Abstract
1,25 dihydroxyvitamin D3 (1,25 (OH)2 D) and its less hypercalcemic analogues have been shown to inhibit the proliferation of vascular smooth muscle cells (VSMC) in culture. However, the mechanism(s) underlying this suppression is not well understood. Here we have shown that 1,25 (OH)2 D and its analogues (RO-25-6760 and RO-23-7553) inhibit endothelin (ET)-dependent DNA synthesis and cell proliferation in neonatal rat aortic VSMC. While ET stimulation of mitogenic activity requires activation of the MEK/ERK signal transduction cascade, 1,25 (OH)2 D neither affected the ET-dependent activation of ERK nor synergized with the MEK inhibitor PD98059 in reducing DNA synthesis in these cultures, implying that the locus of 1,25 (OH)2 D actions lies between ERK and the cell cycle machinery. 1,25 (OH)2 D suppressed ET-induced activation of cyclin dependent kinase 2 (Cdk2), a key cell cycle kinase, but had no effect on the expression of this protein. Collectively, the data identify Cdk2 as the target of 1,25 (OH)2 D in the cell cycle machinery and imply a potential role for 1,25 (OH)2 D, or its less hypercalcemic analogues, in the treatment of disorders of VSMC proliferation involving the vascular wall.
Keywords: vitamin D, vascular smooth muscle cells, cell cycle and cyclin dependent kinase 2
INTRODUCTION
Vitamin D is a secosteroid hormone precursor whose levels are determined by dietary intake or de novo synthesis following interaction of ultraviolet light with cholesterol precursors in the epidermis [1]. The most polar metabolite of vitamin D, 1,25 dihydroxyvitamin D3 (1,25 (OH)2 D), is believed to be the bioactive form of the hormone that binds to the vitamin D receptor (VDR) in the nuclei of target cells and, along with its heterodimeric partner the retinoid X receptor (RXR), triggers the cascade of downstream events that lead to predictable phenotypic changes in these cells.
While most early investigation dealing with vitamin D and its metabolites focused on the capacity of this hormone to mobilize calcium across the intestinal mucosa, thereby providing substrate for mineralization of bone, a growing number of studies have demonstrated both the presence of vitamin D receptors and 1,25 (OH)2 D-dependent biological activity in a variety of tissues and cell types that might be regarded as atypical targets. These include breast [2, 3], colon [3, 4], pancreatic [5, 6] and prostate [2, 3, 7] cancer cells, pancreatic islets [8, 9], cells of the immune system [10], parathyroid cells [11, 12], cardiac myocytes [13, 14] and VSMC [15].
While 1,25 (OH)2 D, as well as it less hypercalcemic analogues [3, 16], have been shown to exert a wide spectrum of effects in these atypical targets, it is, perhaps, its effects on the growth and differentiation of these cells that have attracted the most attention. While exceptions have been noted [15, 17], the preponderance of published data support a growth suppressant role for 1,25 (OH)2 D, as well as other VDR ligands. These ligands inhibit proliferation of breast [2, 3], colon [3, 4], pancreatic [5, 6] and prostate [2, 3, 7] cancer cells. They suppress growth of parathyroid cells [11, 12], prevent hypertrophy of cardiac myocytes [18] and inhibit proliferation of VSMC [15, 19, 20], The latter effect is of particular interest in that it suggests potential utility of these agents in the management of atherosclerosis, post-transplant vasculopathy, re-stenosis post-angioplasty and other disorders characterized by growth and remodeling in the vascular wall.
While the growth suppressant effect of vitamin D in the VSMC is clear, very little information has been published describing the underlying mechanism(s). Parenthetically, in other cell types 1,25 (OH)2 D effects on the cell cycle machinery have been heterogeneous with a number of proteins involved in cell cycle regulation shown to be targets of 1,25 (OH)2 D action [16, 21].
In the present study, we have investigated the effects of 1,25 (OH)2 D and less hypercalcemic analogues of 1,25 (OH)2 D (RO-25-6760 and RO-23-7553) on DNA synthesis and cell proliferation in cultured neonatal rat aortic VSMC. We demonstrate that the VDR agonists uniformly suppress endothelin (ET)-stimulated, but not basal, DNA synthesis and mitogenesis, that the suppression is linked to a reduction in the activity, but not the levels, of cyclin dependent kinase 2 (Cdk2), a key cell cycle kinase that operates in G1 and at the G1/S transition.
MATERIALS AND METHODS
Materials
ET was purchased from Peninsula Laboratories, Inc. (Belmont, CA). Anti- Cdk2, anti-cyclin A, anti-cyclin E, anti- Cdk4, anti- Cdk6, anti-cyclin D1, anti-ERK2, anti-JNK1, anti-p27, anti-p21, anti-p16, anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and anti-VDR antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). [3H]Thymidine, [α-32P]dCTP, and [γ-32P]ATP were purchased from PerkinElmer Life Sciences (Boston, MA). The CellTiter 96 Aqueous nonradioactive cell proliferation assay kit was purchased from Promega (Madison, WI). PD098059 was from Research Biochemicals International (Natick, MA). Bovine myelin basic protein was from Upstate Biotechnology, Inc. (Lake Placid, NY). RO 23–7553 and RO 25–6760 were kindly provided by M. Uskokovic of Bioxell Inc (Nutley, NJ).
Cell culture
Neonatal VSMC were obtained from P. Jones [22]. Cells were cultured at 37 °C in a 5% CO2-humidified incubator in DME H-21 supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100 mg/ml streptomycin, and 2% (v/v) broth, tryptose phosphate (growth media). Cells were used after reaching 75–80% confluence.
[3H]Thymidine incorporation
Cells were cultured in media containing 10% FBS in 24-well plates for 24 hours and then changed to serum-substitute media [23] for the ensuing 24-hour period. At that point, all cells were treated with different concentrations of 1,25 (OH)2 D, one of two non-hypercalcemic 1,25 (OH)2 D analogues, or vehicle for the next 48 hours. Where indicated, ET was included for the final 24 hours of culture. Four hours before collection, cells were pulsed with [3H]thymidine (1mCi/well) in modified Eagle’s medium (MEM) with Earle’s balanced salt solution. [3H]Thymidine incorporation was determined as described previously [24].
Cell proliferation assay
VSMC were cultured in 96-well plates and growth-arrested in serum substitute/DME for 24 hours. Quiescent cells were treated with different concentrations of 1,25 (OH)2 D or its analogues for 48 hours in the presence or absence of ET for the final 24 hours of culture. To terminate the experiment, media was evacuated and MTS solution was added to each well for the final 4 hours. Cell numbers were determined by the Cell-Titer 96 Aqueous nonradioactive cell proliferation assay kit according to the instructions provided by the manufacturer.
Expression and purification of recombinant glutathione S-transferase (GST) –C-JUN
GST-c-JUN1–79 was transformed into Escherichia coli strain HB101. Synthesis of the recombinant protein was induced with 100 μM isopropyl-β-D-galactoside for 6 hours. Bacteria were collected by centrifugation and sonicated in buffer containing 50 mM Tris-HCl (pH 7.5), 2 mM EDTA, 2 mM DTT, and protease inhibitors (1 Complete™ tablet/50 ml buffer, Roche Applied Science). The supernatant was incubated with GSH-Sepharose (Amersham Biosciences) for 2 hours at 4 °C, and then washed with 3 volumes of phosphate-buffered saline containing 2 mM DTT and 0.1% Triton X-100. GST-c-JUN was eluted with 5 mM glutathione in 50 mM Tris-HCl, pH 8.0, and 2 mM DTT.
Immunoprecipitation and immune complex kinase assay
Cells were incubated with the agents indicated for different time intervals and lysed with lysis buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 10% glycerol, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM glycerophosphate) containing protease inhibitors (1 Complete™ tablet/50 ml buffer). 200 μg of soluble protein was incubated with 1 μg of anti-ERK2, anti-JNK1, anti-Cdk2, anti-cyclin A or anti-cyclin E antibodies and 10 μl of protein G-Sepharose for 1–2 hours at 4 °C. Immunoprecipitation and immune complex kinase assay were carried out as described previously [24] with appropriate substrate [2 μg of histone 1 for measurement of Cdk2, cyclin A-, and cyclin E-associated kinase; 5 μg of myelin basic protein (MBP) for measurement of ERK; 5 μg of GST-c-JUN for c-Jun N-terminal kinase (JNK) [24]. Reaction products were electrophoresed on SDS-polyacrylamide gels which were then dried and exposed to X-ray film.
Immunoblot analysis
Cells were treated with 10−8 M 1,25 (OH)2 D for different time intervals. Cellular lysates were generated, subjected to 12.5% SDS-PAGE and transferred onto nitrocellulose membranes (Amersham Chemical Corp., Amersham, IL). Membranes were blocked with 5% nonfat milk in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl and 0.1% Tween 20 (TBST), and probed with anti-cyclin E, anti-cyclin A, anti-cyclin D1, anti- Cdk4, anti- Cdk6, anti- Cdk2, anti-p27, anti-p21, anti-p16, anti-GAPDH and anti-VDR antibodies, each diluted 1: 50–100 in TBST. A horseradish peroxidase–conjugated secondary antibody (diluted 1:1000–2000 in TBST) was employed to detect immunoreactive bands using the enhanced chemiluminescence (ECL) Western blotting detection system (Amersham Chemical Corp.).
Statistical analysis
Data were evaluated using one-way analysis of variance and the Newman-Keuls post-hoc test to assess significance.
RESULTS
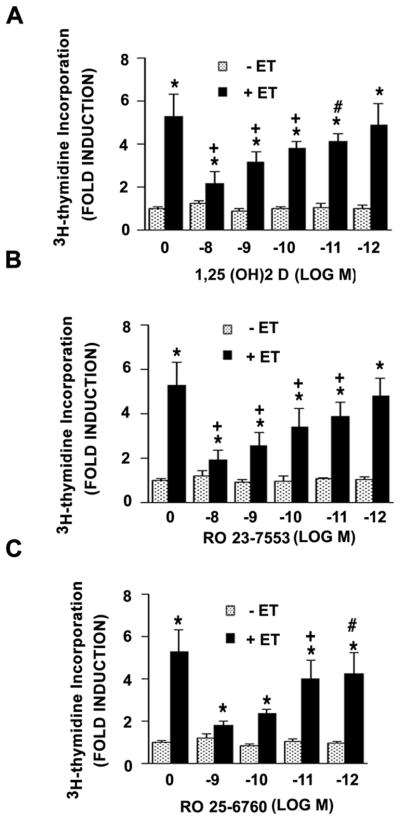
Treatment of neonatal VSMC with 1,25 (OH)2 D resulted in a dose-dependent reduction in ET-dependent 3H-thymidine incorporation in these cultures. As shown in Fig. 1A, ET treatment led to a 5–6 fold increment in 3H-thymidine incorporation while treatment with 1,25 (OH)2 D led to a stepwise reduction. At 10−8 M 1,25 (OH)2 D, ET treatment resulted in only a 2-fold increase in DNA synthesis. 1,25 (OH)2 D had no effect on basal incorporation of 3H-thymidine in the absence of ET. The ability to suppress DNA synthesis was shared by two synthetic analogues of 1,25 (OH)2 D with reduced hypercalcemic activity, RO 23-7553 and RO 25-6760 (Figs. 1B and 1C respectively). Each of these agents effected a similar dose-dependent reduction in ET-dependent DNA synthesis. RO 25-6760, based on its dose response, appeared to be somewhat more effective than either 1,25 (OH)2 D or RO 23-7553 in promoting this inhibition.
Fig. 1.
1,25 (OH)2 D or less hypercalcemic analogues inhibit ET-stimulated [3H]thymidine incorporation in VSMC. Quiescent cells were incubated with different concentrations of 1,25 (OH)2 D (A), RO 23-7553 (B) or RO 25-6760 (C) for 48 hours in the presence or absence of 10−7 M ET for the final 24 hours. [3H]thymidine incorporation was carried out as described in Methods. Pooled data were from 4 separate experiments. *P<0.01 vs. control; +P<0.01; #P<0.05 vs. ET alone.
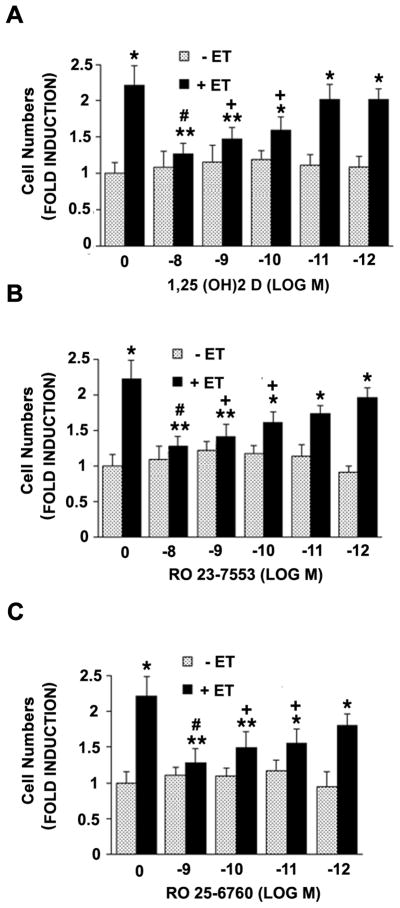
The reductions in DNA synthesis were matched by qualitatively similar effects on cell number, implying that the aforementioned inhibition of 3H-thymidine incorporation is linked to a bonafide reduction in cell division (versus repair synthesis) in these cultures. As shown in Fig. 2A, ET treatment led to a 2-fold increment in cell number which was, again, reduced to levels only slightly above baseline at 10−8 M 1,25 (OH)2 D. Similar reductions were seen with the less hypercalcemic analogues of 1,25 (OH)2 D (RO 23-7553 and RO 25-6760) and, again, RO-25-6760 appeared to be slightly more effective than the other two agents (Figs. 2B and 2C respectively).
Fig. 2.
Effect of 1,25 (OH)2 D or less hypercalcemic analogues on ET-induced increment in cell number. VSMC were treated with indicated concentration of 1,25 (OH)2 D (A), RO 23-7553 (B) or RO 25-6760 (C) for 48 hours in the presence or absence of 10−7 M ET-1 for final 24 hours. Cell number was determined as described in Methods. The results are derived from 3 different experiments. **P<0.05, *P<0.01 vs. control; #P<0.01, +P<0.05 vs. ET alone.
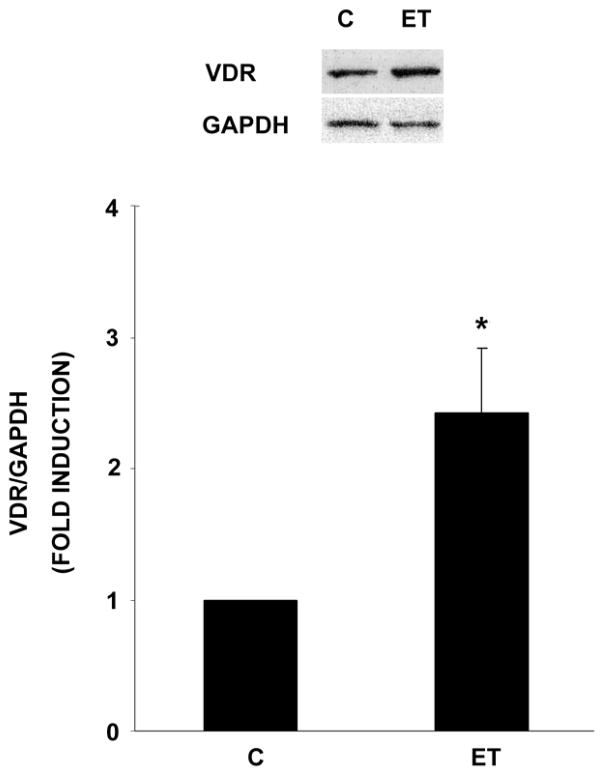
The selectivity of 1,25 (OH)2 D’s inhibition of ET-dependent (vs. basal) 3H-thymidine incorporation and mitogenic activity in these VSMC led us to examine VDR levels in the presence and absence of ET. As shown in Fig. 3, ET (10−7 M) treatment led to more than a 2-fold induction of VDR protein levels, suggesting greater sensitivity to the ligand (1,25 (OH)2 D) following ET (vs. basal) treatment.
Fig. 3.
ET increases expression of the VDR. Cells were treated for 24 hrs in the absence or presence of ET (10−7 M). Cellular extracts were generated and analyzed for VDR or GAPDH by Western blot analysis. Representative blot and pooled results from three experiments are presented. *P<0.01 vs. control.
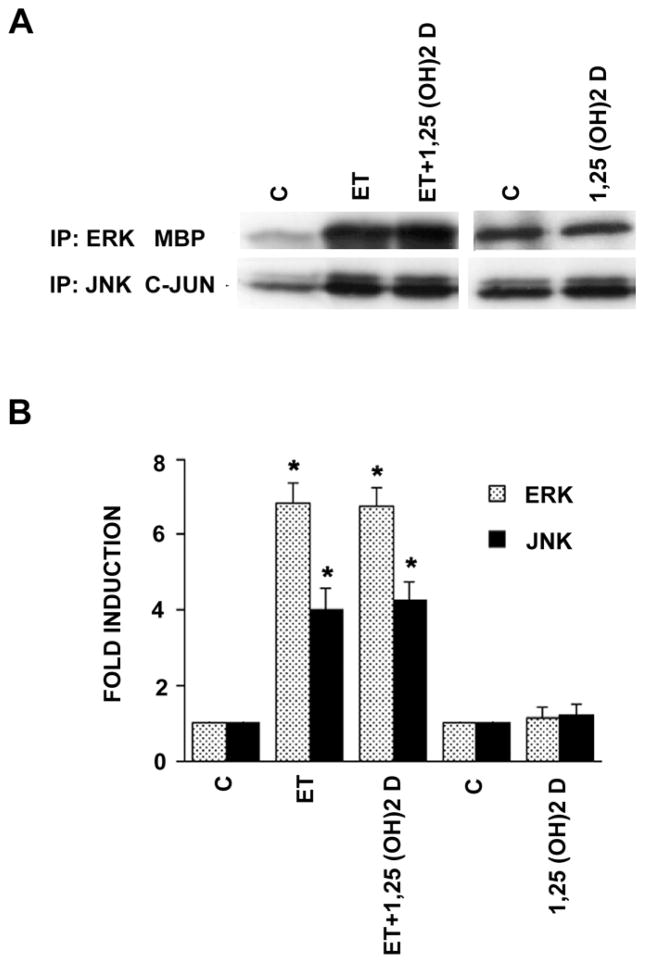
We and others have shown previously that agonists signaling through traditional mitogen activated protein kinase (MAPK)- dependent pathways have the capacity to promote DNA synthesis and cell division in vascular smooth muscle cells [24–27]. In fact, we reported that ET stimulation of DNA synthesis and cell replication in these neonatal VSMC is heavily dependent on stimulation of the extracellular signal regulated kinase(s) (ERK). We examined the possibility that 1,25 (OH)2 D might exert its anti-mitogenic effect through reductions in either ERK or JNK activity. As shown in Fig. 4, using an immune complex kinase assay, we demonstrated that ET activated both ERK (6–7 fold) and JNK (4-fold) activity in these cells. In neither case was the induction reversed or altered by 1,25 (OH)2 D arguing that 1,25 (OH)2 D operates over a pathway that does not directly involve ERK or JNK.
Fig. 4.
1,25 (OH)2 D does not affect ET-induced ERK or JNK activities. Cells were exposed to 10−8 M 1,25 (OH)2 D for 48 hours and then treated with ET (10−7 M) or vehicle alone, for 5 min (ERK activity) or 15 min (JNK activity). ERK and JNK kinases were immunoprecipitated and their activities were measured using MBP and GST-c-JUN as substrates, respectively. Representative autoradiograph and pooled data (n=3) are shown. *P<0.01 vs. control.
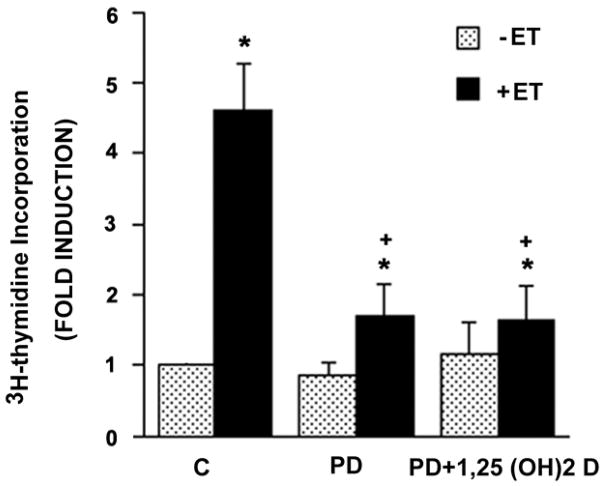
We then asked whether inhibition of the MEK/ERK pathway with the MEK inhibitor PD98059 might synergize with or amplify the inhibitory response to 1,25 (OH)2 D. As expected, PD98059 reduced the ET-dependent increment in 3H-thymidine incorporation by ~70% (Fig. 5). Addition of 1,25 (OH)2 D (10−8 M) to the MEK inhibitor resulted in no further increase in the inhibition, implying that the MEK/ERK pathway and 1,25 (OH)2 D are likely to signal over a shared final common pathway in reducing ET-dependent DNA synthesis in VSMC.
Fig. 5.
MEK inhibitor PD098059 and 1,25 (OH)2 D are not additive in inhibiting ET- induced [3H]thymidine-uptake. Where indicated, cells were treated with 10−8 M 1,25 (OH)2 D (or vehicle) for 48 hours as described in Fig. 1, then incubated with vehicle or 2× 10−5 M PD 098059 for 1 hour prior to exposure to vehicle or 10−7 M ET for the final 24 hours. [3H]-Thymidine incorporation was then measured. Pooled data were derived from three separate experiments. *P<0.01 vs. control. +P<0.01 vs. ET alone.
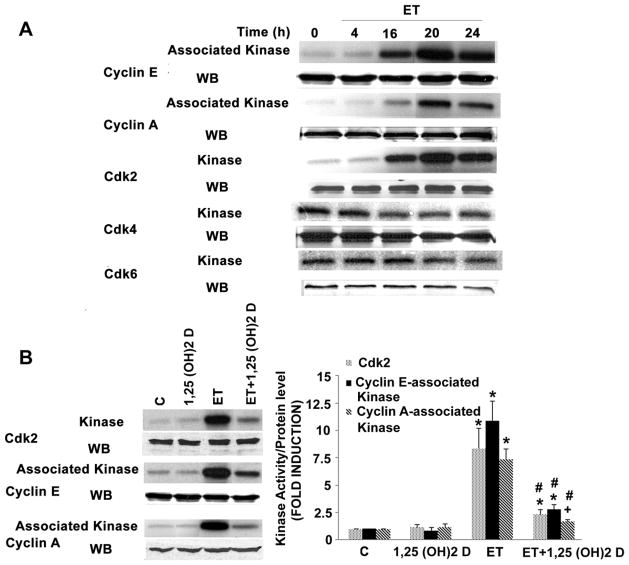
We have also shown previously that ET-dependent stimulation of the MEK/ERK cascade activates the cell cycle at the level of Cdk2 [25]. This, in turn, is assumed to drive the increase in DNA synthesis and subsequent cell proliferation in VSMC. We asked whether 1,25 (OH)2 D would prove capable of inhibiting this activation of Cdk2. As shown in Fig. 6A, using three independent immune complex kinase assays (i.e. direct immunoprecipitation of Cdk2, or co-immunoprecipitation of Cdk2 with cyclin E or cyclin A), we showed that ET increased Cdk2 activity between 7–10 fold without affecting Cdk2, cyclin A or cyclin E protein levels. Simultaneous analyses showed no effect of ET on activity or levels of Cdk4 or Cdk6 (Fig. 6A). While 1,25 (OH)2 D treatment had no effect on basal Cdk2 activity (Fig. 6B), it did reduce ET-dependent activity by more than 80%, again without affecting Cdk2, cyclin A or cyclin E protein levels.
Fig. 6.
1,25 (OH)2 D blocks ET-stimulated Cdk2 activity. Cells were treated with ET (10−7 M) for varying time intervals (A), cell extracts were generated and immunoprecipated with anti- Cdk2, anti-cyclin E, anti-cyclin A, anti-Cdk4 or anti-Cdk6 antibodies. Kinase activity of individual immunoprecipitates was determined using histone 1 as the substrate (top panel). Extracts were then subjected to Western blot analysis to assess levels of individual cyclins and kinases (lower panel) in the cells. Cells were treated with 1,25 (OH)2 D for 48 h and ET for the final 20 hours (B). Cdk2 was immunoprecipated with anti-Cdk2, anti-cyclin E or anti-cyclin A antibodies and kinase activity was measured as described above. Representative kinase assay (left) and pooled data (right) were from 3 independent experiments are shown. +P<0.05, *P<0.01 vs. control; #P<0.01 vs. ET alone.
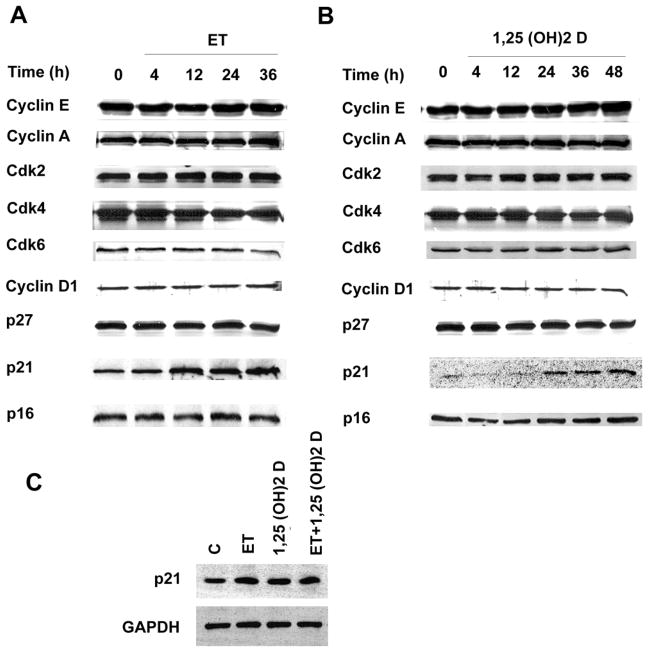
Cdk2 can be activated through an increase in Cdk2 gene expression, through increased expression of its associated activating cyclins (e.g. cyclin D or cyclin E), through reductions in the Cdk2 inhibitors p21Cip or p27 [28], as well as through a variety of post-translational modifications produced by proteins involved in cell cycle regulation. As shown in Fig. 7A, there was no increase in levels of the Cdk2 protein, cyclin D1 or cyclin E over 48 hours of treatment with ET. Levels of the Cdk2 inhibitor p21Cip, appeared to increase consistently after 12–36 hours of ET treatment. Levels of other cyclin dependent kinases, including Cdk4 and Cdk6, and the Cdk inhibitors p27 and p16ink were unchanged by ET treatment relative to controls. 1,25 (OH)2 D, similarly, had relatively little effect on levels of cyclin E, A or D1, Cdk2,4 or 6, or p27 or p16ink (Fig. 7B). Like ET, however, it effected a moderate increase in levels of p21Cip. The combination of ET plus 1,25 (OH)2 D together led to an increase in p21Cip protein levels which was less than additive when compared with either agent used in isolation (Fig. 7C). Collectively, these data document the presence of a 1,25 (OH)2 D-dependent increase in levels of the Cdk inhibitor p21Cip; however, given the similar magnitude and timing of the induction by ET, the data fall short of supporting p21Cip as a candidate mediator of 1,25 (OH)2 D’s anti-proliferative activity.
Fig. 7.
ET and 1,25 (OH)2 D regulate p21Cip but not G1 cyclins, Cdk’s, p27 or p16 in VSMC. A and B. Cells were incubated with 10−7 M ET or 10−8 M 1,25 (OH)2 D for the time intervals indicated. C. Cells were incubated with 10−7 M ET for 24hrs or 10−8 M 1,25 (OH)2 D for 48 hrs alone or in combination. Extracts were generated and analyzed by Western blot using the relevant antibodies. Each experiment was repeated two to three times with qualitatively comparable results.
DISCUSSION
The major new findings of the current study are 1) that the 1,25 (OH)2 D-dependent suppression of neonatal VSMC proliferative activity is linked to a reduction in Cdk2 activity, 2) that this suppression is not associated with a reduction in Cdk2 gene expression (nor expression of other key regulatory G1 cyclins or cyclin-dependent kinases, 3) that while the suppression appears to operate over shared circuitry with the MEK/ERK signaling system, it does not involve a direct suppression of ERK (or JNK) activity.
We have shown previously that the ET-dependent stimulation of DNA synthesis in these VSMC is heavily dependent on activation of the MEK/ERK signal transduction pathway [24–27]. Studies presented in Fig. 4 indicate that 1,25 (OH)2 D does not exert a direct effect on ERK or JNK activity in these cells; however, when 1,25 (OH)2 D and the MEK inhibitor PD90859 are used in combination (see Fig. 5), their respective inhibitory effects are not additive. This would suggest that ERK and 1,25 (OH)2 D operate over shared regulatory circuitry to control proliferative activity in these cells with 1,25 (OH)2 D acting at some locus downstream from ERK, presumably (see below) at the level of Cdk2.
Previous studies in a variety of cell types have pinpointed G1 and the G1/S transition as primary 1,25 (OH)2 D targets in executing its anti-proliferative activity [16, 21]. Several of these studies have suggested reductions in Cdk2 activity [29, 30], reductions in levels of the regulatory G1 cyclins (cyclin A and cyclin E) [29], increases in levels of the Cdk inhibitors, p21Cip [7, 31] and p27 [32, 33] and reduced phosphorylation of the retinoblastoma gene product [34] as potential explanations for the G1 arrest. The present study documents the 1,25 (OH)2 D-dependent reduction in Cdk2 activity in neonatal VSMC, but there is no concomitant reduction in cyclin A or cyclin E, nor any change in levels of the Cdk’s operating in G1 (Cdk4 or Cdk6). The regulatory protein whose expression does change uniquely is p21Cip. This Cdk inhibitor has been linked to 1,25 (OH)2 D in other contexts and is regarded as the potential mediator of 1,25 (OH)2 D’s anti-proliferative activity in prostate [7] and pancreatic [6] cancer cells. The nature of the induction of p21Cip expression by 1,25 (OH)2 D remains controversial. Some investigators have provided evidence that the liganded VDR binds directly to the p21 gene promoter in vitro [31] or in vivo [35] and drives transcriptional activity. Others have argued that 1,25 (OH)2 D operates through an intermediate like TGF® [36] or the IGF binding protein 3 [37] to promote increased p21Cip expression. Our own data shows quite clearly that 1,25 (OH)2 D increases expression of p21Cip in these VSMC with induction of protein levels (Fig. 7B) mRNA and promoter activity (data not shown). However, demonstration of a similar induction by the pro-mitogenic agonist ET argues rather strongly against p21Cip playing a major role in mediating the reduction in Cdk2 activity and ensuing anti-proliferative activity of 1,25 (OH)2 D in VSMC. The induction of p21Cip by ET is curious but not without precedent. Weiss et al have reported that p21Cip expression is required for platelet-derived growth factor(PDGF)-induced rat and bovine aortic smooth muscle cell proliferation [38], implying that this Cdk inhibitor may play important roles in supporting proliferative as well as anti-proliferative effects.
In summary, 1,25 (OH)2 D, as well as less hypercalcemic ligands of the VDR, is capable of inhibiting ET-induced proliferative activity of neonatal VSMC, an actively proliferating VSMC population in culture. This is accompanied by a decrease in ET-stimulated Cdk2 activity, albeit not by decreased expression of Cdk2 or its associated cyclins. This anti-proliferative activity suggests a potentially important therapeutic role for the use of these agents in the management of disorders characterized by dysfunctional smooth muscle cell growth and proliferation in the vascular wall (e.g. atherosclerosis or hypertension).
Acknowledgments
This work was supported by HL 45637 (D.G.G.) from the National Institutes of Health and Grant-in-Aid 0565016Y (S.C.) from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Banwell CM, Singh R, Stewart PM, Uskokovic MR, Campbell MJ. Antiproliferative signalling by 1,25(OH)2D3 in prostate and breast cancer is suppressed by a mechanism involving histone deacetylation. Recent Results Cancer Res. 2003;164:83–98. doi: 10.1007/978-3-642-55580-0_5. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RG, Mehta RR. Vitamin D and cancer. J Nutr Biochem. 2002;13(5):252–264. doi: 10.1016/s0955-2863(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 4.Pendas-Franco N, Garcia JM, Pena C, Valle N, Palmer HG, Heinaniemi M, Carlberg C, Jimenez B, Bonilla F, Munoz A, Gonzalez-Sancho JM. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D(3) Oncogene. 2008 doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 5.Kawa S, Yoshizawa K, Nikaido T, Kiyosawa K. Inhibitory effect of 22-oxa-1,25-dihydroxyvitamin D3, maxacalcitol, on the proliferation of pancreatic cancer cell lines. J Steroid Biochem Mol Biol. 2005;97(1–2):173–177. doi: 10.1016/j.jsbmb.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG, Eads D, Naczki C, Northrup S, Chen T, Koumenis C. 19-nor-1alpha,25-Dihydroxyvitamin D2 (Paricalcitol) inhibits the proliferation of human pancreatic cancer cells in vitro and in vivo. Cancer Biol Ther. 2007;7(3) doi: 10.4161/cbt.7.3.5418. [DOI] [PubMed] [Google Scholar]
- 7.Rao A, Coan A, Welsh JE, Barclay WW, Koumenis C, Cramer SD. Vitamin D receptor and p21/WAF1 are targets of genistein and 1,25-dihydroxyvitamin D3 in human prostate cancer cells. Cancer Res. 2004;64(6):2143–2147. doi: 10.1158/0008-5472.can-03-3480. [DOI] [PubMed] [Google Scholar]
- 8.Kajikawa M, Ishida H, Fujimoto S, Mukai E, Nishimura M, Fujita J, Tsuura Y, Okamoto Y, Norman AW, Seino Y. An insulinotropic effect of vitamin D analog with increasing intracellular Ca2+ concentration in pancreatic beta-cells through nongenomic signal transduction. Endocrinology. 1999;140(10):4706–4712. doi: 10.1210/endo.140.10.7025. [DOI] [PubMed] [Google Scholar]
- 9.Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17(3):509–511. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- 10.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97(1–2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Beckerman P, Silver J. Vitamin D and the parathyroid. Am J Med Sci. 1999;317(6):363–369. doi: 10.1097/00000441-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez ME, Almaden Y, Canadillas S, Canalejo A, Siendones E, Lopez I, Aguilera-Tejero E, Martin D, Rodriguez M. The calcimimetic R-568 increases vitamin D receptor expression in rat parathyroid glands. Am J Physiol Renal Physiol. 2007;292(5):F1390–1395. doi: 10.1152/ajprenal.00262.2006. [DOI] [PubMed] [Google Scholar]
- 13.Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol. 2007;103(3–5):533–537. doi: 10.1016/j.jsbmb.2006.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walters MR, Wicker DC, Riggle PC. 1,25-Dihydroxyvitamin D3 receptors identified in the rat heart. J Mol Cell Cardiol. 1986;18(1):67–72. doi: 10.1016/s0022-2828(86)80983-x. [DOI] [PubMed] [Google Scholar]
- 15.Mitsuhashi T, Morris RC, Jr, Ives HE. 1,25-dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J Clin Invest. 1991;87(6):1889–1895. doi: 10.1172/JCI115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eelen G, Gysemans C, Verlinden L, Vanoirbeek E, De Clercq P, Van Haver D, Mathieu C, Bouillon R, Verstuyf A. Mechanism and potential of the growth-inhibitory actions of vitamin D and ana-logs. Curr Med Chem. 2007;14(17):1893–1910. doi: 10.2174/092986707781058823. [DOI] [PubMed] [Google Scholar]
- 17.Cardus A, Parisi E, Gallego C, Aldea M, Fernandez E, Valdivielso JM. 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int. 2006;69(8):1377–1384. doi: 10.1038/sj.ki.5000304. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97(7):1577–1588. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13(6 Pt 2):954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 20.Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186(1):20–28. doi: 10.1016/j.atherosclerosis.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 21.Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. Vitamin D and cancer. J Steroid Biochem Mol Biol. 2006;102(1–5):156–162. doi: 10.1016/j.jsbmb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Jones PA, Scott-Burden T, Gevers W. Glycoprotein, elastin, and collagen secretion by rat smooth muscle cells. Proc Natl Acad Sci U S A. 1979;76(1):353–357. doi: 10.1073/pnas.76.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer RF, Arthur LO, Fine DL. Propagation of mouse mammary tumor cell lines and production of mouse mammary tumor virus in a serum-free medium. In Vitro. 1976;12(8):558–563. doi: 10.1007/BF02797439. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Gardner DG. Retinoic acid uses divergent mechanisms to activate or suppress mitogenesis in rat aortic smooth muscle cells. J Clin Invest. 1998;102(4):653–662. doi: 10.1172/JCI3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Gardner DG. Suppression of WEE1 and stimulation of CDC25A correlates with endothelin-dependent proliferation of rat aortic smooth muscle cells. J Biol Chem. 2004;279(14):13755–13763. doi: 10.1074/jbc.M310064200. [DOI] [PubMed] [Google Scholar]
- 26.Morey AK, Pedram A, Razandi M, Prins BA, Hu RM, Biesiada E, Levin ER. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology. 1997;138(8):3330–3339. doi: 10.1210/endo.138.8.5354. [DOI] [PubMed] [Google Scholar]
- 27.Whelchel A, Evans J, Posada J. Inhibition of ERK activation attenuates endothelin-stimulated airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 1997;16(5):589–596. doi: 10.1165/ajrcmb.16.5.9160841. [DOI] [PubMed] [Google Scholar]
- 28.Morgan DO. Principles of CDK regulation. Nature. 1995;374(6518):131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 29.Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J. Inhibitory effects of 1alpha,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol. 2001;15(8):1370–1380. doi: 10.1210/mend.15.8.0673. [DOI] [PubMed] [Google Scholar]
- 30.Scaglione-Sewell BA, Bissonnette M, Skarosi S, Abraham C, Brasitus TA. A vitamin D3 analog induces a G1-phase arrest in CaCo-2 cells by inhibiting cdk2 and cdk6: roles of cyclin E, p21Waf1, and p27Kip1. Endocrinology. 2000;141(11):3931–3939. doi: 10.1210/endo.141.11.7782. [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10(2):142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 32.Huang YC, Chen JY, Hung WC. Vitamin D3 receptor/Sp1 complex is required for the induction of p27Kip1 expression by vitamin D3. Oncogene. 2004;23(28):4856–4861. doi: 10.1038/sj.onc.1207621. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Li C, Zhao X, Zhang X, Nicosia SV, Bai W. p27(Kip1) stabilization and G(1) arrest by 1,25-dihydroxyvitamin D(3) in ovarian cancer cells mediated through down-regulation of cyclin E/cyclin-dependent kinase 2 and Skp1-Cullin-F-box protein/Skp2 ubiquitin ligase. J Biol Chem. 2004;279(24):25260–25267. doi: 10.1074/jbc.M311052200. [DOI] [PubMed] [Google Scholar]
- 34.Segaert S, Garmyn M, Degreef H, Bouillon R. Retinoic acid modulates the anti-proliferative effect of 1,25-dihydroxyvitamin D3 in cultured human epidermal keratinocytes. J Invest Dermatol. 1997;109(1):46–54. doi: 10.1111/1523-1747.ep12276488. [DOI] [PubMed] [Google Scholar]
- 35.Saramaki A, Banwell CM, Campbell MJ, Carlberg C. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 2006;34(2):543–554. doi: 10.1093/nar/gkj460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verlinden L, Verstuyf A, Convents R, Marcelis S, Van Camp M, Bouillon R. Action of 1,25(OH)2D3 on the cell cycle genes, cyclin D1, p21 and p27 in MCF-7 cells. Mol Cell Endocrinol. 1998;142(1–2):57–65. doi: 10.1016/s0303-7207(98)00117-8. [DOI] [PubMed] [Google Scholar]
- 37.Boyle BJ, Zhao XY, Cohen P, Feldman D. Insulin-like growth factor binding protein-3 mediates 1 alpha,25-dihydroxyvitamin d(3) growth inhibition in the LNCaP prostate cancer cell line through p21/WAF1. J Urol. 2001;165(4):1319–1324. [PubMed] [Google Scholar]
- 38.Weiss RH, Joo A, Randour C. p21(Waf1/Cip1) is an assembly factor required for platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J Biol Chem. 2000;275(14):10285–10290. doi: 10.1074/jbc.275.14.10285. [DOI] [PubMed] [Google Scholar]