Abstract
The von Hippel-Lindau protein pVHL suppresses renal tumorigenesis in part by promoting degradation of hypoxia-inducible HIF-alpha transcription factors1, and additional mechanisms have been proposed2. pVHL also stabilizes plant homeodomain (PHD) protein Jade-1, which is a candidate renal tumor suppressor that may correlate with renal cancer risk3-5. We show here that Jade-1 binds the oncoprotein β-catenin in Wnt-responsive fashion. Moreover, Jade-1 destabilizes wild-type β-catenin, but not a cancer-causing form of β-catenin. While β-TrCP ubiquitinates only phosphorylated β-catenin6, Jade-1 ubiquitinates both phosphorylated and non-phosphorylated β-catenin and therefore regulates canonical Wnt signaling in both Wnt-off and Wnt-on phases. Thus, the different characteristics of β-TrCP and Jade-1 may ensure optimal Wnt pathway regulation. Furthermore, pVHL down-regulates β-catenin in a Jade-1-dependent manner and inhibits Wnt signaling, supporting a role for Jade-1 and Wnt signaling in renal tumorigenesis. The pVHL tumor suppressor and the Wnt tumorigenesis pathway are therefore directly linked through Jade-1.
To identify molecular mediators of Jade-1 activity, we screened a human kidney cDNA library with a yeast two-hybrid approach using a transcriptionally inactive truncation of Jade-1 lacking both PHDs (Jade-1 dd) as bait (Supplementary Information, Fig. S1a). Nine strong interactors were found, including β-catenin, an oncoprotein and the key transcriptional co-activator of canonical Wnt signaling7.
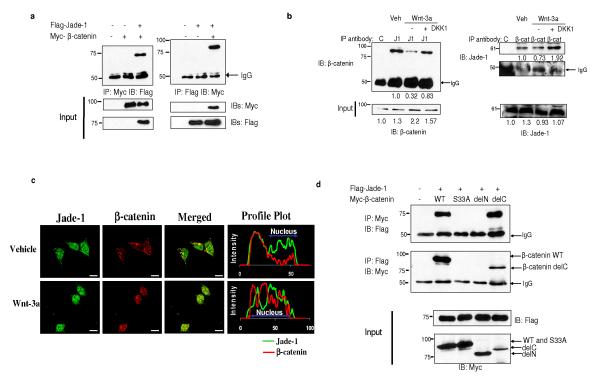
The Jade-1-β-catenin interaction was confirmed in mammalian cells by coimmunoprecipitation (Fig. 1a). The localization and fate of β-catenin depend on Wnt status7. Constitutively, in Wnt-off phase, β-catenin is phosphorylated by GSK-3β, binds to the destruction complex in the cytosol and gets degraded. In Wnt-on phase, GSK-3β is inhibited; β-catenin dissociates from the destruction complex and translocates to the nucleus. We therefore examined the binding of endogenous Jade-1 and β-catenin during the different states of Wnt signaling. Wnt signaling was activated using Wnt-3a ligand or lithium chloride (an inhibitor of GSK-3β that mimics Wnt activation) and inhibited using Wnt-3a plus DKK1, a competitive antagonist of Wnt-3a (Fig. 1b and Supplementary Information, Fig. S1b). Endogenous Jade-1 co-immunoprecipitated with endogenous β-catenin and vice-versa (Fig. 1b). However, the Jade-1-β-catenin interaction was increased in vehicle and Wnt-3a-DKK1 treated cells (Wnt-off phase) compared with Wnt-3a treated cells (Wnt-on phase). Co-localization and profile plots were performed to demonstrate the distribution and abundance of the proteins (Fig. 1c). In Wnt-off phase (Fig. 1c, Vehicle treated), β-catenin was predominantly in the cytosol and cell membrane. Jade-1 was in the cytosol and nucleus, exclusive of nucleoli3,8. Co-localization of Jade-1 and β-catenin was found in the cytosol. Wnt-3a treatment resulted in nuclear translocation of β-catenin. However, Jade-1 and β-catenin exhibited different sub-compartmental localization in the nucleus (Fig. 1c, Wnt-3a treated), resulting in reduction in co-localization. Thus, endogenous Jade-1 and endogenous β-catenin interact, and the interaction is greater in Wnt-off phase than in Wnt-on phase.
Figure 1.
Jade-1 and β-catenin interact. (a) In vivo interaction of Jade-1 and β-catenin. Extracts (600 μg protein) from transiently transfected 293T cells were immunoprecipitated (IP) with 1 μg monoclonal Myc-tag or Flag-tag antibodies. Co-immunoprecipitated β-catenin or Jade-1 was detected by immunoblotting. Whole cell lysates (WCL) (10%) were probed for input. Representative immunoblot of 4 experiments. (b) The interaction of endogenous Jade-1 and endogenous β-catenin is increased in Wnt-off phase. IPs were performed with WCL (500 μg protein) of 293T cells pretreated with vehicle (PBS + 0.1% bovine serum albumin-BSA) or 50 ng Wnt-3a ligand in PBS + 0.1% BSA, with or without 50 ng DKK1, using 1 μg of either rabbit polyclonal Jade-1 antibody (J1) or pre-immune rabbit serum (C). The co-immunoprecipitated β-catenin was detected by immunoblot with monoclonal β-catenin antibody. β-catenin was immunoprecipitated as described above using monoclonal β-catenin antibody (β-cat) and isotype control (C). Jade-1 was detected by immunoblot using Jade-1 antiserum. WCL (10%) were probed for input. Densitometry was performed to quantitate β-catenin and Jade-1. The amount of Jade-1 and β-catenin immunoprecipitated was normalized using IgG. Representative immunoblot of 3 experiments. (c) Co-localization of endogenous Jade-1 and endogenous β-catenin is increased in Wnt-off status. The 293T cells pretreated with vehicle or Wnt-3a (200 ng) for 4 h were fixed and incubated with monoclonal β-catenin and polyclonal Jade-1 antibodies followed by Alexa 594 donkey anti-mouse and Alexa 488 goat anti-rabbit as secondary antibodies. Profile plots were generated using NIH ImageJ to demonstrate quantitative Jade-1 and β-catenin protein distribution. The profile plot represents the signal intensity of each fluorophore along a single line across the midpoint of a representative cell. The X axis represents the distance in pixels through the length of a single cell, and the intensity of each fluorophore is plotted on the Y axis. A representative image from 4 experiments is shown. Scale bar = 10 μm. (d) Identification of the domain of β-catenin required for Jade-1 binding. Extracts from transiently transfected 293T cells were immunoprecipitated. WCL (10%) were probed for input. β-catenin delC and delN constructs lack the C terminus and N terminus of the protein, respectively (Supplementary Fig. S1a). β-catenin S33A is a naturally occurring, cancer-causing amino acid substitution mutant. Representative immunoblot of 3 experiments.
Jade-1 specifically interacted with the N terminus of β-catenin (Fig. 1d). Interestingly, Jade-1 showed reduced binding to a naturally occurring, cancer-causing, constitutively active (CA) S33A mutant of β-catenin lacking this GSK-3β phosphorylation site (Fig. 1d). These findings were confirmed by immunofluorescence microscopy of cells expressing Flag-tagged Jade-1 and a Myc-tagged β-catenin series (Supplementary Information, Fig. S1c-e). In Wnt-off phase, Jade-1 co-localized extensively with wild-type β-catenin and the C terminus truncation of β-catenin predominantly in cytosol, but not with β-catenin S33A or the N terminus truncation of β-catenin (Supplementary Information, Fig. S1d). In contrast in Wnt-on phase, wild-type β-catenin localized to the nucleus, thereby reducing co-localization with Jade-1 (Supplementary information, Fig. S1d versus S1e), consistent with the reduction in endogenous Jade-1-β-catenin binding with Wnt activation. These data indicate that β-catenin N-terminal serine residue 33, or its phosphorylation, is important for optimal binding to Jade-1. We examined the binding of purified recombinant GST-tagged Jade-1 and GST-tagged β-catenin in in vitro GST pull-down assays. GST-tagged Jade-1 associated with GST-tagged β-catenin (Supplementary Information, Fig. S1f). However, this binding was substantially increased after in vitro phosphorylation of β-catenin by kinases CK1 and GSK-3β (Supplementary Information, Fig. S1f). GST-tagged Jade-1 did not bind to the N terminus deletion of β-catenin. Overall, Jade-1 directly binds the N terminus of β-catenin, and the interaction is enhanced with β-catenin phosphorylation in Wnt-off phase.
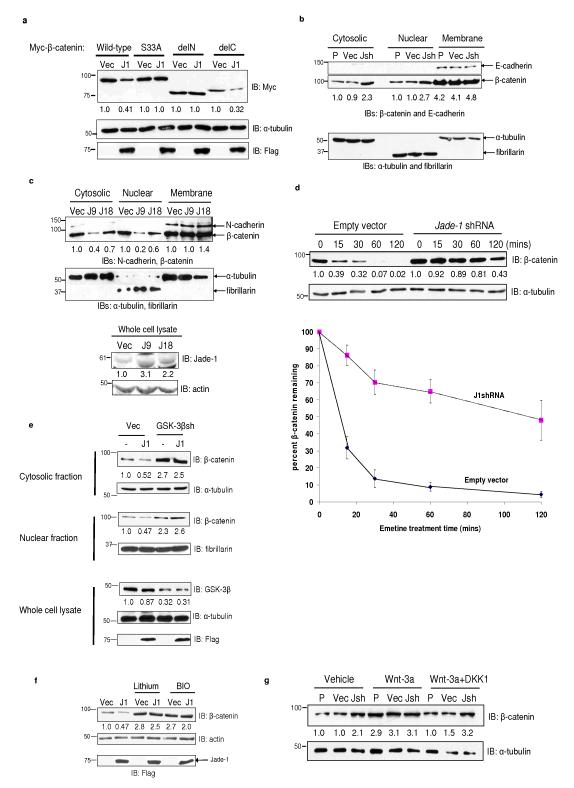
In Wnt-off phase, β-catenin undergoes degradation, a process that depends on the β-catenin N terminus or ‘degron’7,9. Jade-1 interacts with the N terminus of β-catenin and, in particular, residue S33, (Supplementary Information, Fig. S1a). We therefore examined whether Jade-1 regulates β-catenin abundance. Jade-1 down-regulated wild-type β-catenin and a C terminus deletion (Fig. 2a). However, Jade-1 had little effect on an N terminus deletion of β-catenin or β-catenin S33A, consistent with the binding pattern of Jade-1 with β-catenin (Fig. 1d).
Figure 2.
Jade-1 reduces β-catenin protein abundance. (a) Differential regulation of β-catenin constructs by Jade-1. Myc-tagged β-catenin and Flag-tagged Jade-1 (J1) constructs were transiently transfected into 293T cells. β-catenin construct schematics are shown in Supplementary Information, Fig. S1a. WCL were probed for β-catenin and α-tubulin protein. Expression of Jade-1 was detected by immunoblotting using Flag antibody. Densitometry was performed with NIH ImageJ using α-tubulin as loading control. Representative immunoblot of 5 experiments. (b) Jade-1 regulates the cytosolic and nuclear fractions but not the membrane fraction of endogenous β-catenin. The 293 uninfected parental cells (P) or cells infected with empty vector (Vec) or Jade-1 shRNA lentivirus (Jsh) were subjected to cell fractionation. Cytosolic, nuclear and membrane fractions were probed for β-catenin. α-tubulin, fibrillarin and E-cadherin served as loading controls and markers of cytosolic, nuclear and membrane fractions, respectively. These control blots were generated by cutting the membrane at the expected molecular weights of the controls and immunoblotting separately. One of 2 similar experiments is shown. (c) Jade-1 reduces cytosolic and nuclear pools of endogenous β-catenin. Extracts of clonal 786-O renal cancer cell lines stably expressing empty vector (Vec) or Jade-1 (J9 and J18)4,5 were probed for β-catenin. α-tubulin, fibrillarin and N-cadherin served as markers of cytosolic, nuclear and membrane fractions and as loading controls, respectively. These control blots were generated by cutting the membrane at the expected molecular weights of the controls and immunoblotting separately. Jade-1 was detected using polyclonal Jade-1 antibody. Representative immunoblot of 3 experiments. The effect of stably-transfected Jade-1 on β-catenin abundance appears to be dose dependent. (d) Jade-1 silencing substantially prolongs the half-life of endogenous cytosolic β-catenin. Digitonin was used to extract the cytosolic pool of β-catenin. Digitonin is a gently acting detergent that at low concentrations selectively punctures 8-10 nm holes in the sterol rich plasma membrane, allowing leakage of cytosolic proteins of up to 200 kDa mass10 (Upper panels), Digitonin extracts of 293 cells infected with empty vector or JshRNA lentiviral vector and pretreated with 20 μM emetine were probed for β-catenin by immunoblotting. (Lower panel), Percent β-catenin remaining in the digitonin-extracted fraction was analyzed by densitometry after normalizing to α-tubulin. Graph shows mean result of 4 experiments. Error bars = SEM. (e) GSK-3β silencing mitigates Jade-1 regulation of endogenous β-catenin. The 293T cells were transiently cotransfected with Flag-tagged Jade-1 (J1) and GSK-3β shRNA plasmid (GSK-3βsh) or empty vector (Vec) and GFP. One day post transfection, cells were sorted using fluorescence-activated cell sorting (FACS) and were replated for 48 hrs. Protein extracts from cells were probed for β-catenin using monoclonal β-catenin antibody. GSK-3β and Jade-1 proteins were examined by immunoblotting with polyclonal GSK-3β and Jade-1 antibodies, respectively. α-tubulin, fibrillarin and E-cadherin served as loading controls and markers of cytosolic, nuclear and membrane fractions, respectively. One of 2 similar experiments is shown. (f) Chemical inhibition of GSK-3β mitigates Jade-1 regulation of endogenous β-catenin. Transiently transfected 293T cells were serum starved for 12-16 h and treated with lithium chloride (10 mM) or BIO (100 nM) for an additional 12 h. β-catenin was detected using β-catenin antibody. Representative immunoblot of 4 experiments. (g) Jade-1 regulation of endogenous β-catenin in Wnt-on and Wnt-off status. HeLa parental cells (P) or cells infected with empty vector (Vec) or Jade-1 shRNA (Jsh) lentivirus were treated for 4 h with Wnt-3a (100 ng) with or without DKK1 (100 ng). The cytosolic fractions were probed for β-catenin and α-tubulin protein. Representative immunoblot of 4 experiments.
Endogenous β-catenin exists in three distinct cellular pools7. Endogenous β-catenin protein in the cytosolic and nuclear fractions was at least 2-fold higher in 3 different Jade-1 silenced cell lines than in empty vector lines (Fig. 2b and Supplementary Information, Fig. S2b-S2d). The membrane pool of β-catenin was unchanged. Conversely, the amount of β-catenin in the cytosolic and nuclear fractions was substantially lower in Jade-1-expressing stable cell lines than in the empty vector cell lines (Fig. 2c). We then examined the half-life of cytosolic β-catenin. A digitonin-extracted fraction10 was enriched for cytosol, as evidenced by the increase in cytosolic markers, but had no membrane contamination (Supplementary Information, Fig. S2e). The half-life of the digitonin-extracted cytosolic β-catenin was increased from 10 mins to 90 mins in Jade-1 silenced 293 cell lines (Fig. 2d). Thus, Jade-1 silencing substantially stabilized cytosolic β-catenin. β-catenin half-life was reduced in the Jade-1-expressing renal cancer cell lines (Supplementary Information, Fig. S2f). Thus, Jade-1 regulates the stability of the Wnt-responsive pool of β-catenin.
β-catenin degradation depends on GSK-3β. In Wnt-off phase, β-catenin undergoes sequential phosphorylation at threonine 41 and serine 37 and 33 by GSK-3β. Preferential binding of Jade-1 to phospho-β-catenin and lack of binding to β-catenin S33A suggest a possible role for GSK-3β in Jade-1 regulation of β-catenin (Fig. 1d and Supplementary Information, Fig. S1f). Moreover, full-length Jade-1 reduced total β-catenin due predominantly to reduction in phospho-β-catenin (Supplementary Information, Fig. S3a). Thus, Jade-1 preferentially regulates phospho-β-catenin. This observation further suggests that GSK-3β may be particularly important for Jade-1 regulation of β-catenin. Indeed, Jade-1 regulation of β-catenin was mitigated by silencing or chemical inhibition of GSK-3β (Figs. 2e and 2f). Similarly, the effect of Jade-1 silencing on β-catenin abundance was reduced in Wnt-on phase, when GSK-3β activity is inhibited (Fig. 2g and Supplementary Information, Fig. S3b). Overall, these data indicate that Jade-1 requires intact GSK-3β kinase activity for full inhibition of β-catenin.
β-catenin undergoes proteasomal degradation11. Proteasome inhibition with MG132 completely abrogated the effect of Jade-1 on β-catenin abundance (Supplementary Information, Fig. S3c). Moreover, in the presence of Jade-1 and proteasomal inhibition, very high molecular weight species of β-catenin accumulated (Supplementary Information, Fig. S3d), suggesting that Jade-1 may enhance ubiquitination and degradation of β-catenin.
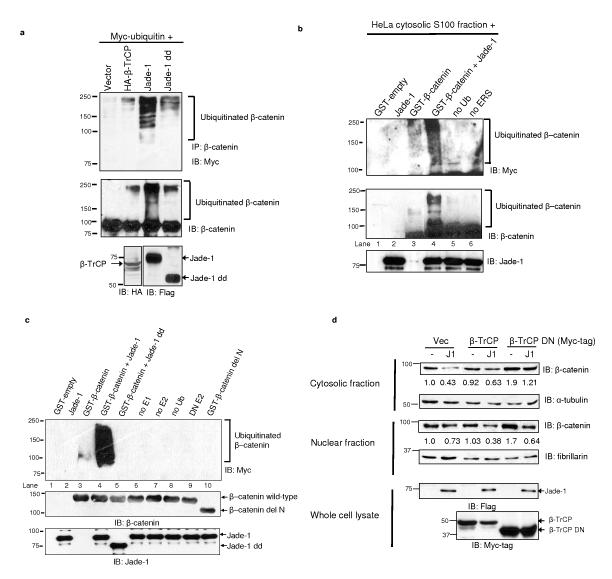
Protein ubiquitination depends on substrate recognition by a highly selective E3 ubiquitin ligase. PHD proteins such as MEKK1 and MIR exhibit E3 ubiquitin ligase activity12,13. Jade-1 has two PHDs3 that align well with the PHDs of MEKK1, MIR1, MIR2 and c-MIR (Supplementary Information, Fig. S4a). Therefore, we reasoned that Jade-1, through its PHDs, might ubiquitinate β-catenin. Deletion of the PHDs reduced the effect of Jade-1 on β-catenin (Supplementary Information, Fig. S4b). Next, endogenous β-catenin was immunoprecipitated, and its ubiquitination was examined in the presence of Myc-tagged ubiquitin and Jade-1, Jade-1 dd or the well-established β-catenin E3 ubiquitin ligase component β-TrCP6 (Fig. 3a). Minimal β-catenin ubiquitination was observed with β-TrCP under these conditions, possibly due to lower expression of β-TrCP or the fact that the other components of the β-TrCP SCF complex were not co-expressed. Interestingly, robust β-catenin polyubiquitination was observed with full-length Jade-1, while deletion of the PHDs substantially reduced β-catenin ubiquitination (Fig 3a). β-catenin ubiquitination appeared as a smear, most prominently in the presence of full-length Jade-1. Since 293T cells are in Wnt-off status under basal conditions, these data indicate that Jade-1 promotes endogenous β-catenin ubiquitination in Wnt-off phase.
Figure 3.
Jade-1 ubiquitinates β-catenin. (a) Deletion of the Jade-1 PHDs substantially reduces endogenous β-catenin ubiquitination. Extracts (600 μg protein) of 293T cells transfected and treated with MG132 (10 μM for 1 h) were immunoprecipitated with β-catenin antibody and protein A beads. The eluate was divided into two equal parts and immunoblotted separately using monoclonal Myc-tag antibody and β-catenin antibody. Expression of β-TrCP and Jade-1 protein was detected by immunoblot. One of 2 similar experiments is shown. (b) Ex vivo ubiquitination of purified β-catenin using the HeLa cell cytosolic S100 fraction. Purified recombinant GST-β-catenin on Glutathione Sepharose™ beads was incubated with HeLa cell S100 fraction (pretreated with ubiquitin aldehyde and MG132), Myc-tagged human recombinant ubiquitin (Ub), recombinant Jade-1 and energy regeneration solution (ERS). β-catenin was eluted from the beads using Laemmli buffer. The eluate was divided into two equal parts and immunoblotted separately using monoclonal Myc-tag or β-catenin antibodies. Lanes designated as no Ub or no ERS were reactions without Ub or ERS only, respectively. The eluates were also probed for Jade-1 input using polyclonal Jade-1 antibody. Lane 3 was rearranged from the same blot. β-catenin ubiquitination appears as a smear and higher molecular weight ladder. One of 2 similar experiments is shown. (c) Jade-1 ubiquitination of β-catenin in vitro, and mapping of the E3 ubiquitin ligase domain on Jade-1. A panel of E2 ubiquitin transferases, including UbcH2, UbcH3, UbcH5a, UbcH5b, UbcH5c, UbcH6, UbcH7 and UbcHc10, was screened for Jade-1-mediated β-catenin ubiquitination (data not shown). Jade-1 ubiquitinated β-catenin only in the presence of UbcH6 (Fig. 3c and Supplementary Information, Fig. S4c) or UbcH2 (data not shown). Ubiquitination reactions were reconstituted using GST-β-catenin (wild-type or delN) with E1 activating enzyme, E2 conjugase (UbcH6), E3 ligase 750 nM Jade-1 (full-length or dd), Myc-tagged human recombinant Ub and MgCl2-ATP. Lanes designated as no E1, no E2 and no Ub were reactions with all constituents except E1, E2 or Ub only, respectively. Dominant-negative UbcH6 (DN E2) was used instead of wild-type UbcH6 as a control. β-catenin was eluted from the beads using Laemmli buffer and immunoblotted using monoclonal Myc-tag antibody. GST-β-catenin and GST-Jade-1 beads equivalent to that used in the reactions were probed separately using polyclonal β-catenin C-terminal antibody and polyclonal Jade-1 antibody. Reactions without E1, E2, or ubiquitin, or with DN UbcH6, served as negative controls (lanes, 6-9). Representative immunoblot of 3 experiments. (d) Jade-1-mediated degradation of endogenous β-catenin is independent of β-TrCP. Whole cell extracts of 293T cells transfected with wild-type or DN β-TrCP, with or without Flag-tagged Jade-1, were probed using β-catenin antibody. WCL (10%) were probed using Flag- and Myc-tag antibodies for expression of Jade-1 and β-TrCP protein, respectively. α-tubulin and fibrillarin served as markers of cytosolic and nuclear fractions, respectively, and as loading controls. Representative immunoblot of 4 experiments.
In vitro ubiquitination of β-catenin was then examined. GST-purified β-catenin was incubated with HeLa cell cytosolic S100 fraction. β-catenin ubiquitination was observed in the presence of Jade-1 (Fig. 3b). To address if Jade-1 directly ubiquitinates β-catenin and to map the E3 ubiquitin ligase domain within Jade-1, we reconstituted ubiquitination reactions with all purified components (Fig. 3c and Supplementary Fig. S4c). β-catenin ubiquitination was observed with Jade-1 in a dose-dependent manner (Supplementary Information, Fig. S4c, lanes 4-6). Deletion of the Jade-1 PHDs or the β-catenin N terminus abrogated β-catenin ubiquitination (Fig. 3c, lanes 5 and 10; and Supplementary Information, Fig. S4c, lanes 7 and 12). Thus, purified Jade-1 ubiquitinates non-phosphorylated GST-tagged β-catenin, and the Jade-1 PHDs are necessary for E3 ubiquitin ligase activity.
In order to examine the relationship between Jade-1- and β-TrCP-mediated β-catenin degradation, DN β-TrCP lacking the F box was used to antagonize both β-TrCP1 and β-TrCP214. DN β-TrCP increased endogenous β-catenin expression by 2 fold (Fig. 3d). Interestingly, Jade-1 could still down-regulate β-catenin in the presence of DN β-TrCP (Fig. 3d), which suggests that Jade-1 regulates β-catenin independently of β-TrCP.
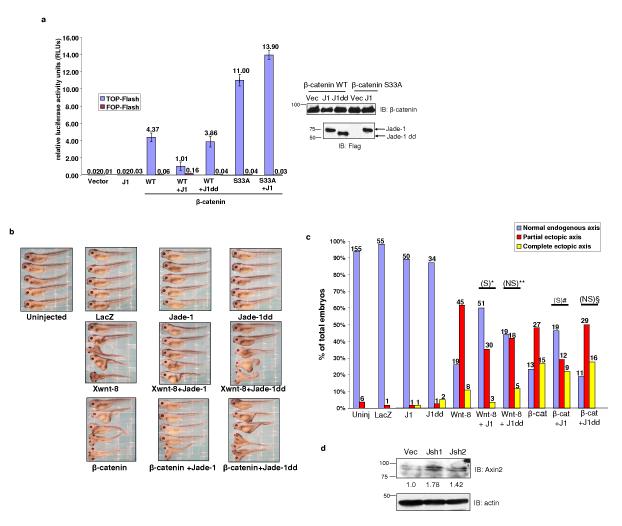
The biological significance of the Jade-1-β-catenin interaction was evaluated in TCF/β-catenin transcription assays. Full-length Jade-1, but not Jade-1 dd, inhibited a TOP-Flash promoter-reporter by 3.5 fold (Fig. 4a). Jade-1 had no effect on β-catenin S33A transcriptional activity (Fig. 4a). These observations are consistent with the effect of Jade-1 on protein levels of wild-type β-catenin and β-catenin S33A (Fig. 2a). Intriguingly, significant inducible endogenous Wnt activity was observed in Jade-1 silenced cell lines (Supplementary information, Fig. S5a), consistent with the stabilization of endogenous β-catenin in these lines.
Figure 4.
Jade-1 inhibits canonical Wnt signaling. (a) Jade-1 suppresses TCF/β-catenin reporter activity. TCF-responsive promoter-reporter TOP-Flash and nonresponsive control reporter FOP-Flash were used. Activity of the Wnt signaling pathway was quantified by measuring relative firefly luciferase activity units (RLUs) normalized to renilla luciferase. The 293T cells were transfected with wild-type β-catenin (β-cat) or β-catenin S33A and Jade-1 (J1) full-length or Jade-1 dd (J1dd). Mean results of 3 experiments are shown. Error bars = SEM. Protein extracts were probed using monoclonal Flag and β-catenin antibodies to examine the expression of constructs. (b) Jade-1 inhibits Xwnt8- and β-catenin-induced ectopic axis formation in Xenopus laevis embryos. Xwnt-8 (0.34-0.45 pg) or β-catenin (100-120 pg) mRNA, with or without Jade-1 (full-length 0.8 ng or dd 1.2 ng) mRNA, was injected into ventral blastomeres. LacZ (1.5 ng) served as a negative control. A total of 7 experiments was performed to compare full-length Jade-1 and Jade-1 dd injected embryos. (c) X-wnt-8- and β-catenin-induced ectopic axis is inhibited by Jade-1 in Xenopus laevis embryos. Histogram depicting Xenopus laevis embryo phenotypes is shown. Numbers shown at the top of each bar indicate the number of embryos in each category. Chi square test was applied to determine statistical significance. *= Xwnt-8 with Jade-1 versus Xwnt-8 (p <0.0001). # = Xwnt-8 with Jade-1 dd versus Xwnt-8 (p= 0.096), **= β-catenin with Jade-1 versus β-catenin (p<0.046), §=β-catenin with Jade-1 dd versus β-catenin (p=0.087). S = statistically significant, NS = not significant. (d) Jade-1 regulates endogenous Wnt targets. WCLs of 293 cells infected with empty vector (Vec) or Jade-1 silencing lentiviral constructs (Jsh1 and Jsh2) were probed for endogenous Axin2 and actin. One of 2 similar experiments is shown.
Since Jade-1 destabilizes β-catenin and inhibits β-catenin-mediated transactivation, we reasoned that Jade-1 might inhibit the canonical Wnt pathway in vivo. We used a functional assay for canonical Wnt signaling, formation of an ectopic axis in Xenopus laevis embryos by ventral injection of Xwnt-8 or β-catenin mRNA 15. Full-length Jade-1, but not Jade-1 dd, significantly inhibited both Xwnt-8- and β-catenin-induced ectopic axis formation in developing Xenopus laevis embryos (Fig. 4b, 4c and Supplementary Information, Fig. S5b). These data suggest that Jade-1 has the capacity to suppress Wnt activity during Wnt-on phase in vivo. This is plausible in view of the appreciable interaction of Jade-1 and β-catenin in Wnt-on phase (Figs. 1b and Supplementary Information Fig. S1b) and Jade-1 binding and ubiquitination of non-phosphorylated β-catenin (Supplementary Information, Fig. S1f and Figs. 3b and 3c). The specific Wnt target Axin216 was also increased in Jade-1 silenced 293 cell lines (Fig. 4d). Other Wnt targets, such as cyclin D1 and c-Myc, were increased in Jade-1 silenced cell lines (Supplementary Information, Fig. S5c). Full-length Jade-1, but not Jade-1 dd, reduced protein levels of c-Myc (Supplementary Information, Fig. S5d). Thus, Jade-1 is an inhibitor of canonical Wnt signaling.
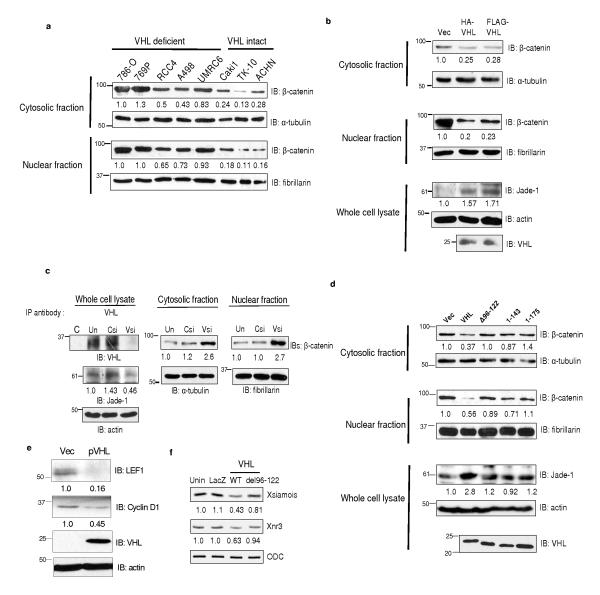
Jade-1 protein is stabilized by wild-type pVHL, but not by mutated pVHL associated with renal cancer3,4. We hypothesized that pVHL might regulate β-catenin through Jade-1. First, we compared β-catenin abundance in VHL-deficient and VHL-intact renal cancer cell lines (Fig. 5a). Interestingly, endogenous cytosolic and nuclear pools of β-catenin were several-fold lower in VHL-intact cell lines compared to VHL-deficient cell lines (Fig. 5a). This result can be explained on the basis of our previous observations that Jade-1 levels are significantly lower in VHL-deficient cell lines5. Next, pVHL reintroduction in 786-O cells increased endogenous Jade-1 4,5. and substantially reduced endogenous β-catenin levels (Fig. 5b and Supplementary Information, Fig. S6a). Knock-down of VHL with siRNA oligonucleotides resulted in down-regulation of Jade-1 and accumulation of β-catenin (Fig. 5c and Supplementary Information, Fig. S6b)17.
Figure 5.
pVHL regulates endogenous β-catenin through Jade-1. (a) β-catenin protein levels depend on VHL status in renal cancer cell lines. Cytosolic and nuclear fractions of renal-cell carcinoma cell lines were probed for endogenous β-catenin protein. One of 2 similar experiments is shown. (b) pVHL down-regulates endogenous β-catenin. Extracts from 786-O cells stably expressing empty vector (Vec) or pVHL were probed for endogenous β-catenin protein. Jade-1 and pVHL expression was detected using polyclonal Jade-1 and monoclonal pVHL antibodies. Representative immunoblot of 3 experiments. (c) pVHL knock-down increases endogenous β-catenin abundance. To confirm pVHL knock-down, pVHL was immunoprecipitated using polyclonal pVHL or control (C) antibody from untransfected (Un) 293T cells or 293T cells transfected with VHL (VHLsi) or control siRNA (Csi) oligonucleotides because endogenous pVHL abundance is low17. Cell fractions were probed for endogenous Jade-1 and endogenous β-catenin protein. Representative immunoblot of 3 experiments. (d) Differential regulation of endogenous β-catenin by wild-type pVHL and pVHL truncations that do not stabilize Jade-1. Extracts of 293T cells transfected with wild-type pVHL, pVHL del96-122 (Δ96-122), pVHL 1-143 and pVHL 1-175 were probed for pVHL, endogenous β-catenin and endogenous Jade-1 by immunoblot. One of 2 similar experiments is shown. (e) pVHL suppresses endogenous Wnt targets. WCLs of empty vector (Vec) or pVHL-expressing 786-O cell lines were probed for pVHL and endogenous LEF1 and cyclin D1. Representative immunoblot of 3 experiments. (f) Wild-type pVHL, but not pVHL del96-122, suppresses β-catenin/Tcf target genes. In vitro transcribed, capped LacZ (0.288 ng), wild-type VHL (WT) (0.194 ng) or VHL del96-122 (0.293 ng) mRNA was injected into dorsal blastomeres of Xenopus laevis embryos. Injected embryos were harvested at stage 10 for RT-PCR. Semiquantitative RT-PCR analysis of the Wnt targets Xsiamois and Xnr3 was performed in whole embryos. ODC served as control. Unin.= uninjected.
To specifically examine whether Jade-1 mediates pVHL down-regulation of β-catenin, we compared β-catenin regulation by wild-type pVHL and by truncated forms of pVHL that do not stabilize Jade-14(Fig. 5d). pVHL del96-122 and naturally occurring, cancer-causing truncations like pVHL 1-143 and pVHL 1-175, which have little or no effect on Jade-1 stability4, had minimal effect on β-catenin (Fig. 5d). We also knocked down Jade-1 in the presence of pVHL. Endogenous Jade-1 levels were increased by pVHL, an effect blocked by Jade-1 knock-down (Supplementary Information, Fig. S6c). Importantly, down-regulation of β-catenin by pVHL was substantially mitigated by Jade-1 knock-down (Supplementary Information, Fig. S6c). Furthermore, pVHL wild-type, but not pVHL del96-122, suppressed β-catenin transcriptional activity (Supplementary Information, Fig. S6d). Specific Wnt targets such as LEF118 and cyclin D1 were reduced in pVHL-expressing renal cancer cell lines (Fig. 5e). Wild-type pVHL, but not pVHL del96-122, also reduced Axin2 in 293T cells (Supplementary Information, Fig. S6e). Overall, these data indicate that pVHL inhibits β-catenin and canonical Wnt signaling and that Jade-1 is a critical mediator of these effects.
To determine if pVHL is able to reduce endogenous Wnt signaling in vivo, and to examine the difference in the suppression of Wnt activity by wild-type pVHL and pVHL del96-122, we injected wild-type VHL or VHL del96-122 mRNA into the dorsal blastomeres of Xenopus laevis embryos, in which canonical Wnt signaling is necessary for dorsal development19. Inhibition of dorsal axis formation in Xenopus laevis embryos is evidenced by reduction of dorsoanterior structures (small eyes, microcephaly, or anencephaly) measured with a dorsoanterior index (DAI) scale19. Wild-type pVHL suppressed dorsal axis formation significantly more than pVHL del96-122 at the same level of protein abundance (Supplementary Information, Figs. S6f and S6g). As expected, inhibition of endogenous axis by wild-type pVHL was associated with greater suppression of β-catenin/Tcf target genes Xsiamois and Xnr3 than by pVHL del96-122 (Fig. 5f). Thus, pVHL inhibits canonical Wnt signaling in vivo, and pVHL del96-122, which binds and regulates Jade-1 only minimally4,5, had little effect on dorsal axis formation and Wnt target genes in Xenopus laevis embryos. These data further support the role of Jade-1 as a critical mediator of pVHL regulation of β-catenin.
In this study, we have demonstrated that Jade-1 is a single-subunit E3 ubiquitin ligase for β-catenin and that the Jade-1 PHDs are essential for E3 ubiquitin ligase function. Moreover, Jade-1 is a critical mediator of pVHL inhibition of β-catenin and canonical Wnt signaling. Jade-1 is anti-proliferative and pro-apoptotic, while β-catenin is pro-proliferative, anti-apoptotic and oncogenic7. Therefore, the tumor suppressor activity of Jade-1 may be due in part to inhibition of β-catenin.
Several ubiquitin ligases for β-catenin have now been identified9,20,21, but only β-TrCP and Jade-1 show Wnt responsiveness, suggesting they are both important in the physiology and pathophysiology of canonical Wnt signaling. Endogenous β-TrCP resides in the cytosol and is capable of binding and ubiquitinating phosphorylated β-catenin6,9,22,23. In contrast, endogenous Jade-1 resides in the cytosol but is found primarily in the nucleus3,8 and is capable of binding and ubiquitinating both phosphorylated and non-phosphorylated β-catenin. Thus, Jade-1 and β-TrCP have only partially overlapping subcellular locations and have differing specificities for the forms of β-catenin. These differences may explain why Jade-1 silencing cannot be completely compensated for by β-TrCP (Fig. 2b, 2d and Supplementary Information, Figs. S2b-d). Moreover, Jade-1 seems to act more distally in the canonical Wnt cascade, affecting β-catenin in the nucleus. This may make Jade-1 responsible for fine control of β-catenin levels.
Jade-1 functions as a single-subunit E3 ubiquitin ligase for β-catenin, whereas β-TrCP requires formation of a multi-subunit protein complex. The PHD functions as an adaptor-type E3 ubiquitin ligase directly transferring the ubiquitin moiety to the substrate13. Control of Jade-1 may therefore be a simpler and perhaps more efficient way of regulating β-catenin abundance. It is likely that the cell exploits the distinct functional and contextual differences between β-TrCP and Jade-1 to ensure effective regulation of Wnt signaling.
β-catenin is emerging as a key molecule in the pathogenesis of renal cancer and renal cystic disease. For example, increased β-catenin activity in renal epithelium in mice results in robust renal cyst and tumor formation24-26. In renal cancer, methylation of the APC gene promoter is common27. pVHL was recently shown to inhibit HGF-mediated tyrosine phosphorylation of β-catenin28. These observations further strengthen the role of Wnt signaling in renal-cell carcinoma. Jade-1 and pVHL may also participate in other forms of cystic kidney disease, in which evidence of dysregulated Wnt signaling is mounting26,29.
Our data suggest that Jade-1 inhibition of Wnt signaling represents a new tumor suppressor axis for pVHL. Furthermore, these findings directly link the kidney-specific pVHL tumor suppressor pathway and the Wnt signaling cascade, a more general tumorigenesis pathway. Jade-1 and beta-catenin may therefore represent therapeutic targets in renal-cell carcinoma.
METHODS
See Supplementary Information for details of cells lines, constructs, antibodies, yeast 2-hybrid screen, immunoblotting, immunoprecipitation, cellular fractionation, GST purification, in vivo ubiquitination, synthesis of capped RNAs, Xenopus injection and phenotype evaluation.
In vitro ubiquitination reaction
Ubiquitination reactions were reconstituted in 30 μl with ubiquitination buffer (50 mM Tris-HCl pH 7.5, 0.5 mM DTT) containing 225 nM E1 activating enzyme (Boston Biochem), 500 nM E2 conjugase, 600 μM Myc-tagged ubiquitin (Boston Biochem), 1 mM MgCl2-ATP, 750 nM Jade-1 and 2.8 μM GST-β-catenin on glutathione beads and incubated at 37 °C for 60 min. Reactions without E1, E2 or ubiquitin or with a DN E2 (mutation of active site cystine to serine) served as negative controls.
Xenopus embryo injection and phenotype evaluation
Embryos were fertilized in vitro, dejellied in 2% cysteine (pH 7.8) and maintained in 0.1x Marc’s Modified Ringer’s medium (MMR). For microinjection, embryos were transferred to 1.5% Ficoll (Pharmacia) in 0.5x MMR and injected at stages 2 or 3. Two blastomeres were injected ventrally with 10 nl of LacZ or Xwnt-8 mRNA with or without Jade-1 mRNA (full-length or dd), or dorsally with LacZ or VHL (wild-type or VHL del96-122) mRNA. Embryos were fixed and analyzed as described previously30-32.
RT-PCR
RNA extraction, reverse transcription, PCR, primer sequence for Xsiamois, Xnr3 and ODC, and PCR conditions have been described31. RNA (2.8 μg measured by Nanodrop, Thermo Fischer Scientific) was used for cDNA preparation.
Supplementary Material
Figure S1 Jade-1 directly binds β-catenin, and the interaction is enhanced in Wnt-off phase. a, Schematic of Jade-1 and β-catenin constructs. Full-length Jade-1 and Jade-1 double deletion of PHDs (Jade-1 dd) are shown. Jade-1 dd lacks both PHDs but has an intact inter-PHD region and contains aa 1-202, 254-311, and 372-509 3,8. β-catenin constructs have an N-terminal Myc tag and include β-catenin wild-type (WT), N-terminal deletion (delN) and C-terminal deletion (delC). b, Jade-1 binds to β-catenin in a Wnt-responsive manner. Immunoprecipitations (IP) were performed with whole cell lysates (WCL) (500 μg protein) of 293T cells pretreated with vehicle (water) (Veh) or 20 mM lithium chloride (Li) for 4 h using 1 μg of either rabbit polyclonal Jade-1 antibody or pre-immune rabbit serum (data not shown). The co-immunoprecipitated β-catenin was detected by immunoblot with monoclonal β-catenin antibody. β-catenin was immunoprecipitated as described above using monoclonal β-catenin antibody and isotype control (data not shown). Jade-1 was detected by immunoblot using polyclonal Jade-1 antiserum. WCL (10%) were probed for input. One of 2 similar experiments is shown. c, Expression of Jade-1 and β-catenin in Wnt-off phase. The 293 cells transfected with Flag-tagged Jade-1 or Myc-tagged β-catenin constructs were pretreated with vehicle (PBS + 0.1% bovine serum albumin) for 4 h, fixed and incubated with monoclonal Myc and polyclonal Flag antibodies, followed by Alexa Fluor 594 donkey anti-mouse and Oregon green 488 goat anti-rabbit as secondary antibodies. A single representative confocal image is shown. Wild-type β-catenin and the C terminus (delC) truncations of β-catenin localized to the cytosol and membrane. The N terminus (delN) truncation of β-catenin localized predominantly to the cytosol while β-catenin S33A localized predominantly to the nucleus. Jade-1 localized to the cytosol and to lesser extent to the nucleus. Scale bar = 10 μm. d, Jade-1 and β-catenin co-localize in Wnt-off phase. The 293 cells transfected with Flag-tagged Jade-1 and Myc-tagged β-catenin constructs pretreated with Wnt-3a (200 ng) for 4 h were processed as above. A single representative confocal image is shown. The co-localization was performed using the NIH ImageJ program in cells having comparable signal level for both constructs. Randomly selected representative cells were analyzed. At least 15 confocal images comprising one Z-stack were generated for each cell. For the scatter plots, co-localization was performed using the entire Z-stack. Scatter plot points along the X or Y axis represent absence of co-localization, whereas scatter plot points along a diagonal represent evidence of co-localization. Images were background subtracted from a randomly chosen region of interest. Scale bar = 10 μm. e, Loss of Jade-1-β-catenin co-localization in Wnt-on status. The transfected 293 cells were pretreated with Wnt-3a (200 ng) for 4 h. The cells were fixed, and co-localization was performed as in d. Scale bar = 10 μm. f, Jade-1 preferentially binds phospho-β-catenin in in vitro GST pull-down assays. (Left panels), Glutathione Sepharose™ beads or purified recombinant GST-tagged Jade-1 bound to glutathione Sepharose™ beads was incubated with cleaved, purified recombinant β-catenin (with or without in vitro phosphorylation then washed, followed by elution of glutathione Sepharose™ beads in Laemmli buffer. In vitro phosphorylation of β-catenin was performed by incubating GST-tagged β-catenin glutathione Sepharose™ beads with ATP, kinases CK1 and GSK-3β for 30 mins at 30 °C. The immunoblot was probed for β-catenin then reprobed using phospho-β-catenin antibody. Ten percent of glutathione Sepharose™ beads was probed as input. (Right panels), Glutathione Sepharose™ beads or purified recombinant GST-tagged β-cateninbound to glutathione Sepharose™ beads was incubated with cleaved, purified recombinant Jade-1 as above. In vitro phosphorylation of GST-tagged-β-catenin was performed as above. Ten percent of glutathione Sepharose™ beads was probed as input. The immunoblot was reprobed using phospho-β-catenin antibody. Representative immunoblot of 3 experiments. G = GST, G-J = GST-Jade-1, G-β = GST-β-catenin, G-phosphoβ = GST-phospho-β-catenin, G-delN = GST-β-catenin delN.
Figure S2 Jade-1 regulates endogenous β-catenin. a, Jade-1 silencing by a lentiviral shRNA system in different cell lines. HeLa, 293 and HK-2 cells lines were infected with empty vector (Vec), non-silencing control (Non) or one of two Jade-1 shRNA lentiviral vectors (Jsh). WCL from parental cells (P) or infected cells were probed for Jade-1 and actin protein. Densitometry results are normalized to actin. One of 2 similar experiments is shown. b, Jade-1 silencing by shRNA plasmid vector upregulates endogenous β-catenin. Extracts of empty vector (Vec) or Jade-1 shRNA (JshRNA) plasmid vector transiently transfected 293T cells were probed for Jade-1 and β-catenin protein. Representative immunoblot of 3 experiments. c, Endogenous β-catenin is upregulated with both Jade-1 shRNA lentiviral constructs. Cytosolic protein extracts from HeLa cells infected with one of two lentiviral Jade-1 shRNA constructs (Jsh1 or Jsh2) were probed for β-catenin. One of 2 similar experiments is shown. d, Jade-1 silencing increases the cytosolic and nuclear fractions but not the membrane fraction of β-catenin. Extracts of parental HK-2 cells (P) or cells infected with empty vector (Vec) or Jade-1 shRNA lentivirus (Jsh) were subjected to cell fractionation. Cytosolic, nuclear and membrane fractions were probed for β-catenin. α-tubulin, fibrillarin and E-cadherin served as loading controls and markers of cytosolic, nuclear and membrane fractions, respectively. These control blots were generated by cutting the membrane at the expected molecular weights of the controls and immunoblotting separately. One of 2 similar experiments is shown. e, The digitonin-extracted fraction is enriched for cytosol and devoid of membrane contamination. Equal amounts of protein from the digitonin-extracted fractions of 293 cells pretreated with protein translation inhibitor emetine (20 μM), harvested at different time points, were probed for β-catenin. E-cadherin served as a marker for the membrane fraction, and α-tubulin and IkappaB-α served as markers of cytosol. These control blots were generated by cutting the membrane at the expected molecular weights of the controls and immunoblotting separately. One of 2 similar experiments is shown. f, Jade-1 expression destabilizes β-catenin in renal cancer cells. Cytosolic fractions from clonal 786-O cell lines stably expressing empty vector or Jade-1 (J9, J22)4,5 harvested after 20 μM emetine treatment were probed for β-catenin. Percent β-catenin remaining was analyzed by densitometry after normalizing to α-tubulin. β-catenin half-life was reduced from 8 hrs to 3.5 hrs in the Jade-1-expressing 786-O cell lines. Graph shows mean result of 3 experiments. Error bars = SEM.
Figure S3 Jade-1 preferentially regulates phospho-β-catenin in Wnt-off phase and enhances proteasomal degradation of β-catenin. a, Jade-1 down-regulates total β-catenin by reducing predominantly phospho-β-catenin. Cytosolic protein extracts from 293T cells transiently transfected with full-length Jade-1 (J1) or Jade-1 dd (J1dd) were probed for total β-catenin. The membrane was stripped and reprobed for phospho-β-catenin (β-catenin phosphorylated at serine 33, 37 and threonine 41) and active β-catenin (non-phosphorylated at serine 37 and threonine 41). Cytosolic fractions were probed for the Myc tag to confirm equivalent expression of Jade-1 and Jade-1 dd (data not shown). One of 2 similar experiments is shown. b, Jade-1 regulation of β-catenin during Wnt-on and Wnt-off phase. HK-2 parental cells (P) or cells infected with empty vector (Vec) or Jade-1 shRNA (Jsh) lentivirus were treated for 4 h with Wnt-3a (200 ng), with or without DKK1 (200 ng). The cytosolic fractions were probed for β-catenin and α-tubulin. Representative immunoblot of 3 experiments. c, Proteasome inhibitor treatment abrogates the effect of Jade-1 on β-catenin. Transfected 293T cells were incubated with proteasome inhibitor (PI) MG132 at 10 μM for 12 hrs. WCL were probed with β-catenin, α-tubulin or Flag-tag antibodies. One of 2 similar experiments is shown. d, Jade-1 increases accumulation of high molecular weight polyubiquitinated β-catenin in the presence of PI. The 293T cells transiently transfected with Jade-1 and β-catenin were treated with PI MG132 at 10 μM for 12 h. WCL were probed as shown. One of 2 similar experiments is shown.
Figure S4 Jade-1 ubiquitinates β-catenin. a, The PHDs of Jade-1 align well with the PHDs of proteins with known E3 ubiquitin ligase activity. The MacVector (Accelrys) sequence analysis program was used to align the PHDs of human Jade-1, MEKK1 (Q13233), viral MIR1 (K3), MIR2 (K5) (U93872) and c-MIR (hCP36279). b, Full-length Jade-1, but not Jade-1 dd, reduces abundance of the cytosolic and nuclear fractions of β-catenin. Protein extracts of 293T cells transiently transfected with full-length Jade-1 (J1) or Jade-1 dd (J1dd) were probed for β-catenin. α-tubulin, fibrillarin or E-cadherin served as loading controls and markers of cytosolic, nuclear and membrane fractions, respectively. These control blots were generated by cutting the membrane at the expected molecular weights of the controls and immunoblotting separately. Cytosolic fractions were probed to examine expression of Jade-1 and Jade-1 dd. One of 2 similar experiments is shown. c, Dose-dependent in vitro ubiquitination of β-catenin by Jade-1. Ubiquitination reactions were reconstituted by incubating GST-β-catenin beads with E1 activating enzyme, E2 conjugase (UbcH6), E3 ligase Jade-1 (in increasing amounts, 250, 750, or 1200 nM) or Jade-1 (full-length or dd) (750 nM), Myc-tagged human recombinant ubiquitin (Ub) and MgCl2-ATP. No E1, no E2, no Ub and DN E2 reactions served as negative controls. β-catenin ubiquitination was detected using polyclonal β-catenin C-terminal antibody. Reactions without E1, E2, or ubiquitin, or with dominant negative (DN) UbcH6, served as negative controls (lanes 8-11). Jade-1 input was detected by immunoblot. Lane 7 was rearranged from the same blot. One of 2 similar experiments is shown.
Figure S5 Jade-1 regulates β-catenin transcriptional target genes. a, Jade-1 silencing results in a significant spontaneous endogenous Wnt activity. The 293 cells stably infected with empty vector or Jade-1 shRNA lentiviral vectors were transiently transfected with TOP-Flash or FOP-Flash and renilla luciferase vectors. Activity of the Wnt signaling pathway was quantified by measuring relative firefly luciferase activity units (RLUs) normalized to renilla luciferase. Mean results of 3 experiments are shown. Error bars = SEM. Student’s t-test was applied to determine statistical significance, p = 0.033. b, Comparable expression of Myc-tagged Jade-1 and Jade-1 dd protein in Xenopus laevis embryos injected also with capped mRNAs for Xwnt-8 or β-catenin. Embryos were harvested at stage 10, and the extracts were resolved by SDS-PAGE. The membrane was probed using Myc and α-tubulin antibodies. c, Wnt targets are upregulated in Jade-1 silenced cell lines. WCL from cells infected with empty vector or from Jade-1 silenced cells were probed for cyclin D1 or c-Myc. Representative immunoblot of 4 experiments. d, Full-length Jade-1, but not Jade-1 dd, down-regulates endogenous c-Myc. Whole cell extracts of 293T cells transfected with Myc-tagged β-catenin with or without Flag-Jade-1 or Flag-Jade-1 dd were probed with c-Myc antibody detecting endogenous c-Myc protein. Flag- and Myc-tag antibodies were used to detect transfected Flag-tagged Jade-1 and Myc-tagged β-catenin, respectively. One of 2 similar experiments is shown.
Figure S6 pVHL regulates β-catenin through Jade-1. a, pVHL down-regulates endogenous β-catenin. Cytosolic and nuclear fractions of VHL-deficient renal cancer cell lines stably expressing pVHL were probed for endogenous β-catenin. The UMRC6 cell line has a lower level of expression of pVHL compared to the A498 cell line (data not shown). One of 2 similar experiments is shown. b, pVHL knock-down increases endogenous β-catenin. VHL-intact renal cancer cell lines were transfected with control (Csi) and VHL siRNA (VHLsi) oligonucleotide. Cytosolic fractions prepared from cells harvested 72 h after siRNA transfection were probed for β-catenin. One of 2 similar experiments is shown. c, Jade-1 silencing in the presence of pVHL mitigates β-catenin regulation. The 293T cells were transiently transfected with empty vector (Vec), pVHL expression vector and Jade-1 shRNA (Jsh). Extracts were probed for Jade-1 and β-catenin protein. pVHL was detected using monoclonal pVHL antibody. One of 2 similar experiments is shown. d, Wild-type pVHL, but not pVHL del96-122, suppresses β-catenin-mediated transcriptional activation. The 293 cells transiently transfected with empty vector or β-catenin, with wild-type pVHL, pVHL del96-122 or empty vector, and TOP-Flash or FOP-Flash were subjected to luciferase assay. Activity of the Wnt signaling pathway was quantified by measuring relative firefly luciferase activity units (RLUs) normalized to renilla luciferase. Mean results of 3 experiments are shown. Error bars = SEM. Student’s t-test was applied to determine statistical significance. p = 0.041 (* = statistically significant), p = 0.067 (NS). Extracts were probed using monoclonal pVHL antibody to show equivalent expression of the pVHL constructs. e, Wild-type pVHL, but not pVHL del96-122, suppresses Axin2. WCLs of 293T cells transfected with empty vector (Vec), wild-type pVHL (WT) and pVHL del96-122 (del96-122) were immunoblotted for Axin2 and actin. One of 2 similar experiments is shown. f, Wild-type pVHL, but not pVHL del96-122, significantly inhibits endogenous axis in Xenopus laevis embryos. In vitro transcribed capped LacZ (0.145-0.288 ng) (N = 73), wild-type VHL (0.130-0.194 ng) (N = 115) or VHL del96-122 (0.146-0.293 ng) (N = 100) mRNA was injected into dorsal blastomeres of Xenopus laevis embryos. Uninjected (N = 135) and LacZ injected embryos served as controls. In uninjected embryos, 96% showed normal dorsoanterior index (DAI) and 3% of embryos had partial axis duplication. In LacZ injected embryos, 92% had normal DAI, 3% of embryos had DAI 3-4, 3% of embryos showed gastrulation defects (GD) and 3% showed partial axis duplication. In wild-type VHL injected embryos, 49% had normal DAI, 30% had DAI 3-4, and 5% had DAI 0-2. Twelve percent of embryos showed GD and 3% showed partial double axis. In del96-122VHL injected embryos, 75% had normal DAI, 15% had DAI 3-4 and none had DAI 0-2. Seven percent of embryos had GD and 3% had partial axis duplication. Chi square test was applied to determine statistical significance. Wild-type pVHL suppressed the endogenous axis formation significantly more than pVHL del96-122 (p = 0.0046). A total of 6 experiments was performed to compare VHL wild-type and VHL del96-122 injected embryos. g, Comparable expression of Myc-tagged pVHL wild-type and pVHL del96-122 protein in Xenopus laevis embryos. Xenopus laevis embryos injected with capped mRNAs were harvested at stage 10. Protein extracts were resolved by SDS-PAGE. The membrane was probed using pVHL and α-tubulin antibodies. Representative immunoblot of 6 experiments.
Acknowledgements
We would like to thank Z.-X. Xiao (Boston University) for insightful suggestions and careful review of the manuscript; K. Symes, M. Malikova and E. Smith (all of Boston University) for Xenopus laevis embryos; R. Kemler (Max-Planck Institute for Immunobiology, Germany) for providing the β-catenin S33A construct; W. Birchmeier (Max-Delbruck-Center for Molecular Medicine, Germany) for β-catenin C and N terminus deletion constructs; and R. Benarous (Institute Pasteur, France) for β-TrCP wild type and DN in pcDNA3.1 myc/His vector. This work was supported by fellowship grants from the National Kidney Foundation and Polycystic Kidney Disease Foundation (to V.C.C.) and by National Institute of Health (NIH) Training Grant T32 DK 07053 (for V.C.C. and R.L.F.); American Heart Association SDG 0535485T and American Cancer Society Grant IRG-72-001-32-IRG (to M.V.P.); by a pilot research grant from the Department of Medicine at Boston University School of Medicine and a Karin Grunebaum Junior Faculty Cancer Research Award (to I.D.); and by NIH Grants R01 CA71796 (to D.C.S.) and R01 CA79830 and R01 DK67569 (to H.T.C.).
End notes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature
Part of this work was presented at the American Society of Nephrology annual meeting in San Diego, CA, USA, November 2006 and the American Society of Nephrology annual meeting in San Francisco, CA, USA, November 2007.
Reprints and permission information are available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
References
- 1.Maxwell PH, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 2.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–90. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 3.Zhou MI, et al. The von Hippel-Lindau tumor suppressor stabilizes novel plant homeodomain protein Jade-1. J Biol Chem. 2002;277:39887–98. doi: 10.1074/jbc.M205040200. [DOI] [PubMed] [Google Scholar]
- 4.Zhou MI, Wang H, Foy RL, Ross JJ, Cohen HT. Tumor suppressor von Hippel-Lindau (VHL) stabilization of Jade-1 protein occurs through plant homeodomains and is VHL mutation dependent. Cancer Res. 2004;64:1278–86. doi: 10.1158/0008-5472.can-03-0884. [DOI] [PubMed] [Google Scholar]
- 5.Zhou MI, et al. Jade-1, a candidate renal tumor suppressor that promotes apoptosis. Proc Natl Acad Sci U S A. 2005;102:11035–40. doi: 10.1073/pnas.0500757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winston JT, et al. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–83. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 8.Panchenko MV, Zhou MI, Cohen HT. von Hippel-Lindau partner Jade-1 is a transcriptional co-activator associated with histone acetyltransferase activity. J Biol Chem. 2004;279:56032–41. doi: 10.1074/jbc.M410487200. [DOI] [PubMed] [Google Scholar]
- 9.Latres E, Chiaur DS, Pagano M. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene. 1999;18:849–54. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- 10.Schulz I. Permeabilizing cells: some methods and applications for the study of intracellular processes. Methods Enzymol. 1990;192:280–300. doi: 10.1016/0076-6879(90)92077-q. [DOI] [PubMed] [Google Scholar]
- 11.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. Embo J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coscoy L, Sanchez DJ, Ganem D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J Cell Biol. 2001;155:1265–73. doi: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Z, Xu S, Joazeiro C, Cobb MH, Hunter T. The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol Cell. 2002;9:945–56. doi: 10.1016/s1097-2765(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 14.Belaidouni N, et al. Overexpression of human beta TrCP1 deleted of its F box induces tumorigenesis in transgenic mice. Oncogene. 2005;24:2271–6. doi: 10.1038/sj.onc.1208418. [DOI] [PubMed] [Google Scholar]
- 15.McCrea PD, Brieher WM, Gumbiner BM. Induction of a secondary body axis in Xenopus by antibodies to beta-catenin. J Cell Biol. 1993;123:477–84. doi: 10.1083/jcb.123.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iliopoulos O, Ohh M, Kaelin WG., Jr. pVHL19 is a biologically active product of the von Hippel-Lindau gene arising from internal translation initiation. Proc Natl Acad Sci U S A. 1998;95:11661–6. doi: 10.1073/pnas.95.20.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hovanes K, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–7. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 19.Larabell CA, et al. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J Cell Biol. 1997;136:1123–36. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, et al. Siah-1 mediates a novel beta-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001;7:927–36. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- 21.Nastasi T, et al. Ozz-E3, a muscle-specific ubiquitin ligase, regulates beta-catenin degradation during myogenesis. Dev Cell. 2004;6:269–82. doi: 10.1016/s1534-5807(04)00020-6. [DOI] [PubMed] [Google Scholar]
- 22.Hart M, et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–10. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 23.Kitagawa M, et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. Embo J. 1999;18:2401–10. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sansom OJ, Griffiths DF, Reed KR, Winton DJ, Clarke AR. Apc deficiency predisposes to renal carcinoma in the mouse. Oncogene. 2005 doi: 10.1038/sj.onc.1208956. [DOI] [PubMed] [Google Scholar]
- 25.Qian CN, et al. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem. 2005;280:3938–45. doi: 10.1074/jbc.M410697200. [DOI] [PubMed] [Google Scholar]
- 26.Saadi-Kheddouci S, et al. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene. 2001;20:5972–81. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 27.Battagli C, et al. Promoter hypermethylation of tumor suppressor genes in urine from kidney cancer patients. Cancer Res. 2003;63:8695–9. [PubMed] [Google Scholar]
- 28.Peruzzi B, Athauda G, Bottaro DP. The von Hippel-Lindau tumor suppressor gene product represses oncogenic beta-catenin signaling in renal carcinoma cells. Proc Natl Acad Sci U S A. 2006;103:14531–6. doi: 10.1073/pnas.0606850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simons M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–43. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieuwkoop J, Faber J. Normal Table of Xenopus laevis. North-Holland; Amsterdam: 1967. [Google Scholar]
- 31.Dominguez I, et al. Protein kinase CK2 is required for dorsal axis formation in Xenopus embryos. Dev Biol. 2004;274:110–24. doi: 10.1016/j.ydbio.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Kao KR, Elinson RP. The entire mesodermal mantle behaves as Spemann’s organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Jade-1 directly binds β-catenin, and the interaction is enhanced in Wnt-off phase. a, Schematic of Jade-1 and β-catenin constructs. Full-length Jade-1 and Jade-1 double deletion of PHDs (Jade-1 dd) are shown. Jade-1 dd lacks both PHDs but has an intact inter-PHD region and contains aa 1-202, 254-311, and 372-509 3,8. β-catenin constructs have an N-terminal Myc tag and include β-catenin wild-type (WT), N-terminal deletion (delN) and C-terminal deletion (delC). b, Jade-1 binds to β-catenin in a Wnt-responsive manner. Immunoprecipitations (IP) were performed with whole cell lysates (WCL) (500 μg protein) of 293T cells pretreated with vehicle (water) (Veh) or 20 mM lithium chloride (Li) for 4 h using 1 μg of either rabbit polyclonal Jade-1 antibody or pre-immune rabbit serum (data not shown). The co-immunoprecipitated β-catenin was detected by immunoblot with monoclonal β-catenin antibody. β-catenin was immunoprecipitated as described above using monoclonal β-catenin antibody and isotype control (data not shown). Jade-1 was detected by immunoblot using polyclonal Jade-1 antiserum. WCL (10%) were probed for input. One of 2 similar experiments is shown. c, Expression of Jade-1 and β-catenin in Wnt-off phase. The 293 cells transfected with Flag-tagged Jade-1 or Myc-tagged β-catenin constructs were pretreated with vehicle (PBS + 0.1% bovine serum albumin) for 4 h, fixed and incubated with monoclonal Myc and polyclonal Flag antibodies, followed by Alexa Fluor 594 donkey anti-mouse and Oregon green 488 goat anti-rabbit as secondary antibodies. A single representative confocal image is shown. Wild-type β-catenin and the C terminus (delC) truncations of β-catenin localized to the cytosol and membrane. The N terminus (delN) truncation of β-catenin localized predominantly to the cytosol while β-catenin S33A localized predominantly to the nucleus. Jade-1 localized to the cytosol and to lesser extent to the nucleus. Scale bar = 10 μm. d, Jade-1 and β-catenin co-localize in Wnt-off phase. The 293 cells transfected with Flag-tagged Jade-1 and Myc-tagged β-catenin constructs pretreated with Wnt-3a (200 ng) for 4 h were processed as above. A single representative confocal image is shown. The co-localization was performed using the NIH ImageJ program in cells having comparable signal level for both constructs. Randomly selected representative cells were analyzed. At least 15 confocal images comprising one Z-stack were generated for each cell. For the scatter plots, co-localization was performed using the entire Z-stack. Scatter plot points along the X or Y axis represent absence of co-localization, whereas scatter plot points along a diagonal represent evidence of co-localization. Images were background subtracted from a randomly chosen region of interest. Scale bar = 10 μm. e, Loss of Jade-1-β-catenin co-localization in Wnt-on status. The transfected 293 cells were pretreated with Wnt-3a (200 ng) for 4 h. The cells were fixed, and co-localization was performed as in d. Scale bar = 10 μm. f, Jade-1 preferentially binds phospho-β-catenin in in vitro GST pull-down assays. (Left panels), Glutathione Sepharose™ beads or purified recombinant GST-tagged Jade-1 bound to glutathione Sepharose™ beads was incubated with cleaved, purified recombinant β-catenin (with or without in vitro phosphorylation then washed, followed by elution of glutathione Sepharose™ beads in Laemmli buffer. In vitro phosphorylation of β-catenin was performed by incubating GST-tagged β-catenin glutathione Sepharose™ beads with ATP, kinases CK1 and GSK-3β for 30 mins at 30 °C. The immunoblot was probed for β-catenin then reprobed using phospho-β-catenin antibody. Ten percent of glutathione Sepharose™ beads was probed as input. (Right panels), Glutathione Sepharose™ beads or purified recombinant GST-tagged β-cateninbound to glutathione Sepharose™ beads was incubated with cleaved, purified recombinant Jade-1 as above. In vitro phosphorylation of GST-tagged-β-catenin was performed as above. Ten percent of glutathione Sepharose™ beads was probed as input. The immunoblot was reprobed using phospho-β-catenin antibody. Representative immunoblot of 3 experiments. G = GST, G-J = GST-Jade-1, G-β = GST-β-catenin, G-phosphoβ = GST-phospho-β-catenin, G-delN = GST-β-catenin delN.
Figure S2 Jade-1 regulates endogenous β-catenin. a, Jade-1 silencing by a lentiviral shRNA system in different cell lines. HeLa, 293 and HK-2 cells lines were infected with empty vector (Vec), non-silencing control (Non) or one of two Jade-1 shRNA lentiviral vectors (Jsh). WCL from parental cells (P) or infected cells were probed for Jade-1 and actin protein. Densitometry results are normalized to actin. One of 2 similar experiments is shown. b, Jade-1 silencing by shRNA plasmid vector upregulates endogenous β-catenin. Extracts of empty vector (Vec) or Jade-1 shRNA (JshRNA) plasmid vector transiently transfected 293T cells were probed for Jade-1 and β-catenin protein. Representative immunoblot of 3 experiments. c, Endogenous β-catenin is upregulated with both Jade-1 shRNA lentiviral constructs. Cytosolic protein extracts from HeLa cells infected with one of two lentiviral Jade-1 shRNA constructs (Jsh1 or Jsh2) were probed for β-catenin. One of 2 similar experiments is shown. d, Jade-1 silencing increases the cytosolic and nuclear fractions but not the membrane fraction of β-catenin. Extracts of parental HK-2 cells (P) or cells infected with empty vector (Vec) or Jade-1 shRNA lentivirus (Jsh) were subjected to cell fractionation. Cytosolic, nuclear and membrane fractions were probed for β-catenin. α-tubulin, fibrillarin and E-cadherin served as loading controls and markers of cytosolic, nuclear and membrane fractions, respectively. These control blots were generated by cutting the membrane at the expected molecular weights of the controls and immunoblotting separately. One of 2 similar experiments is shown. e, The digitonin-extracted fraction is enriched for cytosol and devoid of membrane contamination. Equal amounts of protein from the digitonin-extracted fractions of 293 cells pretreated with protein translation inhibitor emetine (20 μM), harvested at different time points, were probed for β-catenin. E-cadherin served as a marker for the membrane fraction, and α-tubulin and IkappaB-α served as markers of cytosol. These control blots were generated by cutting the membrane at the expected molecular weights of the controls and immunoblotting separately. One of 2 similar experiments is shown. f, Jade-1 expression destabilizes β-catenin in renal cancer cells. Cytosolic fractions from clonal 786-O cell lines stably expressing empty vector or Jade-1 (J9, J22)4,5 harvested after 20 μM emetine treatment were probed for β-catenin. Percent β-catenin remaining was analyzed by densitometry after normalizing to α-tubulin. β-catenin half-life was reduced from 8 hrs to 3.5 hrs in the Jade-1-expressing 786-O cell lines. Graph shows mean result of 3 experiments. Error bars = SEM.
Figure S3 Jade-1 preferentially regulates phospho-β-catenin in Wnt-off phase and enhances proteasomal degradation of β-catenin. a, Jade-1 down-regulates total β-catenin by reducing predominantly phospho-β-catenin. Cytosolic protein extracts from 293T cells transiently transfected with full-length Jade-1 (J1) or Jade-1 dd (J1dd) were probed for total β-catenin. The membrane was stripped and reprobed for phospho-β-catenin (β-catenin phosphorylated at serine 33, 37 and threonine 41) and active β-catenin (non-phosphorylated at serine 37 and threonine 41). Cytosolic fractions were probed for the Myc tag to confirm equivalent expression of Jade-1 and Jade-1 dd (data not shown). One of 2 similar experiments is shown. b, Jade-1 regulation of β-catenin during Wnt-on and Wnt-off phase. HK-2 parental cells (P) or cells infected with empty vector (Vec) or Jade-1 shRNA (Jsh) lentivirus were treated for 4 h with Wnt-3a (200 ng), with or without DKK1 (200 ng). The cytosolic fractions were probed for β-catenin and α-tubulin. Representative immunoblot of 3 experiments. c, Proteasome inhibitor treatment abrogates the effect of Jade-1 on β-catenin. Transfected 293T cells were incubated with proteasome inhibitor (PI) MG132 at 10 μM for 12 hrs. WCL were probed with β-catenin, α-tubulin or Flag-tag antibodies. One of 2 similar experiments is shown. d, Jade-1 increases accumulation of high molecular weight polyubiquitinated β-catenin in the presence of PI. The 293T cells transiently transfected with Jade-1 and β-catenin were treated with PI MG132 at 10 μM for 12 h. WCL were probed as shown. One of 2 similar experiments is shown.
Figure S4 Jade-1 ubiquitinates β-catenin. a, The PHDs of Jade-1 align well with the PHDs of proteins with known E3 ubiquitin ligase activity. The MacVector (Accelrys) sequence analysis program was used to align the PHDs of human Jade-1, MEKK1 (Q13233), viral MIR1 (K3), MIR2 (K5) (U93872) and c-MIR (hCP36279). b, Full-length Jade-1, but not Jade-1 dd, reduces abundance of the cytosolic and nuclear fractions of β-catenin. Protein extracts of 293T cells transiently transfected with full-length Jade-1 (J1) or Jade-1 dd (J1dd) were probed for β-catenin. α-tubulin, fibrillarin or E-cadherin served as loading controls and markers of cytosolic, nuclear and membrane fractions, respectively. These control blots were generated by cutting the membrane at the expected molecular weights of the controls and immunoblotting separately. Cytosolic fractions were probed to examine expression of Jade-1 and Jade-1 dd. One of 2 similar experiments is shown. c, Dose-dependent in vitro ubiquitination of β-catenin by Jade-1. Ubiquitination reactions were reconstituted by incubating GST-β-catenin beads with E1 activating enzyme, E2 conjugase (UbcH6), E3 ligase Jade-1 (in increasing amounts, 250, 750, or 1200 nM) or Jade-1 (full-length or dd) (750 nM), Myc-tagged human recombinant ubiquitin (Ub) and MgCl2-ATP. No E1, no E2, no Ub and DN E2 reactions served as negative controls. β-catenin ubiquitination was detected using polyclonal β-catenin C-terminal antibody. Reactions without E1, E2, or ubiquitin, or with dominant negative (DN) UbcH6, served as negative controls (lanes 8-11). Jade-1 input was detected by immunoblot. Lane 7 was rearranged from the same blot. One of 2 similar experiments is shown.
Figure S5 Jade-1 regulates β-catenin transcriptional target genes. a, Jade-1 silencing results in a significant spontaneous endogenous Wnt activity. The 293 cells stably infected with empty vector or Jade-1 shRNA lentiviral vectors were transiently transfected with TOP-Flash or FOP-Flash and renilla luciferase vectors. Activity of the Wnt signaling pathway was quantified by measuring relative firefly luciferase activity units (RLUs) normalized to renilla luciferase. Mean results of 3 experiments are shown. Error bars = SEM. Student’s t-test was applied to determine statistical significance, p = 0.033. b, Comparable expression of Myc-tagged Jade-1 and Jade-1 dd protein in Xenopus laevis embryos injected also with capped mRNAs for Xwnt-8 or β-catenin. Embryos were harvested at stage 10, and the extracts were resolved by SDS-PAGE. The membrane was probed using Myc and α-tubulin antibodies. c, Wnt targets are upregulated in Jade-1 silenced cell lines. WCL from cells infected with empty vector or from Jade-1 silenced cells were probed for cyclin D1 or c-Myc. Representative immunoblot of 4 experiments. d, Full-length Jade-1, but not Jade-1 dd, down-regulates endogenous c-Myc. Whole cell extracts of 293T cells transfected with Myc-tagged β-catenin with or without Flag-Jade-1 or Flag-Jade-1 dd were probed with c-Myc antibody detecting endogenous c-Myc protein. Flag- and Myc-tag antibodies were used to detect transfected Flag-tagged Jade-1 and Myc-tagged β-catenin, respectively. One of 2 similar experiments is shown.
Figure S6 pVHL regulates β-catenin through Jade-1. a, pVHL down-regulates endogenous β-catenin. Cytosolic and nuclear fractions of VHL-deficient renal cancer cell lines stably expressing pVHL were probed for endogenous β-catenin. The UMRC6 cell line has a lower level of expression of pVHL compared to the A498 cell line (data not shown). One of 2 similar experiments is shown. b, pVHL knock-down increases endogenous β-catenin. VHL-intact renal cancer cell lines were transfected with control (Csi) and VHL siRNA (VHLsi) oligonucleotide. Cytosolic fractions prepared from cells harvested 72 h after siRNA transfection were probed for β-catenin. One of 2 similar experiments is shown. c, Jade-1 silencing in the presence of pVHL mitigates β-catenin regulation. The 293T cells were transiently transfected with empty vector (Vec), pVHL expression vector and Jade-1 shRNA (Jsh). Extracts were probed for Jade-1 and β-catenin protein. pVHL was detected using monoclonal pVHL antibody. One of 2 similar experiments is shown. d, Wild-type pVHL, but not pVHL del96-122, suppresses β-catenin-mediated transcriptional activation. The 293 cells transiently transfected with empty vector or β-catenin, with wild-type pVHL, pVHL del96-122 or empty vector, and TOP-Flash or FOP-Flash were subjected to luciferase assay. Activity of the Wnt signaling pathway was quantified by measuring relative firefly luciferase activity units (RLUs) normalized to renilla luciferase. Mean results of 3 experiments are shown. Error bars = SEM. Student’s t-test was applied to determine statistical significance. p = 0.041 (* = statistically significant), p = 0.067 (NS). Extracts were probed using monoclonal pVHL antibody to show equivalent expression of the pVHL constructs. e, Wild-type pVHL, but not pVHL del96-122, suppresses Axin2. WCLs of 293T cells transfected with empty vector (Vec), wild-type pVHL (WT) and pVHL del96-122 (del96-122) were immunoblotted for Axin2 and actin. One of 2 similar experiments is shown. f, Wild-type pVHL, but not pVHL del96-122, significantly inhibits endogenous axis in Xenopus laevis embryos. In vitro transcribed capped LacZ (0.145-0.288 ng) (N = 73), wild-type VHL (0.130-0.194 ng) (N = 115) or VHL del96-122 (0.146-0.293 ng) (N = 100) mRNA was injected into dorsal blastomeres of Xenopus laevis embryos. Uninjected (N = 135) and LacZ injected embryos served as controls. In uninjected embryos, 96% showed normal dorsoanterior index (DAI) and 3% of embryos had partial axis duplication. In LacZ injected embryos, 92% had normal DAI, 3% of embryos had DAI 3-4, 3% of embryos showed gastrulation defects (GD) and 3% showed partial axis duplication. In wild-type VHL injected embryos, 49% had normal DAI, 30% had DAI 3-4, and 5% had DAI 0-2. Twelve percent of embryos showed GD and 3% showed partial double axis. In del96-122VHL injected embryos, 75% had normal DAI, 15% had DAI 3-4 and none had DAI 0-2. Seven percent of embryos had GD and 3% had partial axis duplication. Chi square test was applied to determine statistical significance. Wild-type pVHL suppressed the endogenous axis formation significantly more than pVHL del96-122 (p = 0.0046). A total of 6 experiments was performed to compare VHL wild-type and VHL del96-122 injected embryos. g, Comparable expression of Myc-tagged pVHL wild-type and pVHL del96-122 protein in Xenopus laevis embryos. Xenopus laevis embryos injected with capped mRNAs were harvested at stage 10. Protein extracts were resolved by SDS-PAGE. The membrane was probed using pVHL and α-tubulin antibodies. Representative immunoblot of 6 experiments.