Abstract
Understanding how a small brain region, the suprachiasmatic nucleus (SCN), can synchronize the body's circadian rhythms is an ongoing research area. This important time-keeping system requires a complex suite of peptide hormones and transmitters that remain incompletely characterized. Here, capillary liquid chromatography and FTMS have been coupled with tailored software for the analysis of endogenous peptides present in the SCN of the rat brain. After ex vivo processing of brain slices, peptide extraction, identification, and characterization from tandem FTMS data with <5-ppm mass accuracy produced a hyperconfident list of 102 endogenous peptides, including 33 previously unidentified peptides, and 12 peptides that were post-translationally modified with amidation, phosphorylation, pyroglutamylation, or acetylation. This characterization of endogenous peptides from the SCN will aid in understanding the molecular mechanisms that mediate rhythmic behaviors in mammals.
Central nervous system neuropeptides function in cell-to-cell signaling and are involved in many physiological processes such as circadian rhythms, pain, hunger, feeding, and body weight regulation (1–4). Neuropeptides are produced from larger protein precursors by the selective action of endopeptidases, which cleave at mono- or dibasic sites and then remove the C-terminal basic residues (1, 2). Some neuropeptides undergo functionally important post-translational modifications (PTMs),1 including amidation, phosphorylation, pyroglutamylation, or acetylation. These aspects of peptide synthesis impact the properties of neuropeptides, further expanding their diverse physiological implications. Therefore, unveiling new peptides and unreported peptide properties is critical to advancing our understanding of nervous system function.
Historically, the analysis of neuropeptides was performed by Edman degradation in which the N-terminal amino acid is sequentially removed. However, analysis by this method is slow and does not allow for sequencing of the peptides containing N-terminal PTMs (5). Immunological techniques, such as radioimmunoassay and immunohistochemistry, are used for measuring relative peptide levels and spatial localization, but these methods only detect peptide sequences with known structure (6). More direct, high throughput methods of analyzing brain regions can be used.
Mass spectrometry, a rapid and sensitive method that has been used for the analysis of complex biological samples, can detect and identify the precise forms of neuropeptides without prior knowledge of peptide identity, with these approaches making up the field of peptidomics (7–12). The direct tissue and single neuron analysis by MALDI MS has enabled the discovery of hundreds of neuropeptides in the last decade, and the neuronal homogenate analysis by fractionation and subsequent ESI or MALDI MS has yielded an equivalent number of new brain peptides (5). Several recent peptidome studies, including the work by Dowell et al. (10), have used the specificity of FTMS for peptide discovery (10, 13–15). Here, we combine the ability to fragment ions at ultrahigh mass accuracy (16) with a software pipeline designed for neuropeptide discovery. We use nanocapillary reversed-phase LC coupled to 12 Tesla FTMS for the analysis of peptides present in the suprachiasmatic nucleus (SCN) of rat brain.
A relatively small, paired brain nucleus located at the base of the hypothalamus directly above the optic chiasm, the SCN contains a biological clock that generates circadian rhythms in behaviors and homeostatic functions (17, 18). The SCN comprises ∼10,000 cellular clocks that are integrated as a tissue level clock which, in turn, orchestrates circadian rhythms throughout the brain and body. It is sensitive to incoming signals from the light-sensing retina and other brain regions, which cause temporal adjustments that align the SCN appropriately with changes in environmental or behavioral state. Previous physiological studies have implicated peptides as critical synchronizers of normal SCN function as well as mediators of SCN inputs, internal signal processing, and outputs; however, only a small number of peptides have been identified and explored in the SCN, leaving unresolved many circadian mechanisms that may involve peptide function.
Most peptide expression in the SCN has only been studied through indirect antibody-based techniques (19–29), although we recently used MS approaches to characterize several peptides detected in SCN releasates (30). Previous studies indicate that the SCN expresses a rich diversity of peptides relative to other brain regions studied with the same techniques. Previously used immunohistochemical approaches are not only inadequate for comprehensively evaluating PTMs and alternate isoforms of known peptides but are also incapable of exhaustively examining the full peptide complement of this complex biological network of peptidergic inputs and intrinsic components. A comprehensive study of SCN peptidomics is required that utilizes high resolution strategies for directly analyzing the peptide content of the neuronal networks comprising the SCN.
In our study, the SCN was obtained from ex vivo coronal brain slices via tissue punch and subjected to multistage peptide extraction. The SCN tissue extract was analyzed by FTMS/MS, and the high resolution MS and MS/MS data were processed using ProSightPC 2.0 (16), which allows the identification and characterization of peptides or proteins from high mass accuracy MS/MS data. In addition, the Sequence Gazer included in ProSightPC was used for manually determining PTMs (31, 32). As a result, a total of 102 endogenous peptides were identified, including 33 that were previously unidentified, and 12 PTMs (including amidation, phosphorylation, pyroglutamylation, and acetylation) were found. The present study is the first comprehensive peptidomics study for identifying peptides present within the mammalian SCN. In fact, this is one of the first peptidome studies to work with discrete brain nuclei as opposed to larger brain structures and follows up on our recent report using LC-ion trap for analysis of the peptides in the supraoptic nucleus (33); here, the use of FTMS allows a greater range of PTMs to be confirmed and allows higher confidence in the peptide assignments. This information on the peptides in the SCN will serve as a basis to more exhaustively explore the extent that previously unreported SCN neuropeptides may function in SCN regulation of mammalian circadian physiology.
EXPERIMENTAL PROCEDURES
Materials
All reagents were obtained from Sigma-Aldrich unless otherwise noted. Siliconized microcentrifuge tubes (1.5 ml) were purchased from Thermo Fisher Scientific (San Jose, CA). Microcon YM-10 centrifugal filter devices were purchased from Millipore (Billerica, MA).
Animals and Circadian Time
An inbred strain of 8–10-week-old female Long-Evans rats, LE-BluGill, demonstrated to be genetically homogeneous by high density genome scan (34) was used for these studies. Animals were fed ad libitum and were housed under constant temperature and humidity conditions in a 12:12 h light/dark cycle environment. Animals were entrained to this lighting schedule for at least 10 days prior to tissue collection. All collections of ex vivo SCN tissue samples were conducted during mid-subjective daytime ∼6–7 h following onset of normal lights-on conditions, referred to as Zeitgeber time (ZT) 6–7. All vertebrate animal procedures were carried out with protocols approved by the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee and in full compliance with National Institutes of Health guidelines for humane animal care.
Preparation of SCN Brain Punch Samples
Animal subjects were decapitated, and the brain was immediately removed from the skull. The hypothalamus was blocked, and using a mechanical tissue chopper, coronal brain sections (500-μm thickness) were prepared. A brain section containing the mid-SCN was retained. A 2-mm-diameter sample corer was used to excise the paired SCN from the surrounding hypothalamus, aligning the top edge of the corer with the dorsal SCN border (see supplemental Fig. S1). This punch technique results in minimal harvest of extra-SCN hypothalamic tissue. Optic nerve tissue at the level of the optic chiasm is contained within the SCN-containing punch. Peptidome analysis of rat optic nerve tissue produces a peptidomic profile distinct from our SCN peptidomics data (unpublished data). The SCN punch preparation was performed in glucose-/bicarbonate-/gentamicin-supplemented Earle's balanced salt solution (Invitrogen) perfused with 95% O2, 5% CO2. SCN-containing punches were immediately transferred to a siliconized microcentrifuge tube that remained submerged in powered dry ice until the time of peptide extraction.
Peptide Extraction from SCN Punches
Either 12 or 24 SCN punches were pooled and subjected to multistage peptide extraction as described in the recent work by Bora et al. (33). First, 150 or 300 μl of deionized water, preheated to 90 °C, was added to the SCN punches. The sample was boiled for 10 min and centrifuged at 14,000 × g for 10 min. The resulting tissue pellet was subjected to the second stage of extraction, whereas the supernatant was retained in a new microcentrifuge tube. After addition of 150 or 300 μl of ice-cold acidified acetone (40:6:1 acetone/water/HCl), the sample was homogenized with ultrasonic cleaner FS30 (Thermo Fisher Scientific) for 30 s, vortexed for 1 min, and kept on ice for 1 h. The sample was vortexed again for 1 min and centrifuged at 14,000 × g for 20 min at 4 °C, and the supernatant was saved. Then, a third extraction was performed by adding 150 or 300 μl of ice-cold 0.25% acetic acid to the tissue pellet and incubating on ice for 1 h. The acidified acetone extract was neutralized by 1 m NaOH and dried to 10–20 μl to remove the acetone. All of the extracts were combined and filtered through a Microcon centrifugal filter device (10-kDa-molecular mass cutoff). Finally, the filtered extract was concentrated using a SpeedVac and used for nanocapillary FTMS/MS injection.
Mass Spectral Analysis (LC-FTMS/MS)
The extracted peptides from the SCN punches were analyzed using a 12 Tesla LTQ-FT Ultra (Thermo Fisher Scientific) interfaced with a 1D NanoLC pump from Eksigent Technologies (Dublin, CA). The sample was loaded with helium bomb pressure (500 p.s.i.) to a trap column (75-μm inner diameter), 5 cm of which was fritted with LiChrosorb (EM Separations, Gibbstown, NJ) and packed with a C18 solid phase (10 μm; YMC Co., Ltd., Allentown, PA). The analytical column used ProteoPep II medium (C18, 300 Å, 5 μm) and was purchased from New Objective (Woburn, MA). The operating flow rate was 300 nl/min with the following gradient conditions: 0–20 min, 0–15% B; 20–90 min, 15–35% B; 90–180 min, 35–60% B; 180–220 min, 60–80% B; 220–240 min, 80–100% B; 240–250 min, 100–0% B; and 250–260 min, 0–5% B. Data acquisition on the LTQ-FTMS instrument consisted of a full scan event (290–2000 m/z; resolving power, m/Δm50% = 90,000 in which Δm50% is the mass spectral peak full width at half-maximum peak height) and data-dependent CID MS/MS scans (40,000 resolving power) of the five most abundant peaks from the previous full scan. MS/MS settings were as follows: isolation width, m/z 5; minimum signal threshold, 1000 counts; normalized collision energy, 35%; activation Q, 0.4; and activation time, 50 ms. Dynamic exclusion was enabled with a repeat count of 4, an exclusion duration of 180 s, and a repeat duration of 30 s.
Data Analysis
Resulting LC-FTMS/MS files (*.raw) were analyzed using ProSightPC 2.0 (Thermo Fisher Scientific) (16), which has several software component algorithms including cRAWler 2.0, which interprets resolved isotopic distributions based on the Xtract or thorough high resolution analysis of spectra by Horn (THRASH) algorithms. The cRAWler program first determines all precursor mass values according to user-specified tolerances such as ranges of m/z and retention time or signal-to-noise ratio and fitting parameters. The precursor and fragmentation scans corresponding to these precursors are then separately averaged and interpreted to provide a list of monoisotopic masses. This information is compiled into a ProSight upload file (.puf). In multiplexing mode, cRAWler can capture multiple precursor masses within the isolation range as multiple precursors based on an intensity cutoff (set at 10% here) relative to the base peak of the analysis window. This allows for cases where multiple precursors are fragmented together (see below).
Database Searching
Each .puf file, which typically contained hundreds of experiments from a single nano-LC-MS/MS run, was first searched in absolute mass mode (MS1 and MS2 tolerances of ±10 ppm) against a database of predicted rat neuropeptides (with and without predicted modifications) generated by taking the set of known rat prohormones processed in silico via the NeuroPred algorithm (35–37). For the searches that did not identify a peptide below an E-value cutoff of 10−4, a search in “neuropeptide” mode was initiated against an intact rat database (UniProt 15.0, 4,318,021 protein forms) with ±100-Da intact mass and ±10-ppm fragment tolerance. Neuropeptide mode scans across sequences to find candidate subsequences whose masses are within tolerance of a precursor mass (no protease specificity); experimental fragment masses are then matched with theoretical fragment masses from these candidate subsequences. Neuropeptide searches along with the other mode described in this work are available through neuroProSight over the internet. A Sequence Gazer tool in neuroProSight software was used for manually determining PTMs on the peptides. The peptides identified from multiplexing mode were manually validated.
RESULTS
Two-millimeter-diameter punches of ventral hypothalamic tissue (500-μm thickness) containing the bilaterally paired SCN were excised from rat coronal brain slices. At least six SCN punches, which contained ∼360 μg of total protein amount based on BCA assay, were needed for a high content nanocapillary FTMS/MS run. From a total of 10 LC-MS/MS runs for the SCN peptidome analysis, 102 endogenous peptides derived from 27 precursor proteins were identified along with 12 PTMs (amidation, phosphorylation, pyroglutamylation, and acetylation) (see Table 1). The average E-value for identification was 4 × 10−21, 17 orders of magnitude below the conservative threshold of 10−4 used here. This remarkable certainty of identification arises from the use of fragmentation scans with high mass accuracy and a scoring/software system that converts these data into peptide identifications with high fidelity. Thirty-three peptides (Table I, denoted with Footnote c) were not previously identified in either mouse or rat brain studies. The references for the identified peptides found in the prior studies of brain as well as SCN are included in a column of Table I. For example, the peptides derived from the prohormones gastrin-releasing peptide (GRP) and vasoactive intestinal peptide (VIP) are intrinsic SCN peptides that have received considerable attention (17, 22, 23, 25, 28, 38–55). Surprisingly, peptides from 12 precursor proteins found in our SCN peptidome study, including cocaine- and amphetamine-regulated transcript protein (CART), cerebellin-1, and proenkephalin B, were not reported in prior SCN studies. Finally, information from mRNA expression data from the mouse SCN reported in the Allen Brain Atlas (56) is included in Table I and highlights localization of prohormone synthesis for the prohormones identified from our present study. In addition to the endogenous peptides derived from prohormones, 66 peptide fragments from proteins like hemoglobin subunit β-1 and myelin basic protein S were also identified (supplemental Table 1). Although peptides that are protein fragments could result from post-mortem degradation during sample preparation, they may be the products of prohormone processing that are physiologically relevant. For example, small peptides formed from hemoglobin, the hemopressins, have known bioactivity and are likely enzymatically produced and are not formed during post-mortem degradation (57–59).
Table I. Peptides identified from SCN peptidome analysis.
PTMs are shown in bold. NPY, neuropeptide Y; MCH, melanin-concentrating hormone; POMC, proopiomelanocortin.
| Precursor | Peptide name | Sequence | Observed mass | Mass difference | E-valuea | UniProt accession number | Refs. of brain studies | Refs. of SCN studiesb | Allen Brain Atlas mRNA expression data |
|---|---|---|---|---|---|---|---|---|---|
| Da | ppm | ||||||||
| CART (AA 37–55) | ALDIYSAVDDASHEKELPR | 2128.05 | 4.1 | 4 × 10−18 | P49192 | 12 | mouse.brain-map.org/brain/gene/72077479.html?ispopup=1 | ||
| CART (AA 60–77) | APGAVLQIEALQEVLKKL | 1919.15 | 3.6 | 6 × 10−6 | P49192 | 102 | |||
| CART (AA 60–79) | APGAVLQIEALQEVLKKLKS | 2134.28 | 3.4 | 6 × 10−29 | P49192 | 102 | |||
| Cerebellin-1 (AA 57–71) | SGSAKVAFSAIRSTN | 1494.78 | 0.6 | 1 × 10−10 | P63182 | 8 | |||
| Cerebellin-1 (AA 57–72) | Cerebellin | SGSAKVAFSAIRSTNH | 1631.84 | 2.3 | 7 × 10−17 | P63182 | 8 | ||
| GRP (AA 24–41) | APVSTGAGGGTVLAKMYP | 1675.87 | 4.6 | 6 × 10−7 | P24393 | SwePep | 17, 22, 23, 25, 38–47 | mouse.brain-map.org/brain/gene/1363.html?ispopup=1 | |
| Pro-MCH (AA 32–55)c | NVEDDIVFNTFRMGKAFQKEDTAE | 2803.32 | −1.3 | 4 × 10−10 | P14200 | 103 | |||
| Pro-MCH (AA 131–143) | Neuropeptide-glutamic acid-isoleucine | EIGDEENSAKFPI(amidation) | 1446.70 | 2.8 | 5 × 10−35 | P14200 | 63 | ||
| Neuropeptide Y (AA 69–97)c | SSPETLISDLLMRESTENAPRTRLEDPSM | 3274.59 | 6.9 | 7 × 10−7 | P07808 | 77–86, 88–91 | mouse.brain-map.org/brain/gene/717.html?ispopup=1 | ||
| Neuropeptide Y (AA 69–98) | C-flanking peptide of NPY (CPON) | SSPETLISDLLMRESTENAPRTRLEDPSMW | 3460.67 | 8.2 | 3 × 10−13 | P07808 | 8 | ||
| Neurosecretory protein VGF (AA 24–60) | APPGRSDVYPPPLGSEHNGQVAEDAVSRPKDDSVPEV | 3867.88 | 4.5 | 3 × 10−21 | P20156 | 104 | 24, 105–108 | mouse.brain-map.org/brain/gene/71924165.html?ispopup=1 | |
| Neurosecretory protein VGF (AA 24–63) | APPGRSDVYPPPLGSEHNGQVAEDAVSRPKDDSVPEVRAA | 4166.06 | 3.5 | 1 × 10−13 | P20156 | 104 | |||
| Neurosecretory protein VGF (AA 238–282)c | MSENVPLPETHQFGEGVSSPKTHLGETLTPLSKAYQSLSAPFPKV | 4835.45 | 7.9 | 2 × 10−15 | P20156 | ||||
| Neurosecretory protein VGF (AA 285–309) | LEGSFLGGSEAGERLLQQGLAQVEA(amidation) | 2557.32 | 1.0 | 1 × 10−21 | P20156 | 109 | |||
| Neurosecretory protein VGF (AA 487–507) | KKNAPPEPVPPPRAAPAPTHV | 2170.21 | 3.6 | 3 × 10−3 | P20156 | 102 | |||
| Neurosecretory protein VGF (AA 489–507) | NAPPEPVPPPRAAPAPTHV | 1914.02 | 3.0 | 7 × 10−18 | P20156 | 102 | |||
| Neurosecretory protein VGF (AA 601–617) | EQEELENYIEHVLLHRP | 2147.08 | 5.6 | 6 × 10−6 | P20156 | 104 | |||
| Neuroendocrine protein 7B2 (AA 25–49)c | YSPRTP DRVSETDIQR LLHGVMEQL | 2939.51 | 5.3 | 8 × 10−10 | P27682 | ||||
| PACAP (AA 111–128) | GMGENLAAAAVDDRAPLT | 1770.87 | 5.5 | 2 × 10−27 | P13589 | 10, 63 | 64–66, 68–76, 110 | mouse.brain-map.org/brain/gene/74511882.html?ispopup=1 | |
| POMC (AA 124–136) | Melanotropin α | SYSMEHFRWGKPV(amidation) | 1621.79 | 3.0 | 2 × 10−27 | Q8K422 | 8, 30, 61, 111 | 17, 112, 113 | mouse.brain-map.org/brain/gene/80517122.html?ispopup=1 |
| POMC (AA 141–158) | RPVKVYPNVAENESAEAF | 2505.26 | 4.6 | 3 × 10−7 | Q8K422 | 8, 61 | |||
| POMC (AA 165–202) | Lipotropin gamma | ELEGEQPDGLEHVLEPDTEKADGPYRVEHFRWGNPPKD | 4385.09 | 6.4 | 9 × 10−10 | Q8K422 | 12, 61 | ||
| POMC (AA 205–235) | Beta-endorphin | YGGFMTSEKSQTPLVTLFKNAIIKNAHKKGQ | 3435.85 | 4.4 | 6 × 10−7 | Q8K422 | 102 | ||
| Proenkephalin A (AA 114–133) | MDELYPVEPEEEANGGEILA | 2204.00 | 5.4 | 5 × 10−8 | P04094 | 33 | 114, 115 | mouse.brain-map.org/brain/gene/74881286.html?ispopup=1 | |
| Proenkephalin A (AA 143–185) | DADEGDTLANSSDLLKELLGTGDNRAKDSHQQESTNNDEDSTS | 4592.04 | −2.8 | 2 × 10−34 | P04094 | 116 | |||
| Proenkephalin A (AA 188–195) | Met-enkephalin-Arg-Gly-Leu | YGGFMRGL | 899.44 | 3.5 | 4 × 10−18 | P04094 | 63 | ||
| Proenkephalin A (AA 198–209) | SPQLEDEAKELQ | 1385.67 | 2.0 | 6 × 10−31 | P04094 | 12, 63 | |||
| Proenkephalin A (AA 219–229) | VGRPEWWMDYQ | 1465.65 | 2.6 | 6 × 10−15 | P04094 | 12, 33, 63 | |||
| Proenkephalin A (AA 239–259)c | FAESLPSDEEGE SYSKEVPEM | 2359.02 | 7.1 | 2 × 10−3 | P04094 | ||||
| Proenkephalin A (AA 263–269) | Met-enkephalin-Arg-Phe | YGGFMRF | 876.40 | 3.4 | 5 × 10−22 | P04094 | 63 | ||
| Proenkephalin B (AA 235–248) | SQENPNTYSEDLDV | 1609.68 | 4.2 | 1 × 10−16 | P06300 | 33 | |||
| Protachykinin 1 (AA 58–68) | Substance P | RPKPQQFFGLM(amidation) | 1346.73 | 3.5 | 9 × 10−19 | P06767 | 30, 63 | 29, 117–123 | mouse.brain-map.org/brain/gene/1038.html?ispopup=1 |
| Protachykinin 1 (AA 72–94) | DADSSIEKQVALLKALYGHGQIS | 2442.29 | 4.8 | 4 × 10−9 | P06767 | 11 | |||
| Da | ppm | ||||||||
| Pro-SAAS (AA 34–40) | KEP | ARPVKEP | 795.46 | −0.6 | 1 × 10−2 | Q9QXU9 | 124 | 30 | mouse.brain-map.org/brain/gene/777.html?ispopup=1 |
| Pro-SAAS (AA 34–59) | Big SAAS | ARPVKEPRSLSAASAPLAETSTPLRL | 2448.34 | 3.5 | 4 × 10−32 | Q9QXU9 | 8 | ||
| Pro-SAAS (AA 42–57) | SLSAASAPLAETSTPL | 1514.79 | 3.3 | 5 × 10−27 | Q9QXU9 | 33 | |||
| Pro-SAAS (AA 42–59) | Little SAAS | SLSAASAPLAETSTPLRL | 1783.98 | 4.0 | 4 × 10−41 | Q9QXU9 | 30, 33 | ||
| Pro-SAAS (AA 44–57) | SAASAPLAETSTPL | 1314.67 | 1.2 | 4 × 10−13 | Q9QXU9 | SwePep | |||
| Pro-SAAS (AA 44–59) | SAASAPLAETSTPLRL | 1583.86 | 4.4 | 2 × 10−28 | Q9QXU9 | 33 | |||
| Pro-SAAS (AA 48–59) | APLAETSTPLRL | 1267.72 | 3.5 | 4 × 10−32 | Q9QXU9 | 10 | |||
| Pro-SAAS (AA 62–75) | AVPRGEAAGAVQEL | 1366.72 | 4.3 | 8 × 10−27 | Q9QXU9 | 10, 33 | |||
| Pro-SAAS (AA 62–89) | AVPRGEAAGAVQELARALAHLLEAERQE | 2954.57 | 2.1 | 8 × 10−50 | Q9QXU9 | 8 | |||
| Pro-SAAS (AA 62–120)c | AVPRGEAAGAVQELARALAHLLEAERQERARAEAQEAEDQQARVLAQLLRAWGSPRASD | 6385.36 | 5.1 | 1 × 10−14 | Q9QXU9 | ||||
| Pro-SAAS (AA 62–143)c | AVPRGEAAGAVQELARALAHLLEAERQERARAEAQEAEDQQARVLAQLLRAWGSPRASDPPLAPDDDPDAPAAQLARALLRA | 8720.54 | −2.2 | 1 × 10−23 | Q9QXU9 | ||||
| Pro-SAAS (AA 113–143)c | WGSPRASDPPLAPDDDPDAPAAQLARALLRA | 3209.62 | −1.0 | 6 × 10−9 | Q9QXU9 | ||||
| Pro-SAAS (AA 121–143)c | PPLAPDDDPDAPAAQLARALLRA | 2353.25 | 2.3 | 1 × 10−8 | Q9QXU9 | ||||
| Pro-SAAS (AA 174–218)c | GPTGPDVEDAADETPDVDPELLRYLLGRILTGSSEPEAAPAPRRL | 4755.42 | 3.9 | 2 × 10−10 | Q9QXU9 | ||||
| Pro-SAAS (AA 221–239)c | AVDQDLGPEVPPENVLGAL | 1931.99 | 2.8 | 1 × 10−37 | Q9QXU9 | ||||
| Pro-SAAS (AA 221–240) | PEN-20 | AVDQDLGPEVPPENVLGALL | 2045.08 | 0.0 | 1 × 10−12 | Q9QXU9 | 8 | ||
| Pro-SAAS (AA 221–241)c | AVDQDLGPEVPPENVLGALLR | 2201.18 | 3.7 | 1 × 10−17 | Q9QXU9 | ||||
| Pro-SAAS (AA 221–242) | PEN | AVDQDLGPEVPPENVLGALLRV | 2300.25 | 3.5 | 4 × 10−32 | Q9QXU9 | 30, 33 | ||
| Pro-SAAS (AA 245–260) | Big LEN | LENSSPQAPARRLLPP | 1744.96 | 5.3 | 6 × 10−39 | Q9QXU9 | 30, 33 | ||
| Prothyroliberin (AA 25–50) | LPEAAQEEGAVTPDLPGLENVQVRPE | 2757.40 | 5.0 | 9 × 10−22 | P01150 | 8 | 125, 126 | ||
| Prothyroliberin (AA 83–103)c | EEEEKDIEAEERGDLGEGGAW | 2347.02 | 4.6 | 7 × 10−5 | P01150 | ||||
| Prothyroliberin (AA 178–199) | FIDPELQRSWEEKEGEGVLMPE | 2617.25 | 4.8 | 1 × 10−20 | P01150 | 8 | |||
| Secretogranin 1 (AA 372–380) | SEESQEKEY | 1127.46 | 1.0 | 4 × 10−13 | O35314 | 11 | |||
| Secretogranin 1 (AA 416–432) | GRGREPGAYPALDSRQE | 1857.91 | 1.3 | 2 × 10−9 | O35314 | 127 | |||
| Secretogranin 1 (AA 513–532) | LGALFNPYFDPLQWKNSDFE | 2400.14 | 4.2 | 2 × 10−35 | O35314 | 128 | |||
| Secretogranin 1 (AA 585–594) | SFAKAPHLDL | 1097.59 | 4.5 | 4 × 10−17 | O35314 | 63 | |||
| Secretogranin 1 (AA 597–611) | Q(pyroglutamylation)YDDGVAELDQLLHY | 1760.79 | 2.8 | 4 × 10−23 | O35314 | 97 | |||
| Secretogranin 2 (AA 168–181)c | FPLMYEENSRENPF | 1771.80 | 4.5 | 6 × 10−19 | P10362 | 94 | mouse.brain-map.org/brain/gene/934.html?ispopup=1 | ||
| Secretogranin 2 (AA 169–181)c | PLMYEENSRENPF | 1624.73 | 4.5 | 2 × 10−8 | P10362 | ||||
| Secretogranin 2 (AA 184–216) | Secretoneurin | TNEIVEEQYTPQSLATLESVFQELGKLTGPSNQ | 3649.81 | 3.2 | 1 × 10−17 | P10362 | 102 | ||
| Secretogranin 2 (AA 188–216)c | ATLESVFQELGKLTGPSNQ | 2018.04 | 5.1 | 8 × 10−11 | P10362 | ||||
| Secretogranin 2 (AA 205–216) | QELGKLTGPSNQ | 1270.65 | 2.2 | 2 × 10−5 | P10362 | 61 | |||
| Secretogranin 2 (AA 287–316)c | SGHLGLPDEGNRKESKDQLSEDAS KVITYL | 3285.66 | 4.3 | 5 × 10−29 | P10362 | ||||
| Secretogranin 2 (AA 495–517)c | PYDNLNDKDQELGEYLARMLVKY | 2786.37 | 4.4 | 4 × 10−13 | P10362 | ||||
| Secretogranin 2 (AA 529–566)c | VPSPGSSEDDLQEEEQLEQAIKEHLGQGSSQEMEKLAK | 4179.99 | 3.8 | 2 × 10−20 | P10362 | ||||
| Secretogranin 2 (AA 529–568) | Manserin | VPSPGSSEDDLQEEEQLEQAIKEHLGQGSSQEMEKLAKVS | 4366.09 | 2.7 | 6 × 10−50 | P10362 | 33 | ||
| Secretogranin 2 (AA 529–568)c | Manserin | VPSPGS(phosphorylation) SEDDLQEEEQLEQAIKEHLGQGSSQEMEKLAKVS | 4446.06 | 4.8 | 4 × 10−23 | P10362 | |||
| Secretogranin 2 (AA 571–583) | IPAGSLKNEDTPN | 1354.67 | 1.6 | 5 × 10−46 | P10362 | 11 | |||
| Secretogranin 2 (AA 571–584)c | IPAGSLKNEDTPNR | 1510.78 | 3.1 | 1 × 10−30 | P10362 | ||||
| Secretogranin 2 (AA 571–585)c | IPAGSLKNEDTPNRQ | 1638.84 | 2.5 | 2 × 10−28 | P10362 | ||||
| Da | ppm | ||||||||
| Secretogranin 2 (AA 571–611)c | IPAGSLKNEDTPNRQYLDEDMLLKVLEYLNQEQAEQGREHL | 4796.38 | 3.0 | 1 × 10−71 | P10362 | ||||
| Secretogranin 2 (AA 571–612)c | IPAGSLKNEDTPNRQYLDEDMLLKVLEYLNQEQAEQGREHLA | 4867.43 | 1.4 | 2 × 10−76 | P10362 | ||||
| Secretogranin 2 (AA 595–611)c | VLEYLNQEQAEQGREHL | 2055.01 | 2.6 | 5 × 10−24 | P10362 | ||||
| Secretogranin 3 (AA 23–36) | FPKPEGSQDKSLHN | 1582.78 | 2.4 | 3 × 10−14 | P47868 | 33 | mouse.brain-map.org/brain/gene/73718057.html?ispopup=1 | ||
| Somatostatin (AA 25–87)c | APSDPRLRQFLQKSLAAATGKQELAKYFLAELLSEPNQTENDALEPEDLPQAAEQDEMRLELQ | 7093.62 | 7.4 | 6 × 10−10 | P60042 | 17, 20, 27, 129–131 | mouse.brain-map.org/brain/gene/1001.html?ispopup=1 | ||
| Tachykinin 3 (AA 95–115)c | NSQPDT PADVVEENTPSFGVL | 2215.04 | 4.4 | 9 × 10−40 | P08435 | ||||
| Provasopressin (AA 24–32) | Arginine-vasopressin | CdYFQNCdPRG(amidation) | 1083.44 | 0.0 | 8 × 10−10 | P01186 | 30, 33, 61 | 132 | mouse.brain-map.org/brain/gene/131.html?ispopup=1 |
| Provasopressin (AA 26–32)c | FQNCPRG(amidation) | 819.38 | 1.6 | 4 × 10−16 | P01186 | ||||
| Provasopressin (AA 151–165) | VQLAGTQESVDSAKP | 1528.77 | −1.3 | 3 × 10−17 | P01186 | 102 | |||
| Provasopressin (AA 151–166) | VQLAGTQESVDSAKPR | 1684.88 | 1.3 | 1 × 10−27 | P01186 | 133 | |||
| Provasopressin (AA 151–167) | VQLAGTQESVDSAKPRV | 1783.95 | 4.2 | 6 × 10−27 | P01186 | SwePep | |||
| Provasopressin (AA 151–168) | VQLAGTQESVDSAKPRVY | 1947.01 | 1.8 | 5 × 10−38 | P01186 | 33, 63, 102 | |||
| Provasopressin (AA 152–168)c | QLAGTQESVDSAKPRVY | 1847.94 | 3.4 | 8 × 10−20 | P01186 | ||||
| Provasopressin (AA 153–168) | LAGTQESVDSAKPRVY | 1719.88 | 2.3 | 9 × 10−32 | P01186 | SwePep | |||
| Provasopressin (AA 154–168) | AGTQESVDSAKPRVY | 1606.80 | 0.5 | 2 × 10−31 | P01186 | 33 | |||
| Provasopressin (AA 155–168) | GTQESVDSAKPRVY | 1535.76 | 1.6 | 4 × 10−23 | P01186 | 33 | |||
| VIP peptides (AA 125–137) | HSDAVFTDNYTRL | 1537.72 | 4.2 | 1 × 10−15 | P01283 | SwePep | 25, 28, 48–55 | mouse.brain-map.org/brain/gene/77371835.html?ispopup=1 | |
| Acyl-CoA-binding protein (AA 2–87) | S(acetylation)QADFDKAAEEVKRLKTQPTDEEMLFIYSHFKQATVGDVNTDRPGLLDLKGKAKWDSWNKLKGTSKENAMKTYVEKVEELKKKYGI | 9932.13 | 1.1 | 3 × 10−43 | P11030 | 134, 135 | |||
| Brain-specific polypeptide PEP-19 (AA 2–62) | S(acetylation)ERQSAGATNGKDKTSGDNDGQKKVQEEFDIDMDAPETERAAVAIQSQFRKFQKKKAGSQS | 6714.25 | −1.9 | 7 × 10−13 | P63055 | 136 | |||
| PEBP-1 (AA 9–25) | AGPLSLQEVDEPPQHAL | 1799.92 | 5.8 | 3 × 10−22 | P31044 | 10 | |||
| PEBP-1 (AA 11–25)c | PLSLQEVDEPPQHAL | 1671.85 | 0.4 | 3 × 10−18 | P31044 | ||||
| PEBP-1 (AA 28–46) | DYGGVTVDELGKVLTPTQV | 1990.03 | 2.8 | 6 × 10−29 | P31044 | 10 | |||
| PEBP-1 (AA 50–66) | PSSISWDGLDPGKLYTL | 1847.93 | 1.6 | 2 × 10−5 | P31044 | 137 | |||
| PEBP-1 (AA 174–187) | DDSVPKLHDQLAGK | 1521.78 | 3.5 | 2 × 10−7 | P31044 | 10 | |||
| GABA(A) receptor subunit α-6 (AA 38–55)c | NLLEGYDNRLRPGFGGAV | 1947.01 | 8.0 | 5 × 10−7 | P30191 | 87 | mouse.brain-map.org/brain/gene/75551467.html?ispopup=1 | ||
| Dihydropyrimidinase-related protein 2 (AA 518–572)c | SAKTSPAKQQAPPVRNLHQSGFSLSGAQIDDNIPRRTTQRIVAPPGGRANITSLG | 5761.09 | 4.8 | 3 × 10−48 | P47942 | ||||
| Dihydropyrimidinase-related protein 2 (AA 560–572)c | APPGGRANITSLG | 1209.65 | 1.9 | 3 × 10−13 | P47942 | ||||
| Dihydropyrimidinase-related protein 3 (AA 558–570)c | APPGGRSNITSLS | 1255.65 | 1.9 | 6 × 10−12 | Q62952 |
a E-values above 1 × 10−4 were manually validated.
b References found in SCN studies were for the prohormones, which were previously reported in the studies.
c Novel peptides.
d Cys-Cys bonds.
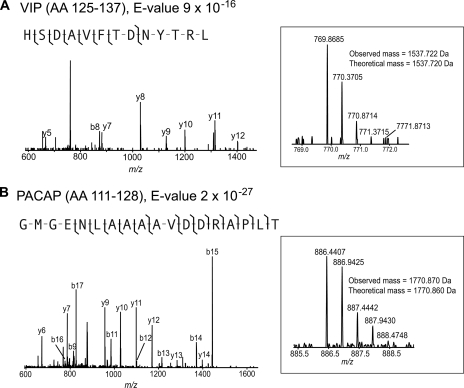
Fig. 1 depicts the examples of FTMS and MS/MS spectra for prohormone-derived peptide forms of VIP and pituitary adenylate cyclase-activating polypeptide (PACAP) identified with E-values of 9 × 10−16 and 2 × 10−27, respectively. Although the sequence of VIP is HSDAVFTDNYTRLRKQMAVKKYLNSI (AA 125–152), another peptide from the VIP prohormone was identified in this study: HSDAVFTDNYTRL (AA 125–137). Because the observed peptide sequence results from cleavage of the prohormone at a dibasic cleavage site (RK), it appears to be a bona fide intracellular processing product from the VIP prohormone and is not expected to arise from extracellular degradation/processing. This shortened peptide has been reported in SwePep. The observed peptide derived from the PACAP prohormone was GMGENLAAAAVDDRAPLT (AA 111–128), whereas the previously confirmed bioactive PACAP-derived peptides are PACAP-27 (AA 131–157) and PACAP-38 (AA 131–168). Because there are dibasic residues (KR) between the observed peptide and the PACAP-27 and -38, again we assume that the observed peptide was produced from the intracellular processing of the PACAP prohormone.
Fig. 1.
FTMS and FTMS/MS data allow identification of peptides present in SCN sample with high confidence. The peptides derived from VIP (A) and PACAP (B) were identified with one b-ion and seven y-ions and with eight b-ions and eight y-ions, respectively. VIP is known to be present in SCN core neurons, and PACAP is synthesized within the retinal ganglion cells that innervate the SCN.
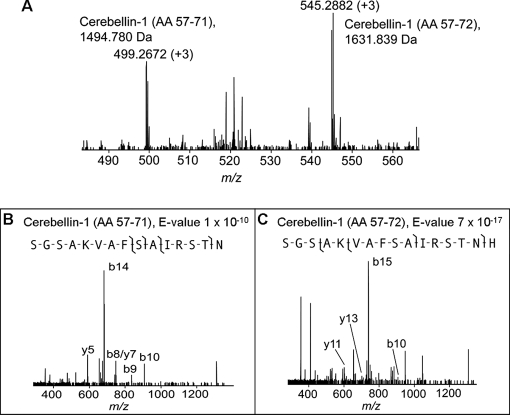
Fig. 2 represents the FTMS and MS/MS spectra for cerebellin (AA 57–72) and a one-amino acid-truncated form (AA 57–71), which are derived from the cerebellin-1 precursor. The two peptides co-eluted, as seen in Fig. 2A, and were identified by the data-dependent top five MS/MS acquisition strategy as seen in Fig. 2B. These two cerebellin forms were previously identified from mouse hypothalamus studies (8); however, there was no report localizing these peptides to the SCN. Interestingly, our previous work on peptide release from the rat SCN demonstrated that an unknown peak at m/z 1495.75 (MH+) changed in abundance with circadian rhythmicity over a 24-h period (30). Here, we confirm that this released peptide corresponds to a shortened form of cerebellin identified here with a 1 × 10−10 E-value.
Fig. 2.
Identification of co-eluted truncated cerebellin and cerebellin. The two peptides derived from cerebellin-1 precursor were detected in the same FTMS scan (A) and fragmented by a data-dependent MS/MS acquisition strategy and identified as SGSAKVAFSAIRSTN (B) and SGSAKVAFSAIRSTNH (C), respectively.
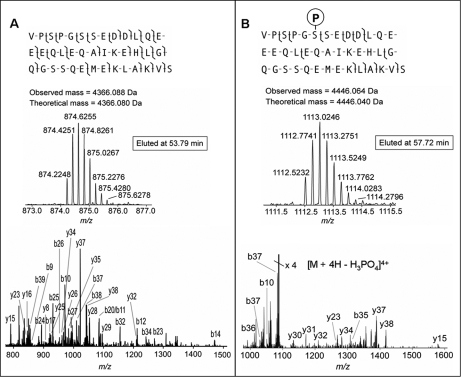
Of the 102 SCN peptides identified, 12 harbored PTMs. One example is depicted in Fig. 3, showing FTMS and MS/MS spectra for two forms of manserin, which is derived from secretogranin 2 precursor. Manserin and phosphorylated manserin were identified with E-values of 6 × 10−50 and 4 × 10−23, respectively, and the integrated intensity values of the peptides were similar at ∼5 × 106 and 1 × 106, respectively. Phosphorylated manserin exhibited the fragment ion generated by neutral loss of H3PO4 as the most prominent signal along with a few fragment ions of low abundance generated by fragmentation of the peptide backbone, which is a typical fragmentation pattern of Ser(P)/Thr(P) phosphopeptides.
Fig. 3.
Identification of manserin with E-value of 6 × 10−50 (A) and phosphorylated manserin with E-value of 4 × 10−23 (B) by tailored software, ProSightPC. The FTMS/MS spectrum of phosphorylated manserin exhibited the fragment ion generated by neutral loss of H3PO4 as the most prominent signal, which is a typical fragmentation pattern of Ser(P)/Thr(P) phosphopeptides by CID.
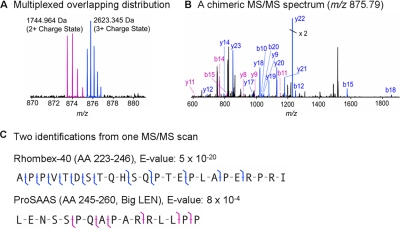
Finally, Fig. 4 represents a search result using multiplexed MS/MS, which resulted from use of high resolution MS/MS data and our tailored software. In Fig. 4A, the isolation of a 5 m/z region for m/z 875.79 in the FTMS scan generates two isotopic distributions, which are 1744.964 and 2623.345 Da. In the data processing of ProSightPC, the two masses were searched independently using the entire fragment ion list derived from the Fig. 4B MS/MS scan and produced the identifications of two peptides that were derived from Rhombex-40 and pro-SAAS precursors, respectively, as seen in Fig. 4C. Rhombex-40 is known as a surface adhesion protein located at the ventral medullary surface (60); there is as yet no report of its expression in hypothalamus. The peptide big LEN, which is derived from pro-SAAS, was identified in our previous SCN studies (30).
Fig. 4.
Multiplexed identification from high resolution FTMS/MS mass spectrum. The two isotopic distributions corresponding to 1744.964 and 2623.345 Da (A) are seen in the isolation window for m/z 875.79 and generate the chimeric FTMS/MS spectrum (B). The tailored software, ProSightPC, produces the two peptides derived from Rhombex-40 and pro-SAAS precursors, respectively (C).
DISCUSSION
Given that the SCN contains endogenous cellular oscillators that control the circadian rhythms of mammals, studying the peptides contained within the SCN is expected to increase our understanding of the circadian mechanisms. With solid-phase extraction collection strategies, we have recently analyzed the secreted peptides from the site of the SCN over a 24-h period and the released peptides from the SCN stimulated via the optic tract (30). We were able to identify several peptides previously reported by indirect studies to be present in the SCN. Furthermore, we discovered four new peptides, three of which are derived from pro-SAAS. One of the pro-SAAS-derived peptides, known as little SAAS, caused phase delays of SCN circadian rhythms in vitro.
However, there have not yet been any comprehensive peptidome studies of the SCN region using MS. Here, we performed the peptidome analysis of the rat hypothalamic SCN, which was prepared during daytime (at ZT 6), and identified 102 endogenous peptides by FTMS/MS, including 33 novel peptides. Although most of the peptides, including the novel peptides, are produced from the cleavage of classical dibasic or monobasic neuropeptide processing sites, a number of peptides have cleavage sites at Leu-Ala or Leu-Leu, which could be products of Leu-X-specific enzyme (61). There were also several peptides with unconventional cleavage sites among the newly identified peptides, for example N-terminal or C-terminal side cleavage of aspartic acid (10, 61) of the peptides from pro-SAAS and C-terminal side cleavage of tryptophan of the peptide derived from neuropeptide Y. These cleavages could occur intracellularly during prohormone processing. Alternatively, these may be occurring during extracellular processing, either endogenously or perhaps during the preparation of tissue extracts. Physiological assessments, such as we have done for little SAAS (30), are necessary to determine the functional role(s) for our novel discovery products.
Many of the identified peptides in the present study were derived from known precursors expressed in the SCN. VIP (AA 125–152), GRP (AA 24–52), and somatostatin (AA 103–116) have been identified immunologically in neurons of the SCN core region. VIP and GRP have established roles in synchronization of the multitude of cell-based clocks in the SCN and also in relay of light information within the SCN to generate phase resetting of SCN tissue (25, 38–41, 48–50). In the present study, we observed shorter peptides derived from the VIP prohormone (AA 125–137) and GRP (AA 24–41) and the other peptide fragment of somatostatin (AA 25–87). The shortened forms of VIP and GRP have been observed in mice and reported in SwePep, whereas somatostatin (AA 103–116) has not been reported. As we stated above, these shortened forms may be from processing within the vesicle or may be from extracellular peptide processing; however, the possibility of degradation during our sample processing cannot be excluded. The reasons for not detecting several expected full-length peptides may be due to short peptide lifetimes, rapid degradation, or detection limits of FTMS/MS. Of course, the prior studies involving the localization of these peptides have used immunohistochemistry and so would not distinguish the full-length and shorter peptide forms. Thus, the unusual shortened forms of these well known peptides appear to be interesting targets for follow-up functional studies. Additionally, arginine-vasopressin (AVP), well known to be released and expressed at the shell neurons of the SCN (62), was identified with the loss of its C-terminal glycine to become an amidated peptide.
In addition to the forms of known intrinsic peptides of the SCN, a PACAP-related peptide, PACAP (AA 111–128) (10, 63), was identified in the present study, whereas the expected and well characterized PACAP peptides include those from AA 131–157 and 131–168. PACAP is known to be synthesized in the retina and released onto the SCN upon stimulation, transmitting photic signals via the retinohypothalamic tract (64–76). The observation of the unique peptide in the present study could indicate that the AA 111–128 fragment has some functional role, that PACAP is synthesized in the SCN, or that there is local translation of PACAP mRNA into its prohormone.
Neuropeptide Y and γ-aminobutyric acid (GABA) are putative transmitters of non-photic signals via the geniculohypothalamic tract to the SCN (77–91). We observed C-flanking peptide of neuropeptide Y, which was reported in geniculohypothalamic tract projections to the SCN (82), and the peptide fragment of GABA receptor subunit α-6. Although we identified three peptides derived from pro-SAAS in our previous releasate study of the SCN (30), here we were able to identify 18 peptides from pro-SAAS, including little SAAS, big SAAS, PEN, PEN-20, and big LEN.
In the present study, we also found multiple peptides that have not been previously reported in SCN circadian studies. CART, cerebellin-1, neuroendocrine protein 7B2, proenkephalin B, secretogranin 1, secretogranin 3, tachykinin 3, acyl-CoA-binding protein, brain-specific polypeptide PEP-19, PEBP-1, and dihydropyrimidinase-related proteins 2 and 3 (DRP-2 and DRP-3) precursors have not been reported in the prior SCN studies. Specifically, DRP-2 and DRP-3 are known to be expressed during neuronal ontogenesis and involve regulation of axon extension (92, 93) and have not been studied in the hypothalamus. Although the peptides derived from these precursors could have functional roles, the functional studies of the peptides are beyond the scope of our study.
Our current peptidome analysis by use of high resolution data and tailored software allowed the full characterization of 12 peptides with PTMs. The phosphorylated manserin (VPSPGS*(phosphorylation)SEDDLQEEEQLEQAIKEHLGQGSSQEMEKLAKVS) derived from secretogranin 2 precursor was identified along with unmodified manserin. Secretogranin 2 is highly expressed in the SCN of mouse (94); however, no endogenous peptides derived from secretogranin 2 have been reported in SCN studies. Recently, Beranova-Giorgianni et al. (95) performed a phosphoproteomics analysis of the human pituitary sample with trypsin digestion followed by IMAC to enrich the phosphopeptides. They observed the phosphopeptide of SPGS(*)S(*)EDDLQEEEQLEQAIK; however, they were unable to determine which Ser site was phosphorylated between the two Ser sites denoted as (*) from their study. We also detected C-terminal amidation forms of neuropeptide-glutamic acid-isoleucine, neurosecretory protein VGF precursor (LEGSFLGGSEAGERLLQQGLAQVEA-NH2), melanotropin α, substance P, AVP, and provasopressin (FQNCPRG-NH2; truncated form of AVP). Specifically, the truncated form of AVP appears not to have been reported in prior studies. An AVP fragment produced from proteolysis in the brain has been reported to be a highly potent neuropeptide (96). In addition, we identified a pyroglutamylated form of secretogranin 1 precursor (Q(pyroglutamylation)YDDGVAELDQLLHY). Although there is no report of this form of peptide in prior SCN studies, the homologous peptide was identified in bovine tissue adrenomedullary chromaffin vesicles (97).
In addition to peptides derived from prohormones, several peptides from non-prohormone-related proteins were detected, specifically four N-terminal acetylated forms of acyl-CoA-binding protein, brain-specific polypeptide PEP-19, thymosin β-4, and thymosin β-10. Many of these protein fragments have been reported in prior peptidome studies, and several, such as the thymosins, have been detected in SCN releasates (30), indicating that these proteins are endogenously processed into these shortened forms and may have some functional significance. Of course, others may represent sample preparation artifacts as the proteins may be degraded during tissue homogenization.
CONCLUSIONS
For identification and characterization of neuropeptides, the overall work flow described here represents a new route to discovery. Using MS/MS data with ≪10-ppm mass accuracy and neuroProSight software, higher quality identification is achieved. This information allows unusual PTMs to be confirmed. The overall sensitivity of the work flow allows such assays to be made on the small nuclei in the brain. Of course, additional developments will streamline this peptide discovery process.
From a neuroscience perspective, what is particularly exciting is combining peptide discovery with approaches optimized to measure peptide release (30, 98–101). The latter approaches provide a functional context for the peptide diversity determined here by allowing the subset of SCN peptides that are released at a particular time of day or under specific stimulation protocols to be uncovered. It is through the combination of peptide discovery and release assays that the functional implications on the complex interplay of a surprising range of peptides can be understood within the SCN.
Although we focused on analyzing the endogenous peptides present in SCN prepared at ZT 6 in the current study, the peptidome study at different ZTs can be considered as an important next step for better understanding how the SCN orchestrates circadian rhythms over a 24-h period. The SCN peptidome study at different ZTs including quantitative analysis of peptide expression is currently in progress.
Supplementary Material
Acknowledgments
We thank Dr. Andrew J. Forbes and Adrianna Bora for helpful discussions on data analysis and the sample processing protocol, respectively.
Footnotes
* This work was supported, in whole or in part, by National Institutes of Health Grant GM 067193-07 (to the laboratory of N. L. K.), Award DE018866 from the NIDCR and the Office of the Director (to J. V. S.), Grant HL092571 from the NHLBI (to M. U. G.), and Award DA018310 from the National Institute on Drug Abuse. This work was also supported by the Packard Foundation and the Sloan Foundation (to the laboratory of N. L. K.).
 The on-line version of this article (available at http://www.mcponline.org) contains supplemental Fig. S1 and Table 1.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental Fig. S1 and Table 1.
1 The abbreviations used are:
- PTM
- post-translational modification
- SCN
- suprachiasmatic nucleus
- ZT
- Zeitgeber time
- .puf
- ProSight upload file
- GRP
- gastrin-releasing peptide
- VIP
- vasoactive intestinal peptide
- PACAP
- pituitary adenylate cyclase-activating polypeptide
- AVP
- arginine-vasopressin
- GABA
- γ-aminobutyric acid
- CART
- cocaine- and amphetamine-regulated transcript protein
- DRP
- dihydropyrimidinase-related protein
- LTQ
- linear trap quadrupole
- AA
- amino acids.
REFERENCES
- 1.Strand F. L. ( 1999) Neuropeptides: Regulators of Physiological Processes, The MIT Press, Cambridge, MA [Google Scholar]
- 2.Kandel E. R., Schwartz J. H., Jessell T. M. ( 2000) Principles of Neural Science, 4th Ed., McGraw-Hill, New York [Google Scholar]
- 3.Burbach J. P. H., de Wied D. (eds) ( 1993) Brain Functions of Neuropeptides: a Current View, Informa HealthCare, London [Google Scholar]
- 4.Hökfelt T., Broberger C., Xu Z. Q., Sergeyev V., Ubink R., Diez M. ( 2000) Neuropeptides—an overview. Neuropharmacology 39, 1337– 1356 [DOI] [PubMed] [Google Scholar]
- 5.Hummon A. B., Amare A., Sweedler J. V. ( 2006) Discovering new invertebrate neuropeptides using mass spectrometry. Mass Spectrom. Rev 25, 77– 98 [DOI] [PubMed] [Google Scholar]
- 6.Rossbach U., Nilsson A., Fälth M., Kultima K., Zhou Q., Hallberg M., Gordh T., Andren P. E., Nyberg F. ( 2009) A quantitative peptidomic analysis of peptides related to the endogenous opioid and tachykinin systems in nucleus accumbens of rats following naloxone-precipitated morphine withdrawal. J. Proteome Res 8, 1091– 1098 [DOI] [PubMed] [Google Scholar]
- 7.Li L., Sweedler J. V. ( 2008) Peptides in the brain: mass spectrometry-based measurement approaches and challenges. Annu. Rev. Anal. Chem 1, 451– 483 [DOI] [PubMed] [Google Scholar]
- 8.Che F. Y., Zhang X., Berezniuk I., Callaway M., Lim J., Fricker L. D. ( 2007) Optimization of neuropeptide extraction from the mouse hypothalamus. J. Proteome Res 6, 4667– 4676 [DOI] [PubMed] [Google Scholar]
- 9.Fricker L. D., Lim J., Pan H., Che F. Y. ( 2006) Peptidomics: Identification and quantification of endogenous peptides in neuroendocrine tissues. Mass Spectrom. Rev 25, 327– 344 [DOI] [PubMed] [Google Scholar]
- 10.Dowell J. A., Heyden W. V., Li L. ( 2006) Rat neuropeptidomics by LC-MS/MS and MALDI-FTMS: enhanced dissection and extraction techniques coupled with 2D RP-RP HPLC. J. Proteome Res 5, 3368– 3375 [DOI] [PubMed] [Google Scholar]
- 11.Fälth M., Sköld K., Svensson M., Nilsson A., Fenyö D., Andren P. E. ( 2007) Neuropeptidomics strategies for specific and sensitive identification of endogenous peptides. Mol. Cell. Proteomics 6, 1188– 1197 [DOI] [PubMed] [Google Scholar]
- 12.Svensson M., Sköld K., Svenningsson P., Andren P. E. ( 2003) Peptidomics-based discovery of novel neuropeptides. J. Proteome Res 2, 213– 219 [DOI] [PubMed] [Google Scholar]
- 13.Taylor S. W., Andon N. L., Bilakovics J. M., Lowe C., Hanley M. R., Pittner R., Ghosh S. S. ( 2006) Efficient high-throughput discovery of large peptidic hormones and biomarkers. J. Proteome Res 5, 1776– 1784 [DOI] [PubMed] [Google Scholar]
- 14.Ramström M., Hagman C., Tsybin Y. O., Markides K. E., Håkansson P., Salehi A., Lundquist I., Håkanson R., Bergquist J. ( 2003) A novel mass spectrometric approach to the analysis of hormonal peptides in extracts of mouse pancreatic islets. Eur. J. Biochem 270, 3146– 3152 [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Ma M., Chen R., Li L. ( 2008) Enhanced neuropeptide profiling via capillary electrophoresis off-line coupled with MALDI FTMS. Anal. Chem 80, 6168– 6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyne M. T., Garcia B. A., Li M., Zamdborg L., Wenger C. D., Babai S., Kelleher N. L. ( 2009) Tandem mass spectrometry with ultrahigh mass accuracy clarifies peptide identification by database retrieval. J. Proteome Res 8, 374– 379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrahamson E. E., Moore R. Y. ( 2001) Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res 916, 172– 191 [DOI] [PubMed] [Google Scholar]
- 18.Gillette M. U., Mitchell J. W. ( 2002) Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res 309, 99– 107 [DOI] [PubMed] [Google Scholar]
- 19.Leak R. K., Moore R. Y. ( 2001) Topographic organization of suprachiasmatic nucleus projection neurons. J. Comp. Neurol 433, 312– 334 [DOI] [PubMed] [Google Scholar]
- 20.Moore R. Y., Speh J. C., Leak R. K. ( 2002) Suprachiasmatic nucleus organization. Cell Tissue Res 309, 89– 98 [DOI] [PubMed] [Google Scholar]
- 21.Morin L. P., Shivers K. Y., Blanchard J. H., Muscat L. ( 2006) Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience 137, 1285– 1297 [DOI] [PubMed] [Google Scholar]
- 22.van den Pol A. N., Tsujimoto K. L. ( 1985) Neurotransmitters of the hypothalamic suprachiasmatic nucleus immunocytochemical analysis of 25 neuronal antigens. Neuroscience 15, 1049– 1086 [DOI] [PubMed] [Google Scholar]
- 23.Karatsoreos I. N., Yan L., LeSauter J., Silver R. ( 2004) Phenotype matters: Identification of light-responsive cells in the mouse suprachiasmatic nucleus. J. Neurosci 24, 68– 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Pol A. N., Decavel C., Levi A., Paterson B. ( 1989) Hypothalamic expression of a novel gene product VGF immunocytochemical analysis. J. Neurosci 9, 4122– 4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Pol A. N., Gorcs T. ( 1986) Synaptic relationships between neurons containing vasopressin gastrin-releasing peptide vasoactive intestinal polypeptide and glutamate decarboxylase immunoreactivity in the suprachiasmatic nucleus dual ultrastructural immunocytochemistry with gold-substituted silver peroxidase. J. Comp. Neurol 252, 507– 521 [DOI] [PubMed] [Google Scholar]
- 26.Yan L., Karatsoreos I., Lesauter J., Welsh D. K., Kay S., Foley D., Silver R. ( 2007) Exploring spatiotemporal organization of SCN circuits. Cold Spring Harb. Symp. Quant. Biol 72, 527– 541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Card J. P., Moore R. Y. ( 1984) The suprachiasmatic nucleus of the golden-hamster: immunohistochemical analysis of cell and fiber distribution. Neuroscience 13, 415– 431 [DOI] [PubMed] [Google Scholar]
- 28.Morin L. P. ( 2007) SCN organization reconsidered. J. Biol. Rhythms 22, 3– 13 [DOI] [PubMed] [Google Scholar]
- 29.van Leeuwen F. W., Swaab D. F., de Raay C. ( 1978) Immunoelectron microscopic localization of vasopressin in rat suprachiasmatic nucleus. Cell Tissue Res 193, 1– 10 [DOI] [PubMed] [Google Scholar]
- 30.Hatcher N. G., Atkins N., Jr., Annangudi S. P., Forbes A. J., Kelleher N. L., Gillette M. U., Sweedler J. V. ( 2008) Mass spectrometry-based discovery of circadian peptides. Proc. Natl. Acad. Sci. U.S.A 105, 12527– 12532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leduc R. D., Kelleher N. L. ( 2007) Using ProSight PTM and related tools for targeted protein identification and characterization with high mass accuracy tandem MS data. Curr. Protoc. Bioinformatics Chapter 13, 13.6.1– 13.6.28 [DOI] [PubMed] [Google Scholar]
- 32.Zamdborg L., LeDuc R. D., Glowacz K. J., Kim Y. B., Viswanathan V., Spaulding I. T., Early B. P., Bluhm E. J., Babai S., Kelleher N. L. ( 2007) ProSight PTM 2.0: improved protein identification and characterization for top down mass spectrometry. Nucleic Acids Res 35, W701– W706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bora A., Annangudi S. P., Millet L. J., Rubakhin S. S., Forbes A. J., Kelleher N. L., Gillette M. U., Sweedler J. V. ( 2008) Neuropeptidomics of the supraoptic rat nucleus. J. Proteome Res 7, 4992– 5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tischkau S. A., Mitchell J. W., Pace L. A., Barnes J. W., Barnes J. A., Gillette M. U. ( 2004) Protein kinase G type II is required for night-to-day progression of the mammalian circadian clock. Neuron 43, 539– 549 [DOI] [PubMed] [Google Scholar]
- 35.Southey B. R., Amare A., Zimmerman T. A., Rodriguez-Zas S. L., Sweedler J. V. ( 2006) NeuroPred: a tool to predict cleavage sites in neuropeptide precursors and provide the masses of the resulting peptides. Nucleic Acids Res 34, W267– W272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amare A., Hummon A. B., Southey B. R., Zimmerman T. A., Rodriguez-Zas S. L., Sweedler J. V. ( 2006) Bridging neuropeptidomics and genomics with bioinformatics: prediction of mammalian neuropeptide prohormone processing. J. Proteome Res 5, 1162– 1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tegge A. N., Southey B. R., Sweedler J. V., Rodriguez-Zas S. L. ( 2008) Comparative analysis of neuropeptide cleavage sites in human, mouse, rat, and cattle. Mamm. Genome 19, 106– 120 [DOI] [PubMed] [Google Scholar]
- 38.Albers H. E., Gillespie C. F., Babagbemi T. O., Huhman K. L. ( 1995) Analysis of the phase-shifting effects of gastrin-releasing peptide when microinjected into the suprachiasmatic region. Neurosci. Lett 191, 63– 66 [DOI] [PubMed] [Google Scholar]
- 39.Brown T. M., Hughes A. T., Piggins H. D. ( 2005) Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC(2) receptor signaling. J. Neurosci 25, 11155– 11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gamble K. L., Allen G. C., Zhou T., McMahon D. G. ( 2007) Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J. Neurosci 27, 12078– 12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McArthur A. J., Coogan A. N., Ajpru S., Sugden D., Biello S. M., Piggins H. D. ( 2000) Gastrin-releasing peptide phase-shifts suprachiasmatic nuclei neuronal rhythms in vitro. J. Neurosci 20, 5496– 5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aida R., Moriya T., Araki M., Akiyama M., Wada K., Wada E., Shibata S. ( 2002) Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol. Pharmacol 61, 26– 34 [DOI] [PubMed] [Google Scholar]
- 43.Aïoun J., Chambille I., Peytevin J., Martinet L. ( 1998) Neurons containing gastrin-releasing peptide and vasoactive intestinal polypeptide are involved in the reception of the photic signal in the suprachiasmatic nucleus of the Syrian hamster: an immunocytochemical ultrastructural study. Cell Tissue Res 291, 239– 253 [DOI] [PubMed] [Google Scholar]
- 44.Antle M. C., Kriegsfeld L. J., Silver R. ( 2005) Signaling within the master clock of the brain: Localized activation of mitogen-activated protein kinase by gastrin-releasing peptide. J. Neurosci 25, 2447– 2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Earnest D. J., DiGiorgio S., Olschowka J. A. ( 1993) Light induces expression of Fos-related proteins within gastrin-releasing peptide neurons in the rat suprachiasmatic nucleus. Brain Res 627, 205– 209 [DOI] [PubMed] [Google Scholar]
- 46.Kallingal G. J., Mintz E. M. ( 2006) Glutamatergic activity modulates the phase-shifting effects of gastrin-releasing peptide and light. Eur. J. Neurosci 24, 2853– 2858 [DOI] [PubMed] [Google Scholar]
- 47.Piggins H. D., Goguen D., Rusak B. ( 2005) Gastrin-releasing peptide induces c-Fos in the hamster suprachiasmatic nucleus. Neurosci. Lett 384, 205– 210 [DOI] [PubMed] [Google Scholar]
- 48.Aton S. J., Colwell C. S., Harmar A. J., Waschek J., Herzog E. D. ( 2005) Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci 8, 476– 483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piggins H. D., Cutler D. J. ( 2003) The roles of vasoactive intestinal polypeptide in the mammalian circadian clock. J. Endocrinol 177, 7– 15 [DOI] [PubMed] [Google Scholar]
- 50.Vosko A. M., Schroeder A., Loh D. H., Colwell C. S. ( 2007) Vasoactive intestinal peptide and the mammalian circadian system. Gen. Comp. Endocrinol 152, 165– 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Card J. P., Brecha N., Karten H. J., Moore R. Y. ( 1981) Immuno-cytochemical localization of vasoactive intestinal polypeptide-containing cells and processes in the suprachiasmatic nucleus of the rat: light and electron-microscopic analysis. J. Neurosci 1, 1289– 1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colwell C. S., Michel S., Itri J., Rodriguez W., Tam J., Lelievre V., Hu Z., Liu X., Waschek J. A. ( 2003) Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R939– R949 [DOI] [PubMed] [Google Scholar]
- 53.Hannibal J., Fahrenkrug J. ( 2003) Circadian rhythm regulation: a central role for the neuropeptide vasoactive intestinal polypeptide. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R935– R936 [DOI] [PubMed] [Google Scholar]
- 54.Kawamoto K., Nagano M., Kanda F., Chihara K., Shigeyoshi Y., Okamura H. ( 2003) Two types of VIP neuronal components in rat suprachiasmatic nucleus. J. Neurosci. Res 74, 852– 857 [DOI] [PubMed] [Google Scholar]
- 55.Sims K. B., Hoffman D. L., Said S. I., Zimmerman E. A. ( 1980) Vasoactive intestinal polypeptide (VIP) in mouse and rat brain: an immunocytochemical study. Brain Res 186, 165– 183 [DOI] [PubMed] [Google Scholar]
- 56.Lein E. S., Hawrylycz M. J., Ao N., Ayres M., Bensinger A., Bernard A., Boe A. F., Boguski M. S., Brockway K. S., Byrnes E. J., Chen L., Chen L., Chen T. M., Chin M. C., Chong J., Crook B. E., Czaplinska A., Dang C. N., Datta S., Dee N. R., Desaki A. L., Desta T., Diep E., Dolbeare T. A., Donelan M. J., Dong H. W., Dougherty J. G., Duncan B. J., Ebbert A. J., Eichele G., Estin L. K., Faber C., Facer B. A., Fields R., Fischer S. R., Fliss T. P., Frensley C., Gates S. N., Glattfelder K. J., Halverson K. R., Hart M. R., Hohmann J. G., Howell M. P., Jeung D. P., Johnson R. A., Karr P. T., Kawal R., Kidney J. M., Knapik R. H., Kuan C. L., Lake J. H., Laramee A. R., Larsen K. D., Lau C., Lemon T. A., Liang A. J., Liu Y., Luong L. T., Michaels J., Morgan J. J., Morgan R. J., Mortrud M. T., Mosqueda N. F., Ng L. L., Ng R., Orta G. J., Overly C. C., Pak T. H., Parry S. E., Pathak S. D., Pearson O. C., Puchalski R. B., Riley Z. L., Rockett H. R., Rowland S. A., Royall J. J., Ruiz M. J., Sarno N. R., Schaffnit K., Shapovalova N. V., Sivisay T., Slaughterbeck C. R., Smith S. C., Smith K. A., Smith B. I., Sodt A. J., Stewart N. N., Stumpf K. R., Sunkin S. M., Sutram M., Tam A., Teemer C. D., Thaller C., Thompson C. L., Varnam L. R., Visel A., Whitlock R. M., Wohnoutka P. E., Wolkey C. K., Wong V. Y., Wood M., Yaylaoglu M. B., Young R. C., Youngstrom B. L., Yuan X. F., Zhang B., Zwingman T. A., Jones A. R. ( 2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168– 176 [DOI] [PubMed] [Google Scholar]
- 57.Lippton H., Lin B., Gumusel B., Witriol N., Wasserman A., Knight M. ( 2006) Hemopressin, a hemoglobin fragment, dilates the rat systemic vascular bed through release of nitric oxide. Peptides 27, 2284– 2288 [DOI] [PubMed] [Google Scholar]
- 58.Heimann A. S., Gomes I., Dale C. S., Pagano R. L., Gupta A., de Souza L. L., Luchessi A. D., Castro L. M., Giorgi R., Rioli V., Ferro E. S., Devi L. A. ( 2007) Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc. Natl. Acad. Sci. U.S.A 104, 20588– 20593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nydahl K. S., Pierson J., Nyberg F., Caprioli R. M., Andrén P. E. ( 2003) In vivo processing of LVV-hemorphin-7 in rat brain and blood utilizing microdialysis combined with electrospray mass spectrometry. Rapid Commun. Mass Spectrom 17, 838– 844 [DOI] [PubMed] [Google Scholar]
- 60.Shimokawa N., Jingu H., Okada J., Miura M. ( 2000) Molecular cloning of Rhombex-40 a transmembrane protein from the ventral medullary surface of the rat brain by differential display. Life Sci 66, 2183– 2191 [DOI] [PubMed] [Google Scholar]
- 61.Che F. Y., Lim J., Pan H., Biswas R., Fricker L. D. ( 2005) Quantitative neuropeptidomics of microwave-irradiated mouse brain and pituitary. Mol. Cell. Proteomics 4, 1391– 1405 [DOI] [PubMed] [Google Scholar]
- 62.Gillette M. U., Reppert S. M. ( 1987) The hypothalamic suprachiasmatic nuclei: circadian patterns of vasopressin secretion and neuronal activity in vitro. Brain Res. Bull 19, 135– 139 [DOI] [PubMed] [Google Scholar]
- 63.Faith M., Skold K., Svensson M., Norrman M., Nilsson A., Fenyo D., Andren P. ( 2006) SWEPEP, a database designed for neuropeptides and mass spectrometry. Mol. Cell. Proteomics 5, 998– 1005 [DOI] [PubMed] [Google Scholar]
- 64.Butcher G. Q., Lee B., Cheng H. Y., Obrietan K. ( 2005) Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism. J. Neurosci 25, 5305– 5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen D., Buchanan G. F., Ding J. M., Hannibal J., Gillette M. U. ( 1999) Pituitary adenylyl cyclase-activating peptide: a pivotal modulator of glutamatergic regulation of the suprachiasmatic circadian clock. Proc. Natl. Acad. Sci. U.S.A 96, 13468– 13473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dziema H., Obrietan K. ( 2002) PACAP potentiates L-type calcium channel conductance in suprachiasmatic nucleus neurons by activating the MAPK pathway. J. Neurophysiol 88, 1374– 1386 [DOI] [PubMed] [Google Scholar]
- 67.Hannibal J. ( 2002) Neurotransmitters of the retino-hypothalamic tract. Cell Tissue Res 309, 73– 88 [DOI] [PubMed] [Google Scholar]
- 68.Hannibal J. ( 2006) Roles of PACAP-containing retinal ganglion cells in circadian timing. Int. Rev. Cytol 251, 1– 39 [DOI] [PubMed] [Google Scholar]
- 69.Hannibal J., Ding J. M., Chen D., Fahrenkrug J., Larsen P. J., Gillette M. U., Mikkelsen J. D. ( 1998) Pituitary adenylate cyclase activating peptide (PACAP) in the retinohypothalamic tract: a daytime regulator of the biological clock. Ann. N.Y. Acad. Sci 865, 197– 206 [DOI] [PubMed] [Google Scholar]
- 70.Hannibal J., Ding J. M., Chen D., Fahrenkrug J., Larsen P. J., Gillette M. U., Mikkelsen J. D. ( 1997) Pituitary adenylate cyclase-activating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J. Neurosci 17, 2637– 2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hannibal J., Fahrenkrug J. ( 2004) Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res 316, 99– 113 [DOI] [PubMed] [Google Scholar]
- 72.Hannibal J., Hindersson P., Ostergaard J., Georg B., Heegaard S., Larsen P. J., Fahrenkrug J. ( 2004) Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest. Ophthalmol. Vis. Sci 45, 4202– 4209 [DOI] [PubMed] [Google Scholar]
- 73.Kopp M. D., Meissl H., Dehghani F., Korf H. W. ( 2001) The pituitary adenylate cyclase-activating polypeptide modulates glutamatergic calcium signalling: investigations on rat suprachiasmatic nucleus neurons. J. Neurochem 79, 161– 171 [DOI] [PubMed] [Google Scholar]
- 74.Kopp M. D., Schomerus C., Dehghani F., Korf H. W., Meissl H. ( 1999) Pituitary adenylate cyclase-activating polypeptide and melatonin in the suprachiasmatic nucleus: effects on the calcium signal transduction cascade. J. Neurosci 19, 206– 219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minami Y., Furuno K., Akiyama M., Moriya T., Shibata S. ( 2002) Pituitary adenylate cyclase-activating polypeptide produces a phase shift associated with induction of mPer expression in the mouse suprachiasmatic nucleus. Neuroscience 113, 37– 45 [DOI] [PubMed] [Google Scholar]
- 76.Beaulé C., Mitchell J. W., Lindberg P. T., Damadzic R., Eiden L. E., Gillette M. U. ( 2009) Temporally restricted role of retinal PACAP: integration of the phase-advancing light signal to the SCN. J. Biol. Rhythms 24, 126– 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Medanic M., Gillette M. U. ( 1993) Suprachiasmatic circadian pacemaker of rat shows 2 windows of sensitivity to neuropeptide Y in vitro. Brain Res 620, 281– 286 [DOI] [PubMed] [Google Scholar]
- 78.Albers H. E., Ferris C. F. ( 1984) Neuropeptide Y role in light-dark cycle entrainment of hamster circadian rhythms. Neurosci. Lett 50, 163– 168 [DOI] [PubMed] [Google Scholar]
- 79.Biello S. M., Mrosovsky N. ( 1996) Phase response curves to neuropeptide Y in wildtype and tau mutant hamsters. J. Biol. Rhythms 11, 27– 34 [DOI] [PubMed] [Google Scholar]
- 80.Brewer J. M., Yannielli P. C., Harrington M. E. ( 2002) Neuropeptide Y differentially suppresses per1 and per2 mRNA induced by light in the suprachiasmatic nuclei of the golden hamster. J. Biol. Rhythms 17, 28– 39 [DOI] [PubMed] [Google Scholar]
- 81.Card J. P., Moore R. Y. ( 1988) Neuropeptide Y localization in the rat suprachiasmatic nucleus and periventricular hypothalamus. Neurosci. Lett 88, 241– 246 [DOI] [PubMed] [Google Scholar]
- 82.Card J. P., Moore R. Y. ( 1989) Organization of lateral geniculate-hypothalamic connections in the rat. J. Comp. Neurol 284, 135– 147 [DOI] [PubMed] [Google Scholar]
- 83.Fukuhara C., Brewer J. M., Dirden J. C., Bittman E. L., Tosini G., Harrington M. E. ( 2001) Neuropeptide Y rapidly reduces Period 1 and Period 2 mRNA levels in the hamster suprachiasmatic nucleus. Neurosci. Lett 314, 119– 122 [DOI] [PubMed] [Google Scholar]
- 84.Harrington M. E., Nance D. M., Rusak B. ( 1985) Neuropeptide-Y immunoreactivity in the hamster geniculo-suprachiasmatic tract. Brain Res. Bull 15, 465– 472 [DOI] [PubMed] [Google Scholar]
- 85.Huhman K. L., Gillespie C. F., Marvel C. L., Albers H. E. ( 1996) Neuropeptide Y phase shifts circadian rhythms in vivo via a Y-2 receptor. Neuroreport 7, 1249– 1252 [DOI] [PubMed] [Google Scholar]
- 86.Lall G. S., Biello S. M. ( 2003) Attenuation of circadian light induced phase advances and delays by neuropeptide Y and a neuropeptide YY1/Y5 receptor agonist. Neuroscience 119, 611– 618 [DOI] [PubMed] [Google Scholar]
- 87.O'Hara B. F., Andretic R., Heller H. C., Carter D. B., Kilduff T. S. ( 1995) GABAA, GABAC, and NMDA receptor subunit expression in the suprachiasmatic nucleus and other brain regions. Brain Res. Mol. Brain Res 28, 239– 250 [DOI] [PubMed] [Google Scholar]
- 88.Prosser R. A. ( 1998) Neuropeptide Y blocks serotonergic phase shifts of the suprachiasmatic circadian clock in vitro. Brain Res 808, 31– 41 [DOI] [PubMed] [Google Scholar]
- 89.van den Pol A. N., Obrietan K., Chen G., Belousov A. B. ( 1996) Neuropeptide Y-mediated long-term depression of excitatory activity in suprachiasmatic nucleus neurons. J. Neurosci 16, 5883– 5895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weber E. T., Rea M. A. ( 1997) Neuropeptide Y blocks light-induced phase advances but not delays of the circadian activity rhythm in hamsters. Neurosci. Lett 231, 159– 162 [DOI] [PubMed] [Google Scholar]
- 91.Yannielli P. C., Brewer J. M., Harrington M. E. ( 2004) Blockade of the NPYY5 receptor potentiates circadian responses to light: complementary in vivo and in vitro studies. Eur. J. Neurosci 19, 891– 897 [DOI] [PubMed] [Google Scholar]
- 92.Minturn J. E., Fryer H. J., Geschwind D. H., Hockfield S. ( 1995) Toad-64, a gene expressed early in neuronal differentiation in the rat, is related to Unc-33, a C-Elegans gene involved in axon outgrowth. J. Neurosci 15, 6757– 6766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quinn C. C., Chen E., Kinjo T. G., Kelly G., Bell A. W., Elliott R. C., McPherson P. S., Hockfield S. ( 2003) TUC-4b, a novel TUC family variant, regulates neurite outgrowth and associates with vesicles in the growth cone. J. Neurosci 23, 2815– 2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hong H. K., Chong J. L., Song W. M., Song E. J., Jyawook A. A., Schook A. C., Ko C. H., Takahashi J. S. ( 2007) Inducible and reversible clock gene expression in brain using the tTA system for the study of circadian behavior. PLoS Genet 3, 324– 338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beranova-Giorgianni S., Zhao Y., Desiderio D. M., Giorgianni F. ( 2006) Phosphoproteomic analysis of the human pituitary. Pituitary 9, 109– 120 [DOI] [PubMed] [Google Scholar]
- 96.Burbach J. P., Kovács G. L., de Wied D., van Nispen J. W., Greven H. M. ( 1983) A major metabolite of arginine vasopressin in the brain is a highly potent neuropeptide. Science 221, 1310– 1312 [DOI] [PubMed] [Google Scholar]
- 97.Flanagan T., Taylor L., Poulter L., Viveros O. H., Diliberto E. J., Jr. ( 1990) A novel 1745-dalton pyroglutamyl peptide derived from chromogranin-B is in the bovine adrenomedullary chromaffin vesicle. Cell. Mol. Neurobiol 10, 507– 523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iannacone J. M., Ren S., Hatcher N. G., Sweedler J. V. ( 2009) Collecting peptide release from the brain using porous polymer monolith-based solid phase extraction capillaries. Anal. Chem 81, 5433– 5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hatcher N. G., Richmond T. A., Rubakhin S. S., Sweedler J. V. ( 2005) Monitoring activity-dependent peptide release from the CNS using single-bead solid-phase extraction and MALDI TOF MS detection. Anal. Chem 77, 1580– 1587 [DOI] [PubMed] [Google Scholar]
- 100.Haskins W. E., Watson C. J., Cellar N. A., Powell D. H., Kennedy R. T. ( 2004) Discovery and neurochemical screening of peptides in brain extracellular fluid by chemical analysis of in vivo microdialysis samples. Anal. Chem 76, 5523– 5533 [DOI] [PubMed] [Google Scholar]
- 101.Li Q., Zubieta J. K., Kennedy R. T. ( 2009) Practical aspects of in vivo detection of neuropeptides by microdialysis coupled off-line to capillary LC with multistage MS. Anal. Chem 81, 2242– 2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X., Che F. Y., Berezniuk I., Sonmez K., Toll L., Fricker L. D. ( 2008) Peptidomics of Cpe(fat/fat) mouse brain regions: implications for neuropeptide processing. J. Neurochem 107, 1596– 1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abrahamson E. E., Leak R. K., Moore R. Y. ( 2001) The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 12, 435– 440 [DOI] [PubMed] [Google Scholar]
- 104.Levi A., Ferri G. L., Watson E., Possenti R., Salton S. R. ( 2004) Processing, distribution, and function of VGF, a neuronal and endocrine peptide precursor. Cell. Mol. Neurobiol 24, 517– 533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Okamura H., Tanaka M., Kanemasa K., Ban Y., Inouye S. I., Ibata Y. ( 1994) In situ hybridization histochemistry of Vghm1f messenger RNA in the rat suprachiasmatic nucleus: co-localization with vasopressin neurophysin and VIP-PHI. Neurosci. Lett 182, 181– 184 [DOI] [PubMed] [Google Scholar]
- 106.Okamura H., Tanaka M., Kanemasa K., Ban Y., Inouye S. I., Ibata Y. ( 1995) In situ hybridization histochemistry of VGF mRNA in the rat suprachiasmatic nucleus: co-localization with vasopressin/neurophysin and VIP/PHI. Neurosci. Lett 189, 181a– 184a [DOI] [PubMed] [Google Scholar]
- 107.Snyder S. E., Salton S. R. ( 1998) Expression of VGF mRNA in the adult rat central nervous system. J. Comp. Neurol 394, 91– 105 [PubMed] [Google Scholar]
- 108.Wisor J. P., Takahashi J. S. ( 1997) Regulation of the VGF gene in the golden hamster suprachiasmatic nucleus by light and by the circadian clock. J. Comp. Neurol 378, 229– 238 [PubMed] [Google Scholar]
- 109.Yamaguchi H., Sasaki K., Satomi Y., Shimbara T., Kageyama H., Mondal M. S., Toshinai K., Date Y., González L. J., Shioda S., Takao T., Nakazato M., Minamino N. ( 2007) Peptidomic identification and biological validation of neuroendocrine regulatory peptide-1 and -2. J. Biol. Chem 282, 26354– 26360 [DOI] [PubMed] [Google Scholar]
- 110.Kopp M., Meissl H., Korf H. W. ( 1997) The pituitary adenylate cyclase-activating polypeptide-induced phosphorylation of the transcription factor CREB (cAMP response element binding protein) in the rat suprachiasmatic nucleus is inhibited by melatonin. Neurosci. Lett 227, 145– 148 [DOI] [PubMed] [Google Scholar]
- 111.Kiss J. Z., Cassell M. D., Palkovits M. ( 1984) Analysis of the ACTH beta-end alpha-MSH-immunoreactive afferent input to the hypothalamic paraventricular nucleus of rat. Brain Res 324, 91– 99 [DOI] [PubMed] [Google Scholar]
- 112.Barden N., Mérand Y., Rouleau D., Garon M., Dupont A. ( 1981) Changes in the beta-endorphin content of discrete hypothalamic nuclei during the estrous-cycle of the rat. Brain Res 204, 441– 445 [DOI] [PubMed] [Google Scholar]
- 113.Tuinhof R., Artero C., Fasolo A., Franzoni M. F., Ten Donkelaar H. J., Wismans P. G., Roubos E. W. ( 1994) Involvement of retinohypothalamic input, suprachiasmatic nucleus, magnocellular nucleus and locus coeruleus in control of melanotrope cells of Xenopus laevis: a retrograde and anterograde tracing study. Neuroscience 61, 411– 420 [DOI] [PubMed] [Google Scholar]
- 114.Marani E., Rietveld W. J., Luiten P. G., van der Veeken J. G. ( 1987) Cell typing and connections of the rat suprachiasmatic nucleus. Prog. Clin. Biol. Res 227A, 199– 213 [PubMed] [Google Scholar]
- 115.Zamir N., Palkovits M., Brownstein M. ( 1985) Distribution of immunoreactive Met-enkephalin-Arg6-Gly7-Leu8 and Leu-enkephalin in discrete regions of the rat brain. Brain Res 326, 1– 8 [DOI] [PubMed] [Google Scholar]
- 116.Bernay B., Gaillard M. C., Guryca V., Emadali A., Kuhn L., Bertrand A., Detraz I., Carcenac C., Savasta M., Brouillet E., Garin J., Elalouf J. M. ( 2009) Discovering new bioactive neuropeptides in the striatum secretome using in vivo microdialysis and versatile proteomics. Mol. Cell. Proteomics 8, 946– 958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abe H., Honma S., Shinohara K., Honma K. ( 1996) Substance P receptor regulates the photic induction of Fos-like protein in the suprachiasmatic nucleus of Syrian hamsters. Brain Res 708, 135– 142 [DOI] [PubMed] [Google Scholar]
- 118.Hannibal J., Fahrenkrug J. ( 2002) Immunoreactive substance P is not part of the retinohypothalamic tract in the rat. Cell Tissue Res 309, 293– 299 [DOI] [PubMed] [Google Scholar]
- 119.Mikkelsen J. D., Larsen P. J. ( 1993) Substance-P in the suprachiasmatic nucleus of the rat: an immunohistochemical and in-situ hybridization study. Histochemistry 100, 3– 16 [DOI] [PubMed] [Google Scholar]
- 120.Otori Y., Tominaga K., Fukuhara C., Yang J., Yamazaki S., Cagampang F. R., Okamura H., Inouye S. T. ( 1993) Substance P-like immunoreactivity in the suprachiasmatic nucleus of the rat. Brain Res 619, 271– 277 [DOI] [PubMed] [Google Scholar]
- 121.Piggins H. D., Rusak B. ( 1997) Effects of microinjections of substance P into the suprachiasmatic nucleus region on hamster wheel-running rhythms. Brain Res. Bull 42, 451– 455 [DOI] [PubMed] [Google Scholar]
- 122.Shibata S., Tsuneyoshi A., Hamada T., Tominaga K., Watanabe S. ( 1992) Effect of substance P on circadian-rhythms of firing activity and the 2-deoxyglucose uptake in the rat suprachiasmatic nucleus in vitro. Brain Res 597, 257– 263 [DOI] [PubMed] [Google Scholar]
- 123.Shigeyoshi Y., Maebayashi Y., Okamura H. ( 1997) Co-localization of preprosomatostatin mRNA and preprotachykinin A mRNA in neurons of the rat suprachiasmatic nucleus. Brain Res. Mol. Brain Res 48, 159– 163 [DOI] [PubMed] [Google Scholar]
- 124.Fricker L. D., McKinzie A. A., Sun J., Curran E., Qian Y., Yan L., Patterson S. D., Courchesne P. L., Richards B., Levin N., Mzhavia N., Devi L. A., Douglass J. ( 2000) Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J. Neurosci 20, 639– 648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gary K. A., Sollars P. J., Lexow N., Winokur A., Pickard G. E. ( 1996) Thyrotropin-releasing hormone phase shifts circadian rhythms in hamsters. Neuroreport 7, 1631– 1634 [DOI] [PubMed] [Google Scholar]
- 126.Lechan R. M., Jackson I. M. D. ( 1982) Immunohistochemical localization of thyrotropin-releasing hormone in the rat hypothalamus and pituitary. Endocrinology 111, 55– 65 [DOI] [PubMed] [Google Scholar]
- 127.Parkin M. C., Wei H., O'Callaghan J. P., Kennedy R. T. ( 2005) Sample-dependent effects on the neuropeptidome detected in rat brain tissue preparations by capillary liquid chromatography with tandem mass spectrometry. Anal. Chem 77, 6331– 6338 [DOI] [PubMed] [Google Scholar]
- 128.Pan H., Che F. Y., Peng B., Steiner D. F., Pintar J. E., Fricker L. D. ( 2006) The role of prohormone convertase-2 in hypothalamic neuropeptide processing: a quantitative neuropeptidomic study. J. Neurochem 98, 1763– 1777 [DOI] [PubMed] [Google Scholar]
- 129.Biemans B. A., Gerkema M. P., Van der Zee E. A. ( 2002) Increase in somatostatin immunoreactivity in the suprachiasmatic nucleus of aged Wistar rats. Brain Res 958, 463– 467 [DOI] [PubMed] [Google Scholar]
- 130.Fukuhara C., Shinohara K., Tominaga K., Otori Y., Inouye S. T. ( 1993) Endogenous circadian rhythmicity of somatostatin-like immunoreactivity in the rat suprachiasmatic nucleus. Brain Res 606, 28– 35 [DOI] [PubMed] [Google Scholar]
- 131.Shinohara K., Isobe Y., Takeuchi J., Inouye S. T. ( 1991) Circadian-rhythms of somatostatin-immunoreactivity in the suprachiasmatic nucleus of the rat. Neurosci. Lett 129, 59– 62 [DOI] [PubMed] [Google Scholar]
- 132.Vandesande F., Dierickx K., DeMey J. ( 1975) Identification of vasopressin-neurophysin producing neurons of rat suprachiasmatic nuclei. Cell Tissue Res 156, 377– 380 [DOI] [PubMed] [Google Scholar]
- 133.Che F. Y., Yuan Q., Kalinina E., Fricker L. D. ( 2005) Pedtidomics of Cpe(fat/fat) mouse hypothalamus: effect of food deprivation and exercise on peptide levels. J. Biol. Chem 280, 4451– 4461 [DOI] [PubMed] [Google Scholar]
- 134.Alho H., Fremeau R. T., Jr., Tiedge H., Wilcox J., Bovolin P., Brosius J., Roberts J. L., Costa E. ( 1988) Diazepam binding inhibitor gene expression: location in brain and peripheral tissues of rat. Proc. Natl. Acad. Sci. U.S.A 85, 7018– 7022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guidotti A., Toffano G., Costa E. ( 1978) Endogenous protein modulates affinity of GABA and benzodiazepine receptors in rat brain. Nature 275, 553– 555 [DOI] [PubMed] [Google Scholar]
- 136.Ziai R., Pan Y. C., Hulmes J. D., Sangameswaran L., Morgan J. I. ( 1986) Isolation, sequence, and developmental profile of a brain-specific polypeptide, Pep-19. Proc. Natl. Acad. Sci. U.S.A 83, 8420– 8423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wei H., Dean S. L., Parkin M. C., Nolkrantz K., O'Callaghan J. P., Kennedy R. T. ( 2005) Microscale sample deposition onto hydrophobic target plates for trace level detection of neuropeptides in brain tissue by MALDI-MS. J. Mass Spectrom 40, 1338– 1346 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.