in Aipl1−/− mice by expression of the Nrlp-hAipl1 transgene in rods. Immunoblots of retinal extracts probed with rod PDE6 antibody (MOE) show restoration of rod PDE6 levels in tg hAipl1; Aipl1−/− retina (Fig. 3C). Consistent with previous studies, PDE6 is absent in Aipl1−/− mice (P8) before the onset of retinal degeneration (Fig. 3C) (6).
Cone photoreceptors depend on AIPL1 for their proper function
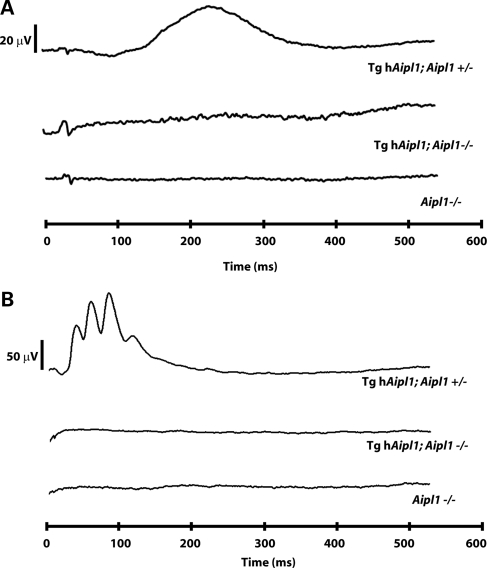
To determine whether cone photoreceptors are functional in the absence of AIPL1, we recorded photoreceptor electrical responses in the presence of a low (30 cd/m2) background light that saturates rods and isolates cone cell ERGs (21,22). Control (tg hAipl1; Aipl1+/−) mice showed a robust cone response from the earliest age (P15) at which we recorded ERGs (Fig. 4A) to adulthood (P30; Fig. 4B). However, mice lacking AIPL1 in cones (tg hAipl1; Aipl1−/−) do not respond to bright light at any age tested (Fig. 4A and B). This lack of cone ERG resembles the responses in Aipl1−/− mice (Fig. 4). In summary, our ERG recordings demonstrate that AIPL1 plays a direct role in cones and is needed for a proper light response from cone photoreceptor cells.
Figure 4.
AIPL1 is needed for the cone photoreceptor light response. Full-field light-adapted ERG recordings in P15 (A) and P30 (B) show a typical cone electrical response in tg hAipl1; Aipl1+/− mice. Tg hAipl1; Aipl1−/− mice have no light-derived cone electrical response, similar to Aipl1−/−. ERGs were recorded at 2.5 cd s m−2 in the presence of a 30 cd m−2 background light. These recordings are an average of at least three different mice. Littermates were used to compare responses.
Functional and viable rod photoreceptors slow cone cell death in the absence of AIPL1
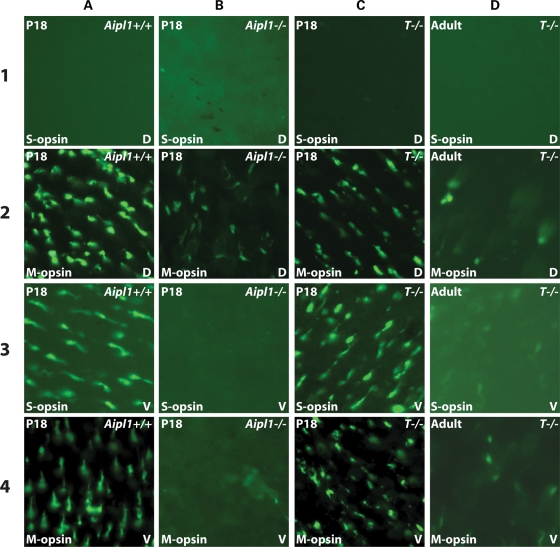
In the Aipl1−/− model, both rods and cones degenerate simultaneously (6). Though it is clear that AIPL1 is essential to the proper functioning of cone photoreceptors, it is not known whether degeneration of rod photoreceptor cells in the absence of AIPL1 contributes to the rapid death of cone cells. To answer this question, we investigated whether AIPL1-deficient cones degenerate in a rapid fashion when rod photoreceptors are protected from cell death in our transgenic mouse model. Whole mount staining of retina with antibodies against S- and M-opsin demonstrates a complete loss of cones in the ventral region of Aipl1−/− retina at P18 (Fig. 5B3 and B4). However, some cones containing M-opsin remain in the dorsal region, suggesting an uneven and more severe degeneration of cones in the ventral region of the retina in the absence of AIPL1. The persistence of M-opsin in the dorsal region of the retina has been observed in several models of retinitis pigmentosa, which exhibit bystander cone photoreceptor cell death (14). In contrast, S- and M-opsin containing cones in both the ventral and dorsal regions of tg hAipl1;Aipl1−/− retina are present at similar numbers as observed in wild-type (Fig. 5C1–C4 and A1–A4). However, these cones degenerate slowly and by P40 the cone photoreceptors are highly reduced in both the dorsal and ventral regions of the retina (Fig. 5D1–D4). Due to the uneven distribution of S-opsin, we do not observe any cones containing S-opsin in the dorsal region of the retina regardless of the mouse genotype (Fig. 5A–D1).
Figure 5.
Cone degeneration is slow in the presence of rescued rod photoreceptors. Whole-mounted retinas from P18 Aipl1+/+ (A), Aipl1−/− (B), tg hAipl1; Aipl1−/− (T−/−) (C) and adult (P40) tg hAipl1; Aipl1−/− (D) were labeled with anti-S- and M-opsin antibodies. Images were taken from the dorsal (D) and ventral (V) regions of the retina. Aipl1+/+ retina (A) shows typical distribution of S-opsin containing cones in the ventral retina (A3) and M-opsin containing cones throughout the dorsal and ventral retina (A2 and A4). The majority of cone photoreceptors degenerate by P18 in Aipl1−/− mice (B) except for a few remaining M-cones in the dorsal retina (B2). However, in tg hAipl1; Aipl1−/− retina, there was no obvious reduction in the number of cones and the cone distribution was similar to that of wild-type at P18 (C). These cones degenerate slowly and at P40 a drastic reduction in cone photoreceptor number was observed in tg hAipl1; Aipl1−/− retina (D). Images were taken at ×40 magnification.
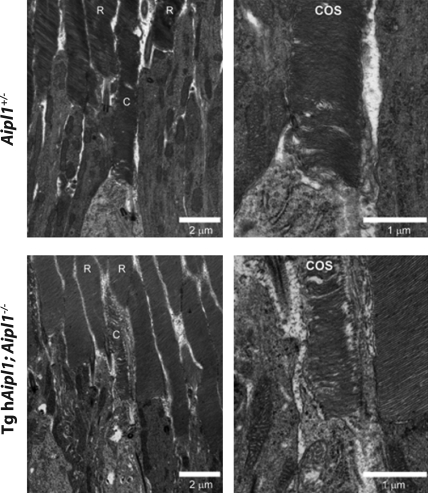
Ultrastructural analysis of photoreceptor cells by electron microscopy in retina lacking AIPL1 showed disheveled, shorter and highly irregular rod and cone outer segments (6). This dramatic disorganization of cone outer segments is likely due to rapidly degenerating rod and cone cells. Our transgenic animal model is an ideal system to examine the morphology of cone outer segments lacking AIPL1, independent of the influence from degenerating rod cells. High-resolution transmission electron microscopy of P18 Aipl1+/− mice demonstrates normal rod and cone outer segment ultrastructure with well-organized outer segment disk membranes present in each cell type (Fig. 6, upper panel). In mice lacking endogenous AIPL1, rod-specific expression of hAIPL1 protein completely rescues rod outer segment ultrastructure (R, Fig. 6, bottom panel). In contrast, the cone outer segments of P18 tg hAipl1; Aipl1−/− mice are short and thin, in comparison to the control, with disorganized disk membranes (Fig. 6, bottom panel). Altogether, these results demonstrate that in the presence of viable rod cells, surviving cone cells that lack AIPL1 possess disorganized outer segments, which may compromise their viability and contribute to their eventual degeneration.
Figure 6.
Cone outer segment morphology is partially disrupted in the absence of AIPL1. The ultrastructure of cone outer segments (COS) was evaluated by transmission electron microscopy of Aipl1+/− (control) and tg hAipl1; Aipl1−/− mouse retina thin sections. Outer segments of both rods (R) and cones (C) in Aipl1+/− control mice showed normal dimensions and organization. Although transgenic expression of hAIPL1 in rods lacking the endogenous protein (tg hAipl1; Aipl1−/−) effectively rescued rod outer segment ultrastructure, intact rods only partially rescued cone outer segment ultrastructure. The cone outer segments observed in tg hAipl1; Aipl1−/− retinas were generally shorter, thinner and less organized than those in Aipl1+/− controls. Right panels show higher magnification views.
Cone PDE6 is destabilized in the absence of AIPL1
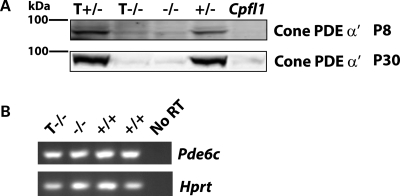
The cones of tg hAipl1; Aipl1−/− mice survive past the age at which Aipl1−/− mice exhibit photoreceptor degeneration, but are functionally quiescent (Figs 4 and 5). The loss of cone photoreceptor electrical responses suggests that there is a disruption of the phototransduction cascade in these cells. Considering the compelling involvement of AIPL1 in rod PDE6 complex formation, it was important to investigate the status of cone PDE6 in our transgenic model (6,18). Immunoblots of retinal extracts probed with cone PDE6 polyclonal antibody illustrate that cone PDE6 is highly (80%, n = 3) reduced in transgenic mice as early as P8 (Fig. 7A). The Cpfl1 mouse, with a mutation in the cone PDE6 gene, serves as a control, and exhibits a complete loss of cone PDE6 (Fig. 7A) (23,24).
Figure 7.
Cone PDE6 is destabilized in the absence of AIPL1. (A) Western blots probed with anti-cone PDE6 α′ indicate that protein levels are highly reduced at all ages, similar to Aipl1−/−. T+/−, transgenic hAipl1 in Aipl1+/− background; +/−, Aipl1+/−; −/−, Aipl1−/−. Cpfl1, a mouse with a mutation in cone PDE6 α′ subunit, serves as a negative control and exhibits no cone PDE6 immunoreactivity (Lane 5). (B) RT–PCR for Pdec6c illustrates that the message levels of Pde6c is not altered in the absence of AIPL1. Hprt (Hypoxanthine-guanine phosphoribosyltransferase), a housekeeping gene, functions as a control for reverse transcription efficiency.
Since defective post-translational processing affects the stability of rod PDE6 in the absence of AIPL1, we investigated whether cone PDE6 levels were affected similarly (18). RT–PCR analysis of retinal homogenates with cone PDE6-specific primers shows that cone PDE6 message levels are not altered due to the lack of AIPL1 (Fig. 7B). This result indicates that the reduction of cone PDE6 protein is due to a post-translational processing defect. This is further supported by the presence of a small amount of cone PDE6 protein in Aipl1−/− cones at P8, while there is no detectable cone PDE6 in Cpfl1 mice (Fig. 7A).
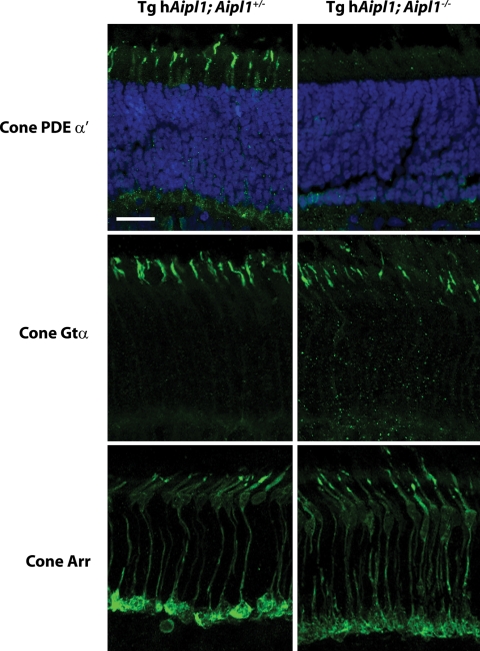
In agreement with our western blot results showing a drastic reduction in cone PDE6 levels, we were unable to detect cone PDE6 in our transgenic mouse model by immunolocalization (Fig. 8). In contrast, we were able to detect cone transducin α (Fig. 8), cone transducin γ (data not shown) and cone arrestin (Fig. 8) at seemingly normal levels in the absence of AIPL1. Our immunohistochemical studies show that the localization of these proteins was not altered due to the lack of AIPL1 in cones (Fig. 8). This result further supports our observations that cones are present at wild-type levels in tg hAipl1; Aipl1−/− mice (Fig. 5). In conclusion, both our western blot and immunohistochemistry results point to a crucial role for AIPL1 in the stability and function of cone PDE6 subunits.
Figure 8.
AIPL1 is necessary for the expression of cone PDE6. In tg hAipl1; Aipl1+/− mice, cone PDE6 α′ is properly localized to the cone outer segments. However, no detectable immunoreactivity for cone PDE6 was observed in the retinas of P18 tg hAipl1; Aipl1−/− mice. The expression pattern of cone Gtα and cone arrestin immunolabelling is similar between tg hAipl1; Aipl1+/− and tg hAipl1; Aipl1−/− mice at P18, further indicating that there is no appreciable reduction in cone number in tg hAipl1; Aipl1−/− mice at this age. Cone PDE6 and cone Gtα antibodies were used at 1:1000 dilution and cone arrestin antibody was used at 1:500 dilution. Scale bar, 20 µm.
DISCUSSION
In this study, using a novel transgenic mouse model that exclusively rescues rod function in a complete knockout of AIPL1, we demonstrate the need for AIPL1 in cone function beyond reasonable doubt. The lack of cone function in the absence of AIPL1 is due to a drastic destabilization of the cone PDE6 subunits. Interestingly, AIPL1-deficient cone photoreceptor cells degenerate at a slower rate when rod photoreceptors are rescued by the transgenic expression of hAIPL1 in comparison to a complete AIPL1 knockout model. Surviving cones in younger animals show structural defects, including outer segments that are thinner and shorter with disorganized disk membranes.
LCA is an inherited autosomal recessive retinal disease linked to mutations in several different genes that are involved in photoreceptor cell development, structure, protein transport and phototransduction (1,2). Despite the different roles these genes play in the photoreceptor cells, LCA ultimately results in dysfunction of both rods and cones. Interestingly, a carrier of a mutation in Aipl1 exhibited defects in the rod-dependent visual response, likely due to haploinsufficiency, but had a normal cone response (3). In agreement with this finding, some children with a mutant Aipl1 allele have been diagnosed with juvenile retinitis pigmentosa, a rod-specific disease (25). These observations that changes in Aipl1 preferentially result in rod dystrophies suggest that the crucial role for AIPL1 is in rod photoreceptor cells. This hypothesis is further supported by our immunolocalization studies showing that in comparison to rod photoreceptors, AIPL1 is present at reduced levels in adult human cone photoreceptors (Fig. 1A). In contrast to the reduced levels of AIPL1 in adult cone cells, robust expression of AIPL1 was observed in early cone photoreceptors at fetal week 11, before differentiation of rod photoreceptor cells (7,9). The reason behind the down-regulation of AIPL1 in adult human cone cells is not clear. Although these observations suggest a role for AIPL1 in early development of cone photoreceptor cells, our study does not support this hypothesis. In our transgenic mouse model, cones lacking AIPL1 develop normally (Fig. 5C1–C4) and late markers of cone photoreceptor development such as cone opsins (Fig. 5C1–C4) and arrestin (Fig. 8) are expressed normally. Furthermore, we observe no reduction in the number of cones at post-natal day 18 demonstrating that AIPL1 is not important for early cone photoreceptor development (Fig. 5C1–C4).
Despite normal development, cone photoreceptor cells degenerate in the absence of AIPL1 (Fig. 5). Surprisingly, the degeneration of cone cells is slower in the presence of functional rods in comparison to a complete knockout of AIPL1 where rods also degenerate (Supplementary Material, Fig. S1). By post-natal day 18 in Aipl1−/− mice that exhibit rod cell death, degeneration of cones was complete in the ventral retina (Fig. 5B3 and B4). However, in our transgenic mouse model with rescued rod cells, cone photoreceptors do not exhibit severe degeneration prior to post-natal day 40, but do show signs of outer segment shortening and disorganization (Figs 5C, D and 6). The reduced rate of cone photoreceptor degeneration suggests that the rescue of rod photoreceptors by transgenic expression of hAIPL1 supports cone viability by one of several possible mechanisms.
It has been demonstrated that rod cells produce a protein, rod-derived cone viability factor (RdCVF), which is essential for the survival of cone photoreceptors (13). It is possible that the presence of a trophic factor such as RdCVF may slow cone photoreceptor death. This is supported by a recent study showing that RdCVF can reduce the cone cell death observed in retinitis pigmentosa (26). By preserving the rod photoreceptors of AIPL1-deficient mice, cone cell survival may be enhanced by the expression of RdCVF or another unknown trophic factor.
It is also possible that the preservation of the majority of the photoreceptor cells in the retina helps stabilize the cones for a period of time. A recent study in zebrafish demonstrated that as cones die due to PDE6 deficiency, rod cells surrounding the cones are also lost (27). This suggests that cell density in the photoreceptor layer may aid in photoreceptor survival. In the case of our model, the preservation of the rod density, which comprises 97% of the photoreceptor cells, may protect the cones from degeneration through an unknown, density-related mechanism. One possible explanation of density-dependent degeneration is illustrated by a recent study in rd1/rd1 mice which proposes that the loss of rod photoreceptors in retinitis pigmentosa disrupts the passage of nutrients between the retinal pigment epithelium and cone photoreceptors resulting in cone malnutrition and the initiation of degeneration (14). Though this hypothesis does not explain the rapid and simultaneous death of rod and cone photoreceptors in the complete knockout of AIPL1, the preservation of rod photoreceptors in our transgenic mouse model should prevent the disruption in the connection between cone photoreceptor outer segments and the retinal pigment epithelium. Interestingly, we do not observe any evidence of rod cell death in the tissue surrounding cone photoreceptors in our transgenic model. This suggests that there is no ‘bystander’ rod cell degeneration influenced by cone photoreceptor cell death.
Even though cone cell survival is enhanced by the presence of functional rod photoreceptor cells, our analysis of tg hAipl1; Aipl1−/− mice shows that the cone photoreceptors are functionally quiescent at all ages tested (Fig. 4). The lack of electrical responses observed in ERG could be due to problems in cone photoreceptor cell development or due to functional deficits in phototransduction. Since AIPL1-deficiency does not cause a developmental defect in cones, the lack of functional cones in our transgenic model likely is the product of disrupted phototransduction. It is clear from our results that in the absence of AIPL1, cone PDE6, a protein essential for phototransduction, is severely reduced (Figs 7A and 8). This lack of PDE6 correlates with the functional deficits we observe in our transgenic mouse model and is similar to another mouse model with defects in cone PDE6, Cpfl1 (Fig. 3) (23,24). Based on a recent study that showed interaction of AIPL1 with the α- subunit of rod PDE6, we predict that AIPL1 interacts with the catalytic subunit of cone PDE6 (α′) and assists its folding and/or assembly (18). The presence of AIPL1 in the soma of adult human and mouse cone cells supports its role as a chaperone protein and strengthens our hypothesis regarding AIPL1's interaction with cone PDE6 (Fig. 1). However, further detailed studies are needed to decipher the need for AIPL1 to promote the stability of cone PDE6.
The loss of cone PDE6 most likely results in the perturbation of cGMP levels in the cone photoreceptors. In the well-characterized rod PDE6 β mutant rd1/rd1 mouse model, rod PDE6 γ knockout and in the complete knockout of AIPL1, the loss of rod PDE6 results in increased cGMP, which then is thought to alter the calcium levels and ultimately leads to rod photoreceptor cell death (6,28,29). Since the endogenous levels of cGMP and resulting calcium levels are higher in cones compared with rods photoreceptors, it is possible that cones are able to tolerate higher calcium levels better than rods, which is reflected in increased survival of cone photoreceptor cells (30–32). Nevertheless, the results from our tg hAipl1; Aipl1−/− mice suggest that rapid cone cell death is caused by the disruption of intrinsic and extrinsic factors essential to cone photoreceptor cells. Our model illustrates that cone cell death due to the absence of AIPL1 is inherently slow, but in Aipl1−/− mice cone photoreceptor death is accelerated by the death of rods. In humans, the cone-rich fovea should be immune to the influence of rod cell death on cone survival in the absence of AIPL1. Therefore, the quiescent and slow-degenerating cones observed in our transgenic model are likely analogous to the fovea of patients with changes in Aipl1. This idea is supported by results from an adult LCA patient with a mutation in AIPL1 who exhibited severe degeneration of the retina and all surviving cells were more cone-like photoreceptors (11). If the cone cells in the central retina of LCA patients can survive for a longer period of time, then the window in which gene therapy may be successful would be extended, allowing for the preservation of cone vision (33,34).
MATERIALS AND METHODS
Generation of transgenic mice
Full-length hAipl1 (1.2 kb) was amplified from a human retinal cDNA library (Dr Jeremy Nathans, Johns Hopkins University) and cloned under a 2.5 kb Nrl promoter (Dr Anand Swaroop, NEI). After linearizing the plasmid construct with restriction enzymes (Age I and Not I), the 3.6 kb insert containing Nrlp-hAipl1 was injected into fertilized C57BL/6 x SJL oocytes and implanted into pseudopregnant mice (University of Washington transgenic core facility). Founders were genotyped by PCR using primers against hAipl1 (5′-CGAGTGCCCTGGGGACGGGGG-3′) and Nrl promoter (5′-TGCCCGTCCTCCACGGAGAGGGA-3′). Four founders were bred into mice lacking Aipl1 (Aipl1−/−) to produce F1 progeny. These mice were then bred into Aipl1−/− mice to produce mice with the transgene in an Aipl1−/− background. Founders were also tested for the absence of the rd allele as described (35).
Immunoblotting
Retinas were homogenized in phosphate buffered saline (PBS) containing 1 mini protease inhibitor tablet (per 6 ml of PBS; Roche Biochemicals) and 10 mm dithiothreitol (DTT) using a pellet pestle (VWR) in a 1.5 ml microcentrifuge tube on ice. Homogenization was repeated for three pulses (15 s each). Samples were solubilized in 2X SDS-loading buffer (62.5 mm Tris–HCl, pH 6.8/2% SDS/10% glycerol/0.005% bromophenol blue/5% 2-mercaptoethanol) and boiled for 5 min, followed by the addition of 25 U benzonase (Pierce). Equal volumes (20 µl) of sample were separated by SDS–PAGE gel electrophoresis, transferred to an Immobilon-FL membrane (Millipore) and probed with antibodies. The primary antibodies for this analysis were used at 1:2000 dilution and include: AIPL1 polyclonal antibody (4365L), rod PDE6 antibody (MOE, Cytosignal) and cone PDE6 antibody (3184P, against amino acids 1–115 of mouse PDE6 α′). The secondary antibodies used in this study were: Odyssey goat anti-rabbit Alexa 680 and Odyssey goat anti-mouse Alexa 680 (LI-COR Biosciences) at 1:50 000. All antibodies were diluted in 1X PBST (1X PBS/0.1% Tween-20). Membranes were scanned using an Odyssey Infrared Imaging System (LI-COR Biosciences). All experiments described in this study were repeated at least three times with identical results.
Electroretinography
ERGs were performed as described (6). Dark-adapted mice were anesthetized with isoflurane (5% in 2.5% oxygen) and placed on a heating block at 37°C with a nose cone supplying isoflurane (1.5% in 2.5% oxygen) during recording. Eyes were dilated (1:1 Phenylephrine:Tropicamide) for 10 min prior to recording and lubricated with methylcellulose. A silver wire electrode rested on the methylcellulose above the cornea with a needle reference electrode inserted under the scalp, between the ears. Mice were placed into a Ganzfeld chamber and light flashes were delivered at varying intensities. Dark-adapted ERGs were performed after mice were kept in the dark for 24 h. Light-adapted ERGs were performed after mice were exposed to constant low level light (30 cd m−2) for 10 min and flashes were administered in the presence of this constant background lighting. ERGs were performed with a UTAS-E4000 Visual Electrodiagnostic Test System using EMWIN 8.1.1 software (LKC Technologies). Analysis was completed using the Grabdata program (Dr Machelle Pardue, Emory University) and Microsoft Excel.
Eyecup preparation and immunohistochemistry
Mouse eyes were enucleated, punctured with a fine needle and incubated for 10 min in 4% paraformaldehyde (Electron Microscopy Sciences) in 1X PBS at room temperature. Eyes were removed from the fixative and the cornea and lens were dissected away. Eyecups were replaced in the fixative for 4–6 h at room temperature, then cryoprotected in 1X PBS containing 20% sucrose overnight at 4°C. The eyecups were embedded in OCT (Tissue-Tek, Sakura) and stored at −80°C. Retinal sections were cut (20 µm thick) using a Leica CM1850 cryostat and were mounted on Superfrost Plus slides (Fisher Scientific).
For immunohistochemistry, slides were washed (three times for 10 min) in 1X PBST (1X PBS with 0.1% Triton X-100) and incubated for 1 h in blocking buffer (2% goat serum (Invitrogen), 0.1% Triton X-100 and 0.05% sodium azide in 1X PBS). Primary antibody was applied to sections and incubated overnight at 4°C. Slides were washed (three times for 10 min) in 1X PBST and incubated with secondary antibody (Odyssey Alexa Fluor-488, or Alexa Fluor-568, LI-COR Biosciences) diluted 1:2,000 for 1 h at room temperature. 4,6-Diamidino-2-phenylindole (DAPI, Invitrogen, 1:5,000 dilution) or propidium iodide (Invitrogen, 2 µg/ml working concentration) were used as a nuclear stain. Slides were mounted with Fluoromount-G (Southern Biotech) and coverslipped. Confocal imaging was performed at the WVU Microscope Imaging Facility with a Zeiss LSM 510 laser scanning confocal on a LSM AxioImager upright microscope using excitation wavelengths of 405, 488 and 543 nm. Primary antibodies were: AIPL1 polyclonal antibody (4365L), Gα transducin (GαT1) polyclonal antibody (Santa Cruz), rhodopsin monoclonal antibody (4D2, Dr Robert Molday, University of British Columbia), S-, and M-cone opsin polyclonal antibodies (Millipore), cone PDE6 polyclonal antibody (3184P), mouse cone arrestin polyclonal antibody (1:500, Dr Cheryl Craft, University of Southern California), cone transducin monoclonal antibody (GαT2, Santa Cruz). All primary antibodies were used at 1:1,000 dilution, unless noted otherwise. Rhodamine-conjugated PNA (Vector Laboratories) was used at 1:250 dilution for 1 h during secondary antibody incubation. All antibodies were diluted in antibody dilution buffer (0.05% goat serum, 0.1% Triton X-100 and 0.05% sodium azide in 1X PBS).
For localization of mAIPL1 in mouse photoreceptors, cryosections were heated to 95°C in 0.1 m Tris–HCl, pH 9.5 for 15 min for antigen retrieval. Sections were processed (as above) using anti-mAIPL1 antibody (1:1,000, Dr Tiansen Li, Harvard Medical School). For localization of AIPL1 in human photoreceptors, cryosections were ‘antigen-retrieved’ by heating (95°C) in 10 mm sodium citrate solution, pH 6, for 5 min. After blocking 1 h in 10% normal donkey serum, the sections were incubated overnight in primary antibodies [hs-AIPL1 diluted 1:10,000 and anti-human cone arrestin monoclonal antibody (7G6, Dr Peter R. MacLeish, Morehouse School of Medicine) diluted 1:1000 in PBST] at 4°C as described (36).
For localization of hAIPL1 in mouse photoreceptors, sections were labeled with AIPL1 polyclonal antibody conjugated to Dylight 488. The conjugated antibody was created using the Dylight 488 antibody labeling kit (Pierce) per manufacturer's instructions. Sections were incubated overnight at 4°C with AIPL1-488 (1:500 dilution) and washed (three times for 10 min) with 1X PBST. The other primary antibodies were applied following these washes utilizing the above-mentioned protocol.
Preparation and immunohistochemistry of whole mount retina
Whole mount tissue was processed as previously described (37). Briefly, whole eyes were enucleated and the dorsal side was marked by puncturing the cornea with a 25G 5/8 precision glide needle for orientation purposes. The whole eye was fixed in 4% paraformaldehyde in 1X PBS for 30 min. The eye was then removed from fixative and the cornea and lens were dissected away, being very careful to preserve the known orientation. Free-floating retinas were returned to 4% paraformaldehyde for 6 h.
For immunohistochemistry of the tissue, the retina was washed with 1X PBS (three times for 30 min each) and then incubated with blocking buffer [2% goat serum (Invitrogen), 0.1% Triton X-100 and 0.05% sodium azide in 1X PBS] for 4 h. After blocking, retinas were incubated for 12 h with primary antibodies (S- and M- opsin polyclonal antibodies, Millipore, diluted 1:500 in antibody dilution buffer). Whole retinal tissues were washed in 1X PBST (two times for 30 min each) and 1X PBS (one time for 30 min) before being incubated overnight in Odyssey goat anti-rabbit Alexa 488 (diluted 1:1000, LI-COR Biosciences). After secondary antibody incubation, retinas were further washed in 1X PBST (two times 30 min each) and 1X PBS (one time for 30 min). Before imaging, the retina was placed on a Superfrost Plus slide (Fisher Scientific) with the outer segments of the photoreceptors facing down. Radial cuts 2–3 mm in length were made to flatten the otherwise concave tissue. Finally, the whole retinal tissue was flat mounted, vitreal side up, on to the slide (lines of nail polish served as spacers to preserve the vertical structure of the outer retina) and were cover slipped for imaging. Images were acquired using a Leica DMIRB inverted fluorescence microscope at ×40 magnification.
Analysis of photoreceptor ultrastructure
Samples were prepared for evaluation by transmission electron microscopy (TEM) essentially as described (38). In brief, enucleated eyes were placed into freshly made fixative (2% paraformaldehyde, 2.5% glutaraldehyde, 100 mm cacodylate, pH 7.4) for 30 min. The cornea and lens were subsequently removed, and the eyecups were sectioned into six to eight pieces, which were fixed for at least 1 week. Fixed tissue was dehydrated in a graded ethanol series and then embedded into Polybed 812 (PolySciences, Inc.). Thin sections were collected onto nickel grids and stained with 2% uranyl acetate and lead citrate, and imaged in an FEI Morgagni transmission electron microscope at 80 kV. Cone outer segment ultrastructure was evaluated by inspection of at least six cones, in each of two animals, for each genotype. Criteria for positive identification of cones included clear continuity between nucleus, inner segment and outer segment.
RT–PCR
Mouse eyes were enucleated, frozen on dry ice in TRIzol reagent (Invitrogen) and total RNA was isolated as per manufacturer's instructions. Oligo(dT)-primed reverse transcription reactions were performed with 1 µg of total RNA by using SuperScript III (Invitrogen) to obtain cDNA, which was used as a template in PCR. Quick-Load PCR master mix (New England Biolabs) was used in all PCR reactions. Conditions for all of the reactions were 95°C for 1 min followed by 95°C for 45 s, 55°C for 45 s and 72°C for 45 s, for 30 cycles. Hprt, which was used as a control, was amplified by using primers 5′-CAAACTTTGCTTTCCCTGGT-3′ and 5′-CAAGGGCATATCCAACAACA-3′, and yielded a 250 bp product. Pde6c was amplified using primers 5′-GCAACTGACCTGGCACTCTATTTCA-3′ and 5′-GGCGCTGCCTCCTAAATCTGTGG-3′, and produced a 540 bp product. All experiments used tissues from littermates.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by National Institutes of Health [RO1EY017035 to V.R., RO1EY08123 to W.B., R01EY019298 to W.B., P30 EY014800-01 to W.B., P20 RR15574 COBRE to Center for Neuroscience, West Virginia University (WVU), S10RR017890 to A.F.X.G. and R01EY013246 to A.F.X.G.]; Lions Eye Bank (WVU), Research to Prevent Blindness (RPB) challenge grant (WVU) and a grant of the Foundation Fighting Blindness to W.B.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs Cheryl Craft (mouse cone arrestin), Tiansen Li (mAIPL1), Robert Molday (rod opsin/4D2) and Peter MacLeish (human cone arrestin/7G6) for their donations of antibodies. The Nrl promoter construct and Cpfl1 mice were generous gifts from Drs Anand Swaroop and Bo Chang, respectively. We also thank Drs Karen Martin and Ching-Kang Chen for their guidance with confocal microscopy and electroretinography, respectively. We thank Dr Machelle Pardue for the Grabdata software patch.
Conflict of Interest statement. None declared.
REFERENCES
- 1.den Hollander A.I., Roepman R., Koenekoop R.K., Cremers F.P. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Wang H., den Hollander A.I., Moayedi Y., Abulimiti A., Li Y., Collin R.W., Hoyng C.B., Lopez I., Bray M., Lewis R.A., et al. Mutations in SPATA7 cause Leber congenital amaurosis and juvenile retinitis pigmentosa. Am. J. Hum. Genet. 2009;84:380–387. doi: 10.1016/j.ajhg.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dharmaraj S., Leroy B.P., Sohocki M.M., Koenekoop R.K., Perrault I., Anwar K., Khaliq S., Devi R.S., Birch D.G., De Pool E., et al. The phenotype of Leber congenital amaurosis in patients with AIPL1 mutations. Arch. Ophthalmol. 2004;122:1029–1037. doi: 10.1001/archopht.122.7.1029. [DOI] [PubMed] [Google Scholar]
- 4.Sohocki M.M., Bowne S.J., Sullivan L.S., Blackshaw S., Cepko C.L., Payne A.M., Bhattacharya S.S., Khaliq S., Qasim Mehdi S., Birch D.G., et al. Mutations in a new photoreceptor-pineal gene on 17p cause Leber congenital amaurosis. Nat. Genet. 2000;24:79–83. doi: 10.1038/71732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyer M.A., Donovan S.L., Zhang J., Gray J., Ortiz A., Tenney R., Kong J., Allikmets R., Sohocki M.M. Retinal degeneration in Aipl1-deficient mice: a new genetic model of Leber congenital amaurosis. Brain Res. Mol. Brain Res. 2004;132:208–220. doi: 10.1016/j.molbrainres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Ramamurthy V., Niemi G.A., Reh T.A., Hurley J.B. Leber congenital amaurosis linked to AIPL1: a mouse model reveals destabilization of cGMP phosphodiesterase. Proc. Natl Acad. Sci. USA. 2004;101:13897–13902. doi: 10.1073/pnas.0404197101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrickson A., Bumsted-O'Brien K., Natoli R., Ramamurthy V., Possin D., Provis J. Rod photoreceptor differentiation in fetal and infant human retina. Exp. Eye Res. 2008;87:415–426. doi: 10.1016/j.exer.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Spuy J., Chapple J.P., Clark B.J., Luthert P.J., Sethi C.S., Cheetham M.E. The Leber congenital amaurosis gene product AIPL1 is localized exclusively in rod photoreceptors of the adult human retina. Hum. Mol. Genet. 2002;11:823–831. doi: 10.1093/hmg/11.7.823. [DOI] [PubMed] [Google Scholar]
- 9.van der Spuy J., Kim J.H., Yu Y.S., Szel A., Luthert P.J., Clark B.J., Cheetham M.E. The expression of the Leber congenital amaurosis protein AIPL1 coincides with rod and cone photoreceptor development. Invest. Ophthalmol. Vis. Sci. 2003;44:5396–5403. doi: 10.1167/iovs.03-0686. [DOI] [PubMed] [Google Scholar]
- 10.Liu X., Bulgakov O.V., Wen X.H., Woodruff M.L., Pawlyk B., Yang J., Fain G.L., Sandberg M.A., Makino C.L., Li T. AIPL1, the protein that is defective in Leber congenital amaurosis, is essential for the biosynthesis of retinal rod cGMP phosphodiesterase. Proc. Natl Acad. Sci. USA. 2004;101:13903–13908. doi: 10.1073/pnas.0405160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Spuy J., Munro P.M., Luthert P.J., Preising M.N., Bek T., Heegaard S., Cheetham M.E. Predominant rod photoreceptor degeneration in Leber congenital amaurosis. Mol. Vis. 2005;11:542–553. [PubMed] [Google Scholar]
- 12.Carter-Dawson L.D., LaVail M.M., Sidman R.L. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest. Ophthalmol. Vis. Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- 13.Leveillard T., Mohand-Said S., Lorentz O., Hicks D., Fintz A.C., Clerin E., Simonutti M., Forster V., Cavusoglu N., Chalmel F., et al. Identification and characterization of rod-derived cone viability factor. Nat. Genet. 2004;36:755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- 14.Punzo C., Kornacker K., Cepko C.L. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat. Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ripps H. Cell death in retinitis pigmentosa: gap junctions and the ‘bystander’ effect. Exp. Eye Res. 2002;74:327–336. doi: 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- 16.Strettoi E., Porciatti V., Falsini B., Pignatelli V., Rossi C. Morphological and functional abnormalities in the inner retina of the rd/rd mouse. J. Neurosci. 2002;22:5492–5504. doi: 10.1523/JNEUROSCI.22-13-05492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramamurthy V., Roberts M., van den Akker F., Niemi G., Reh T.A., Hurley J.B. AIPL1, a protein implicated in Leber's congenital amaurosis, interacts with and aids in processing of farnesylated proteins. Proc. Natl Acad. Sci. USA. 2003;100:12630–12635. doi: 10.1073/pnas.2134194100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolandaivelu S., Huang J., Hurley J.B., Ramamurthy V. AIPL1, a protein associated with childhood blindness, interacts with alpha-subunit of rod phosphodiesterase (PDE6) and is essential for its proper assembly. J. Biol. Chem. 2009;284:30853–30861. doi: 10.1074/jbc.M109.036780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akimoto M., Cheng H., Zhu D., Brzezinski J.A., Khanna R., Filippova E., Oh E.C., Jing Y., Linares J.L., Brooks M., et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc. Natl Acad. Sci. USA. 2006;103:3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohocki M.M., Sullivan L.S., Tirpak D.L., Daiger S.P. Comparative analysis of aryl-hydrocarbon receptor interacting protein-like 1 (Aipl1), a gene associated with inherited retinal disease in humans. Mamm Genome. 2001;12:566–568. doi: 10.1007/s003350020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmor M.F., Fulton A.B., Holder G.E., Miyake Y., Brigell M., Bach M. ISCEV Standard for full-field clinical electroretinography (2008 update) Doc. Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 22.Marmor M.F., Zrenner E. Standard for clinical electroretinography (1999 update). International Society for Clinical Electrophysiology of Vision. Doc. Ophthalmol. 1998;97:143–156. doi: 10.1023/a:1002016531591. [DOI] [PubMed] [Google Scholar]
- 23.Chang B., Grau T., Dangel S., Hurd R., Jurklies B., Sener E.C., Andreasson S., Dollfus H., Baumann B., Bolz S., et al. A homologous genetic basis of the murine cpfl1 mutant and human achromatopsia linked to mutations in the PDE6C gene. Proc. Natl Acad. Sci. USA. 2009;106:19581–19586. doi: 10.1073/pnas.0907720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang B., Hawes N.L., Hurd R.E., Davisson M.T., Nusinowitz S., Heckenlively J.R. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 25.Booij J.C., Florijn R.J., ten Brink J.B., Loves W., Meire F., van Schooneveld M.J., de Jong P.T., Bergen A.A. Identification of mutations in the AIPL1, CRB1, GUCY2D, RPE65 and RPGRIP1 genes in patients with juvenile retinitis pigmentosa. J. Med. Genet. 2005;42:e67. doi: 10.1136/jmg.2005.035121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y., Mohand-Said S., Danan A., Simonutti M., Fontaine V., Clerin E., Picaud S., Leveillard T., Sahel J.A. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol. Ther. 2009;17:787–795. doi: 10.1038/mt.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stearns G., Evangelista M., Fadool J.M., Brockerhoff S.E. A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish. J. Neurosci. 2007;27:13866–13874. doi: 10.1523/JNEUROSCI.3136-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsang S.H., Gouras P., Yamashita C.K., Kjeldbye H., Fisher J., Farber D.B., Goff S.P. Retinal degeneration in mice lacking the gamma subunit of the rod cGMP phosphodiesterase. Science. 1996;272:1026–1029. doi: 10.1126/science.272.5264.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farber D.B. From mice to men: the cyclic GMP phosphodiesterase gene in vision and disease. The Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 1995;36:263–275. [PubMed] [Google Scholar]
- 30.Krizaj D., Copenhagen D.R. Calcium regulation in photoreceptors. Front Biosci. 2002;7:d2023–d2044. doi: 10.2741/a896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng Z., Choi S.Y., Dharia A., Li J., Sterling P., Kramer R.H. Synaptic Ca2+ in darkness is lower in rods than cones, causing slower tonic release of vesicles. J. Neurosci. 2007;27:5033–5042. doi: 10.1523/JNEUROSCI.5386-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takemoto N., Tachibanaki S., Kawamura S. High cGMP synthetic activity in carp cones. Proc. Natl Acad. Sci. USA. 2009;106:11788–11793. doi: 10.1073/pnas.0812781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X., Pawlyk B., Xu X., Liu X., Bulgakov O.V., Adamian M., Sandberg M.A., Khani S.C., Tan M.H., Smith A.J., et al. Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther. 2009 doi: 10.1038/gt.2009.104. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan M.H., Smith A.J., Pawlyk B., Xu X., Liu X., Bainbridge J.B., Basche M., McIntosh J., Tran H.V., Nathwani A., et al. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: effective rescue of mouse models of partial and complete Aipl1 deficiency using AAV2/2 and AAV2/8 vectors. Hum. Mol. Genet. 2009;18:2099–2114. doi: 10.1093/hmg/ddp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gimenez E., Montoliu L. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (Pdeb(rd1)) in FVB/N-derived transgenic mice. Lab Anim. 2001;35:153–156. doi: 10.1258/0023677011911525. [DOI] [PubMed] [Google Scholar]
- 36.Bhosale P., Li B., Sharifzadeh M., Gellermann W., Frederick J.M., Tsuchida K., Bernstein P.S. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry. 2009;48:4798–4807. doi: 10.1021/bi9004478. [DOI] [PubMed] [Google Scholar]
- 37.Lin B., Masland R.H., Strettoi E. Remodeling of cone photoreceptor cells after rod degeneration in rd mice. Exp. Eye Res. 2009;88:589–599. doi: 10.1016/j.exer.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg A.F., Ritter L.M., Khattree N., Peachey N.S., Fariss R.N., Dang L., Yu M., Bottrell A.R. An intramembrane glutamic acid governs peripherin/rds function for photoreceptor disk morphogenesis. Invest. Ophthalmol. Vis. Sci. 2007;48:2975–2986. doi: 10.1167/iovs.07-0049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.