Abstract
By exploiting the abundant tissues available from Populus trees, 3–4 m high, we have been able to isolate plasma membranes of high purity from leaves, xylem, and cambium/phloem at a time (4 weeks after bud break) when photosynthesis in the leaves and wood formation in the xylem should have reached a steady state. More than 40% of the 956 proteins identified were found in the plasma membranes of all three tissues and may be classified as “housekeeping” proteins, a typical example being P-type H+-ATPases. Among the 213 proteins predicted to be integral membrane proteins, transporters constitute the largest class (41%) followed by receptors (14%) and proteins involved in cell wall and carbohydrate metabolism (8%) and membrane trafficking (8%). ATP-binding cassette transporters (all members of subfamilies B, C, and G) and receptor-like kinases (four subfamilies) were two of the largest protein families found, and the members of these two families showed pronounced tissue distribution. Leaf plasma membranes were characterized by a very high proportion of transporters, constituting almost half of the integral proteins. Proteins involved in cell wall synthesis (such as cellulose and sucrose synthases) and membrane trafficking were most abundant in xylem plasma membranes in agreement with the role of the xylem in wood formation. Twenty-five integral proteins and 83 soluble proteins were exclusively found in xylem plasma membranes, which identifies new candidates associated with cell wall synthesis and wood formation. Among the proteins uniquely found in xylem plasma membranes were most of the enzymes involved in lignin biosynthesis, which suggests that they may exist as a complex linked to the plasma membrane.
As a model for trees, the Populus genome was recently sequenced (1), chosen because of its relatively small size. Thus, it is now possible to perform proteomics on poplar material to obtain information on e.g. tissue and intracellular distribution of proteins in a tree. We have used this possibility to determine the protein composition of plasma membranes obtained from different tissues of young poplar trees. The plasma membrane constitutes the interface between the cell and the surrounding environment, a position that imposes a number of important functions on the plasma membrane. These include transport of compounds into and out of the cell, communication with the cell exterior, defense against invading pathogens, and cell wall synthesis, functions that are fulfilled by transport proteins, receptors, glucan synthases, proteins involved in membrane trafficking, etc. (2–8). The explicit demand put on plant plasma membranes to support development of a surrounding cell wall is of particular interest, especially the formation of secondary cell wall, which has important economical value as a renewable source in paper and biofuel production. During leafing of poplar trees in spring, there is a rapid development not only of leaves but also of the wood-forming tissue next to the bark in the stem. Using MS, we have determined the protein composition of plasma membranes isolated from leaves, xylem, and cambium/phloem. The protein composition of plasma membranes obtained from different tissues should give important information on the biological activities in these tissues and reveal which proteins are highly expressed in a particular tissue and which are more evenly expressed in all tissues and therefore may be regarded as “housekeeping” proteins. The plasma membranes were obtained from young poplar trees, 3–4 m high, harvested in early summer about 4 weeks after bud break. At this stage, leaves were essentially fully expanded, and both leaf photosynthesis and wood formation in the xylem were expected to have reached a steady state (9, 10). Our understanding of cell wall synthesis has mainly been gathered using Arabidopsis as a model system (11) where the transition from primary to secondary cell wall synthesis has been analyzed at the transcript level (12, 13). To study secondary cell wall formation without interference of multiple cell types, tracheary element differentiation in cell culture is a useful tool (14). Utilizing the system for proteomics, however, is problematic as described by Millar et al. (15). In Populus, a considerable amount of secondary cell wall is produced, which has made high resolution transcript profiling across the wood-forming zone possible. Thus, the different developmental stages from cambium meristem to phloem or xylem have been resolved at the transcript level (16, 17), but characteristics of different cell types, including vessels, fibers, and ray cells, still need further investigation. These studies relied on frozen tissue and cryosectioning not applicable for membrane isolation. The sample treatment and volumes required for the present proteomics study limited the developmental resolution in stem tissue to xylem and cambium/phloem. Our study has so far resulted in the identification of more than 900 proteins of which more than 20% are predicted to be integral membrane proteins. More than 40% of total proteins were found in the plasma membrane fractions of all three tissues, and about one-third if only integral membrane proteins are considered. Twenty-one percent of the integral proteins were only found in leaf plasma membranes, 12% were unique to xylem plasma membranes, and 11% were only found in plasma membranes isolated from cambium/phloem.
EXPERIMENTAL PROCEDURES
Plant Material
Young poplar (Populus tremula × Populus tremuloides, clone S21K884036) trees, 3–4 m high and with a diameter of about 5 cm at the base, were obtained from a field trial at Ekebo, south Sweden (latitude, 55.95°; longitude, 13.12°), belonging to the Forestry Research Institute of Sweden. The ∼3-year-old trees were harvested on June 1, 2006, about 4 weeks after bud break, when leaves were essentially fully expanded. The lowest 1–1.5 m of the stem from five trees was divided (by saw) into pieces of about 2 dm, and the bark was ripped off. Using a knife, the cambium/phloem layer on the inside of the bark was scraped off (yield, 31 g) into a beaker containing preparation medium: 0.33 m sucrose, 50 mm MOPS-KOH, pH 7.5, 5 mm EDTA, 0.2% (w/v) casein hydrolysate, 10% (w/v) polyethylene glycol, 0.6% (w/v) polyvinylpolypyrrolidone, 5 mm ascorbate, 5 mm DTT (polyvinylpolypyrrolidone, ascorbate, and DTT were added immediately before use). Similarly, the xylem on the outside of the remaining stem pieces was scraped off (yield, 44 g). Finally, 50 g of leaves was taken from branches.
Plasma Membrane Isolation
The three plant materials were homogenized, using a knife blender, in 150 ml of preparation medium. Immediately after homogenization, PMSF was added to a final concentration of 0.5 mm together with 1.5 ml of a “protease inhibitor mixture for plant cell and tissue extracts” (Sigma P 9599) containing 4-(2-aminoethyl)benzenesulfonyl fluoride, bestatin, pepstatin A, E-64, leupeptin, and 1,10-phenantroline in DMSO. The homogenates were filtered through a 200-μm nylon mesh and centrifuged at 10,000 × g for 15 min; the supernatants were saved and centrifuged at 30,000 × g for 55 min. The resulting microsomal pellets were resuspended in 5 (xylem and cambium/phloem) and 10 (leaves) ml, respectively, of resuspension medium: 0.33 m sucrose, 5 mm potassium phosphate, pH 7.8, 0.1 mm EDTA, 1 mm DTT (DTT was added immediately before use). Resuspended membranes (4.50 ml for xylem and cambium/phloem and 9.00 ml for leaves) were added to 13.50 (xylem and cambium/phloem) and 27.00 g (leaves) of phase mixtures to produce two 18.00-g and one 36.00-g aqueous polymer two-phase systems with a final composition of 6.1% (w/w) dextran 500, 6.1% (w/w) polyethylene glycol 3350, 5 mm potassium phosphate, pH 7.8, and 3 mm KCl. Plasma membranes were then purified by aqueous polymer two-phase partitioning as described previously (18). The final upper phases were diluted at least 2-fold with 0.33 m sucrose, 5 mm potassium phosphate, pH 7.8, 0.1 mm EDTA, and plasma membranes were pelleted by centrifugation at 100,000 × g for 1 h. The whole preparation procedure was performed at 4 °C. The plasma membrane pellets were resuspended in 0.3 ml of resuspension medium and stored in liquid nitrogen until used. Similarly, the final lower phases from the phase systems, containing intracellular membranes, as well as the remaining parts of the microsomal fractions were diluted ∼10-fold, pelleted, and stored as above. Protein concentration was determined according to Bearden (19).
The plasma membrane vesicles, which on isolation were largely cytoplasmic side-in, were turned inside-out by treatment with the detergent Brij 58 (20) and pelleted again. This was done by mixing, at room temperature, stock solutions of 2 m KCl and of 20 mg/ml Brij 58 in 0.33 m sucrose, 5 mm potassium phosphate, pH 7.8 with plasma membranes to give a detergent to protein ratio of 5:1 (w/w) and a KCl concentration of 0.2 m. Plasma membranes were then pelleted at 100,000 × g for 2 h at 4 °C and resuspended in half the original volume of resuspension medium. The membranes recovered from the lower phases and the microsomal fractions were washed similarly but with Brij 58 excluded from the wash medium. All membrane fractions were then subjected to SDS-PAGE.
SDS-PAGE and Immunoblotting
Samples were solubilized at room temperature in standard sample buffer, and polypeptides were separated by SDS-PAGE (12% acrylamide, 0.3% bisacrylamide) according to Laemmli (21). Gels were either stained with Coomassie Brilliant Blue R-250, or polypeptides were electrophoretically transferred to an Immobilon PVDF transfer membrane (Millipore) for immunostaining. After blocking in 2% (w/v) BSA in PBS (0.15 m NaCl, 0.01 m potassium phosphate, pH 7.5) overnight, the blots were incubated with one of the following rabbit polyclonal antisera diluted in PBS: 1) anti-Lhcb1 (Agrisera, Vännäs, Sweden), an antiserum raised against a peptide corresponding to a sequence of the Lhcb1 protein (one of three Photosystem II light-harvesting complex (LHCII)1 isoforms) of Arabidopsis; 2) anti-COXII (Agrisera), an antiserum raised against a peptide corresponding to a widely conserved sequence of the mitochondrial cytochrome oxidase subunit II; 3) anti-Arf1 (Agrisera), an antiserum raised against the full-length Arabidopsis protein and therefore probably recognizing all ADP-ribosylation factors; 4) anti-H+-ATPase, an antiserum raised against a polypeptide corresponding to amino acids 851–949 of the C terminus of the Arabidopsis H+-ATPase isoform 2 (AHA2) (a kind gift from Professor R. Serrano (Universidad Politecnica, Valencia, Spain)); 5) anti-sucrose synthase, an antiserum raised against sucrose synthase isoform 2 purified from maize (Zea mays) kernels (22) (a kind gift from Professor P. S. Chourey (University of Florida, Gainesville, FL)); 6) anti-PIP2 aquaporin, an antiserum raised against a peptide corresponding to amino acids 271–281 of the C terminus of the spinach (Spinacia oleracea) aquaporin isoform So PIP2;1; this amino acid sequence is conserved in plant aquaporins belonging to the PIP2 subfamily (23), and the antiserum thus recognizes all PIP2 isoforms (a kind gift from Professor P. Kjellbom (Lund University, Lund, Sweden)); and 7) anti-calreticulin, a serum raised against maize calreticulin and also recognizing the closely related calnexin (24). The horseradish peroxidase-conjugated secondary antibody was visualized by ECL (GE Healthcare).
Digestion of Proteins and Recovery of Peptides
After SDS-PAGE, the three lanes containing plasma membranes were each cut into 34 sections, and samples were also taken from some of the major bands in the intracellular membrane fractions (compare Fig. 1). Bands were further cut into 0.5–1 mm cubes and washed with 100 μl of water in microcentrifuge tubes. Gel pieces were destained by repeated incubation in 100 μl of 35% ACN, 50 mm NH4HCO3 for 10 min each. Dehydration was performed twice using 100 μl of ACN for 5 min; in between, 100 μl of 50 mm NH4HCO3 was used in a 30-min rehydration. The second dehydration was completed by evaporation for 10 min at 40 °C (Labconco CentriVap concentrator). Finally, 19 μl of ice-cold trypsin (Gold MS grade; Promega) was added per tube (5 ng/μl in 50 mm NH4HCO3), and gel pieces were rehydrated on ice for 1 h. Excess trypsin was removed, and 50 mm NH4HCO3 was added to cover the gel pieces. Tubes were transferred to a 37 °C incubator for overnight digestion. Extraction of peptides was started by adding 50 μl of 50 mm NH4HCO3 to each tube for a 10-min incubation with occasional gentle vortexing. Tubes were centrifuged for 30 s at 21,000 × g, and supernatants were collected in fresh tubes. The extraction step was repeated twice, and the supernatants were pooled. Extracted peptides were evaporated until dry, dissolved in 10 μl of 0.1% (v/v) formic acid, and stored at −20 °C until used.
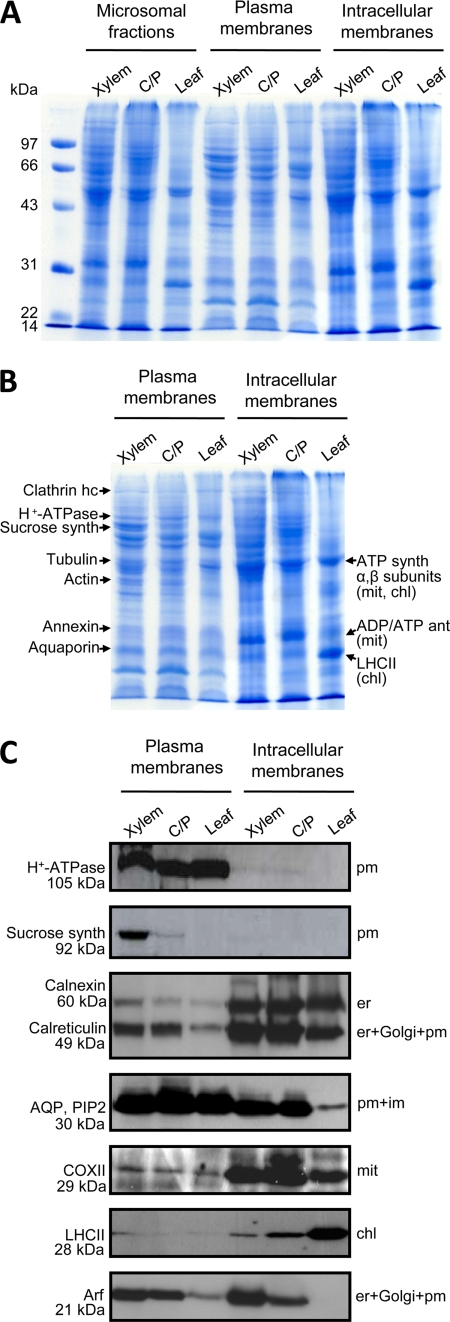
Fig. 1.
Polypeptide patterns of poplar membrane fractions and distribution of marker proteins. A, poplar microsomal fractions obtained from xylem, cambium/phloem (C/P), and leaves, respectively, were subjected to aqueous two-phase partitioning to produce plasma membrane and intracellular membrane fractions. Polypeptides were separated by SDS-PAGE (20 μg of protein/lane) and stained with Coomassie Blue. B, positions in the gel of some of the major proteins identified by mass spectrometry. C, immunoblot using sera directed against the plasma membrane P-type H+-ATPase, sucrose synthase, calnexin and calreticulin, PIP2 subfamily aquaporin, cytochrome oxidase subunit II, Photosystem II light-harvesting complex, and ADP-ribosylation factor. Expected locations for these proteins are indicated to the right. Sucrose synthase is a marker for cellulose/callose synthesis in the plasma membrane, and ADP-ribosylation factor is a marker for vesicle transport, i.e. membrane trafficking. The molecular masses given are the calculated masses and do not necessarily reflect the positions in the gel in A and B. For instance, the P-type H+-ATPase bands is next to the 97-kDa marker (compare A and B), although its isoforms have molecular masses of about 105 kDa. ant, antiporter; AQP, PIP2, PIP2 subfamily aquaporin; chl, chloroplast; COXII, cytochrome oxidase subunit II; er, endoplasmic reticulum; hc, heavy chain; im, intracellular membranes; mit, mitochondria; pm, plasma membrane; synth, synthase.
Identification of Proteins by Peptide Fragmentation
Extracted peptides were separated and analyzed by reversed-phase liquid chromatography-MS/MS using a nanoACQUITY UPLCTM system (Waters) and a Q-Tof UltimaTM (Waters). Solvent gradients and columns used with the UPLC system were as described previously (25). To optimize peptide ionization parameters and supervise splitting of concentrated samples, 0.5 μl of each sample was first analyzed in MS mode. The complexity of the samples was assessed by inspecting the resulting data set (e.g. file size), and some samples were then chosen to be analyzed in the mass range m/z 400–1500, and others were split in two (m/z 400–650 and 650–1500) or three (m/z 400–500, 500–650, and 650–1500) ranges using the include list option. Peptide fragmentation data were generated by automated data-dependent acquisition. The three most abundant signals of a survey scan (400–1500 m/z range, 0.8-s scan time, 0.2-s interdelay) were selected by include list; charge state and collision energy were applied accordingly for sequential MS/MS fragmentation scanning (50–2000 m/z range, 1-s scan time, 0.1-s interdelay). Conversion of raw data to peak lists for the database search was performed with the ProteinLynx Global Server (v2.0.5) software. The following criteria were applied: MS channel: background subtraction (third order, 30% below curve), smoothing (two iterations, Savitzky-Golay, four-channel window), and centering (minimum, six channels; 80% peak height); MS/MS channels: same settings as the MS channel except for background subtraction (55%), centering (minimum four channels), and deisotoping. Two different database searches were performed using a Populus protein database (45,555 entries, assembly release v1.1) created from predicted and translated gene models from the Populus trichocarpa genomic sequence. Searches were performed individually for each sample with analyzed mass ranges merged using a local version of the Mascot search program (v2.2.04; Matrix Science Ltd.) and the Mascot Daemon application (v2.1.6). The settings used for the database search were: trypsin-specific digestion with one missed cleavage allowed, propionamide cysteine (26) set as fixed modification, acetylated N-terminal and oxidized methionine set as variable modifications, peptide tolerance set to 80 ppm, and fragment tolerance set to 0.06 Da. In a primary database search, only proteins that contained one or more top ranking peptide (“bold red” filtering) was exported from Mascot to csv files using a threshold for statistical significance of <0.05 (referred to as “top rank” proteins). A second search on the top 100 proteins was exported including “same set” and “subset” proteins. Multidimensional protein identification technology scoring was applied in both searches. Files were merged by in-house software and arranged in Excel before removal of overlapping peptides within each sample (tissue) as well as peptides with a score below 25. To limit the number of subset proteins with a single peptide hit in supplemental Table 2, a peptide score above 40 was set as the threshold. A false positive rate of 4.7% (47/(947 + 47)) was estimated by decoy database (randomized Populus protein database, 45,555 entries) search. Contamination by keratin was determined by searching a human database downloaded from the International Protein Index (September 25, 2009, 84,032 entries) and linked to Populus data. Assessing the result, we found one protein (ID 493) possible to be background contamination.
Phylogenetic Analysis
Alignments and the phylogenetic tree based on amino acid sequences were computed by MEGA version 4 (27) and presented by TreeView 1.6.6. The following settings were used: Gonnet matrix series, gap opening penalty of 10, gap extension penalty of 0.2, neighbor-joining method, and 2000 bootstrap replicates.
RESULTS
Plasma Membrane Preparations
In the preparation procedure used, the membrane vesicles in the microsomal fraction are partitioned between the upper and lower phases of an aqueous polymer two-phase system (28). The composition of the phase system is adjusted such that plasma membranes partition to the upper phase, whereas intracellular membranes partition to the interface and to the lower phase (18). The yield of plasma membrane protein was 1.7, 1.2, and 1.4 mg from leaves, xylem, and cambium/phloem, respectively, which corresponds to 2, 3, and 6% of total microsomal membrane protein, respectively. SDS-PAGE of the membrane fractions (Fig. 1A) showed very similar polypeptide patterns for the microsomal fraction and the intracellular membrane fraction of each tissue, which was expected because very small proportions of the membranes in the microsomal fractions ended up in the upper phases, i.e. in the plasma membrane fractions. Also, the plasma membrane fractions showed similar polypeptide patterns, although they were derived from different tissues. In Fig. 1B, the positions of some of the major proteins in the plasma membrane and intracellular membrane fractions are indicated, assuming that the number of peptides identified by MS reflects abundance (29) (Table I and supplemental Table 3; data not shown for the intracellular membrane fractions). H+-ATPase isoforms and aquaporins are well known major integral proteins of the plasma membrane (30, 31), but several of the major proteins were peripheral proteins involved in membrane trafficking and as components of the cytoskeleton, such as clathrin heavy chain, annexin, tubulin, and actin. Sucrose synthase was found in a major band in the xylem plasma membranes. The intracellular membrane fractions derived from xylem and cambium/phloem were dominated by mitochondrial membranes, and the ADP/ATP antiporter as well as the α- and β-subunits of the mitochondrial ATP synthase were major polypeptide bands in these fractions. The leaf intracellular membrane fraction was instead dominated by chloroplast membranes, and major bands were due to LHCII protein and the α- and β-subunits of the chloroplast ATP synthase.
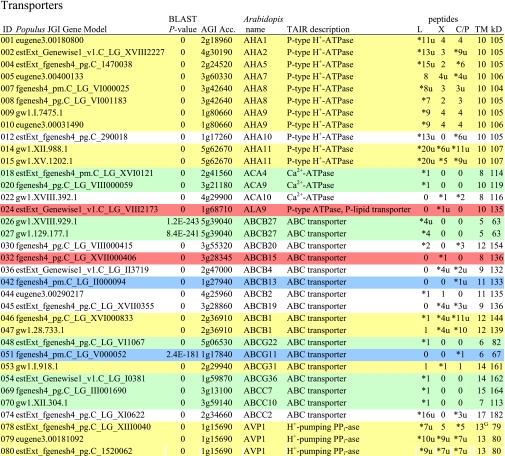
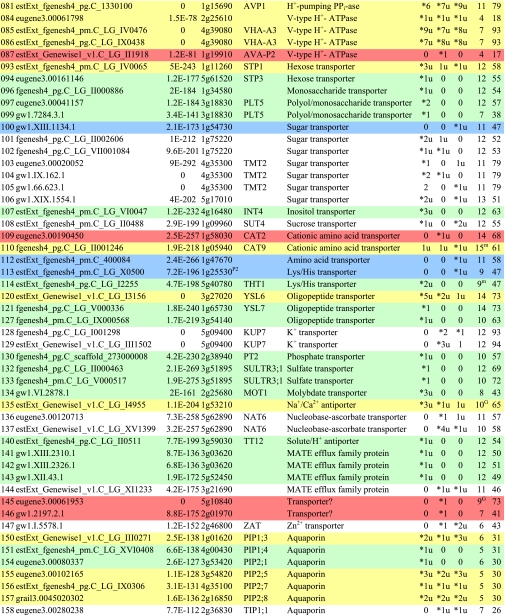
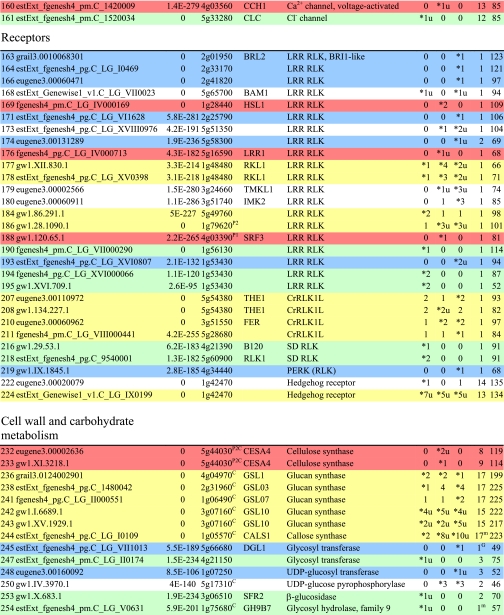
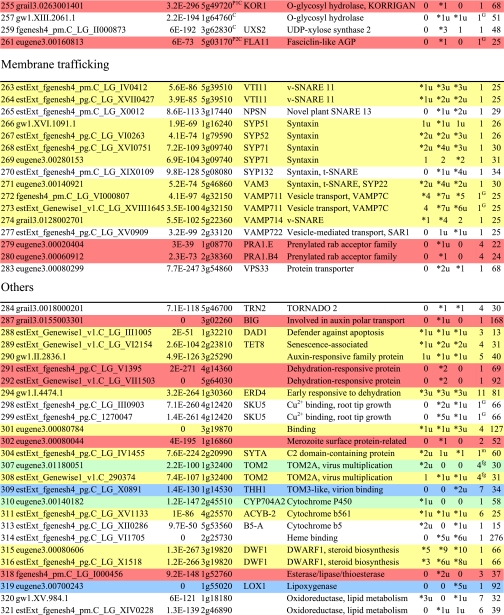
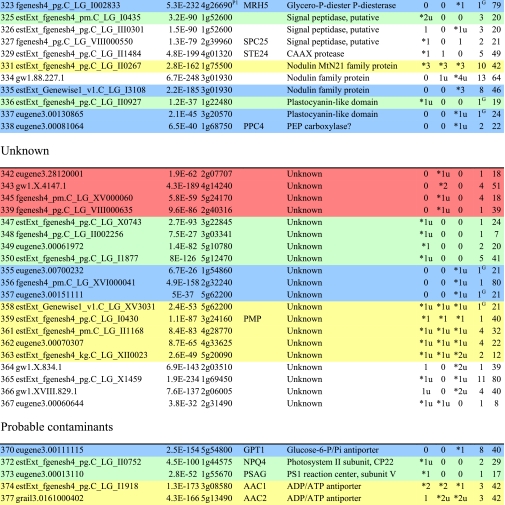
Table I. Integral membrane proteins detected by mass spectrometry in plasma membranes from leaves, xylem, and cambium/phloem.
Integral membrane proteins were identified by Phobius (38), and the number of predicted transmembrane domains (TM) are given in column 10. Proteins are grouped according to function and identified by an ID in column 1, also used in figures and text. Only top rank proteins are included, and the number of peptides identifying each protein is given in columns 7–9 for plasma membranes from leaves (L), xylem (X), and cambium/phloem (C/P), respectively; a number followed by “u” indicates that at least one peptide unique to the protein was found, and a star indicates that the protein is classified as top rank in that tissue. The color code is yellow for proteins found in the plasma membranes from all three tissues and green, red, and blue for proteins found only in leaf, xylem, and cambium/phloem plasma membranes, respectively. All annotation is via the Arabidopsis database at TAIR. Thus, amino acid sequences corresponding to identified gene models in the poplar database (column 2) were blasted against the Arabidopsis database to identify the closest Arabidopsis homolog of each protein, which is identified by its AGI accession number (column 4) followed by its short name in column 5 and description in column 6. Blast p values (column 3) are included to indicate how well the poplar and Arabidopsis amino acid sequences agree. The calculated molecular mass in column 11 is from Mascot. Arabidopsis genes previously suggested to be involved in cell wall formation are indicated in column 4. Predicted lipid anchors are indicated in column 10. For ABC transporters, the new nomenclature according to Verrier et al. (2) was used, and for RLK receptors, we used the nomenclature in Shiu and Bleecker (5). For a complete list of integral proteins including the subset, see supplemental Table 2. PM, plasma membrane; PEP, phosphoenolpyruvate; MATE, multidrug and toxin extrusion.
C Gene present in the Cell Wall Navigator database (11).
P1 Highly coregulated gene for At CESA1, -3, and -6, i.e. primary cell wall formation (12).
P2 Highly coregulated gene for At CESA4, -7, and -8, i.e. secondary cell wall formation (12).
G GPI.
m Myristoyl.
g Geranylgeranyl.
f Farnesyl (predictions described in Ref. 49).
Plasma membranes prepared by aqueous two-phase partitioning are usually of high purity (about 95%) and consist mainly of right side-out (cytoplasmic side-in) vesicles (Ref. 4 and references therein). A very low degree of contamination by other membranes was supported also by the present investigation. Immunoblotting (Fig. 1C) showed a strong enrichment of the P-type H+-ATPase, a canonical plasma membrane protein, and also of PIP2 aquaporin in the plasma membrane fractions. However, substantial amounts of PIP2 aquaporin were also found in the intracellular membrane fractions, particularly those from xylem and cambium/phloem, in agreement with previous reports on the presence of plasma membrane intrinsic proteins (PIPs) also in intracellular membranes (32). By contrast, LHCII, a major component of chloroplast thylakoid membranes, and cytochrome oxidase (subunit II), a major component of mitochondrial inner membranes, were almost exclusively found in the intracellular membrane fractions. Calreticulin and calnexin, which are usually regarded to be located in the endoplasmic reticulum (33), were both mainly found in the intracellular membrane fractions. Notably, calreticulin has also been located to both Golgi and plasma membranes in tobacco using immunogold labeling (34). Calnexin is probably a more reliable marker for the endoplasmic reticulum because calnexin, unlike calreticulin, is an integral membrane protein. Sucrose synthase, a soluble enzyme that associates with cellulose synthase (35), was used as a marker for cellulose synthesis and was clearly most abundant in the xylem plasma membrane fraction where wood formation occurs. The antiserum raised against the full-length Arabidopsis Arf1 protein will most probably detect all ADP-ribosylation factors because they form a closely related family with 21 members in Arabidopsis (36). The ADP-ribosylation factors are small GTPases that are regulators of vesicular trafficking and thus present in all membranes involved in that process (37). Judging from the staining by the Arf1 antiserum, membrane trafficking was most intense in the xylem engaged in wood formation and least intense in the essentially fully expanded leaves (Fig. 1C). A low degree of contamination of the plasma membrane fractions was also suggested by the MS data (Table I); only peptides identifying the ADP/ATP antiporter (compare Fig. 1B), one Photosystem I and one Photosystem II subunit, and the glucose 6-phosphate/Pi antiporter, markers for the mitochondrial inner membrane, the chloroplast thylakoid membrane, and the plastid envelope inner membrane, respectively, were found in the plasma membrane fractions. As a final step in the purification of the plasma membranes, they were treated with the detergent Brij 58 and 0.2 m KCl to turn the cytoplasmic side-in vesicles inside-out and thus remove soluble proteins enclosed in the vesicles as well as loosely bound proteins. Plasma membranes are not solubilized by this detergent, but the vesicles are simply turned cytoplasmic side-out (20). However, we do not know to what extent other membranes are solubilized by Brij 58, and the detergent was therefore excluded from the wash medium for the microsomal and intracellular membrane fractions. If some of the intracellular membranes were solubilized, the Brij treatment of the plasma membrane fractions would constitute an additional purification step also with respect to contaminating integral membrane proteins. Proteins remaining after this procedure were separated by SDS-PAGE, sections of the lanes were excised, and the excised proteins were further processed for analysis by nano-LC-MS/MS.
Protein Identification
A total of 956 proteins was identified by matching peptide fragment ion mass data to the Populus sequence database (Fig. 2). These proteins were either identified by one or more peptides unique to a particular protein, or they were ranked as first choice (top rank) by the identification software used (Mascot v2.2.04, Matrix Science Ltd.) based on one or more peptides shared by e.g. several members of a protein family. Thus, in addition to the 956 top rank proteins, about 800 proteins ranked as subset (proteins also matching peptide fragment ion mass data but with lower ranking) were also identified. In Figs. 2, 3, 4, and 6; Table I, and supplemental Table 3, only top rank proteins are included. However, in Fig. 5, displaying a large protein family, and in supplemental Table 2, subset proteins are also included. This should be justified because already the Arabidopsis genome contains a large number of closely related genes coding for members of protein families, a feature that should be even more emphasized in the 40–60% larger protein-coding genome of Populus (1). In supplemental Tables 2 and 3, each protein is identified with a number running from 1 to 1122. These IDs are kept in Table I and used in Figs. 5 and 6 and in the text to refer to the respective proteins. All annotations in Table I and supplemental Tables 2 and 3 were via the Arabidopsis database at TAIR. Thus, amino acid sequences corresponding to identified gene models in the Populus v1.1 (45,555 entries) database were blasted against the Arabidopsis TAIR8 (32,825 entries) data set to identify the closest Arabidopsis homolog of each protein, which in Table I and supplemental Tables 2 and 3 is identified by its AGI accession number. p values are included to give an indication of how well the poplar and Arabidopsis amino acid sequences agree and thus of the reliability of the annotation for the poplar protein.
Fig. 2.
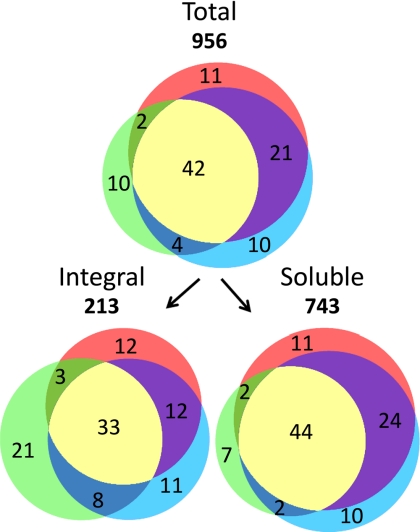
Tissue distribution of plasma membrane proteins detected by mass spectrometry. The distribution (%) of proteins among plasma membranes from leaves (green), xylem (red), and cambium/phloem (blue) is shown. Only the 956 proteins classified as top rank are included. These are further divided into 213 integral membrane proteins, i.e. proteins predicted to have one or more transmembrane domain using the program Phobius (38), and 743 soluble proteins.
Fig. 3.
Integral protein composition of plasma membranes from leaves, xylem, and cambium/phloem. The 213 top rank integral membrane proteins (Fig. 2) have been divided into classes, mainly according to function (Table I).
Fig. 4.
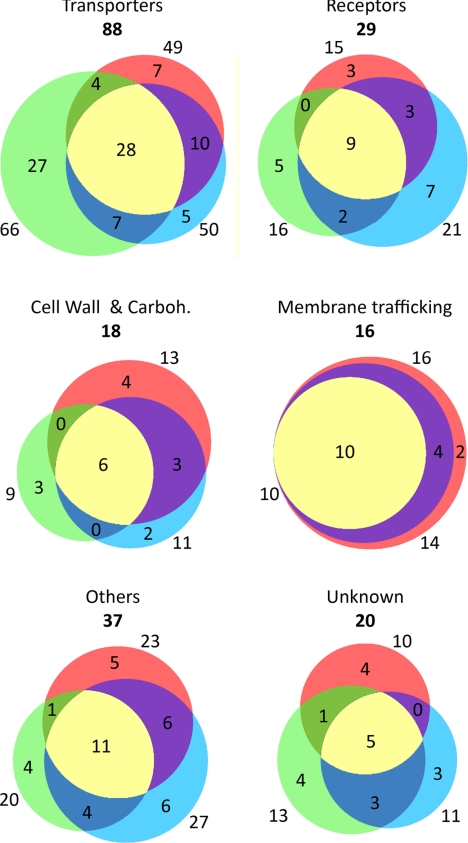
Distribution of major classes of integral proteins between plasma membranes from leaves, xylem, and cambium/phloem. The distribution (number of proteins) of integral membrane proteins among plasma membranes from leaves (green), xylem (red), and cambium/phloem (blue) is shown. Only the 213 integral proteins (Fig. 2) classified as top rank are included. Carboh, carbohydrate metabolism.
Fig. 6.
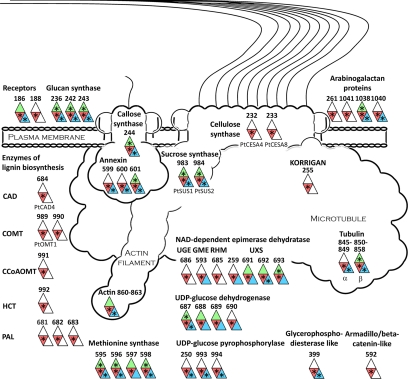
Proteins involved in wood formation. A schematic model of the cellulose-synthesizing complex and other proteins associated with wood formation, such as enzymes involved in lignin biosynthesis, is shown. The numbers outside each symbol refer to the gene model ID in Table I for integral proteins and supplemental Table 3 for soluble proteins. Only proteins detected in the xylem plasma membranes are included, and the color code is green for leaf, red for xylem, and blue for cambium/phloem plasma membranes. A star in a colored field indicates that the protein is classified as top rank in that tissue. When available, Populus names are stated below the symbol. Abbreviations for the NAD-dependent epimerase dehydratase family (11) are: UGE, UDP-d-glucose 4-epimerase; GME, GDP-d-mannose 3,5-epimerase; RHM, UDP-l-rhamnose synthase; UXS, UDP-d-apiose/xylose synthase. Abbreviations for the lignin enzymes are: CAD, cinnamyl alcohol dehydrogenase; COMT, caffeic acid O-methyltransferase; CCoAOMT, caffeoyl-CoA 3-O-methyltransferase; HCT, hydroxycinnamoyltransferase; PAL, phenylalanine ammonia-lyase.
Fig. 5.
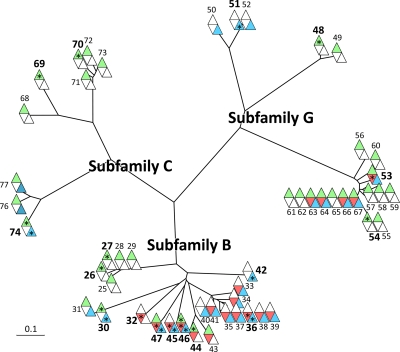
Phylogenetics analysis of plasma membrane ABC transporters and their tissue localization. ABC transporters detected by mass spectrometry, both top rank and subset, are included (one truncated sequence, ID 75 in Table I, was excluded). The numbers outside each symbol refer to the gene model ID in Table I (top rank integral proteins; numbers in bold) and supplemental Table 2 (both top rank and subset integral proteins). The phylogenetic analysis (27) was supervised by using all Arabidopsis proteins annotated to subfamilies B, C, and G (2). The color code is green for leaf, red for xylem, and blue for cambium/phloem plasma membranes. A star in a colored field indicates that the protein is classified as top rank in that tissue.
A relatively large part (42%) of the 956 top rank proteins was found in the plasma membranes of all three tissues, and only 10–11% were unique to a particular tissue (Fig. 2). Using the software Phobius (38) to predict transmembrane domains, 213 integral proteins were identified, and the remaining 743 were classified as soluble proteins (Fig. 2). Among these 743 soluble proteins, seven are predicted to have transmembrane domains but judged by us to be soluble proteins based on previous knowledge of these proteins (protein IDs 622, 623, 628, 629, 963, 964, and 1104 in supplemental Table 3); most of them are subunits of the proteasome. Many of the soluble proteins are likely to be true peripheral proteins of the plasma membranes. However, because of the difficulties involved in differentiating between true peripheral proteins and contaminating soluble proteins, the present study is focused on the integral membrane proteins.
Tissue Distribution of Integral Membrane Proteins
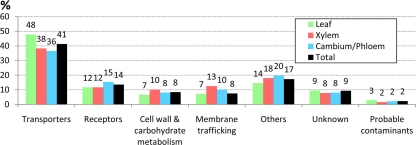
The 213 top rank proteins predicted to be integral membrane proteins are listed in Table I where they are divided according to function into “transporters,” “receptors,” “cell wall and carbohydrate metabolism,” “membrane trafficking,” “others,” “unknown,” and “probable contaminants.” The integral protein composition of plasma membranes from leaves, xylem, and cambium/phloem is shown in Fig. 3, and the overlap between tissues for each class of integral protein is shown in Fig. 4. Notably, almost half of the identified leaf plasma membrane integral proteins are transporters (Fig. 3), partly reflecting the photosynthetic activity, which not only involves CO2 fixation and hence carbohydrate transporters but also reduction of nitrate to produce amino acids and reduction of sulfate to produce thiol. Thus, 75% of the transporters were found in the leaf plasma membranes, 32% were found in the plasma membranes of all three tissues, and only 25% were exclusively found in the plasma membranes of xylem and/or cambium/phloem (Fig. 4). The plasma membranes isolated from cambium/phloem had the highest proportion of receptors (Figs. 3 and 4), possibly reflecting the position of this tissue in the periphery of the stem. More than 70% of the proteins involved in cell wall and carbohydrate metabolism and all of the proteins engaged in membrane trafficking were identified in the xylem plasma membranes (Figs. 3 and 4) in agreement with the role of the xylem in wood formation. Furthermore, about 60% of the proteins involved in membrane trafficking were identified in the plasma membranes of all three tissues, and not a single protein was uniquely identified in the leaf plasma membranes (Fig. 4). This suggests that membrane trafficking is a process that relies mainly on housekeeping proteins in agreement with the data of Uemura et al. (8). Very few obvious contaminants were found (Fig. 3).
Transporters
Transporters were by far the largest class of integral proteins identified in the plasma membranes of all three tissues (Table I and Fig. 3). In Table I and supplemental Table 2, they are listed in the following order: pumps (IDs 1–92), carriers (IDs 93–149), and channels (IDs 150–161).
Assuming that the number of identified peptides reflects protein abundance, the H+-ATPase (IDs 1–15; Table I) was the most abundant transporter. This is expected from its role in the plasma membrane where it creates the electrochemical gradient driving secondary transport and therefore should be an abundant housekeeping protein. It is also in agreement with earlier findings that the H+-ATPase constitutes several percent of total integral protein in spinach leaf plasma membranes (calculated from recovery of the protein upon isolation (30)). At least 13 H+-ATPase isoforms were detected. Based upon data from Arabidopsis, tobacco, and rice, which all contain 10 or more H+-ATPase isoforms, the isoforms have been divided into five subfamilies, and subfamily I and II members have been suggested to be the most highly expressed (3). AHA1, -2, and -5 belong to subfamily II, and AHA11 belongs to subfamily I, and indeed, homologs to these isoforms are among the most highly expressed in poplar. However, homologs to AHA7, -8, -9 and -10 also show relatively high levels of expression in poplar in contrast to what is known for Arabidopsis where these isoforms are weakly expressed and/or show a very tissue-specific expression: AHA8 and -9 are highly expressed only in the flower, and AHA10 is only expressed in the seed coat (Genevestigator (39)). The Arabidopsis AHA10 is not even localized to the plasma membrane but seems to have a role in acidifying transport vesicles in the seed coat (40). Notably, the poplar homolog of AHA10 (ID 12) was the only isoform that was not identified in all three tissues.
ATP-binding cassette (ABC) transporters constitute the largest family of pumps found in the present investigation (IDs 25–77; Table I and supplemental Table 2). ABC transporters are able to transport a broad array of compounds. Substrates ranging from lipids to ions have been reported, and ABC transporters are involved in many different processes, such as resistance to pathogens, detoxification, and cutin accumulation at the plant surface (2). According to a recent inventory and new nomenclature for plant ABC proteins (2), they can be divided into nine subfamilies, ABCA–I, with all subfamilies except H found in plants. A phylogenetic analysis of the ABC transporters identified by us (both top rank and subset; Table I and supplemental Table 2) showed that they all fall within three subfamilies, B, C, and G (Fig. 5), with a rather “specific” tissue distribution. Thus, only a few of the ABC transporters were identified in the plasma membranes of all three tissues, two in subfamily B (IDs 46 and 47), five in G (IDs 53, 63, 64, 66, and 67), and none in C. The two subfamily B members (IDs 46 and 47) are homologs to At ABCB1 (earlier PGP1) reported to catalyze auxin efflux (41). Most of the ABC transporters were identified in leaves and many of these in leaves only; notably all were in subfamily C (IDs 68–77) except one (ID 74), which was also present in cambium/phloem. Just a few were identified in cambium/phloem only, one in subfamily B (ID 42) and three in G (IDs 50–52). Only one, a subfamily B member (ID 32), was specific to xylem, and its Arabidopsis homolog, ABCB15 (MDR13), has been suggested to excrete lignin monomers into the apoplast (42). Subfamily B is a large and diverse subfamily not only involved in export of secondary metabolites. Thus, At ABCB4 (PGP4; homolog to IDs 34, 36–39, and 41) appears to catalyze import of auxin (43), and At ABCB27 (TAP2/ALS1; homolog to IDs 25–29) has been suggested to have a role in aluminum resistance, albeit in root tissue (44). Subfamily G is markedly expanded in plants with more than 40 members in both Arabidopsis and rice (2). At ABCG36 (PEN3/PDR8; homolog to IDs 54 and 61) is suggested to export antifungal materials at attempted invasion sites and in that way confer resistance to powdery mildews (45), and the wheat homolog, LR34, is associated with resistance to multiple fungal pathogens (46). Both poplar homologs (IDs 54 and 61) were only identified in leaves. At ABCG11 (WBC11; homolog to IDs 50–52) is involved in the export of wax components and is required for normal cutin accumulation at the cell surface (47). Notably, the poplar ABCG11 homologs (IDs 50–52) were only detected in the plasma membranes from cambium/phloem, i.e. in a tissue that should be involved in secretion of cuticular lipids.
Two pumps usually associated with the vacuolar membrane were also detected: H+-pumping PPi-ase (IDs 78–81; Table I) and V-type ATPase (IDs 84–87). Both pumps have been reported earlier in proteomics studies of plasma membranes from e.g. Arabidopsis leaves and cell cultures (48, 49). Both pumps have also been localized to plant plasma membranes by immunostaining in situ (50, 51), and it was suggested that the plasma membrane constitutes “a temporary repository for tonoplast proteins en route to the vacuole” (50).
Most of the carriers (IDs 93–147; Table I) are predicted to have 9–15 transmembrane domains and are therefore likely to belong to the “major facilitator superfamily” (4), the members of which typically have around 12 transmembrane domains. Only a few of the carriers were found in all three tissues (IDs 93, 110, 120, and 135), and as few were specific to xylem (IDs 109, 145, and 146) or cambium/phloem (IDs 100, 112, and 113). Most of the carriers were found in the leaf plasma membranes and are likely associated with the photosynthetic reduction of CO2 to carbohydrate, nitrate to amino nitrogen, and sulfate to thiol. Thus, a number of carriers of carbohydrates (IDs 93–108), amino acids and oligopeptides (IDs 110 and 114–127), and sulfate (IDs 132 and 133) were localized to the leaf plasma membranes as well as a putative molybdate transporter (ID 134). The Arabidopsis homolog to this protein was recently identified as a high affinity molybdate transporter and named MOT1 (52) and shown to be present in the plasma membrane of both roots and shoots in agreement with the localization of its poplar homolog (ID 134). The largest sink for molybdate in plants is probably nitrate reductase, which catalyzes the first step in the mainly photosynthetic conversion of nitrate to amino nitrogen.
Only a few channels (IDs 150–161; Table I) were detected. Eight aquaporins, six PIPs (IDs 150–157) expected to be localized in the plasma membrane as indicated by the name, and one tonoplast intrinsic protein (TIP) (ID 158) rather expected to be found in the vacuolar membrane. TIPs have been recorded earlier in proteomics studies of plant plasma membranes (e.g. Ref. 48) and also localized to plasma membranes by immunostaining in situ (50). Thus, the PIP and TIP nomenclature as an indication of location has been questioned (32). Aquaporins should be housekeeping proteins; still some “tissue specificity” was observed, and two aquaporins were only detected in leaf tissue (IDs 151 and 154). Aquaporins are also really major integral proteins of plant plasma membranes, and PIPs may constitute about 20% of total integral protein (31) in contrast to ion channels, which because of their high transport capacity are only needed in small amounts and therefore easily escape detection by MS. Thus, only two ion channels, a “xylem-specific” Ca2+ channel (ID 160) and a “leaf-specific” Cl− channel (ID 161), were found. Chloride channels are involved in turgor regulation and may therefore be relatively abundant.
Receptors
Two types of receptors (Table I), receptor-like kinases (RLKs; IDs 163–219) and Hedgehog receptors (IDs 222 and 224) were identified. The RLKs constitute by far the largest family of integral proteins found in the present survey and indeed the largest family of receptors in plants with over 600 members in Arabidopsis and over 1100 in rice (5, 6). They typically have an N-terminal extracellular domain for signal perception followed by a transmembrane domain and a C-terminal intracellular protein kinase domain for propagation of the signal. All RLKs detected by us fall within four subfamilies according to the classification of their Arabidopsis homologs (IDs 163–219; Table I). The largest subfamily, the leucine-rich repeat (LRR) RLKs (IDs 163–195), have an extracellular domain with 1–32 LRRs, which e.g. may interact with pathogen-specific molecules (so-called pathogen-associated molecular pattern) to trigger innate immunity (53) or with brassinosteroids and thus serve as hormone receptors (e.g. At BRI1 (54)). A clear tissue specificity was observed, and only four of the 20 LRR RLKs were detected in the plasma membranes of all three tissues.
The CrRLK1L (Catharanthus roseus RLK1-like) receptors are the second largest group (IDs 207–211), and all four members were found in the plasma membranes of all three tissues. The N-terminal extracellular domains of this group do not show any similarity to domains of known function. However, the functions of two members of the group, At THE1 (THESEUS1; homolog to IDs 207 and 208) and At FER (FERONIA; homolog to ID 210), have recently been elucidated. Both are involved in growth regulation; THESEUS inhibits cell expansion by somehow affecting cellulose synthesis, and FERONIA inhibits the growth of pollen tubes at the target, and it is suggested that also other members of the CrRLK1L subfamily control cell growth during development and perhaps upon pathogen attack (55).
A member of the proline extensin-like receptor kinase (PERK) subfamily was found in cambium/phloem plasma membranes only (ID 219). The extracellular domain is proline-rich, but there is no information on ligands or the function of these receptors. The two SD RLKs (IDs 216 and 218) were both found in leaf plasma membranes only. SD is for S-locus glycoprotein-like domain, and this extracellular domain has lectin properties and specifically binds α-d-mannose. The Brassica S-locus receptor kinase mediates the self-incompatibility response (56), and a recent report suggests that SD RLKs may function as negative regulators in plant defense responses (57). An additional seven putative RLKs can be found among the soluble proteins in supplemental Table 3 (IDs 458–460, 470, 471, 515, and 608). The reason that these RLKs have not been classified as integral proteins could either be due to the program used to predict transmembrane domains (38) or be due to mistakes in the gene model. These additional RLKs all fall within the subfamilies already identified (Table I).
Receptors belonging to the patched family, and hence with putative Hedgehog receptor activity, were also detected (IDs 222 and 224; Table I). The Hedgehog signaling pathway was first discovered in Drosophila and is a fundamental signal transduction pathway in animal development, controlling axial patterning and stem cell fate (58).
Cell Wall and Wood Formation
To identify proteins involved in cell wall formation, 660 Arabidopsis genes were extracted from the Cell Wall Navigator database (11) and matched to our data set. In addition, a number of genes identified to be involved in wood formation by analyzing coexpression of genes during secondary cell wall formation (12) were added. Several proteins, both integral and peripheral, corresponding to such genes were identified in this study, particularly in the xylem plasma membranes (Table I and supplemental Table 3). Fig. 6 shows a schematic model of the cellulose-synthesizing complex and other proteins associated with wood formation (modified from Refs. 59–61) where proteins identified by us are indicated.
The cellulose synthase complex is highly integrated with the cytoskeleton (59, 61), and an elevated expression of α- and β-tubulin genes in xylem tissue compared with leaf tissue was shown by Oakley et al. (62). Our results confirm these findings at the protein level, and essentially all tubulins were identified in xylem (IDs 845–859; supplemental Table 3). Notably, no α-tubulin (IDs 845–849) was identified in the leaf plasma membranes. Furthermore, the large (>220-kDa) homolog (ID 813) of the At MOR1 protein was found, at the top of the gel, in the xylem plasma membranes. This protein has been suggested to have a role in microtubule stabilization and in linking microtubules to the plasma membrane via plasma membrane-associated proteins (63). We also detected an enrichment of actin (IDs 860–864) in the stem tissues, all homologs of At ACT7 and -11, as well as actin-binding villin (IDs 777 and 778), profilin (IDs 779 and 780), and actin-depolymerizing factor (IDs 781 and 782). Cellulose synthases of the CESA (GT2 family (7)) subfamily should have a central role in the complex, and homologs of At CESA1 (IDs 229–231), CESA4 (IDs 232 and 233), and CESA7 (IDs 234 and 235) were detected, although only the two At CESA4 homologs, Pt CESA4 (ID 232) and Pt CESA8 (ID 233), were classified as top rank proteins (Table I and supplemental Table 2). At CESA4, -7, and -8 are associated with secondary cell wall formation in Arabidopsis (12).
Sucrose synthase (SUS) is a soluble enzyme that associates with the plasma membrane and has been suggested to provide the substrate, UDP-glucose, for cellulose synthase and callose synthase (35). We identified a large number of peptides from Pt SUS1 (ID 983) and Pt SUS2 (ID 984) in the plasma membranes from the wood-forming tissue and less of Pt SUS3 (ID 985) and another member of the GT4 family (7) (ID 986) in agreement with the gene expression data of Geisler-Lee et al. (7). This is also consistent with the immunolocalization of sucrose synthase predominantly to the xylem plasma membranes (Fig. 1C). Within the GT48 family (7) (IDs 236–244), most peptides were recovered from a likely callose synthase (ID 244) with highest sequence similarity to At CALS1, slightly enriched in cambium/phloem as ID 243. Annexin (IDs 599–601), a regulator of callose synthase activity (64) and a putative membrane-cytoskeleton linker (65), was enriched in the stem tissue. KORRIGAN (ID 255), an O-glycosyl hydrolase required for cellulose microfibril synthesis (66), was only found in the xylem.
Several peptides were recorded for the homologs of At UXS6, UDP-xylulose synthase 6 (IDs 691 and 692), in stem tissues. This gene is coregulated with the secondary cell wall-specific CESA4, -7, and -8 in Arabidopsis (12). A homolog of the membrane-bound Arabidopsis UDP-xylulose synthase 2, At UXS2 (ID 259), was also enriched in stem tissues in agreement with the data of Pattathil et al. (67). Peptides were also recovered from fasciclin-like arabinogalactan (FLA) proteins in the xylem plasma membranes, particularly from the homolog of At FLA10 (ID 1040) with a recognized abundant transcript during tension wood formation in Populus (68). Arabinogalactan proteins are highly glycosylated (69) and would consequently be difficult to digest during sample preparation. The FLA proteins are likely anchored to the plasma membrane by glycosylphosphatidylinositol (GPI), and the GPI anchor might have a central role in cell wall synthesis (70). Coexpression analyses (12) also identified two LRR RLKs (IDs 186 and 188) as proteins linked to wood formation. Interestingly, almost all of the enzymes involved in lignin monomer biosynthesis (defined in Ref. 71) were found in the xylem plasma membranes (IDs 681–684 and 989–992).
Membrane Trafficking
A number of SNARE family proteins (SNAREs, syntaxins, and VAMPs) were found (IDs 263–277; Table I) as well as some other proteins (IDs 279, 280, and 283) probably involved in membrane trafficking. SNAREs mediate membrane fusion and have a key role in endocytotic cycling of membranes, including recycling of receptors and other plasma membrane proteins (72, 73). They are also involved in exocytosis, which is an important feature in cell wall formation (74). Notably, all SNAREs but three (IDs 265, 270, and 277) were found in the plasma membranes of all three tissues, and SNAREs thus seem to be housekeeping proteins as shown by Uemura et al. (8) for Arabidopsis. However, judging from the number of peptides identified, membrane trafficking was most intense in the xylem, a tissue in rapid growth, and least intense in the leaves, which were essentially fully expanded at harvest.
Soluble Proteins
The plasma membranes were subjected to a combined detergent (Brij 58) and salt wash to remove loosely bound proteins. Because the Brij 58 treatment also turns the vesicles inside-out, proteins simply enclosed in the vesicles were also removed. Thus, the soluble proteins that remain associated with the plasma membranes should be enriched in true peripheral plasma membrane proteins. All top rank soluble proteins found in the plasma membrane fractions are listed in supplemental Table 3 (IDs 380–1122) grouped according to their functions in TAIR. About 7% of these proteins are predicted to have a lipid anchor (prediction described in Ref. 49). A number of proteins known to be associated with plasma membranes were identified, such as 14-3-3 protein (IDs 514 and 783–788) and calmodulin (IDs 602–605), activators of e.g. the plasma membrane P-type H+-ATPase (75) and Ca2+-ATPase (76), respectively; components of the cytoskeleton, such as actin (IDs 860–864) and tubulin (IDs 845–859), which are anchored to the plasma membrane, as well as ribosomal proteins (IDs 386, 387, and 865–980) attached to the plasma membrane via the cytoskeleton (77, 78); and proteins involved in membrane trafficking, including endocytosis and exocytosis, such as small (mainly Rab) GTPases (e.g. IDs 516–554, 606, 607, and 789–792), clathrin heavy chain (IDs 1009–1012) and related proteins (IDs 793–797) (61), and annexin (IDs 599–601). Some of these peripheral proteins are abundant in the plasma membrane fractions (compare Fig. 1B), judging from the number of peptides recovered (supplemental Table 3). A set of protein- or lipid-modifying enzymes involved in signal transduction downstream of receptors was detected, such as protein kinases (IDs 453–457); protein phosphatase 2C (ID 400); phospholipases A (ID 401), C (IDs 402–404), and D (IDs 405–408); and phosphatidylinositol 4-kinase (ID 469). Several proteins involved in wood formation were also found, particularly in the xylem plasma membranes, and these are discussed above.
DISCUSSION
We have identified more than 900 proteins in the plasma membranes isolated from leaves, xylem, and cambium/phloem obtained from young poplar trees (Table I and supplemental Table 3). About 22% of these proteins are predicted to be integral membrane proteins (Fig. 2). Thus, the majority of the proteins are soluble proteins and putative peripheral proteins of the plasma membranes. However, because of the problems involved in differentiating between true peripheral proteins and contaminating soluble proteins, we have chosen to focus on the proteins predicted to have one or more transmembrane domains. Among these 213 integral membrane proteins, transporters constitute the largest class (41%) followed by receptors (14%) and proteins involved in cell wall and carbohydrate metabolism (8%) and membrane trafficking (8%) (Fig. 3). Others, of which various stress-induced proteins are the largest group, represent only 17%, and “unknowns” only represent 9% (Fig. 3). These are data similar to those reported earlier for plasma membranes from Arabidopsis leaves (48) and cultured cells (49), rice shoot and root tissue (79), and grape berries (80). Particularly, transporters and receptors dominate in the studies above, and this is also the case in the studies aimed at identifying phosphorylated proteins in Arabidopsis (81–83) and rice (79) plasma membranes. Thus, many transporters are shown to be phosphorylated, probably as a means to regulate their activities, as well as many receptors in the RLK family, not surprisingly, because they undergo autophosphorylation. Using localization of organelle proteins by isotope tagging (LOPIT), the main location for 527 proteins obtained from an Arabidopsis callus culture were determined (84). Of these 527 proteins, 417 are classified as integral membrane proteins by Phobius (38), and 50 of these integral proteins are found among our poplar homologs (Table I). These include 10 of their 130 endoplasmic reticulum proteins, four of the 69 Golgi proteins, two of the 71 mitochondrial/plastid proteins, six of the 68 not classified, eight of the 17 vacuolar proteins, and 20 of the 62 plasma membrane proteins. The LOPIT data should reflect the steady state distribution of proteins, which will vary depending on e.g. tissue and developmental stage. Thus, endomembrane proteins are in a constant flux, and for example, plasma membrane proteins travel through the endoplasmic reticulum and Golgi before they reach the cell surface and may then again be internalized and recycled (73). When 22 of the proteins in the LOPIT study were expressed as green fluorescent protein fusion proteins in tobacco leaves, 16 were targeted as predicted, two were not targeted as predicted, and the localization of four was inconclusive, and it was suggested that location may differ between tissues (84).
In the present study, the aim was to identify plasma membrane proteins common to leaves, xylem, and cambium/phloem of young poplar trees as well as proteins specific to these tissues. The leaves were essentially fully expanded at harvest, and the overlap with published transcript profile clusters (9, 16, 17) suggests sampling of xylem from the secondary cell wall-forming zone and cambium/phloem at earlier stages of cell wall development. One-third of the integral proteins and 44% of the soluble proteins were found in all three tissues (Fig. 2). These proteins, which are common to all tissues, may be regarded as housekeeping proteins providing “everyday service.” A typical example is the P-type H+-ATPase, which creates the electrochemical gradient across the plasma membrane, driving secondary transport (IDs 1–15; Table I), and therefore should be present in virtually all living plant cells. However, many of the proteins only found in one (31%) or two (27%) tissues (Fig. 2) are not necessarily “tissue-specific” proteins. The identification of a protein by MS is partly dependent on protein abundance. Generally, highly expressed proteins will have a larger probability to be detected than lowly expressed proteins. For instance, there are very few ion channels identified because of their very high transport capacities and therefore low copy numbers. Still, tissue-specific is a useful expression if it is understood that it may often mean highly abundant rather than unique and that “absence” of a protein in a tissue often just reflects low abundance. Most of the proteins identified in the present study show tissue specificity, and the tissue distribution largely agrees with what is presently known about the activities in these tissues.
The leaf plasma membranes were characterized by a very high proportion of transporters, constituting almost half of the integral proteins (Fig. 3). This agrees well with the role of the leaves as the source of carbohydrates, amino acids, and other products of photosynthesis to the rest of the plant. Moreover, based on the number of peptides found, leaf plasma membranes harbor more P-type H+-ATPase than the plasma membranes from the other two tissues, which is supported by the immunostaining in Fig. 1C. This reflects well the larger number of carriers (and hence the workload for the P-type H+-ATPase in supporting secondary transport) recorded for the leaf plasma membranes compared with xylem and cambium/phloem plasma membranes (Table I).
Very few proteins were uniquely shared between the plasma membranes from leaves and the other two tissues, respectively (2–4% of total; Fig. 2), whereas xylem and cambium/phloem plasma membranes shared many proteins not found in leaf plasma membranes (21% of total; Fig. 2). This may partly be due to the fact that xylem and cambium/phloem are neighboring tissues with a diffuse border, which makes it difficult to obtain pure preparations of each tissue with the preparation procedure used by which these two tissues are simply torn apart. Few of the integral proteins specific to cambium/phloem plasma membranes have a known function that can be related to known functions of that tissue; the exception is the ABC transporters that are homologs of Arabidopsis ABCG11 (IDs 50–52, Fig. 5, Table I, and supplemental Table 2). In Arabidopsis, ABCG11 has a role in the secretion of cuticular lipids (47), which should be a function for a tissue close to the surface of the stem. This position may also be the reason for the relatively high proportion of receptors in the cambium/phloem plasma membranes (Figs. 3 and 4). Many of these receptors likely have a role in sensing changes in the environment, such as emerging pathogen attacks.
Proteins involved in cell wall and carbohydrate metabolism were most abundant in the xylem plasma membranes (Figs. 3 and 4) in agreement with the role of the xylem in wood formation. Coexpression of genes during secondary cell wall formation has identified a number of proteins likely to be involved in this process (12), and several of these proteins were also identified in this study, particularly in xylem plasma membranes (summarized in Fig. 6). These include a number of integral proteins, such as cellulose synthase, but also peripheral proteins, such as sucrose synthase suggested to provide the substrate, UDP-glucose, for cellulose synthase and callose synthase (35). The model in Fig. 6 also includes both actin filaments and microtubules. Thus, we detected an enrichment of both tubulin and actin in the stem tissues (supplemental Table 3), and notably, α-tubulin was only found in xylem and cambium/phloem plasma membranes and was “absent” in leaf plasma membranes. Using live cell imaging on intact roots of Arabidopsis, Wightman and Turner (85) recently further clarified the important relationship among microtubules, actin, and the cellulose synthase complex during secondary cell wall formation. They found that bundles of microtubules localized the cellulose synthase complex to the edges of developing cell wall thickenings and that actin cables were essential for the rapid trafficking of cellulose synthase complex-containing “organelles” around the cell. Using a tobacco cell culture and transgenic xylogenic cells, the secondary cell wall and secretory proteome were recently analyzed (15). Of the 109 proteins identified in multiple green plants, 26 soluble proteins were similar to proteins identified in our study, including vacuole-associated annexin, vacuolar H+-ATPase, 14-3-3 protein, phospholipase D, phosphoglycerate kinase, fructose-bisphosphate aldolase, glutathione peroxidase, methionine synthase, malate dehydrogenase, and phosphoenolpyruvate carboxylase. Interestingly, one integral protein, a callose synthase (Oryza sativa), was reported with a sequence similarity closest to subset protein ID 237 (supplemental Table 2), although several proteins in the GT48 family (7) are closely related. By BLAST-matching proteins identified in isolated detergent-resistant plasma membrane microdomains (DRMs), also from a tobacco cell culture (86), an overlap was found with several GT48 proteins identified in the present study (IDs 242–244). Other cell wall-related proteins found in these DRMs were similar to KORRIGAN (ID 255) and FLA10 (ID 1040). Novel data on hybrid aspen (P. tremula × P. tremuloides) DRMs (87) showed that more than 70% of total glucan synthase activities present in the original plasma membrane preparation are associated with DRMs. Post-translational processing of the enzymes seemed necessary for catalytic activity, highlighting some of the difficulties in determining the activities of callose (GT48) and cellulose synthases (GT2) (87).
In this study, we have largely focused on cell wall formation in xylem and less on phloem development. A recent proteomics study of the pumpkin phloem sap identified 1121 proteins (88). Our study shows a 28% overlap with these proteins by BLAST matching; all but three (IDs 177, 178, and 287) are non-transmembrane proteins. Among their 45 most abundant and common phloem proteins, we found 19 in leaf, 30 in xylem, and 32 in cambium/phloem plasma membranes. We therefore conclude that few of these proteins are highly enriched in cambium/phloem plasma membranes compared with the xylem plasma membranes. We found only a small number of proteins to be enriched in cambium/phloem plasma membranes, such as homologs to Arabidopsis FLA1 (IDs 1038 and 1039). Transcript data to support a specific cambium/phloem expression in Populus (89) for an FLA1 homolog, however, are not conclusive. One protein only found in cambium/phloem plasma membranes was a homolog to Arabidopsis lipoxygenase LOX1 (ID 319), a component of jasmonate signal transduction, regulating a variety of processes (90). Jasmonic acid has for instance been shown to induce rapid changes in carbon transport and partitioning in Populus (91), probably regulated by changes in phloem cell wall structure and composition (92–94).
A large number of proteins involved in membrane trafficking were recorded in the xylem plasma membranes (Figs. 3 and 4). This is in agreement with the important role of exocytosis in cell wall formation where, e.g. matrix polysaccharides made in the Golgi are delivered to the cell wall via secretory vesicles (74). Indeed, judging from the number of peptides recovered (Table I) and the presence of ADP-ribosylation factors (Fig. 1C), membrane trafficking was most intense in xylem and least intense in leaves, which were essentially fully expanded at harvest.
Of particular interest is the finding of almost all enzymes involved in lignin biosynthesis in the xylem plasma membranes. These are soluble proteins and have earlier been located to the xylem but intracellularly to the cytosol, polysomes, endoplasmic reticulum, and the Golgi apparatus (95–98). The organization of these enzymes into complexes was suggested already in 1974 (99), and biochemical and genetic data supporting the presence of complexes have been reviewed (100). Our data suggest that the lignin-forming enzymes may exist as a complex linked to the plasma membrane, possibly in close proximity to a transporter translocating lignin monomers across the plasma membrane. The Arabidopsis ABC transporter ABCB15 has been suggested to have such a role based on its expression profile, which closely resembles those of known monolignol biosynthetic genes (42), and its poplar homolog (ID 32) was exclusively found in the xylem plasma membranes.
Analyses of genes coexpressed during wood formation have identified many proteins likely to be involved in secondary cell wall formation (12). Several of these candidate proteins were found in the present proteomics study (Fig. 6), which identifies 25 integral proteins (Table I) and 83 soluble proteins (supplemental Table 3) exclusively found in xylem plasma membranes and thereby also provides additional candidates to the list of proteins putatively involved in wood formation.
Supplementary Material
Acknowledgments
We thank Adine Karlsson for skillful technical assistance and senior researcher Lars-Göran Stener at the Forestry Research Institute of Sweden (Ekebo, Svalöv, Sweden) for the supply of poplar trees. We are in debt to Professor R. Serrano (Universidad Politecnica, Valencia, Spain), Professor P. S. Chourey (University of Florida, Gainesville, Florida), and Professor P. Kjellbom (Lund University, Lund, Sweden) for gifts of antisera.
Footnotes
* This work was supported by grants from the Swedish Foundation for Strategic Research (to C. L.) and the Formas excellence center FUNCFIBER, Kempe foundation, Troëdssons foundation (to G. W.).
 The on-line version of this article (available at http://www.mcponline.org) contains supplemental Tables 2–5.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental Tables 2–5.
1 The abbreviations used are:
- LHCII
- Photosystem II light-harvesting complex
- DRMs
- detergent-resistant plasma membrane microdomains
- FLA
- fasciclin-like arabinogalactan
- GPI
- glycosylphosphatidylinositol
- GT
- glycosyltransferase
- LOPIT
- localization of organelle proteins by isotope tagging
- UPLC
- ultraperformance LC
- PIP
- plasma membrane intrinsic protein
- TAIR
- The Arabidopsis Information Resource
- AGI
- Arabidopsis Genome Initiative
- ABC
- ATP-binding cassette
- ID
- identification number
- PPi-ase
- inorganic pyrophosphatase
- TIP
- tonoplast intrinsic protein
- RLK
- receptor-like kinase
- LRR
- leucine-rich repeat
- PERK
- proline extensin-like receptor kinase
- SD
- S-locus glycoprotein-like domain
- SUS
- sucrose synthase
- SNARE
- soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- VAMP
- vesicle-associated membrane protein
- CESA
- Cellulose synthase.
REFERENCES
- 1.Tuskan G. A., Difazio S., Jansson S., Bohlmann J., Grigoriev I., Hellsten U., Putnam N., Ralph S., Rombauts S., Salamov A., Schein J., Sterck L., Aerts A., Bhalerao R. R., Bhalerao R. P., Blaudez D., Boerjan W., Brun A., Brunner A., Busov V., Campbell M., Carlson J., Chalot M., Chapman J., Chen G. L., Cooper D., Coutinho P. M., Couturier J., Covert S., Cronk Q., Cunningham R., Davis J., Degroeve S., Déjardin A., Depamphilis C., Detter J., Dirks B., Dubchak I., Duplessis S., Ehlting J., Ellis B., Gendler K., Goodstein D., Gribskov M., Grimwood J., Groover A., Gunter L., Hamberger B., Heinze B., Helariutta Y., Henrissat B., Holligan D., Holt R., Huang W., Islam-Faridi N., Jones S., Jones-Rhoades M., Jorgensen R., Joshi C., Kangasjärvi J., Karlsson J., Kelleher C., Kirkpatrick R., Kirst M., Kohler A., Kalluri U., Larimer F., Leebens-Mack J., Leplé J. C., Locascio P., Lou Y., Lucas S., Martin F., Montanini B., Napoli C., Nelson D. R., Nelson C., Nieminen K., Nilsson O., Pereda V., Peter G., Philippe R., Pilate G., Poliakov A., Razumovskaya J., Richardson P., Rinaldi C., Ritland K., Rouzé P., Ryaboy D., Schmutz J., Schrader J., Segerman B., Shin H., Siddiqui A., Sterky F., Terry A., Tsai C. J., Uberbacher E., Unneberg P., Vahala J., Wall K., Wessler S., Yang G., Yin T., Douglas C., Marra M., Sandberg G., Van de Peer Y., Rokhsar D. ( 2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313, 1596– 1604 [DOI] [PubMed] [Google Scholar]
- 2.Verrier P. J., Bird D., Burla B., Dassa E., Forestier C., Geisler M., Klein M., Kolukisaoglu U., Lee Y., Martinoia E., Murphy A., Rea P. A., Samuels L., Schulz B., Spalding E. J., Yazaki K., Theodoulou F. L. ( 2008) Plant ABC proteins—a unified nomenclature and updated inventory. Trends Plant Sci 13, 151– 159 [DOI] [PubMed] [Google Scholar]
- 3.Arango M., Gévaudant F., Oufattole M., Boutry M. ( 2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216, 355– 365 [DOI] [PubMed] [Google Scholar]
- 4.Pao S. S., Paulsen I. T., Saier M. H., Jr. ( 1998) Major facilitator superfamily. Microbiol. Mol. Biol. Rev 62, 1– 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiu S. H., Bleecker A. B. ( 2001) Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. U.S.A 98, 10763– 10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiu S. H., Karlowski W. M., Pan R., Tzeng Y. H., Mayer K. F., Li W. H. ( 2004) Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16, 1220– 1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geisler-Lee J., Geisler M., Coutinho P. M., Segerman B., Nishikubo N., Takahashi J., Aspeborg H., Djerbi S., Master E., Andersson-Gunnerås S., Sundberg B., Karpinski S., Teeri T. T., Kleczkowski L. A., Henrissat B., Mellerowicz E. J. ( 2006) Poplar carbohydrate-active enzymes. Gene identification and expression analyses. Plant Physiol 140, 946– 962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uemura T., Ueda T., Ohniwa R. L., Nakano A., Takeyasu K., Sato M. H. ( 2004) Systematic analysis of SNARE molecules in Arabidopsis: Dissection of the post-Golgi network in plant cells. Cell Struct. Funct 29, 49– 65 [DOI] [PubMed] [Google Scholar]
- 9.Sjödin A., Wissel K., Bylesjö M., Trygg J., Jansson S. ( 2008) Global expression profiling in leaves of free-growing aspen. BMC Plant Biol 8, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luquez V., Hall D., Albrectsen B. R., Karlsson J., Ingvarsson P., Jansson S. ( 2008) Natural phenological variation in aspen (Populus tremula): the SwAsp collection. Tree Genet. Genomes 4, 279– 292 [Google Scholar]
- 11.Girke T., Lauricha J., Tran H., Keegstra K., Raikhel N. ( 2004) The cell wall navigator database. A systems-based approach to organism-unrestricted mining of protein families involved in cell wall metabolism. Plant Physiol 136, 3003– 3008; discussion 3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson S., Wei H., Milne J., Page G. P., Somerville C. R. ( 2005) Identification of genes required for cellulose synthesis by regression analysis of public microarray data sets. Proc. Natl. Acad. Sci. U.S.A 102, 8633– 8638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao C., Craig J. C., Petzold H. E., Dickerman A. W., Beers E. P. ( 2005) The xylem and phloem transcriptomes from secondary tissues of the Arabidopsis root-hypocotyl. Plant Physiol 138, 803– 818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner S., Gallois P., Brown D. ( 2007) Tracheary element differentiation. Annu. Rev. Plant Biol 58, 407– 433 [DOI] [PubMed] [Google Scholar]
- 15.Millar D. J., Whitelegge J. P., Bindschedler L. V., Rayon C., Boudet A. M., Rossignol M., Borderies G., Bolwell G. P. ( 2009) The cell wall and secretory proteome of a tobacco cell line synthesising secondary wall. Proteomics 9, 2355– 2372 [DOI] [PubMed] [Google Scholar]
- 16.Hertzberg M., Aspeborg H., Schrader J., Andersson A., Erlandsson R., Blomqvist K., Bhalerao R., Uhlén M., Teeri T. T., Lundeberg J., Sundberg B., Nilsson P., Sandberg G. ( 2001) A transcriptional roadmap to wood formation. Proc. Natl. Acad. Sci. U.S.A 98, 14732– 14737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrader J., Nilsson J., Mellerowicz E., Berglund A., Nilsson P., Hertzberg M., Sandberg G. ( 2004) A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. Plant Cell 16, 2278– 2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson C., Sommarin M., Widell S. ( 1994) Isolation of highly purified plant plasma membranes and separation of inside-out and right-side-out vesicles. Methods Enzymol 228, 451– 469 [Google Scholar]
- 19.Bearden J. C., Jr. ( 1978) Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim. Biophys. Acta 533, 525– 529 [DOI] [PubMed] [Google Scholar]
- 20.Johansson F., Olbe M., Sommarin M., Larsson C. ( 1995) Brij 58, a polyoxyethylene ether, creates membrane vesicles of uniform sidedness. A new tool to obtain inside-out (cytoplasmic side-out) plasma membrane vesicles. Plant J 7, 165– 173 [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U. K. ( 1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680– 685 [DOI] [PubMed] [Google Scholar]
- 22.Echt C. S., Chourey P. S. ( 1985) A comparison of two sucrose synthase isozymes from normal and shrunken-1 Maize. Plant Physiol 79, 530– 536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson I., Karlsson M., Johanson U., Larsson C., Kjellbom P. ( 2000) The role of aquaporins in cellular and whole plant water balance. Biochim. Biophys. Acta 1465, 324– 342 [DOI] [PubMed] [Google Scholar]
- 24.Persson S., Rosenquist M., Svensson K., Galvão R., Boss W. F., Sommarin M. ( 2003) Phylogenetic analyses and expression studies reveal two distinct groups of calreticulin isoforms in higher plants. Plant Physiol 133, 1385– 1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bylesjö M., Nilsson R., Srivastava V., Grönlund A., Johansson A. I., Jansson S., Karlsson J., Moritz T., Wingsle G., Trygg J. ( 2009) Integrated analysis of transcript, protein and metabolite data to study lignin biosynthesis in hybrid aspen. J. Proteome Res 8, 199– 210 [DOI] [PubMed] [Google Scholar]
- 26.Bonaventura C., Bonaventura J., Stevens R., Millington D. ( 1994) Acrylamide in polyacrylamide gels can modify proteins during electrophoresis. Anal. Biochem 222, 44– 48 [DOI] [PubMed] [Google Scholar]
- 27.Tamura K., Dudley J., Nei M., Kumar S. ( 2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol 24, 1596– 1599 [DOI] [PubMed] [Google Scholar]
- 28.Albertsson P.-Å. ( 1986) Partition of Cell Particles and Macromolecules, 3rd Ed., John Wiley & Sons, New York [Google Scholar]
- 29.Ishihama Y., Oda Y., Tabata T., Sato T., Nagasu T., Rappsilber J., Mann M. ( 2005) Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics 4, 1265– 1272 [DOI] [PubMed] [Google Scholar]
- 30.Johansson F., Sommarin M., Larsson C. ( 1994) Rapid purification of the plasma membrane H+-ATPase in its nonactivated form using FPLC. Physiol. Plant 92, 389– 396 [Google Scholar]
- 31.Johansson I., Larsson C., Ek B., Kjellbom P. ( 1996) The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 8, 1181– 1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barkla B. J., Vera-Estrella R., Pantoja O., Kirch H. H., Bohnert H. J. ( 1999) Aquaporin localization—how valid are the TIP and PIP labels? Trends Plant Sci 4, 86– 88 [DOI] [PubMed] [Google Scholar]
- 33.Michalak M., Mariani P., Opas M. ( 1998) Calreticulin, a multifunctional Ca2+ binding chaperone of the endoplasmic reticulum. Biochem. Cell Biol 76, 779– 785 [DOI] [PubMed] [Google Scholar]
- 34.Borisjuk N., Sitailo L., Adler K., Malysheva L., Tewes A., Borisjuk L., Manteuffel R. ( 1998) Calreticulin expression in plant cells: developmental regulation, tissue specificity and intracellular distribution. Planta 206, 504– 514 [DOI] [PubMed] [Google Scholar]
- 35.Amor Y., Haigler C. H., Johnson S., Wainscott M., Delmer D. P. ( 1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc. Natl. Acad. Sci. U.S.A 92, 9353– 9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vernoud V., Horton A. C., Yang Z., Nielsen E. ( 2003) Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol 131, 1191– 1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Memon A. R. ( 2004) The role of ADP-ribosylation factor and SARI in vesicular trafficking in plants. Biochim. Biophys. Acta 1664, 9– 30 [DOI] [PubMed] [Google Scholar]
- 38.Käll L., Krogh A., Sonnhammer E. L. ( 2004) A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol 338, 1027– 1036 [DOI] [PubMed] [Google Scholar]
- 39.Hruz T., Laule O., Szabo G., Wessendorp F., Bleuler S., Oertle L., Widmayer P., Gruissem W., Zimmermann P. ( 2008) Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv. Bioinformatics 2008, 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baxter I. R., Young J. C., Armstrong G., Foster N., Bogenschutz N., Cordova T., Peer W. A., Hazen S. P., Murphy A. S., Harper J. F. ( 2005) A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A 102, 2649– 2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geisler M., Blakeslee J. J., Bouchard R., Lee O. R., Vincenzetti V., Bandyopadhyay A., Titapiwatanakun B., Peer W. A., Bailly A., Richards E. L., Ejendal K. F., Smith A. P., Baroux C., Grossniklaus U., Müller A., Hrycyna C. A., Dudler R., Murphy A. S., Martinoia E. ( 2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44, 179– 194 [DOI] [PubMed] [Google Scholar]
- 42.Ehlting J., Mattheus N., Aeschliman D. S., Li E., Hamberger B., Cullis I. F., Zhuang J., Kaneda M., Mansfield S. D., Samuels L., Ritland K., Ellis B. E., Bohlmann J., Douglas C. J. ( 2005) Global transcript profiling of primary stems from Arabidopsis thaliana identifies candidate genes for missing links in lignin biosynthesis and transcriptional regulators of fiber differentiation. Plant J 42, 618– 640 [DOI] [PubMed] [Google Scholar]
- 43.Terasaka K., Blakeslee J. J., Titapiwatanakun B., Peer W. A., Bandyopadhyay A., Makam S. N., Lee O. R., Richards E. L., Murphy A. S., Sato F., Yazaki K. ( 2005) PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 17, 2922– 2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen P. B., Cancel J., Rounds M., Ochoa V. ( 2007) Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 225, 1447– 1458 [DOI] [PubMed] [Google Scholar]
- 45.Stein M., Dittgen J., Sánchez-Rodríguez C., Hou B. H., Molina A., Schulze-Lefert P., Lipka V., Somerville S. ( 2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18, 731– 746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krattinger S. G., Lagudah E. S., Spielmeyer W., Singh R. P., Huerta-Espino J., McFadden H., Bossolini E., Selter L. L., Keller B. ( 2009) A Putative ABC Transporter Confers Durable Resistance to Multiple Fungal Pathogens in Wheat. Science 323, 1360– 1363 [DOI] [PubMed] [Google Scholar]
- 47.Bird D., Beisson F., Brigham A., Shin J., Greer S., Jetter R., Kunst L., Wu X., Yephremov A., Samuels L. ( 2007) Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J 52, 485– 498 [DOI] [PubMed] [Google Scholar]
- 48.Alexandersson E., Saalbach G., Larsson C., Kjellbom P. ( 2004) Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant Cell Physiol 45, 1543– 1556 [DOI] [PubMed] [Google Scholar]
- 49.Marmagne A., Ferro M., Meinnel T., Bruley C., Kuhn L., Garin J., Barbier-Brygoo H., Ephritikhine G. ( 2007) A high content in lipid-modified peripheral proteins and integral receptor kinases features in the Arabidopsis plasma membrane proteome. Mol. Cell. Proteomics 6, 1980– 1996 [DOI] [PubMed] [Google Scholar]
- 50.Robinson D. G., Haschke H. P., Hinz G., Hoh B., Maeshima M., Marty F. ( 1996) Immunological detection of tonoplast polypeptides in the plasma membrane of pea cotyledons. Planta 198, 95– 103 [Google Scholar]
- 51.Ratajczak R., Hinz G., Robinson D. G. ( 1999) Localization of pyrophosphatase in membranes of cauliflower inflorescence cells. Planta 208, 205– 211 [DOI] [PubMed] [Google Scholar]
- 52.Tomatsu H., Takano J., Takahashi H., Watanabe-Takahashi A., Shibagaki N., Fujiwara T. ( 2007) An Arabidopsis thaliana high-affinity molybdate transporter required for efficient uptake of molybdate from soil. Proc. Natl. Acad. Sci. U.S.A 104, 18807– 18812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nürnberger T., Kemmerling B. ( 2006) Receptor protein kinases—pattern recognition receptors in plant immunity. Trends Plant Sci 11, 519– 522 [DOI] [PubMed] [Google Scholar]
- 54.Belkhadir Y., Chory J. ( 2006) Brassinosteroid signaling: a paradigm for steroid hormone signaling from the cell surface. Science 314, 1410– 1411 [DOI] [PubMed] [Google Scholar]
- 55.Hématy K., Höfte H. ( 2008) Novel receptor kinases involved in growth regulation. Curr. Opin. Plant Biol 11, 321– 328 [DOI] [PubMed] [Google Scholar]
- 56.McCubbin A. G., Kao T. H. ( 2000) Molecular recognition and response in pollen and pistil interactions. Annu. Rev. Cell Dev. Biol 16, 333– 364 [DOI] [PubMed] [Google Scholar]
- 57.Kim H. S., Jung M. S., Lee S. M., Kim K. E., Byun H., Choi M. S., Park H. C., Cho M. J., Chung W. S. ( 2009) An S-locus receptor-like kinase plays a role as a negative regulator in plant defense responses. Biochem. Biophys. Res. Commun 381, 424– 428 [DOI] [PubMed] [Google Scholar]
- 58.Bürglin T. R. ( 2008) The Hedgehog protein family. Genome Biol 9, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salnikov V. V., Grimson M. J., Delmer D. P., Haigler C. H. ( 2001) Sucrose synthase localizes to cellulose synthesis sites in tracheary elements. Phytochemistry 57, 823– 833 [DOI] [PubMed] [Google Scholar]
- 60.Scheible W. R., Pauly M. ( 2004) Glycosyltransferases and cell wall biosynthesis: novel players and insights. Curr. Opin. Plant Biol 7, 285– 295 [DOI] [PubMed] [Google Scholar]
- 61.Wasteneys G. O. ( 2004) Progress in understanding the role of microtubules in plant cells. Curr. Opin. Plant Biol 7, 651– 660 [DOI] [PubMed] [Google Scholar]
- 62.Oakley R. V., Wang Y. S., Ramakrishna W., Harding S. A., Tsai C. J. ( 2007) Differential expansion and expression of α- and β-tubulin gene families in Populus. Plant Physiol 145, 961– 973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wasteneys G. O. ( 2002) Microtubule organization in the green kingdom: chaos or self-order? J. Cell Sci 115, 1345– 1354 [DOI] [PubMed] [Google Scholar]
- 64.Andrawis A., Solomon M., Delmer D. P. ( 1993) Cotton fiber annexins: a potential role in the regulation of callose synthase. Plant J 3, 763– 772 [DOI] [PubMed] [Google Scholar]
- 65.Konopka-Postupolska D. ( 2007) Annexins: putative linkers in dynamic membrane-cytoskeleton interactions in plant cells. Protoplasma 230, 203– 215 [DOI] [PubMed] [Google Scholar]
- 66.Robert S., Bichet A., Grandjean O., Kierzkowski D., Satiat-Jeunemaître B., Pelletier S., Hauser M. T., Höfte H., Vernhettes S. ( 2005) An Arabidopsis endo-1,4-β-D-glucanase involved in cellulose synthesis undergoes regulated intracellular cycling. Plant Cell 17, 3378– 3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pattathil S., Harper A. D., Bar-Peled M. ( 2005) Biosynthesis of UDP-xylose: characterization of membrane-bound At Uxs2. Planta 221, 538– 548 [DOI] [PubMed] [Google Scholar]
- 68.Andersson-Gunnerås S., Mellerowicz E. J., Love J., Segerman B., Ohmiya Y., Coutinho P. M., Nilsson P., Henrissat B., Moritz T., Sundberg B. ( 2006) Biosynthesis of cellulose-enriched tension wood in Populus: global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. Plant J 45, 144– 165 [DOI] [PubMed] [Google Scholar]
- 69.Nothnagel E. A. ( 1997) Proteoglycans and related components in plant cells. Int. Rev. Cytol 174, 195– 291 [DOI] [PubMed] [Google Scholar]
- 70.Gillmor C. S., Lukowitz W., Brininstool G., Sedbrook J. C., Hamann T., Poindexter P., Somerville C. ( 2005) Glycosylphosphatidylinositol-anchored proteins are required for cell wall synthesis and morphogenesis in Arabidopsis. Plant Cell 17, 1128– 1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raes J., Rohde A., Christensen J. H., Van de Peer Y., Boerjan W. ( 2003) Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiol 133, 1051– 1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geldner N., Jürgens G. ( 2006) Endocytosis in signalling and development. Curr. Opin. Plant Biol 9, 589– 594 [DOI] [PubMed] [Google Scholar]
- 73.Murphy A. S., Bandyopadhyay A., Holstein S. E., Peer W. A. ( 2005) Endocytotic cycling of PM proteins. Annu. Rev. Plant Biol 56, 221– 251 [DOI] [PubMed] [Google Scholar]
- 74.Lerouxel O., Cavalier D. M., Liepman A. H., Keegstra K. ( 2006) Biosynthesis of plant cell wall polysaccharides—a complex process. Curr. Opin. Plant Biol 9, 621– 630 [DOI] [PubMed] [Google Scholar]
- 75.Svennelid F., Olsson A., Piotrowski M., Rosenquist M., Ottman C., Larsson C., Oecking C., Sommarin M. ( 1999) Phosphorylation of Thr-948 at the C terminus of the plasma membrane H+-ATPase creates a binding site for the regulatory 14–3-3 protein. Plant Cell 11, 2379– 2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boursiac Y., Harper J. F. ( 2007) The origin and function of calmodulin regulated Ca2+ pumps in plants. J. Bioenerg. Biomembr 39, 409– 414 [DOI] [PubMed] [Google Scholar]
- 77.Davies E., Fillingham B. D., Oto Y., Abe S. ( 1991) Evidence for the existence of cytoskeleton-bound polysomes in plants. Cell Biol. Int. Rep 15, 973– 981 [DOI] [PubMed] [Google Scholar]
- 78.Medalia O., Weber I., Frangakis A. S., Nicastro D., Gerisch G., Baumeister W. ( 2002) Macromolecular architecture in eukaryotic cells visualized by cryoelectron tomography. Science 298, 1209– 1213 [DOI] [PubMed] [Google Scholar]
- 79.Whiteman S. A., Nühse T. S., Ashford D. A., Sanders D., Maathuis F. J. M. ( 2008) A proteomic and phosphoproteomic analysis of Oryza sativa plasma membrane and vacuolar membrane. Plant J 56, 146– 156 [DOI] [PubMed] [Google Scholar]
- 80.Zhang J., Ma H., Feng J., Zeng L., Wang Z., Chen S. ( 2008) Grape berry plasma membrane proteome analysis and its differential expression during ripening. J. Exp. Bot 59, 2979– 2990 [DOI] [PubMed] [Google Scholar]
- 81.Nühse T. S., Stensballe A., Jensen O. N., Peck S. C. ( 2004) Phosphoproteomics of the Arabidopsis plasma membrane and a new phosphorylation site database. Plant Cell 16, 2394– 2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hem S., Rofidal V., Sommerer N., Rossignol M. ( 2007) Novel subsets of the Arabidopsis plasmalemma phosphoproteome identify phosphorylation sites in secondary active transporters. Biochem. Biophys. Res. Commun 363, 375– 380 [DOI] [PubMed] [Google Scholar]
- 83.Benschop J. J., Mohammed S., O'Flaherty M., Heck A. J., Slijper M., Menke F. L. H. ( 2007) Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol. Cell. Proteomics 6, 1198– 1214 [DOI] [PubMed] [Google Scholar]
- 84.Dunkley T. P., Hester S., Shadforth I. P., Runions J., Weimar T., Hanton S. L., Griffin J. L., Bessant C., Brandizzi F., Hawes C., Watson R. B., Dupree P., Lilley K. S. ( 2006) Mapping the Arabidopsis organelle proteome. Proc. Natl. Acad. Sci. U.S.A 103, 6518– 6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wightman R., Turner S. R. ( 2008) The roles of the cytoskeleton during cellulose deposition at the secondary cell wall. Plant J 54, 794– 805 [DOI] [PubMed] [Google Scholar]
- 86.Morel J., Claverol S., Mongrand S., Furt F., Fromentin J., Bessoule J. J., Blein J. P., Simon-Plas F. ( 2006) Proteomics of plant detergent-resistant membranes. Mol. Cell. Proteomics 5, 1396– 1411 [DOI] [PubMed] [Google Scholar]
- 87.Bessueille L., Sindt N., Guichardant M., Djerbi S., Teeri T. T., Bulone V. ( 2009) Plasma membrane microdomains from hybrid aspen cells are involved in cell wall polysaccharide biosynthesis. Biochem. J 420, 93– 103 [DOI] [PubMed] [Google Scholar]
- 88.Lin M. K., Lee Y. J., Lough T. J., Phinney B. S., Lucas W. J. ( 2009) Analysis of the pumpkin phloem proteome provides insights into angiosperm sieve tube function. Mol. Cell. Proteomics 8, 343– 356 [DOI] [PubMed] [Google Scholar]
- 89.Sjödin A., Street N. R., Sandberg G., Gustafsson P., Jansson S. ( 2009) The Populus Genome Integrative Explorer (PopGenIE): a new resource for exploring the Populus genome. New Phytol 182, 1013– 1025 [DOI] [PubMed] [Google Scholar]
- 90.Wasternack C. ( 2007) Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot 100, 681– 697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Babst B. A., Ferrieri R. A., Gray D. W., Lerdau M., Schlyer D. J., Schueller M., Thorpe M. R., Orians C. M. ( 2005) Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol 167, 63– 72 [DOI] [PubMed] [Google Scholar]
- 92.Amiard V., Demmig-Adams B., Mueh K. E., Turgeon R., Combs A. F., Adams W. W., 3rd ( 2007) Role of light and jasmonic acid signaling in regulating foliar phloem cell wall ingrowth development. New Phytol 173, 722– 731 [DOI] [PubMed] [Google Scholar]
- 93.McCurdy D. W., Patrick J. W., Offler C. E. ( 2008) Wall ingrowth formation in transfer cells: novel examples of localized wall deposition in plant cells. Curr. Opin. Plant Biol 11, 653– 661 [DOI] [PubMed] [Google Scholar]
- 94.Le Hir R., Beneteau J., Bellini C., Vilaine F., Dinant S. ( 2008) Gene expression profiling: keys for investigating phloem functions. Trends Plant Sci 13, 273– 280 [DOI] [PubMed] [Google Scholar]
- 95.Takabe K., Takeuchi M., Sato T., Ito M., Fujita M. ( 2001) Immunocytochemical localization of enzymes involved in lignification of the cell wall. J. Plant Res 114, 509– 515 [Google Scholar]
- 96.Ro D. K., Mah N., Ellis B. E., Douglas C. J. ( 2001) Functional characterization and subcellular localization of poplar (Populus trichocarpa x Populus deltoides) cinnamate 4-hydroxylase. Plant Physiol 126, 317– 329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruelland E., Campalans A., Selman-Housein G., Puigdomenech P., Rigau J. ( 2003) Cellular and subcellular localization of the lignin biosynthetic enzymes caffeic acid-O-methyltransferase, cinnamyl alcohol dehydrogenase and cinnamoyl-coenzyme A reductase in two monocots, sugarcane and maize. Physiol. Plant 117, 93– 99 [Google Scholar]
- 98.Sato T., Takabe K., Fujita M. ( 2004) Immunolocalization of phenylalanine ammonia-lyase and cinnamate-4-hydroxylase in differentiating xylem of poplar. C. R. Biol 327, 827– 836 [DOI] [PubMed] [Google Scholar]
- 99.Stafford H. A. ( 1974) Possible multienzyme complexes regulating the formation of C6-C3 phenolic compounds and lignins in higher plants. Recent Adv. Phytochem 8, 53– 79 [Google Scholar]
- 100.Winkel-Shirley B. ( 1999) Evidence for enzyme complexes in the phenylpropanoid and flavonoid pathways. Physiol. Plant 107, 142– 149 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.