Abstract
In vivo systemic absorption of the amino acid prodrugs of acyclovir (ACV) after oral administration was evaluated in rats. Stability of the prodrugs, L-Alanine-ACV (AACV), L-Serine-ACV (SACV), L-Isoleucine-ACV (IACV), γ-Glutamate-ACV (EACV) and L-Valine-ACV (VACV) was evaluated in various tissues. Interaction of these prodrugs with the transporters on Caco-2 cells was studied. In vivo systemic bioavailability of these prodrugs upon oral administration was evaluated in jugular vein cannulated rats. The amino acid ester prodrugs showed affinity towards various amino acid transporters as well as the peptide transporter on the Caco-2 cells. In terms of stability, EACV was most enzymatically stable compared to other prodrugs especially in liver homogenate. In oral absorption studies, ACV and AACV showed high terminal elimination rate constants (λz). SACV and VACV exhibited approximately five fold increase in area under the curve (AUC) values relative to ACV (p<0.05). Cmax(T) (maximum concentration) of SACV was observed to be 39 ± 22 µM in plasma which is 2 times better than VACV and 15 times better than ACV. Clast(T) (concentration at the last time point) of SACV was observed to be 0.18 ± 0.06 µM in plasma which is 2 times better than VACV and 3 times better than ACV. Amino acid ester prodrugs of ACV were absorbed at varying amounts (Cmax) and eliminated at varying rates (λz) thereby leading to varying extents (AUC). The amino acid ester prodrug SACV owing to its enhanced stability, higher AUC and better concentration at last time point seems to be a promising candidate for the oral treatment of herpes infections.
Keywords: acyclovir, amino acid prodrugs, oral bioavailability, Caco-2, transporters
INTRODUCTION
Intestinal absorption of drugs, nutrients, and other compounds is mediated by uptake transporters expressed at the apical enterocyte membrane. These transporters play a significant role in drug absorption and distribution to organic systems. A host of transporters have been discovered at the brush-border membrane of intestinal epithelium, which can be targeted for drug delivery. Transport systems for peptide (Daniel, 2004), amino acid (Frenhani and Burini, 1999), monocarboxylic acids (Enerson and Drewes, 2003), bile acids (Lack, 1979) and vitamins (Said and Mohammed, 2006) are such carriers that have been discovered on the intestinal epithelium and utilized for targeted drug delivery (Tsuji and Tamai, 1996; Tolle-Sander et al., 2004). Among nutrient transporters, amino acid and peptide transporters are preferred for drug delivery due to their ubiquitous nature and overlapping substrate specificity.
Amino acid transporters have been classified on the basis of their functional differences such as sodium dependence and substrate specificity. In terms of specificity they can be divided into three types namely anionic, cationic and neutral (Christensen, 1990). Small neutral amino acids, are transported predominantly by Na+- dependent transport system ASC (for Ala-, Ser-, and Cys-preferring), system A (for Ala-preferring) and B0,+ (neutral and cationic amino acids preferring) and also by Na+- independent transport system ASC, LAT (large neutral amino acids preferring) and b0,+. Amino acid transport systems, b0,+, y+L and B0,+, translocate a wider range of substrates, including cationic and neutral amino acids, differing however in their interactions with inorganic monovalent ions such as Na+.
Depending upon their affinity or capacity, amino acid transporters have been known to transport not only naturally occurring amino acids but also amino acid–related drug compounds such as L-dopa, a therapeutic agent for Parkinsonism; melphalan (Goldenberg et al., 1979), an anticancer Phe mustard; triiodothyronine (Blondeau et al., 1993) and thyroxine (Lakshmanan et al., 1990), two thyroid hormones; and gabapentin (Su et al., 1995), an anticonvulsant and valacyclovir (Hatanaka et al., 2004), an anti-viral drug. A recent report suggests that the ability of ATB(0,+) to transport valacyclovir is comparable to that of the peptide transporter PEPT1 (Hatanaka et al., 2004).
Peptide transporters (PepT1 and PepT2) are perhaps the drug transporters that have captured the most recent attention in drug delivery. Small peptides such as di- and tripeptides are transported by PepT1 and PepT2 in intestinal and renal epithelial cells, respectively. Structure, function, mechanism, and substrate specificity of the peptide transporters have been extensively studied (Ganapathy and Leibach, 1982; Dantzig and Bergin, 1990; Ganapathy et al., 1995; Hidalgo et al., 1995; Hu et al., 1995; Liang et al., 1995; Han et al., 1998). Due to their broad substrate specificity, PepT1 and PepT2 contribute to the intestinal absorption of several drug compounds such as β-lactam antibiotics, cephalosporins, angiotensin- converting enzyme, and renin inhibitors (Dantzig and Bergin, 1990; Han et al., 1998). These findings suggest that amino acid and peptide transporters can have significant potential as a delivery targets for amino acid-based drugs and prodrugs.
Strategies have been used to design prodrugs of various poorly absorbed drugs targeted toward receptors/transporters to improve systemic bioavailability (Anand et al., 2004a; Anand et al., 2004b; Steffansen et al., 2004; Tolle-Sander et al., 2004). Valacyclovir (VACV) is such a prodrug that is derived from acyclovir (ACV) by esterifying 3-hydroxyl group of ACV with L-valine. Acyclovir, an antiviral nucleoside, possesses activity against human herpes viruses. Owing to its limited bioavailability, ACV has shown moderate antiviral efficacy after oral administration (Steingrimsdottir et al., 2000). VACV has been reported to increase the oral bioavailability of acyclovir by 3-to 5-fold in humans (Purifoy et al., 1993; Weller et al., 1993). Enhanced oral absorption of acyclovir after administration of valacyclovir has been attributed to the amino acid (B0,+) (Hatanaka et al., 2004) and peptide (PepT1) (Ganapathy et al., 1998) mediated intestinal translocation.
A series of novel water-soluble amino acid ester prodrugs of acyclovir were synthesized previously (Anand et al., 2004b; Katragadda et al., 2008). These compounds were designed to target the transporters on the cornea and intestinal epithelial cells for improved ocular and oral absorption of acyclovir, respectively. Previous results indicated that the amino acid ester prodrugs of ACV exhibited affinity toward the amino acid transporters, and the uptake of these prodrugs was efficiently mediated by ASCT1 and B0,+ (Anand et al., 2004b; Katragadda et al., 2008). In this report, we describe pharmacokinetics of these prodrugs after oral administration in Sprague-Dawley rats. The interaction of these amino acid prodrugs with the transporters on Caco-2 cells is also discussed. The transport of these prodrugs across Caco-2 monolayers was compared with that of VACV to establish the transport of these compounds across enterocytic cell membranes.
MATERIALS AND METHODS
Materials
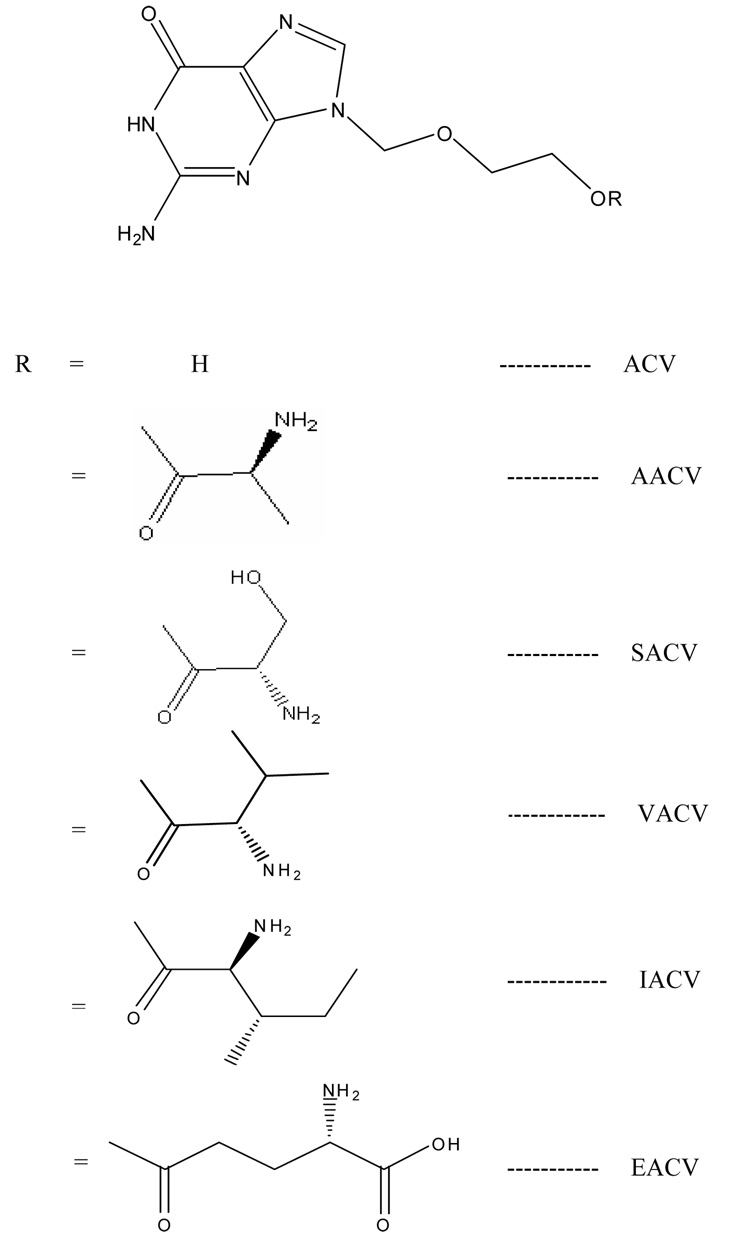
[3H] alanine (66 Ci/mmol), [3H] phenylalanine (50 Ci/mmol), and [3H] arginine (42 Ci/mmol) were obtained from NEN Biochemicals (Boston, MA, USA). [3H]Glycylsarcosine (4 Ci/mmol) was purchased from Moravek Biochemicals (Brea, CA). Valacyclovir was a gift from GlaxoSmithKline (Research Triangle Park, NC). Human colon carcinoma derived Caco-2 cells were obtained from American Type Culture Collection (Rockville, MD). The growth medium Dulbecco’s modified Eagle’s medium was obtained from Invitrogen (Carlsbad, CA). Minimal essential medium, nonessential amino acids, penicillin, streptomycin, sodium bicarbonate, and HEPES were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was obtained from JRH Biosciences (Lenexa, KS). Culture flasks (75-cm2 growth area), 12 wells (3.8 cm2 growth area per well), and polyester transwells (pore size 0.4 µM with diameter of 6.5 mm) were procured from Costar (Bedford, MA). The buffer components and solvents were obtained from Fisher Scientific (St. Louis, MO). All the amino acid prodrugs of acyclovir (Figure 1) were synthesized in our laboratory according to previously published procedures (Anand et al., 2004b; Katragadda et al., 2008). Four amino acid prodrugs were used in this study: L-Alanine-ACV (AACV), L-Serine-ACV (SACV), L-Isoleucine-ACV (IACV), and γ-Glutamate-ACV (EACV).
Figure 1.
Structures of amino acid prodrugs of acyclovir.
Animals
Jugular vein cannulated male Sprague-Dawley rats weighing 200 to 250 g were obtained from Charles River Laboratories (Wilmington, MA). Animal care and treatment in this investigation was in compliance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institute of Health.
Cell culture
All cell cultures were maintained in humidified incubator at 37°C with a 5% carbon dioxide in air atmosphere. Caco-2 cells were obtained from American Type Culture Collection and grown in plastic tissue culture flasks. The passage number used for this study ranged from 40–45. Conventional culture medium containing Dulbecco’s modified Eagle’s medium, 10% FBS (heat inactivated), 1% nonessential amino acids, 100 IU/ml penicillin, 100 µg/ml streptomycin, 44 mM sodium bicarbonate and 20 mM HEPES at pH 7.4 was used according to the protocol established in our laboratory for maintaining the cell line. Upon reaching 80% confluence, cells were removed by trypsin/EDTA treatment and plated at a density of 200,000 cells/cm2 on 12 well plates and 65,000 cells/cm2 on transwells containing clear polyester membranes (1.1 cm2, 0.4 µm mean pore size). Cells were then grown in medium containing 10% FBS (heat-inactivated). Caco-2 cells used in our studies were grown for 17 to 20 days.
Uptake studies
All uptake studies were conducted on Caco-2 cells after 17 to 20 days seeding. The medium was removed and cells were washed twice with DPBS (pH 7.4). In typical uptake experiments, cells were incubated with substrates ([3H] alanine (7.6 nM); [3H] arginine (11.9 nM); [3H] phenylalanine (10 nM); and [3H] glysar (125 nM)) prepared in DPBS for 30 minutes. Following incubation, cells were washed three times with ice-cold HEPES (4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid) buffer to terminate the uptake experiment. Then cells were lysed overnight with 1 ml 0.05% (w/v) Triton X-100 in 1N NaOH at room temperature. Aliquots (500 µl) from each well were transferred to scintillation vials containing 5 ml scintillation cocktail (Fisher Scientific, Fairlawn, NJ). Samples were then analyzed by liquid scintillation spectrophotometry using scintillation counter (Beckman Instruments Inc., Model LS-6500) and the rate of uptake was normalized to the protein content of each well. The amount of protein in the cell lysate was measured by BioRad protein estimation kit using bovine serum albumin as the standard (BioRad Protein estimation Kit, Hercules, CA).
Transport studies
Transport experiments were carried out with 12 well transwells (Costar, Bedford, MA). Before the experiment, Caco-2 cell monolayer grown on the clear polyester membranes was washed with DPBS (pH 7.4) and incubated at 37°C. Freshly prepared drug solution (1 mM) in DPBS (pH 7.4) was placed in the donor chamber and the receiver chamber was filled with DPBS (pH 7.4). The volumes of donor and receiver chambers were 0.5 and 1.5 ml each respectively. Receiver chamber was sampled at predetermined time intervals and an equal volume of fresh DPBS solution was added to maintain sink conditions. All samples were stored at −80°C until further HPLC analysis. All experiments were performed at least in triplicates at 37°C.
Preparation of homogenates
Caco-2 homogenate
Confluent Caco-2 cells grown in tissue culture flasks were isolated with the aid of mechanical scraper and washed thrice with Dulbecco’s phosphate-buffered saline (DPBS). Cells were homogenized in 2 ml of chilled (4°C) DPBS for about 10 min with a mechanical homogenizer in an ice bath. Subsequently, the homogenates were centrifuged at 12,500 rpm for 25 min at 4°C to remove cellular debris, and the supernatant was used for hydrolysis studies.
Plasma
Noncannulated male Sprague-Dawley rats were anesthetized by administering ketamine (50mg/kg) and xylazine (5mg/kg) subcutaneously and subsequently euthanized by puncturing the heart. Blood was collected from the heart. Plasma was immediately separated from the blood by centrifugation at 6,500 rpm for 10 min to remove the debris and then the supernatant (plasma) was stored at −80°C until the hydrolysis studies were conducted.
Intestinal and liver homogenate
Noncannulated male Sprague-Dawley rats were euthanized by a lethal injection of sodium pentobarbital through the tail vein. Intestinal segments and liver were isolated and stored at −80°C before use. Tissues were homogenized in 5 ml of chilled (4°C) DPBS for about 4 min with a tissue homogenizer (Tissue Tearor model 985-370; Biospec Products Inc., Bartlesville, OK) in an ice bath. Subsequently, the homogenates were centrifuged at 12,500 rpm for 25 min at 4°C to remove cellular debris and the supernatant was used for hydrolysis studies.
Metabolism studies
The supernatant obtained from the above described procedure was equilibrated at 37°C for about 15 min before an experiment. Supernatant (0.8 ml) was incubated with 0.2 ml of 1 mM solutions of prodrugs at 37°C in a shaking water bath for the length of the study. The control consisted of 0.8 ml of DPBS instead of the supernatant. Aliquots (50 µl) were withdrawn at appropriate time intervals for up to 24 h. The samples were immediately diluted with 50 µl of chilled acetonitrile/methanol (4:5 mixture) to precipitate the proteins and stored at −80°C until further analysis. Apparent first order rate constants were calculated and corrected for any chemical hydrolysis observed with the control. The protein content of the supernatant was determined by the method of Bradford (1976) (Bradford, 1976) using bovine serum albumin as the standard (protein estimation kit; Bio-Rad, Hercules, CA).
In vivo studies with Sprague-Dawley rats
Oral absorption studies of ACV and its prodrugs were carried out at an equivalent dose of 20.0 mg/kg. Animals were fasted overnight (12–18 h) with free access to water. Freshly prepared drug solutions in water were administered by oral gavage (0.8 ml). Blood samples (200 µl) were collected from the jugular vein at predetermined time intervals over a period of 5 h. Heparinized saline (200 µl) was injected through the vein to maintain a fairly constant fluid volume. Plasma was immediately separated by centrifugation and then stored at −80°C until further analysis.
Sample preparation
Using a simple protein precipitation method ACV and its prodrugs were extracted from rat plasma. Plasma samples were thawed at room temperature and 0.2 ml of acetonitrile was added to 0.1 ml of plasma and 0.01 ml of internal standard (GCV- 0.5 µg/ml) in a 1.5 mL centrifuge tube. The sample was then vortexed vigorously for 1 min and the samples were allowed to rest for 10 min at room temperature prior to centrifugation. Samples were centrifuged at approximately 12,500 rpm for 30 min at 4°C. The supernatant was then separated and evaporated using Speedvac (SAVANT Instruments, Inc., Holbrook, NY). The dry residues were dissolved in 100 µl of water (0.1 % formic acid) and then the sample was vortexed for 2 mins and centrifuged at 12,500 rpm for 15 min at 4°C. Calibration standards were prepared by spiking plasma with the standard solutions. The supernatant was analyzed using a LC/MS/MS.
Analytical procedures
HPLC
The HPLC system was comprised of a Rainin Dynamax Pump SD-200 and a Rainin Dynamax UV Detector UV-C at 254 nm. The column used was a C18 Luna column 4.6 × 250mm (Phenomenex, Torrance, CA). Mobile phase consisted of a mixture of buffer and an organic modifier. The percentage of organic phase was varied in order to elute the compounds of interest. This method gave rapid and reproducible results. HPLC conditions for the various compounds have been described previously (Anand et al., 2004b; Katragadda et al., 2008). Intra- and interday precision (measured by coefficient of variation, CV%) was less than 3 and 5%, respectively.
LC/MS/MS
The analysis of the plasma samples was performed using a triple quadrupole mass spectrometer with electrospray ionization (ESI) on a turbo ionspray source (API 2000; Applied Biosystems, Foster City, CA, USA) coupled to a liquid chromatography system (Agilent HP1100, Agilent Technology Inc., Palo Alto, CA, USA) and C18-column 100×2.0 mm (Phenomenex, Torrance, CA). The mobile phase consisted of 25% methanol and 75 % water with 0.1% formic acid, and was delivered at a flow rate of 0.15 mL/min. The sample volume injected was 50 µL and the analysis time was 5–6 min. Multiple reaction monitoring (MRM) mode was utilized to detect the compound of interest. The mass spectrometer was operated in the positive-ion detection mode at collision energy of 18 V. The turbo ionspray temperature was optimized at 150°C. Nitrogen gas was used to obtain collision-induced detection (CID). Ions were detected by monitoring the decay of the m/z 225 precursor ion to the m/z 152 product ion for acyclovir, the decay of the m/z 256 precursor ion to the m/z 152 product ion for ganciclovir, internal standard (IS). The typical ion source parameters were, declustering potential (DP) 36V; collision energy (CE) 18 eV; entrance potential (EP) 6 V; and collision cell exit potential (CXP) 4V. Peak areas for all components were automatically integrated by using Analyst™ software and peak-area ratios (area of analytes to area of IS) were plotted vs concentration by weighted linear regression (1/concentration). The analytical data resulted from prodrugs with MRM method show a significant linearity which extends to picomolar range. This method gave rapid and reproducible results. The limits of quantification were found to be ACV 1 ng/ml; AACV 5 ng/ml; SACV 5 ng/ml; EACV 5 ng/ml; IACV 5 ng/ml and VACV 5 ng/ml. Intra- and interday precision (measured by coefficient of variation, CV%) was less than 3 and 5%, respectively.
Permeability measurements
Steady state fluxes (SSF) were determined from the slope of the cumulative amount of drug transported vs time plot and expressed per unit of Caco-2 cell surface area as described by Eq. 1. The cumulative amount of drug transported is considered as the sum of the receptor cell prodrug and regenerated drug.
| Eq. 1 |
M is the cumulative amount of drug transported and A is the Caco-2 cell surface area exposed to permeant. Caco-2 cell membrane permeabilities are determined by normalizing the SSF to the donor concentration, Cd according to Eq. 2.
| Eq. 2 |
Statistical analysis
All experiments were conducted at least in triplicate and results are expressed as mean ± S.D. Student’s t test was used to detect statistical significance between the parameters of the prodrugs and ACV and p<0.05 was considered to be statistically significant. Statistical comparisons between the parameters of the prodrugs were performed using the analysis of variance (SPSS for Windows, release 10.0.7; SPSS Inc., Chicago, IL).
All relevant pharmacokinetic parameters were calculated using noncompartmental analyses of plasma-time curves after oral administration of ACV and the amino acid prodrugs of ACV using a pharmacokinetic software package WinNonlin, version 2.1 (Pharsight, Mountain View, CA). Data Fit was examined by observing R2, correlation or coefficient of variance (CV), weighted residuals and predicted Vs observed values. Maximum plasma concentrations (Cmax) and area under the plasma concentration time curves (AUC0-last and AUC0-inf) were obtained from the plasma-concentration time profiles using noncompartmental analysis. The slopes of the terminal phase of plasma profiles were estimated by log-linear regression and the terminal rate constant (λz) was derived from the slope. The terminal plasma half-lives were calculated from the equation: t1/2 = 0.693 / λz. The total concentration (TC) parameters were calculated by adding the concentrations of the administered prodrug (PD) and the regenerated ACV.
RESULTS
Uptake of [3H] alanine in the presence of prodrugs
[3H] ala (model substrate for ASC system) uptake was carried out in the presence of 5 mM ala, AACV, SACV, EACV, IACV, and VACV. [3H] ala uptake was significantly inhibited in the presence of 5 mM ala and AACV (Table 1).
Table 1.
Uptake of [3H] L-Ala (7.6 nM), [3H] L-Arg (11.9 nM), [3H] L-Phenylalanine (10 nM) and [3H] Glysar (125 nM) by Caco-2 in the presence of corresponding nonradioactive substrate and prodrugs.
| Units = fraction uptake of control per mg of protein |
[3H] Alanine | [3H] Arginine | [3H] Phenylalanine |
[3H] Gly-Sar |
|---|---|---|---|---|
| control | 6.58 ± 0.75 | 7.81 ± 1.23 | 16.27 ± 2.66 | 2.38 ± 0.25 |
| corresponding nonradioactive substrate‡ |
2.63 ± 0.67* | 3.60 ± 0.37* | 2.21 ± 0.16* | 0.45 ± 0.04* |
| AACV† | 1.90 ± 0.29* | 6.50 ± 1.17 | 9.22 ± 2.11 | 2.49 ± 0.32 |
| SACV† | 7.64 ± 0.79 | 8.44 ± 0.67 | 12.72 ± 1.12 | 0.35 ± 0.05* |
| IACV† | 4.93 ± 0.75 | 5.05 ± 0.67* | 4.06 ± 0.33* | 0.70 ± 0.06* |
| EACV† | 6.59 ± 1.65 | 8.54 ± 0.24 | 14.58 ± 2.60 | 2.18 ± 0.09 |
| VACV† | 6.25 ± 1.40 | 8.50 ± 1.17 | 12.92 ± 0.17 | 0.87 ± 0.1* |
Values are mean ± S.D. (n=4 to 8).
indicates p<0.05.
Uptake of radioactive substrate in the presence of corresponding nonradioactive competitive substrate (5 mM)
Uptake of radioactive substrate in the presence of prodrugs (5 mM)
Uptake of [3H] arginine in the presence of prodrugs
[3H] arg (model substrate for B0,+ system) uptake was carried out in the presence of 5 mM arg, AACV, SACV, EACV, IACV, and VACV. [3H] arg uptake was significantly inhibited in the presence of 5 mM arg and partially inhibited in the presence of 5 mM IACV (Table 1).
Uptake of [3H] phenylalanine in the presence of prodrugs
[3H] phenylalanine (model substrate for LAT system) uptake was performed in the presence of 5 mM phenylalanine, AACV, SACV, EACV, IACV, and VACV. [3H] phenylalanine uptake was significantly inhibited in the presence of 5 mM phenylalanine and IACV (Table 1).
Uptake of [3H] glysar in the presence of prodrugs
[3H] Glysar (model substrate for peptide transporter system) uptake was performed in the presence of 5 mM Glysar, AACV, SACV, EACV, IACV, and VACV. [3H] Glysar uptake was significantly inhibited in the presence of 5 mM Glysar, SACV and partially inhibited in the presence of 5 mM IACV and VACV (Table 1).
Transport across Caco-2 monolayers
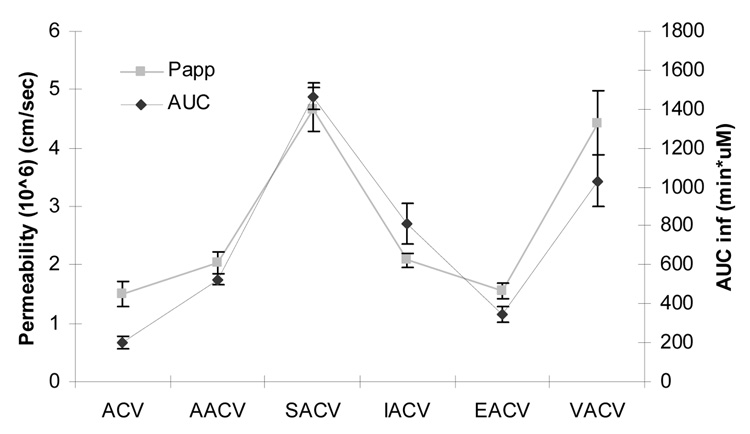
Transport of 1 mM ACV, AACV, SACV, EACV, IACV and VACV was investigated across Caco-2 monolayers. Papp values were determined from the linear portion of the cumulative amount transported versus time plot. The Papp values of ACV, AACV, SACV, EACV, IACV and VACV were calculated at pH 7.4 (Table 2).
Table 2.
Permeability values of ACV, AACV, SACV, EACV, IACV, and VACV across Caco-2 cell monolayer.
| Permeability (106) (cm/sec) |
|
|---|---|
| ACV | 1.5 ± 0.2 |
| AACV | 2.04 ± 0.18 |
| SACV | 4.66 ± 0.37 |
| EACV | 1.56 ± 0.13 |
| IACV | 2.08 ± 0.11 |
| VACV | 4.43 ± 0.56 |
Values are mean ± S.D. (n = 3–6).
Metabolism studies
Caco-2 homogenate
The half-lives of the amino acid prodrugs SACV and IACV were calculated as 6.1 and 6.9 hrs, respectively, in comparison with 1.6 and 1.15 hrs for VACV and AACV, respectively. The amino acid ester prodrugs AACV and VACV are rapidly hydrolyzed after incubation with the homogenate as compared to other prodrugs (Table 3). EACV showed moderate hydrolysis in Caco-2 homogenate.
Table 3.
In vitro enzymatic stability of ACV prodrugs in Caco-2, rat plasma, intestine and liver homogenates.
| AACV | SACV | EACV | VACV | IACV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate (k* 103) | Half life (hrs) | Rate (k* 103) | Half life (hrs) | Rate (k* 103) | Half life (hrs) | Rate (k* 103) | Half life (hrs) | Rate (k* 103) | Half life (hrs) | |
| Caco2 | 10.1 ± 0.6 | 1.15 ± 0.07 | 2.1 ± 0.6 | 6.1 ± 2.2 | 2.82 ± 0.54 | 4.2 ± 0.7 | 11.9 ± 0.7 | 1.6 ± 0.15 | 1.8 ± 0.6 | 6.9 ± 1.9 |
| Plasma | 2.45 ± 0.35 | 4.8 ± 0.7 | 0.06 ± 0.02 | 195 ± 57 | 0.28 ± 0.04 | 42.4 ± 6.1 | 0.05 ± 0.02 | 226 ± 67 | 0.08 ± 0.006 | 139 ± 9.2 |
| Intestine | 105 ± 2.7 | 0.11 ± 0.002 | 5.7 ± 0.7 | 2.1 ± 0.2 | 1.42 ± 0.08 | 8.2 ± 0.5 | 18.5 ± 0.7 | 0.6 ± 0.02 | 8.8 ± 0.5 | 1.32 ± 0.07 |
| Liver | -- | a | 27 ± 3 | 0.44 ± 0.06 | 0.05 ± 0.002 | 223 ± 8.8 | 163 ± 9 | 0.07 ± 0.004 | 165 ± 0.9 | 0.07 ± 0.004 |
Values are mean ± S.D. (n = 3–6).
min−1.mg protein−1
No intact prodrug detected after 1 min.
Plasma
Amino acid ester prodrugs of ACV exhibited appreciable stability in plasma. VACV exhibited the highest half-life of 226 ± 67 hrs compared with other amino acid prodrugs. AACV rapidly hydrolyzed after incubation with plasma compared to other prodrugs. Among the amino acid prodrugs, SACV generated a half-life of 195 ± 57 hrs (Table 3). It was observed during the experiments that all the amino acid prodrugs (except AACV) showed intact prodrug followed by hydrolysis to ACV.
Intestinal homogenate
The prodrugs hydrolyzed to yield the parent drug ACV in intestinal homogenates. The half-lives of the amino acid prodrugs EACV and SACV were calculated as 8.2 and 2.1 hrs, respectively, in comparison with 1.3 hrs for IACV. The amino acid ester prodrugs VACV and AACV rapidly hydrolyzed to ACV (36 and 6 min, respectively) after incubation with the intestinal homogenate (Table 3).
Liver homogenate
The prodrugs were rapidly and completely hydrolyzed to yield the parent drug ACV in liver homogenates. EACV showed exceptional stability in liver homogenate compared to other prodrugs. The half-life of the amino acid prodrug EACV was calculated as 223 hrs, where as other remaining prodrugs were completely hydrolysed before 0.5 hrs. The amino acid ester prodrug AACV was rapidly hydrolyzed (no intact prodrug detected after 1 min) after incubation with the homogenate (Table 3).
In vivo oral absorption
Analyses of the metabolites after administration of amino acid prodrugs revealed that the prodrugs were rapidly cleaved to the parent drug, ACV. After administration of AACV, the intact AACV could not be detected at any time point, possibly due to extensive metabolism by intestine and/or liver. Remaining prodrugs showed intact prodrug for the entire duration of the experiment in very small amounts compared with the regenerated ACV.
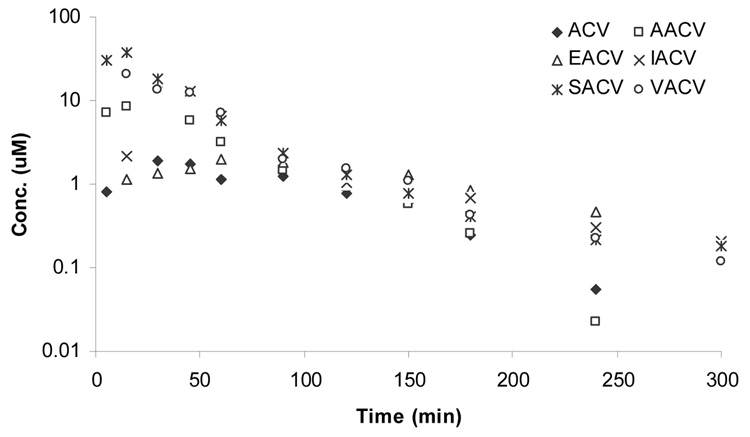
Total concentration
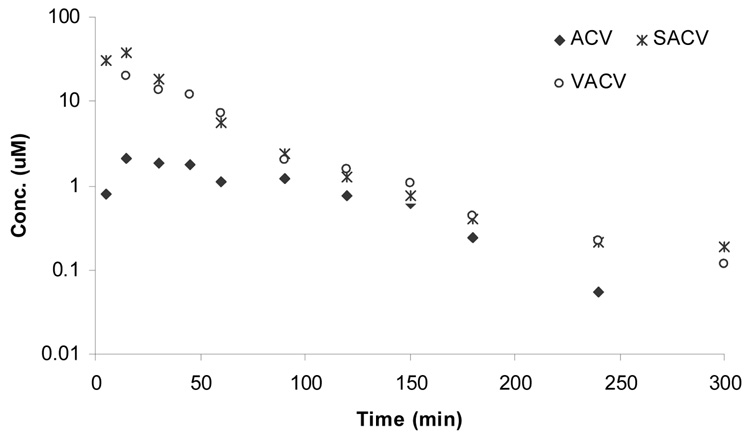
In vivo oral absorption profiles of all the prodrugs were analyzed by the total concentration (prodrug and regenerated ACV) (Figure 2) and by the individual profiles as intact prodrug (Figure 3) and regenerated ACV (Figure 4). In this section we will be discussing the total concentration profiles of ACV and its prodrugs. The systemic absorption of the drugs and prodrugs upon oral administration was determined by sampling from the jugular vein. The systemic absorption of total plasma-concentration of ACV vs time profiles of ACV, AACV, SACV, EACV, IACV and VACV are depicted in Figure 2. Pharmacokinetic parameters have been summarized in Table 4. Oral administration of amino acid prodrugs led to an increase in intestinal absorption of ACV compared with ACV alone. SACV and VACV led to approximately 5-fold elevation of area under the curve (AUCinf(T)) over ACV (Figure 5), whereas IACV and AACV increased AUCinf(T) by 3 and 2 folds, respectively. Cmax(T) values for total concentration of ACV after administration of ACV, AACV, SACV, EACV, IACV and VACV were observed to be 2.3 ± 0.3, 12.1 ± 1.8, 39 ± 22, 2.5 ± 0.1, 20 ± 5 and 22 ± 0.3 µM, respectively, with SACV exhibiting the highest maximum concentration (Cmax). Time to reach maximum concentration (Tmax(T)) for ACV, AACV, SACV, IACV and VACV did not vary significantly (p<0.05) except EACV. The mean residence time for total concentration of EACV [MRTlast(T)] was higher than that of ACV. The elimination rate constants of total concentration of ACV (λz(T)) for ACV, AACV, SACV, IACV, EACV, and VACV were calculated as 0.04 ± 0.006, 0.04 ± 0.004, 0.01 ± 0.002, 0.01 ± 0.002, 0.01 ± 0.00003 and 0.02 ± 0.002 min−1, respectively indicating ACV and AACV had high elimination rate and then followed was VACV. Clast(T) (concentration at the last time point) of SACV observed to be 0.18 ± 0.06 µM in plasma which is 2 times better than VACV and 3 times better than ACV.
Figure 2.
Plasma-concentration time profile of total concentration of ACV upon oral administration of (♦) ACV, (□) AACV, (*) SACV, (x) IACV, (∆) EACV, and (○) VACV.
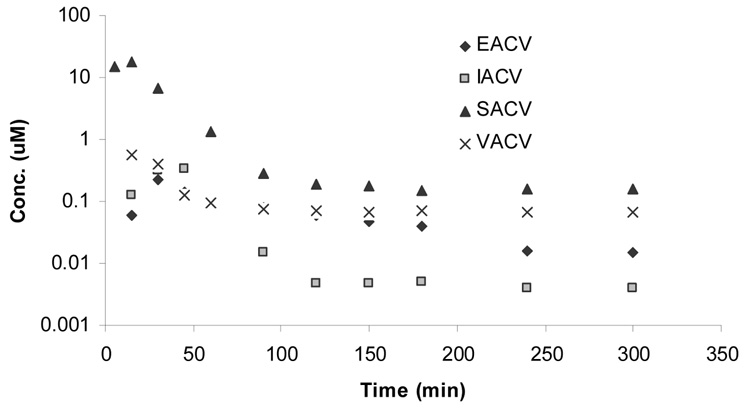
Figure 3.
Plasma-concentration time profile of intact prodrug upon oral administration of (▲) SACV, (■) IACV, (♦) EACV, and (x) VACV.
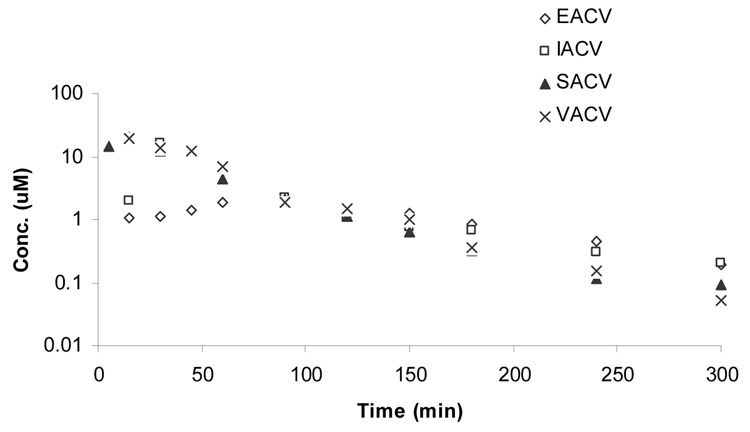
Figure 4.
Plasma-concentration time profile of regenerated ACV upon oral administration of (▲) SACV, (□) IACV, (◊) EACV, and (x) VACV.
Table 4.
Pharmacokinetic Parameters for systemic absorption of amino acid prodrugs of ACV. Values presented herewith are for total concentration.
| Parameter | ACV | AACV | SACV | IACV | EACV | VACV |
|---|---|---|---|---|---|---|
| λz (T) (1/min) | 0.04 ± 0.006 | 0.04 ± 0.004 | 0.01± 0.002 | 0.01 ± 0.002 | 0.01 ± 0.0003 | 0.02 ± 0.002 |
| Tmax (T) (min) | 19 ± 4 | 20 ± 8.6 | 14 ± 5 | 34 ± 4 | 80 ± 9 | 20 ± 4.3 |
| Cmax (T) (µM) | 2.3 ± 0.3 | 12.1 ± 1.8 | 39 ± 2 | 20 ± 5 | 2.5 ± 0.1 | 22 ± 0.3 |
| Clast (T) (µM) | 0.06 ± 0.04 | 0.03 ± 0.01 | 0.18 ± 0.06 | 0.2 ± 0.01 | 0.2 ± 0.02 | 0.12 ± 0.008 |
| AUCinf (T) (min*µM) | 204 ± 33 | 524 ± 28 | 1466 ± 173 | 815 ± 104 | 345 ± 40 | 1032 ± 130 |
| MRTlast (T) (min) | 72 ± 6.4 | 47 ± 3 | 51 ± 10 | 64 ± 2 | 115 ± 5 | 49 ± 2 |
Values are mean ± S.E. (n=4–6)
a control
p<0.05 compared to control.
T- total concentration in terms of ACV; MRT- mean residence time, AUC- area under curve, Cmax- maximum concentration, Tmax- time to reach maximum concentration, λZ- terminal elimination rate constant, Clast- concentration at the last time point.
Figure 5.
Plasma-concentration time profile of total concentration of ACV upon oral administration of (♦) ACV, (*) SACV, and (○) VACV.
Intact prodrug
Pharmacokinetic parameters of intact SACV, EACV, IACV and VACV upon their oral administration (Figure 3) have been listed in Table 5A. The systemic absorption of intact prodrug plasma-concentration time profiles of SACV, EACV, IACV and VACV are depicted in Figure 3. As mentioned above intact prodrug of AACV was not observed in plasma indicating extensive intestine/hepatic metabolism. Highest systemic exposure was obtained upon administration of SACV relative to EACV, IACV and VACV. The AUC obtained after oral administration (AUCinf(PD)) of SACV was approximately 25-fold higher relative to EACV, IACV and VACV administration. Cmax values for intact prodrug concentration (Cmax(PD)) after administration of SACV, EACV, IACV and VACV were observed to be 18 ± 12, 0.46 ± 0.15, 0.23 ± 0.06, and 0.56 ± 0.2 µM, respectively, with SACV exhibiting the highest Cmax value (p < 0.05). Clast of SACV (Clast(PD)) was observed to be 0.15 ± 0.01 µM in plasma which is 2 times better than VACV.
Table 5.
| Table 5A. Pharmacokinetic Parameters for systemic absorption of amino acid prodrugs of ACV. Values presented herewith are for intact prodrug. | ||||
|---|---|---|---|---|
| Parameter | SACV | IACV | EACV | VACV |
| Cmax (PD) (µM) | 18 ± 12 | 0.46 ± 0.15 | 0.23 ± 0.06 | 0.56 ± 0.2 |
| Clast (PD) (µM) | 0.15 ± 0.01 | 0.004 ± 0.0004 | 0.015 ± 0.0012 | 0.06 ± 0.0006 |
| AUClast (PD) (min*µM) | 547 ± 347 | 15 ± 4 | 17 ± 1 | 34 ± 6 |
| AUCinf (PD) (min*µM) | 573 ± 339 | 16 ± 4 | 18 ± 1 | 37 ± 6 |
| Table 5B. Pharmacokinetic Parameters for systemic absorption of amino acid prodrugs of ACV. Values presented herewith are for regenerated ACV from prodrug. | ||||
|---|---|---|---|---|
| Parameter | SACV | IACV | EACV | VACV |
| Cmax (ACV) (µM) | 21 ± 10 | 19 ± 5 | 2.3 ± 0.2 | 22 ± 0.5 |
| Clast (ACV) (µM) | 0.09 ± 0.02 | 0.2 ± 0.01 | 0.2 ± 0.03 | 0.05 ± 0.008 |
| AUClast (ACV) (min*µM) | 897 ± 321 | 779 ± 106 | 297 ± 45 | 991 ± 126 |
| AUCinf (ACV) (min*µM) | 903 ± 322 | 800 ± 103 | 313 ± 47 | 994 ± 126 |
Values are mean ± S.E. (n=4–6)
PD - oncentration of prodrug; AUC- area under curve, Cmax- maximum concentration, Clast- concentration at the last time point
Values are mean ± S.E. (n=4–6)
ACV - concentration of generated parent drug; AUC- area under curve, Cmax- maximum concentration, Clast- concentration at the last time point
Parent drug
Pharmacokinetic parameters of regenerated ACV upon oral administration of SACV, EACV, IACV and VACV (Figure 4) have been listed in Table 5B. In contrast to intact prodrug, AUC values of the regenerated ACV (AUCinf(ACV)) from SACV, IACV and VACV were similar. Lower systemic exposure of regenerated ACV was obtained upon administration of EACV relative to SACV, IACV and VACV. The AUC obtained after oral administration of EACV was approximately 3-fold lesser relative to SACV, IACV and VACV administration. Cmax values for regenerated ACV concentration (Cmax(ACV)) after administration of SACV, EACV, IACV and VACV were observed to be 21 ± 10, 2.3 ± 0.2, 19 ± 5, and 22 ± 0.5 µM, respectively, with EACV exhibiting the lowest Cmax value (p < 0.05). Clast (Clast(ACV)) value of EACV and IACV (0.2 ± 0.01 µM) was better than VACV and SACV and the reason may be these prodrugs degrade faster than VACV and SACV in plasma resulting in more regenerated ACV at the last time point.
DISCUSSION
The incidence of genital herpes infections caused by HSV-1 and 2 has increased significantly in the past 20 years (Fleming et al., 1997). Since the introduction of antiviral drugs in the early 1980s (Balfour, 1999), the management of genital herpes infections has improved considerably although it is not yet possible to cure herpes virus infections. Although genital herpes is self-limiting in healthy adults, the disease is painful and distressing, with severe psychosocial impact (Manne and Sandler, 1984; Goldmeier et al., 1988). Acyclovir was the first effective antiviral drug approved for use and is still extensively prescribed, particularly in the treatment of immunocompetent patients with genital HSV disease (Perry and Faulds, 1996). Although acyclovir is a well tolerated and effective antiviral drug, its bioavailability after oral administration is low. As a result, up to 5 times administration per day is often necessary for the management of genital HSV disease. In this study amino acid prodrugs of acyclovir were studied to improve the bioavailability of acyclovir thereby improving the therapy.
The amino acid ester prodrugs of ACV have been studied for their affinity toward amino acid and peptide transporters expressed in colon carcinoma cell line, Caco-2. These compounds (especially IACV) exhibited affinity toward various transporters by inhibiting various model substrates. In general terms, inhibition studies may not be a good predictor for the actual cellular transport of drug candidates, because the substrates might only bind to the transporter without being translocated by it. Hence, the affinity of these prodrugs may not be translated into transepithelial transport and oral delivery. Therefore, transport experiments with the amino acid prodrugs AACV, SACV, EACV, IACV, and VACV were carried out across Caco-2 monolayers at pH 7.4 (Table 2). The above argument was proved in case of IACV, despite exhibiting inhibition in uptake studies, the transport of IACV did not improve much. To delineate the mechanistic details further studies are needed.
Caco-2, plasma, intestinal and liver homogenate hydrolysis studies were carried out to evaluate the hydrolysis characteristics of ACV amino acid ester prodrugs. Hydrolysis kinetics of the prodrugs in Caco-2 cell suspensions indicated that these prodrugs can (AACV, VACV) undergo hydrolysis by enterocytic enzymes, which could limit their bioavailability upon oral administration. The rank order stability of the prodrugs (except EACV) seem to follow a trend as more enzymatically active tissues (liver) caused more hydrolysis compared to less active tissues (plasma). Intestine exhibited more hydrolysis of the prodrugs compared to Caco-2 homogenate confirming improved and varied enzymatic activity. In plasma, VACV and SACV were found to be the most stable compounds with a half-life of 195 ± 57 and 226 ± 67 hrs respectively. These in vitro studies further suggest that upon oral administration, hydrolysis of the prodrugs by intestinal/hepatic system will be extensive than by systemic circulation.
In liver, EACV was found to be the most stable compound with a half-life of 223 ± 8.8 hrs and AACV the least, because no intact prodrug was detected 1 min after the beginning of an experiment (Table 3). The less enzymatic liability of EACV can be explained by the lack of amino terminus near the ester bond (hydrolysis site) which is formed between γ-carboxy terminus of glutamic acid and hydroxyl group of ACV. Further studies were needed to confirm the importance of amino terminus in facilitating the enzymatic hydrolysis of the ester bond (Figure 1).
Oral absorption studies of ACV and the amino acid prodrugs AACV, SACV, EACV, IACV and VACV were carried out in Sprague-Dawley rats with cannulated jugular vein. After oral administration, AACV is rapidly and completely hydrolyzed by both intestinal and hepatic enzymes as no intact AACV could be detected in the systemic circulation. Upon oral administration of other prodrugs, intact prodrug was observed for the entire duration of the experiment in varying amounts. However these prodrugs, after being absorbed from the gastrointestinal tract, underwent extensive first pass intestinal and/or hepatic metabolism. This was evident by higher levels of ACV generated compared to intact prodrug in systemic circulation (Tables 5A and 5B).
AUCinf(T) values obtained after oral administration of the amino acid ester prodrugs of ACV were significantly higher (p<0.05) than ACV itself. This increase in bioavailability of ACV upon oral administration can be attributed to the interaction of these prodrugs with intestinal amino acid transporters (ASC and B0,+) and peptide transporter (PEPT1) that mediates their transport across intestinal epithelium to blood (Table 1). SACV and VACV yielded the highest AUCinf(T), which is at least 5-fold higher than ACV after systemic absorption (Figure 5). Cmax value of SACV was 2 times better than VACV and 15 times better than ACV. Clast(T) values obtained after administration of SACV, EACV and IACV are 3 times better than the Clast(T) value obtained after administration of ACV. The same is not the case with VACV which may be due to rapid metabolism of VACV to ACV (Table 3). As previously reported, there is high accumulation of ACV after the administration of VACV at the recommended dosage regimens of 250 mg, 500 mg, and 1 g of VALTREX (valacyclovir hydrochloride) administered four times daily for 11 days in volunteers with normal renal function (Weller et al., 1993).
Even though EACV did not result in appreciable increase in AUCinf(T) and Cmax(T) values, the notable difference in Tmax(T), Clast(T) and MRTlast(T) values can be attributed to slower absorption due to less lipophilic nature (Tmax(T)) and higher enzymatic stability (Clast(T), MRTlast(T)) of EACV compared to other prodrugs (Table 4, Figure 2). In our studies, the plasma elimination half-life of ACV after administration of ACV and AACV ranged from 15 to 20 min upon systemic absorption. The reason for similar elimination pattern for AACV and ACV was due to the fact that only regenerated ACV from AACV was observed in the plasma. The plasma elimination half-life of total ACV after administration of VACV ranged from 30 to 40 min whereas SACV, IACV and EACV had a half-life of 60 to 90 min. Higher elimination rate of VACV compared to SACV and EACV again can be attributed to the enzymatic liability of the drug.
One of the notable differences observed between intact prodrug profiles of prodrugs was Cmax (Cmax(PD)), Clast (Clast(PD)) and AUC (AUCinf(PD)) values of SACV were significantly higher compared to all other prodrugs (Table 5A, Figure 3). The reason can be the combination of factors given below. It had higher absorption (Cmax(PD)), higher stability and lower elimination rate of SACV (λ(T)). Even though EACV exhibits comparable elimination rate and higher stability than SACV, the limiting factor for lower AUC of EACV should be lower absorption (Cmax(PD)). This was again confirmed by the regenerated ACV profiles of prodrugs as AUC and Cmax values of all the prodrugs (AUCinf(ACV)) were similar except EACV (Table 5B, Figure 4).
IACV seem to possess affinity towards peptide transporter (PepT1) and also with various amino acid transporters (Table 1). Interestingly SACV also seem to interact with peptide transporter (PepT1) on Caco-2 cells. In terms of enzymatic stability, the prodrugs can be ranked in a descending order as EACV>>SACV>IACV>VACV>AACV and in lipophilicity scale the compounds can be ranked as IACV>VACV>AACV>SACV>EACV. Besides stability and lipophilicity, ionization state of the prodrug also can limit the permeability/bioavailability of a prodrug as reported with VACV (Balimane and Sinko, 2000). Further studies need to be performed to confirm the effect of ionization on the permeability of other prodrugs. AACV being a highly unstable and EACV being highly hydrophilic seem to limit their bioavailability. So the improved bioavailability of other prodrugs may be the result of interplay of the factors given above.
In conclusion, oral administration of amino acid ester prodrugs of ACV led to an increase in systemic absorption of ACV compared with direct administration of ACV. There seems to be a very good correlation between the permeability of these prodrugs across Caco-2 cell monolayer and AUC values after oral administration confirming that Caco-2 cell line can be a very good screening model to predict the oral absorption of amino acid ester prodrugs (Figure 6). The amino acid prodrugs of ACV except EACV are rapidly metabolized to the regenerated ACV due to intestinal/hepatic first pass effect. Despite their rapid metabolism, the amino acid prodrugs are efficiently absorbed by the intestinal amino acid and peptide transporters, leading to an increase in intestinal absorption of ACV relative to ACV administered as such. High Clast value of SACV can improve the therapy by maintaining higher systemic concentrations of drug for longer periods thereby requiring lower dosage or lower frequency of dosing. Therefore, the amino acid prodrugs of ACV particularly SACV owing to its enhanced stability, enhanced AUC and high concentration at last time point (Clast) seems to be a promising candidate for the treatment of oral herpes infections.
Figure 6.
Correlation between apparent permeability (Papp) across Caco-2 cells and area under the curve (AUCinf(T)) obtained after oral administration of ACV and its amino acid ester prodrugs. Errors bars represent S.D (n = 6).
Acknowledgments
We would like to acknowledge Dr. Yasser E. Nashed and Dr. Zhu Xiaodong for synthesizing the amino acid prodrugs of acyclovir. We would like to thank GlaxoSmithKline for the generous supply of valacyclovir. This work was supported by NIH grants RO1 EY09171 and RO1 EY10659.
Supported in part by US Public Health Service grants R01EY09171 and R01EY10659 (AKM).
Abbreviations
- ASCT1
Na+ dependent neutral amino acid transporter
- B0,+
Na+ dependent neutral and cationic amino acid transporter
- LAT
Large neutral amino acid transporter
- hPEPT1
human intestinal peptide transporter
- ACV
acyclovir
- VACV
valine-acyclovir
- SACV
serine-acyclovir
- AACV
alanine-acyclovir
- EACV
γ-glutamate-acyclovir
- IACV
isoleucine-acyclovir
- DPBS
Dulbecco’s phosphate-buffered saline
- AUC
area under the curve
- CL/F
clearance
- MRT
mean residence time
- Papp
apparent permeability
- HSV
herpes simplex virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anand BS, Katragadda S, Mitra AK. Pharmacokinetics of novel dipeptide ester prodrugs of acyclovir after oral administration: intestinal absorption and liver metabolism. J Pharmacol Exp Ther. 2004a;311:659–667. doi: 10.1124/jpet.104.069997. [DOI] [PubMed] [Google Scholar]
- Anand BS, Katragadda S, Nashed YE, Mitra AK. Amino acid prodrugs of acyclovir as possible antiviral agents against ocular HSV-1 infections: interactions with the neutral and cationic amino acid transporter on the corneal epithelium. Curr Eye Res. 2004b;29:153–166. doi: 10.1080/02713680490504614. [DOI] [PubMed] [Google Scholar]
- Balfour HH., Jr Antiviral drugs. N Engl J Med. 1999;340:1255–1268. doi: 10.1056/NEJM199904223401608. [DOI] [PubMed] [Google Scholar]
- Balimane P, Sinko P. Effect of ionization on the variable uptake of valacyclovir via the human intestinal peptide transporter (hPepT1) in CHO cells. Biopharm Drug Dispos. 2000;21:165–174. doi: 10.1002/1099-081x(200007)21:5<165::aid-bdd225>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Blondeau JP, Beslin A, Chantoux F, Francon J. Triiodothyronine is a high-affinity inhibitor of amino acid transport system L1 in cultured astrocytes. J Neurochem. 1993;60:1407–1413. doi: 10.1111/j.1471-4159.1993.tb03302.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- Dantzig AH, Bergin L. Uptake of the cephalosporin, cephalexin, by a dipeptide transport carrier in the human intestinal cell line, Caco-2. Biochim Biophys Acta. 1990;1027:211–217. doi: 10.1016/0005-2736(90)90309-c. [DOI] [PubMed] [Google Scholar]
- Enerson BE, Drewes LR. Molecular features, regulation, and function of monocarboxylate transporters: implications for drug delivery. J Pharm Sci. 2003;92:1531–1544. doi: 10.1002/jps.10389. [DOI] [PubMed] [Google Scholar]
- Fleming DT, McQuillan GM, Johnson RE, Nahmias AJ, Aral SO, Lee FK, St Louis ME. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- Frenhani PB, Burini RC. Mechanisms of absorption of amino acids and oligopeptides. Control and implications in human diet therapy. Arq Gastroenterol. 1999;36:227–237. doi: 10.1590/s0004-28031999000400011. [DOI] [PubMed] [Google Scholar]
- Ganapathy ME, Brandsch M, Prasad PD, Ganapathy V, Leibach FH. Differential recognition of beta -lactam antibiotics by intestinal and renal peptide transporters, PEPT 1 and PEPT 2. J Biol Chem. 1995;270:25672–25677. doi: 10.1074/jbc.270.43.25672. [DOI] [PubMed] [Google Scholar]
- Ganapathy ME, Huang W, Wang H, Ganapathy V, Leibach FH. Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun. 1998;246:470–475. doi: 10.1006/bbrc.1998.8628. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Leibach FH. Peptide transport in intestinal and renal brush border membrane vesicles. Life Sci. 1982;30:2137–2146. doi: 10.1016/0024-3205(82)90287-9. [DOI] [PubMed] [Google Scholar]
- Goldenberg GJ, Lam HY, Begleiter A. Active carrier-mediated transport of melphalan by two separate amino acid transport systems in LPC-1 plasmacytoma cells in vitro. J Biol Chem. 1979;254:1057–1064. [PubMed] [Google Scholar]
- Goldmeier D, Johnson A, Byrne M, Barton S. Psychosocial implications of recurrent genital herpes simplex virus infection. Genitourin Med. 1988;64:327–330. doi: 10.1136/sti.64.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, de Vrueh RL, Rhie JK, Covitz KM, Smith PL, Lee CP, Oh DM, Sadee W, Amidon GL. 5'-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm Res. 1998;15:1154–1159. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Haramura M, Fei YJ, Miyauchi S, Bridges CC, Ganapathy PS, Smith SB, Ganapathy V, Ganapathy ME. Transport of amino acid-based prodrugs by the Na+- and Cl(−) -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2004;308:1138–1147. doi: 10.1124/jpet.103.057109. [DOI] [PubMed] [Google Scholar]
- Hidalgo IJ, Bhatnagar P, Lee CP, Miller J, Cucullino G, Smith PL. Structural requirements for interaction with the oligopeptide transporter in Caco-2 cells. Pharm Res. 1995;12:317–319. doi: 10.1023/a:1016259816661. [DOI] [PubMed] [Google Scholar]
- Hu M, Zheng L, Chen J, Liu L, Zhu Y, Dantzig AH, Stratford RE., Jr Mechanisms of transport of quinapril in Caco-2 cell monolayers: comparison with cephalexin. Pharm Res. 1995;12:1120–1125. doi: 10.1023/a:1016247523311. [DOI] [PubMed] [Google Scholar]
- Katragadda S, Xiadong Z, Talluri RS, Mitra AK. Small neutral amino acid ester prodrugs of acyclovir targeting amino acid transporters on the cornea: possible antiviral agents against ocular HSV-1 infections. Manuscript submitted. 2008 [PMC free article] [PubMed] [Google Scholar]
- Lack L. Properties and biological significance of the ileal bile salt transport system. Environ Health Perspect. 1979;33:79–90. doi: 10.1289/ehp.793379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan M, Goncalves E, Lessly G, Foti D, Robbins J. The transport of thyroxine into mouse neuroblastoma cells, NB41A3: the effect of L-system amino acids. Endocrinology. 1990;126:3245–3250. doi: 10.1210/endo-126-6-3245. [DOI] [PubMed] [Google Scholar]
- Liang R, Fei YJ, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem. 1995;270:6456–6463. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- Manne S, Sandler I. Coping and adjustment to genital herpes. J Behav Med. 1984;7:391–410. doi: 10.1007/BF00845272. [DOI] [PubMed] [Google Scholar]
- Perry CM, Faulds D. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs. 1996;52:754–772. doi: 10.2165/00003495-199652050-00009. [DOI] [PubMed] [Google Scholar]
- Purifoy DJ, Beauchamp LM, de Miranda P, Ertl P, Lacey S, Roberts G, Rahim SG, Darby G, Krenitsky TA, Powell KL. Review of research leading to new anti-herpesvirus agents in clinical development: valaciclovir hydrochloride (256U, the L-valyl ester of acyclovir) and 882C, a specific agent for varicella zoster virus. J Med Virol. 1993 Suppl 1:139–145. doi: 10.1002/jmv.1890410527. [DOI] [PubMed] [Google Scholar]
- Said HM, Mohammed ZM. Intestinal absorption of water-soluble vitamins: an update. Curr Opin Gastroenterol. 2006;22:140–146. doi: 10.1097/01.mog.0000203870.22706.52. [DOI] [PubMed] [Google Scholar]
- Steffansen B, Nielsen CU, Brodin B, Eriksson AH, Andersen R, Frokjaer S. Intestinal solute carriers: an overview of trends and strategies for improving oral drug absorption. Eur J Pharm Sci. 2004;21:3–16. doi: 10.1016/j.ejps.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Steingrimsdottir H, Gruber A, Palm C, Grimfors G, Kalin M, Eksborg S. Bioavailability of aciclovir after oral administration of aciclovir and its prodrug valaciclovir to patients with leukopenia after chemotherapy. Antimicrob Agents Chemother. 2000;44:207–209. doi: 10.1128/aac.44.1.207-209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TZ, Lunney E, Campbell G, Oxender DL. Transport of gabapentin, a gamma-amino acid drug, by system l alpha-amino acid transporters: a comparative study in astrocytes, synaptosomes, and CHO cells. J Neurochem. 1995;64:2125–2131. doi: 10.1046/j.1471-4159.1995.64052125.x. [DOI] [PubMed] [Google Scholar]
- Tolle-Sander S, Lentz KA, Maeda DY, Coop A, Polli JE. Increased acyclovir oral bioavailability via a bile acid conjugate. Mol Pharm. 2004;1:40–48. doi: 10.1021/mp034010t. [DOI] [PubMed] [Google Scholar]
- Tsuji A, Tamai I. Carrier-mediated intestinal transport of drugs. Pharm Res. 1996;13:963–977. doi: 10.1023/a:1016086003070. [DOI] [PubMed] [Google Scholar]
- Weller S, Blum MR, Doucette M, Burnette T, Cederberg DM, de Miranda P, Smiley ML. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin Pharmacol Ther. 1993;54:595–605. doi: 10.1038/clpt.1993.196. [DOI] [PubMed] [Google Scholar]