Abstract
Background
Common infections may be associated with stroke risk, though no single infection is likely a major independent predictor.
Objective
To determine the association between a composite measure of serologies to common infections (Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, Herpes Simplex Virus 1 and 2) and stroke risk in a prospective cohort study.
Design
Prospective cohort followed longitudinally for median 8 years.
Patients
Randomly selected stroke-free participants from a multiethnic urban community.
Setting
Northern Manhattan Study (NOMAS).
Main Outcome measure
Incident stroke and other vascular events.
Results
All five infectious serologies were available from baseline samples in 1625 participants (mean age 68.5 ± 10.1 years; 64.9% women). Cox proportional hazards models were used to estimate associations of each positive serology with stroke. Individual parameter estimates were then combined into a weighted index of infectious burden (IB) and used to calculate hazard ratios and confidence intervals (HR, 95% CI) for association with risk of stroke and other outcomes, adjusted for risk factors. Each individual infection was positively though not significantly associated with stroke risk after adjusting for other risk factors. The IB index was associated with an increased risk of all strokes (adjusted HR per standard deviation 1.39, 95% CI 1.02–1.90) after adjusting for demographics and risk factors. Results were similar after excluding those with coronary disease (adjusted HR 1.50, 95% CI 1.05–2.13) and adjusting for inflammatory biomarkers.
Conclusion
A quantitative weighted index of infectious burden was associated with risk of first stroke in this cohort. Future studies are needed to confirm these findings and to further define optimal measures of IB as a stroke risk factor.
Introduction
Stroke is the third leading cause of death in the US, and the leading cause of serious disability.1 Classic modifiable risk factors include hypertension, cardiac disease, dyslipidemia, and smoking, but many strokes occur in patients without any of these risk factors.1 There is therefore interest in identifying additional modifiable stroke risk factors. Seroepidemiological studies in patients with coronary artery disease and stroke have provided evidence of an association of risk with serological evidence of prior infections with various pathogens such as Chlamydia pneumoniae, Helicobacter pylori, and herpesviruses.2,3,4,5,6,7,8 In addition, several of these organisms have been identified in atherosclerotic tissue specimens, and some have been found capable of inducing atherosclerosis in animal models.9,10
Mechanistically, these pathogens are thought to promote inflammation, thus contributing to atherosclerosis.11,12 Prospective studies and meta-analyses, however, have suggested that the association of individual serologies with risk of vascular events is modest.2 A more likely scenario is that a composite measure of multiple serologies, or infectious burden, is associated with risk.13,14,15,16 According to this hypothesis, the greater the number of infectious exposures during one’s lifetime, the higher the chance of promoting atherosclerosis via inflammation, thus increasing the risk of cardiovascular disease. Some investigators have provided support for this concept by correlating the number of positive serologies with the presence of atherosclerosis and carotid plaque progression or risk of vascular events, including stroke.13,14,15,17 In these studies, however, all positive serologies are given equal weight in predictive models. It is plausible, however, that different infections would each carry different weights of association with risk of vascular disease.
We hypothesized that a weighted measure of infectious burden would be associated with risk of incident stroke in a prospective cohort study in a multiethnic urban adult population. We further hypothesized that this same weighted measure would also be associated with other vascular event outcomes.
METHODS
Participant selection
The Northern Manhattan Study (NOMAS) is a community-based prospective cohort study designed to investigate stroke incidence, risk factors, and predictors of severity and outcome,18,19 as described previously. Briefly, this is a stroke-free, multiethnic, urban population with a race-ethnic distribution of 63% Hispanic, 20% non-Hispanic black, and 15% non-Hispanic white participants who were recruited by random digit dialing. A total of 3298 participants were enrolled between 1993 and 2001 if they 1) had no prior diagnosis of stroke, 2) were over 39 years old, and 3) resided in Northern Manhattan for at least 3 months in a household with a telephone. All participants gave informed consent and the study was approved by Columbia University Medical Center Institutional Review Board.
Baseline data were obtained via interviews with bilingual research assistants, physical and neurological examination by study physicians, in-patient assessments, and fasting blood specimen analysis.18 Blood pressure, height, weight, fasting lipid panels, leukocyte count, and glucose were measured by standard techniques. High-sensitivity C-reactive protein (hsCRP) was measured using a BNII nephelometer (Dade-Behring, Deerfield, IL). Hypertension (history, on medications, or SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg) and diabetes mellitus (history, on medications, or fasting blood glucose ≥ 126 mg/dL) were defined as described previously.19 Blood samples collected at enrollment were centrifuged and frozen at −70° C in 1 mL aliquots until the time of analysis. Because not all participants had sufficient blood samples available for the multiple serological analyses required for this study, a subsample of 1625 participants was used for the present analyses.
Serological analyses
Serologies against 5 common pathogens which have each been linked to atherosclerotic disease in prior studies were assessed. Enzyme linked immunoassay (ELISA) was used to measure antibody titers against C. pneumoniae (Sayvon Diagnostics, Israel), H. pylori, CMV (Wampole Laboratories, Princeton, NJ), HSV 1 and HSV 2 (Focus Diagnostics, Cypress, CA). Both IgG and IgA titers were measured for C. pneumoniae, but based on previous results in our population5,6 and others,3 IgA titers were used for further analyses. IgG titers were assessed for the other pathogens. Positive serologies were based upon recommendations from the commercial laboratories providing the assays. All serologic testing was conducted by personnel blinded to clinical outcomes.
Follow-up assessments
Annual follow-up conducted by telephone included assessment of vital status (dead or alive), interval hospitalizations, and presence of symptoms and events consistent with stroke or MI as previously described.18,19 Strokes were independently classified by two neurologists according to a modified Stroke Data Bank scheme,20 with subtype assessments determined by consensus. Disagreements were adjudicated by a third neurologist evaluator. A study cardiologist reviewed MIs for validation. First presentation of any type of stroke, ischemic or hemorrhagic, was defined according to the criteria established by the World Health Organization. MI was defined by criteria specified by the Cardiac Arrhythmia Pilot Study21 and the Lipid Research Clinics Coronary Primary Prevention Trial.22 All deaths were verified by a study physician and classified as vascular (stroke, MI, heart failure, pulmonary embolus, cardiac arrhythmia, and other vascular causes) or non-vascular.
Statistical analyses
The primary endpoint was all strokes; secondary endpoints were ischemic stroke, MI, vascular deaths, non-vascular deaths, all deaths, and a combined endpoint of stroke, MI, and vascular death. First, Cox proportional hazards models were used to estimate the regression coefficients and 95% confidence intervals for the association of each individual serology with risk of stroke in unadjusted models and models adjusted for demographics, blood pressure, coronary artery disease, waist size, alcohol consumption, physical activity, smoking, blood sugar, HDL, and LDL. We included variables in multivariate models that were significant in prior analyses of vascular outcomes in NOMAS and that are traditionally accepted risk factors for stroke. We used continuous measures for blood pressure and blood sugar rather than cruder dichotomous measures such as hypertension and diabetes in our main models based on evidence of continuous relationships between these physiologic measures and risk of vascular disease.23 Additional models were run using hypertension and diabetes mellitus as categorical variables.
Parameter estimates, or β coefficients, from a model containing only the individual serologies were then used to derive a weighted index designated as the infectious burden (IB). Each parameter estimate represents the strength of the association between the individual positive serology and stroke as an outcome. Individual parameter estimates for those serologies for which an individual was positive were added together to form the IB index. Parameter estimates for those serologies for which the individual was negative would not be counted toward the total index score. For example, the IB for a participant with individual positive serologies for CMV and HSV2 would equal the sum of the parameter estimates for only CMV and HSV2. This index was then used as the independent variable in unadjusted and adjusted models to calculate the hazard ratios and confidence intervals (HR, 95% CI) for association with risk of stroke and other outcomes. Further models were calculated among those without history of MI. The final models among those without MI satisfied proportionality assumptions. Associations were expressed per change in standard deviation (SD) of IB.
RESULTS
Participant characteristics among the 1625 participants with serologic data are shown in Table 1, and were similar to those in the overall NOMAS cohort, with the exception that the participants in this study had enrolled later compared to those who did not have all five infectious serologies at the time of enrollment. Mean age of participants was 68.4 ± 10.1 years, and the median follow-up was 7.6 years (interquartile range 6.4–9.0 years). In follow-up, there were 67 strokes, of which 56 were ischemic, 98 MIs, 150 vascular deaths, 215 non-vascular deaths, and 390 deaths of all causes. Frequencies of positive serologies, using pre-specified thresholds, for C. pneumoniae, H. pylori, CMV, HSV 1, and HSV 2 are given in Table 1. Positive titers were common for these organisms, ranging from 55% for H. pylori to 86% for HSV 1.
Table 1.
Participant Characteristics
| Characteristic | N (%) or Mean ± SD |
|---|---|
| n | 1625 |
| Age, y | 68.4 ±10.1 |
| Men, n (%) | 570 (35.1) |
| Race-Ethnicity, n (%) | |
| Hispanic | 946 (58.2) |
| Non-Hispanic Black | 333 (20.5) |
| Non-Hispanic White | 295 (18.2) |
| High School Diploma, n (%) | 707 (43.5) |
| Risk Factors, n (%) | |
| Hypertension | 1195 (73.5) |
| Diabetes mellitus | 340 (21.0) |
| Current Smoker | 272 (16.8) |
| Coronary artery disease | 340(20.9) |
| Hyperlipidemia | 975 (60.0) |
| Atrial fibrillation | 79 (4.9) |
| Systolic blood pressure | 143.6 ± 21.2 |
| Diastolic blood pressure | 83.2 ± 11.1 |
| Low density lipoprotein | 129.8 ± 35.8 |
| High density lipoprotein | 46.5 ±14.1 |
| Positive titers to: | |
| Chlamydia pneumoniae IgA > 1.1 | 1071 (65.9) |
| Chlamydia pneumoniae IgG > 1.1 | 1209 (74.4) |
| Helicobacter pylori IgG ≥ 20 | 898 (55.3) |
| Cytomegalovirus IgG ≥ 1.1 | 1388 (85.4) |
| Herpes simplex virus 1 IgG > 1.1 | 1402 (86.3) |
| Herpes simplex virus 2 IgG > 1.1 | 928 (57.1) |
Each infectious serology was positively associated with an increased risk for all strokes, with hazard ratios adjusted for age, sex, race-ethnicity, high school education, SBP, LDL, HDL, blood sugar, moderate-to-heavy activity level, waist size, and CAD ranging between 1.13 for H. pylori to 2.19 for CMV, although none of these associations reached statistical significance (Table 2).
Table 2.
Risk of Stroke Associated with Positive Serologies for C. pneumoniae, H. Pylori, CMV, HSV 1, and HSV 2
| Positive Serology | Unadjusted HR (95% CI) | Adjusted HR (95 % CI)* |
|---|---|---|
| Chlamydia pneumonia IgA | 1.36 (0.81–2.31) | 1.30 (0.75–2.25) |
| H. pylori IgG | 1.01 (0.62–1.64) | 1.13 (0.68–1.89) |
| CMV IgG | 2.21 (0.89–5.49) | 2.19 (0.84–5.70) |
| HSV 1 IgG | 1.44 (0.66–3.14) | 1.35 (0.59–3.07) |
| HSV 2 IgG | 1.31 (0.80–2.16) | 1.59 (0.91–2.76) |
Adjusted for age, sex, race-ethnicity, high school education, systolic blood pressure, high density lipoprotein, low density lipoprotein, blood sugar, moderate alcohol use, cigarette smoking status, waist circumference, physical activity, and coronary artery disease.
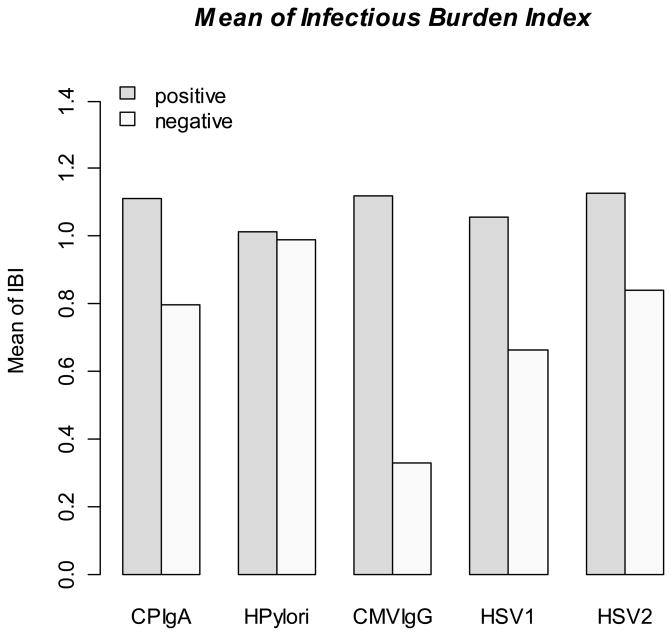
To determine whether composite seropositivity was associated with risk of stroke, individual unadjusted parameter estimates were added, as above, to generate the weighted infectious burden (IB) index (mean 1.00 ± SD 0.33; median 1.08). The mean IB index for those positive for each serology is shown in the Figure. The mean IB index was higher in non-Hispanic blacks (1.05 ± 0.31) and Hispanics (1.07 ± 0.27) compared to non-Hispanic whites (0.75 ± 0.41; p<0.0001 for both comparisons). It was slightly higher in women (1.02 ± 0.31) than men (0.97 ± 0.36; p=0.0016). There was no difference by age.
Figure. Mean infectious burden index by serological status to each of the five infections.
The figure shows the mean infectious burden index (IBI) according to positive or negative status for each of the individual infectious serologies.
IB was associated with risk of stroke (unadjusted HR per SD 1.39, 95% CI 1.04–1.87), and the effect was essentially unchanged after adjusting for demographics (adjusted HR 1.42, 95% CI 1.04–1.94; Table 3). After adjusting for age, sex, race-ethnicity, high school education, SBP, LDL, HDL, blood sugar, moderate-to-heavy activity level, waist size, and CAD, the association remained (adjusted HR 1.39, 95% CI 1.02–1.90). Further adjusting for leukocyte count and hsCRP had no effect on this estimate. The magnitude of the correlation of hsCRP with infectious burden index was only modest (r=0.09, p=0.0001).
Table 3.
Risk of Stroke Associated with Infectious Burden Index
| HR (95 % CI) per SD IB | ||
|---|---|---|
| Among full cohort (n=1625) | Among those without history of MI (n=1525) | |
| Unadjusted | 1.39 (1.04–1.87) | 1.51 (1.08–2.11) |
| Adjusted for demographics* | 1.42 (1.04–1.94) | 1.54 (1.08–2.20) |
| Adjusted for demographics* and risk factors† | 1.39 (1.02–1.90) | 1.50 (1.05–2.13) |
| Adjusted for demographics*, risk factors,† and log hsCRP | 1.39 (1.02–1.90) | 1.52 (1.06–2.17) |
| Adjusted for demographics, risk factors,† and log leukocyte count | 1.40 (1.03–1.91) | 1.51 (1.06–2.16) |
Demographics: age, sex, race-ethnicity, and high school education
Risk factors: Systolic blood pressure, high density lipoprotein, low density lipoprotein, blood sugar, moderate alcohol use, cigarette smoking status, waist circumference, moderate-to-heavy activity level, and CAD
Abbreviations: HR=hazard ratio; CI=confidence interval; SD=standard deviation; IBI=infectious burden
Additional models were run using history of hypertension and diabetes mellitus, as previously defined,19 in place of blood pressure and blood sugar measurements, and the results were essentially unchanged (adjusted HR per SD change in infectious burden 1.42, 1.03–1.97). Further analyses limited to those without history of MI (n=1525) showed a modestly increased magnitude of effect (fully adjusted HR per SD 1.50, 95% CI 1.05–2.13).
Tests for interactions with age, sex, race-ethnicity, and other risk factors demonstrated a significant interaction with diabetes mellitus (p= 0.0195). Among diabetics, there was an increased risk of stroke associated with IB (adjusted HR per SD 1.63, 95% CI 1.16–2.29). The effect in non-diabetics was reduced in magnitude and not statistically significant (adjusted HR per SD 1.29, 95% CI 0.94–1.78). Other interactions were not significant.
All secondary endpoints were positively associated with IB. Non-vascular deaths (adjusted HR per SD 1.23, 95% CI 1.04–1.45) and the combined endpoint of all stroke, MI and deaths (adjusted HR per SD 1.15, 95% CI 1.03–1.29) reached statistical significance (Table 4).
Table 4.
Risk of Secondary Outcomes Associated With Infectious Burden
| Secondary Outcomes | Unadjusted HR (95% CI) per SD IB | Adjusted HR (95 % CI)* per SD IB |
|---|---|---|
| Ischemic stroke | 1.33 (0.97–1.82) | 1.31 (0.94–1.83) |
| All MI | 0.99 (0.82–1.20) | 1.07 (0.85–1.33) |
| Vascular deaths | 0.99 (0.85–1.16) | 0.96 (0.81–1.15) |
| Non-vascular deaths | 1.21 (1.04–1.40) | 1.23 (1.04–1.45) |
| All deaths | 1.12 (1.01–1.24) | 1.12 (0.99–1.26) |
| Ischemic stroke, all MI, and vascular death | 1.05 (0.92–1.19) | 1.05 (0.90–1.21) |
| All stroke, all MI, and vascular death | 1.06 (0.93–1.20) | 1.05 (0.91–1.22) |
| All stroke, all MI, and all deaths | 1.13 (1.03–1.25) | 1.15 (1.03–1.29) |
Adjusted for age, sex, race-ethnicity, high school education, systolic blood pressure, high density lipoprotein, low density lipoprotein, blood sugar, moderate alcohol use, cigarette smoking status, waist circumference, moderate-to-heavy activity level, and coronary artery disease.
Abbreviations: HR=hazard ratio; CI=confidence interval; SD=standard deviation; IBI=infectious burden
DISCUSSION
In this prospective cohort study, a weighted index of exposure to 5 common infections previously implicated in atherosclerotic disease was associated with risk of first stroke. Although individually each infection was positively associated with increased stroke risk, none were individually statistically significant. Our measure of infectious burden considered the association of infections with stroke risk as a weighted measure rather than simply summing up the number of positive serologies, as had been done in prior studies. We therefore did not have an a priori assumption about the strength of association between each of the individual infections and stroke risk. These results need to be validated in independent populations, however, before they can be generalized. These results provide evidence that there is probably no single infectious agent responsible for atherosclerosis or stroke, but that a more likely mechanism for any possible association of infection with stroke is through a more general pro-inflammatory mechanism.
The rationale for investigating these particular five pathogens is multifold. First, each of these common pathogens may persist after an acute infection and thus contribute to perpetuating a state of chronic, low level infection. Second, prior studies demonstrated an association between each of these pathogens and cardiovascular diseases.2,3,4,5,6,7,8,9,10,11,12 C. pneumoniae, the best studied of these infections in relation to atherosclerosis, is an atypical respiratory pathogen which has been investigated as a stroke risk factor in several case-control and prospective studies, with mixed results. In prior case-control studies from the northern Manhattan population, C. pneumoniae IgA titers were associated with increased stroke risk, though IgG titers were not.5,6 Studies in other populations have similarly found IgA to be a better marker of stroke risk than IgG titers.3 These results have not been confirmed in other studies, however, and prospective studies have demonstrated at best a very modest association of C. pneumoniae titers with stroke risk.2 Our present prospective study confirms that the effect of C. pneumoniae IgA titers is likely to be modest. Further studies in large populations using well-standardized assays with generally accepted titer cutoffs may be needed to more definitively elucidate the relationship between C. pneumoniae and stroke.2
Recent studies have implicated H. pylori, which is well-recognized as a cause of chronic gastric inflammation, ulceration, and cancer,24 as another pathogen whose persistence might be associated with increased stroke risk. Several case-control studies have demonstrated an increased risk of stroke in seropositive subjects.25,26,27 A meta-analysis of case-control studies on H. pylori serostatus revealed an increased risk for stroke (OR 1.41, 95% CI 1.11–1.78), and especially for large vessel stroke.28 The stroke risk appeared to be especially apparent in subjects seropositive for strains with the virulence factor CagA toxin.28 Other studies have not found this association, however.8,17,29,30 We did not limit our analyses to large vessel stroke, however, nor did we measure H. pylori CagA toxin serostatus.
CMV is a common viral pathogen, and it can reactivate and cause particularly severe complications in immunocompromised hosts, though even immunocompetent hosts can develop CMV disease. CMV has been implicated as a cause of transplant vasculopathy.31,32 There is also some evidence that CMV is associated with stroke risk in otherwise healthy individuals. In two case-control studies, CMV seropositivity was associated with increased stroke risk,33,34 though this was not confirmed in several prospective studies.7,17,35,36 It has been shown that in immunocompromised hosts such as those with diabetes,37 or in post-renal transplant patients,31 the risk of atherosclerotic events including strokes is increased with CMV seropositivity. CMV was most strongly associated with stroke risk among the 5 pathogens we studied, though it did not reach statistical significance. Many of our participants (21%) were diabetic, and this may have influenced our findings.
HSV 1 and HSV 2 are also viral pathogens with chronic persistence in a latent state. Early work on Marek disease virus, a type of chicken herpesvirus, demonstrated that the virus was associated with atherosclerosis in a chicken model.38 Few studies have investigated the association of HSV with stroke risk, and their findings are conflicting. In one case-control study, HSV 1 was associated with increased stroke risk,34 but in another nested case-control study neither HSV 1 nor HSV 2 increased stroke risk.7 As with CMV, immunocompetent status, even which associated with diabetes, might be a confounding factor.
Our results extend the findings of previous prospective studies aimed at investigating the association of infectious burden and other vascular events. In a secondary analysis of the Heart Outcomes Prevention Evaluation (HOPE trial), combined serostatus for 4 vs. 0–1 pathogens was associated with risk of MI, stroke, or cardiovascular death with a magnitude similar to what we found (HR 1.41, 95% CI 1.02–1.96).15 In that study, however, pathogen burden was not associated with stroke as an independent outcome. In the Framingham study, pathogen burden based on serostatus for C. pneumoniae, H. pylori, and CMV was not associated with pooled primary endpoints of MI, atheroembolic stroke, and CHD death.17 A small case-control study did show a modest association between pathogen burden based on serologies for L. pneumophilla, M. pneumoniae, and C. pneumoniae and risk of stroke and TIA.39 However, other small case-control studies investigating the pathogen burden using similar serologies did not find an increased risk for stroke, though they may have been underpowered.8,34
There are several differences between our study and these other studies. Most importantly, our weighted measure of infectious burden avoided a priori assumptions about the strength of association between each of the individual infections and stroke risk. Our study also included HSV 1 and 2 serostatus in the calculation of the IB index, which may have enhanced the ability to detect its influence on first stroke. Small sample size, selection bias, and case-control study design may account for some of the other differences. The HOPE trial, moreover, was a secondary prevention trial rather than a population-based study.
Our study also explored the association between infectious burden and secondary vascular outcomes. Non-vascular deaths and the combined endpoint of all stroke, MI, and death were associated with the IB index, though MI and vascular death were not. Studies of periodontal infection and vascular disease have similarly found that there may be a stronger association for stroke than for MI.40,41
Our study could have potential clinical implications. For example, treatment and eradication of these chronic pathogens might mitigate future risk of stroke. Antibiotic therapy directed against C. pneumoniae has been tested in randomized, controlled trials without evidence of benefit against heart disease.42,43 Whether the same holds true for stroke has not yet been established. More studies will be required to further explore infectious burden as a potential modifiable risk factor for stroke.
Our study has several strengths. First, this was a large, multi-ethnic, population-based prospective study with routine assessment of risk factors and minimal loss to follow-up. We had a large proportion of Hispanics, a traditionally understudied group, in our population. Incidence of first stroke was assessed after adjusting for traditional risk factors, as well as for hsCRP and WBC counts, variables previously shown to be associated with incident stroke.19,44 The limitations of our study include the fact that participant information regarding the use of specific cholesterol lowering agents such as statins, preexisting inflammatory diseases, anti-inflammatory medication use, immunosuppression status, and infection status at the time of stroke or other outcomes was unavailable. Availability of this data could have better accounted for known and other unknown confounding factors. Further studies will be needed to confirm the independent predictive effect of infectious burden as a stroke risk factor.
Acknowledgments
This research was supported by NIH/NINDS R37 29993 (MSVE/RLS) and NIH/NINDS R01 48134 (MSVE). Dr. Elkind had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL American Heart Association/American Stroke Association Stroke Council; Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; Quality of Care and Outcomes Research Interdisciplinary Working Group; American Academy of Neurology. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 2.Kalayoglu MV, Libby P, Byrne GI. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA. 2002;288:2724–31. doi: 10.1001/jama.288.21.2724. [DOI] [PubMed] [Google Scholar]
- 3.Wimmer MLJ, Sandmann-Strupp R, Saikku P, Haberl RL. Association of chlamydial infection with cerebrovascular disease. Stroke. 1996;27:2207–2210. doi: 10.1161/01.str.27.12.2207. [DOI] [PubMed] [Google Scholar]
- 4.Cook PJ, Honeybourne D, Lip GY, et al. Chlamydia pneumoniae antibody titers are significantly associated with acute stroke and transient cerebral ischemia: the West Birmingham Stroke Project. Stroke. 1998;29:404–10. doi: 10.1161/01.str.29.2.404. [DOI] [PubMed] [Google Scholar]
- 5.Elkind MS, Lin IF, Grayston JT, Sacco RL. Chlamydia pneumoniae and the risk of first ischemic stroke : The Northern Manhattan Stroke Study. Stroke. 2000;31:1521–5. doi: 10.1161/01.str.31.7.1521. [DOI] [PubMed] [Google Scholar]
- 6.Elkind MS, Tondella ML, Feikin DR, et al. Seropositivity to Chlamydia pneumoniae is associated with risk of first ischemic stroke. Stroke. 2006;37:790–5. doi: 10.1161/01.STR.0000202624.89869.e9. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Hennekens CH, Stampfer MJ, Wang F. Prospective study of herpes simplex virus, cytomegalovirus, and the risk of future myocardial infarction and stroke. Circulation. 1998;98:2796–9. doi: 10.1161/01.cir.98.25.2796. [DOI] [PubMed] [Google Scholar]
- 8.Heuschmann PU, Neureiter D, Gesslein M, et al. Association between infection with Helicobacter pylori and Chlamydia pneumoniae and risk of ischemic stroke subtypes: Results from a population-based case-control study. Stroke. 2001;32:2253–8. doi: 10.1161/hs1001.097096. [DOI] [PubMed] [Google Scholar]
- 9.Laitinen K, Laurila A, Pyhala L, et al. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immun. 1997;65:4832–5. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muhlestein JB, Anderson JL, Hammond EH, et al. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation. 1998;97:633–6. doi: 10.1161/01.cir.97.7.633. [DOI] [PubMed] [Google Scholar]
- 11.Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation. 1999;100:e20–8. doi: 10.1161/01.cir.100.4.e20. [DOI] [PubMed] [Google Scholar]
- 12.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518–32. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 13.Espinola-Klein C, Rupprecht HJ, Blankenberg S, et al. Impact of infectious burden on extent and long-term prognosis of atherosclerosis. Circulation. 2002;105:15–21. doi: 10.1161/hc0102.101362. [DOI] [PubMed] [Google Scholar]
- 14.Espinola-Klein C, Rupprecht HJ, Blankenberg S, et al. Impact of infectious burden on progression of carotid atherosclerosis. Stroke. 2002;33:2581–6. doi: 10.1161/01.str.0000034789.82859.a4. [DOI] [PubMed] [Google Scholar]
- 15.Smieja M, Gnarpe J, Lonn E, et al. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2003;107:251–7. doi: 10.1161/01.cir.0000044940.65226.1f. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Nieto FJ, Horne BD, et al. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation. 2001;103:45–51. doi: 10.1161/01.cir.103.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Haider AW, Wilson PW, Larson MG, et al. The association of seropositivity to Helicobacter pylori, Chlamydia pneumoniae, and cytomegalovirus with risk of cardiovascular disease: a prospective study. J Am Coll Cardiol. 2002;40:1408–13. doi: 10.1016/s0735-1097(02)02272-6. [DOI] [PubMed] [Google Scholar]
- 18.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a tri-ethnic cohort: The Northern Manhattan Study. Stroke. 2004;35:2263–9. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 19.Elkind MSV, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Relative Elevation in Leukocyte Count Predicts First Cerebral Infarction. Neurology. 2005;64:2121–2125. doi: 10.1212/01.WNL.0000165989.12122.49. [DOI] [PubMed] [Google Scholar]
- 20.Gan R, Sacco RL, Kargman DE, et al. Testing the validity of the lacunar hypothesis: the Northern Manhattan Stroke Study experience. Neurology. 1997;48:1204–11. doi: 10.1212/wnl.48.5.1204. [DOI] [PubMed] [Google Scholar]
- 21.Greene HL, et al. Classification of deaths after myocardial infarction as arrhythmic or nonarrhythmic (the Cardiac Arrhythmia Pilot Study) Am J Cardiol. 1989;63:1–6. doi: 10.1016/0002-9149(89)91065-5. [DOI] [PubMed] [Google Scholar]
- 22.Morris DL, Kritchevsky SB, Davis CE. Serum carotenoids and coronary heart disease. The Lipid Research Clinics Coronary Primary Prevention Trial and Follow-up Study. JAMA. 1994;272:1439–41. doi: 10.1001/jama.272.18.1439. [DOI] [PubMed] [Google Scholar]
- 23.Law MR, Wald NJ. Risk factor thresholds: their existence under scrutiny. BMJ. 2002;324:1570–6. doi: 10.1136/bmj.324.7353.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veldhuyzen van Zanten SJ, Sherman PM. Helicobacter pylori infection as a cause of gastritis, duodenal ulcer, gastric cancer and nonulcer dyspepsia: a systematic overview. Can Med Assoc J. 1994;150:177–85. [PMC free article] [PubMed] [Google Scholar]
- 25.Park MH, Min JY, Koh SB, et al. Helicobacter pylori infection and the CD14 C(−260)T gene polymorphism in ischemic stroke. Thromb Res. 2006;118:671–7. doi: 10.1016/j.thromres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Sawayama Y, Ariyama I, Hamada M, et al. Association between chronic Helicobacter pylori infection and acute ischemic stroke: Fukuoka Harasanshin Atherosclerosis Trial (FHAT) Atherosclerosis. 2005;178:303–9. doi: 10.1016/j.atherosclerosis.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Masoud SA, Arami MA, Kucheki E. Association between infection with Helicobacter pylori and cerebral noncardioembolic ischemic stroke. Neurol India. 2005;53:303–6. doi: 10.4103/0028-3886.16928. [DOI] [PubMed] [Google Scholar]
- 28.Cremonini F, Gabrielli M, Gasbarrini G, Pola P, Gasbarrini A. The relationship between chronic H. pylori infection, CagA seropositivity and stroke: meta-analysis. Atherosclerosis. 2004;173:253–9. doi: 10.1016/j.atherosclerosis.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Whincup PH, Mendall MA, Perry IJ, Strachan DP, Walker M. Prospective relations between Helicobacter pylori infection, coronary heart disease, and stroke in middle aged men. Heart. 1996;75:568–72. doi: 10.1136/hrt.75.6.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preusch MR, Grau AJ, Buggle F, et al. Association between cerebral ischemia and cytotoxin-associated gene-A-bearing strains of Helicobacter pylori. Stroke. 2004;35:1800–4. doi: 10.1161/01.STR.0000131751.35926.48. [DOI] [PubMed] [Google Scholar]
- 31.Ozdemir FN, Akgul A, Altunoglu A, et al. The association between cytomegalovirus infection and atherosclerotic events in renal transplant recipients. Transplant Proc. 2007;39:990–2. doi: 10.1016/j.transproceed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 32.Ventura HO, Mehra MR, Smart FW, Stapleton DD. Cardiac allograft vasculopathy: current concepts. Am Heart J. 1995;129:791–8. doi: 10.1016/0002-8703(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 33.Tarnacka B, Gromadzka G, Czlonkowska A. Increased circulating immune complexes in acute stroke: the triggering role of Chlamydia pneumoniae and cytomegalovirus. Stroke. 2002;33:936–40. doi: 10.1161/01.str.0000014562.75483.6b. [DOI] [PubMed] [Google Scholar]
- 34.Kis Z, Sas K, Gyulai Z, et al. Chronic infections and genetic factors in the development of ischemic stroke. New Microbiol. 2007;30:213–20. [PubMed] [Google Scholar]
- 35.Coles KA, Knuiman MW, Plant AJ, et al. A prospective study of infection and cardiovascular diseases: the Busselton Health Study. Eur J Cardiovasc Prev Rehabil. 2003;10:278–82. doi: 10.1097/00149831-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Fagerberg B, Gnarpe J, Gnarpe H, Agewall S, Wikstrand J, et al. Chlamydia pneumoniae but not cytomegalovirus antibodies are associated with future risk of stroke and cardiovascular disease: a prospective study in middle-aged to elderly men with treated hypertension. Stroke. 1999;30:299–305. doi: 10.1161/01.str.30.2.299. [DOI] [PubMed] [Google Scholar]
- 37.Guech-Ongey M, Brenner H, Twardella D, Hahmann H, Rothenbacher D. Role of cytomegalovirus sero-status in the development of secondary cardiovascular events in patients with coronary heart disease under special consideration of diabetes. Int J Cardiol. 2006;111:98–103. doi: 10.1016/j.ijcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 38.Fabricant CG, Fabricant J, Minick CR, Litrenta MM. Herpesvirus-induced atherosclerosis in chickens. Federation Proc. 1983;42:2476–9. [PubMed] [Google Scholar]
- 39.Ngeh J, Goodbourn C. Chlamydia pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophila in elderly patients with stroke (C-PEPS, M-PEPS, L-PEPS): a case-control study on the infectious burden of atypical respiratory pathogens in elderly patients with acute cerebrovascular disease. Stroke. 2005;36:259–65. doi: 10.1161/01.STR.0000152961.11730.d9. [DOI] [PubMed] [Google Scholar]
- 40.Wu T, Trevisan M, Genco RJ, et al. Periodontal disease and risk of cerebrovascular disease: The first National Health and Nutrition Examination Survey and its follow-up study. Arch Intern Med. 2000;160:2749–55. doi: 10.1001/archinte.160.18.2749. [DOI] [PubMed] [Google Scholar]
- 41.Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. JAMA. 2000;284:1406–10. doi: 10.1001/jama.284.11.1406. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor CM, Dunne MW, Pfeffer MA, et al. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459–66. doi: 10.1001/jama.290.11.1459. [DOI] [PubMed] [Google Scholar]
- 43.Grayston JT, Kronmal RA, Jackson LA, et al. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637–45. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]
- 44.Di Napoli M, Schwaninger M, Cappelli R, et al. Evaluation of C-Reactive Protein measurement for assessing the risk and prognosis in ischemic stroke: A statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36:1316–1329. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]