Abstract
The Frz chemosensory system is a two-component signal transduction pathway that controls cell reversals and directional movements for the two motility systems in Myxococcus xanthus. To trigger cell reversals, FrzE, a hybrid CheA-CheY fusion protein, autophosphorylates the kinase domain at His-49 and phosphoryl groups are transferred to aspartate residues (Asp-52 and Asp-220) in the two receiver domains of FrzZ, a dual CheY-like protein that serves as the pathway output. The role of the receiver domain of FrzE was unknown. In this paper, we characterize the FrzE protein in vitro and show that the receiver domain of FrzE negatively regulates the autophosphorylation activity of the kinase domain of FrzE. Unexpectedly, it does not appear to play a direct role in phospho-relay as in most other histidine kinase-receiver domain hybrid systems. The regulatory role of the FrzE receiver domain suggests that it may interact with or be phosphorylated by an unknown protein. We also show the dynamics of motility system specific marker proteins in FrzE mutants as cells move forward and reverse. Our studies indicate that the two motility systems are functionally coordinated and that any system specific branching to the pathway most likely occurs downstream of FrzE.
Introduction
Myxococcus xanthus is a rod-shaped Gram negative bacterium that has a complex life cycle that includes vegetative swarming and fruiting body formation. Both of these behaviors require controlled movement on solid surfaces propelled by gliding motility. M. xanthus utilizes two different motility systems to move on surfaces (Hodgkin and Kaiser, 1979a; Hodgkin and Kaiser, 1979b; Zusman et al., 2007). Social motility, or S-motility, utilizes type IV pili that extend from the leading cell pole, attach to the surface and retract, pulling cells toward the point of attachment (Li et al., 2003). This process has been directly observed in Pseudomonas aeruginosa (Skerker and Berg, 2001). The second form of motility is adventurous motility, or A-motility. The mechanism of A-motility is not well understood, but has been hypothesized to involve motor proteins that move on cytoskeletal filaments and focal adhesion complexes located along the length of the cell body or possibly powered by slime extrusion from nozzle-like structures (Mignot, 2007; Mignot et al., 2007b; Sliusarenko et al., 2007; Wolgemuth et al., 2002). M. xanthus cells maintain directed movements by controlling the frequency of cell reversals and their bias (Zusman et al., 2007). Cellular reversal frequency is affected by environmental stimuli such as attractants, repellents and cell density (Kearns and Shimkets, 1998; McBride et al., 1992; Shi et al., 1996). During a reversal, the old lagging pole becomes the new leading pole, thus the machinery for both motility systems must be rapidly inverted in order for pili to extend and retract from the new pole and for the adventurous motility components to cause a change in direction (Mauriello and Zusman, 2007)
The Frz signal transduction system is a chemotaxis pathway that controls cellular reversal frequency in both the A- and the S-motility systems (Blackhart and Zusman, 1985; Bustamante et al., 2004; Zusman, 1982). For example, loss-of-function Frz mutants rarely reverse and gain-of-function Frz mutants hyper-reverse. These Frz mutants are unable to undergo normal group behaviors, such as fruiting body development during starvation or colony swarming. The frz genes encode proteins with domains that are homologous to chemotaxis proteins and are modeled to show similar activities and interactions. Chemotaxis signaling is a specialized form of two-component signal transduction, for a review see (Stock et al., 2000). In enteric bacteria, flagellar clockwise rotation (tumbling) is stimulated by membrane bound methyl-accepting chemotaxis protein (MCP) receptors stimulating a histidine protein kinase, CheA, to autophosphorylate on a conserved histidine residue, a reaction that requires an interaction with a coupling protein, CheW. The phosphoryl group is subsequently transferred to a response regulator, CheY, on a conserved aspartate residue. Phospho-CheY then interacts with the flagellar motor switch to cause clockwise flagellar rotation. CheY-P is dephosphorylated by CheZ, decreasing the intrinsic aspartyl-phosphate half-life and allowing the motor switch to rapidly restore counterclockwise flagellar rotation. CheR and CheB, a methyltransferase and methylesterase respectively, function in adaptation (Baker et al., 2006). M. xanthus does not produce flagella but it has a similar chemosensory system to control cellular reversals, the Frz system. FrzCD is a cytoplasmic receptor that interacts with FrzE, a CheA-CheY fusion protein. In the presence of FrzA, a CheW homologue, FrzCD stimulates FrzE autophosphorylation and transfer of the phosphoryl group from the CheA domain of FrzE (H49) to FrzZ (D52 and D220), a CheY-CheY fusion protein (Inclan et al., 2007). FrzF and FrzG are homologues of a methyltransferase and methylesterase encoded in the Frz operon (McBride and Zusman, 1993). The Frz system does not contain a CheZ homologue.
Chemosensory signal transduction in M. xanthus is unusually complex as cells contain two very different motility systems, 23 MCPs, eight chemosensory systems, and 137 sensor and hybrid histidine kinases (Goldman et al., 2006; Zusman et al., 2007). For example, the Frz chemosensory system contains three CheY-like receiver domains (one in FrzE and two in FrzZ) that regulate the behavior of the two motility systems. We showed previously that the two receiver domains of FrzZ are essential for Frz signaling to both the A- and S-motility systems but the function of the FrzE receiver domain was unclear (Inclan et al., 2007). Mutants containing Asp-to-Ala or -Glu mutations on the highly conserved aspartate-709 residue in the receiver domain of FrzE showed divergent reversal frequency phenotypes when analyzed on A- or S-motility favored surfaces (Li et al., 2005). Since this receiver domain appears to play a significant role in reversal frequency regulation, we undertook an in vitro study of its role in the Frz pathway. In this paper we show that the receiver domain of FrzE plays an inhibitory role on the autophosphorylation activity of the kinase domain of FrzE. We hypothesize that this regulation controls downstream phosphosignaling to FrzZ and thus affects cellular reversal frequency. Additionally, since marker proteins from the A- and S- motility systems localize normally in FrzE receiver domain mutants, the two motility systems must be functionally coordinated and FrzE most likely does not regulate the two motility systems independently in a branched signaling pathway, as previously hypothesized.
Results
FrzE autophosphorylation is inhibited in vitro
FrzE is a hybrid protein consisting of a CheA histidine kinase and a CheY receiver domain. To determine the role of the receiver domain, FrzE and mutant forms of FrzE (see Fig. 1A) were cloned and purified from Escherichia coli and tested in an in vitro phosphorylation assay using purified recombinant FrzE, FrzCD, FrzA and [γ-32P]-ATP as previously described (Inclan et al., 2007). Figure 1B shows that under the conditions used, no autophosphorylation was observed for the full length FrzE (Fig. 1B, lane2); however, FrzE protein containing only the CheA domain of FrzE (FrzECheA) showed a radioactive band at the expected molecular weight (Fig. 1B lane1). To identify the site of phosphorylation, we searched sequence alignments with other CheA proteins; these alignments identified His-49 as the predicted site for FrzECheA autophosphorylation. We therefore constructed a mutant version of FrzECheA where His-49 was replaced with alanine (FrzECheA H49A). FrzECheA H49A did not autophosphorylate (Fig. 1C lane2), confirming His-49 as the site of phosphorylation for FrzECheA.
Figure 1.
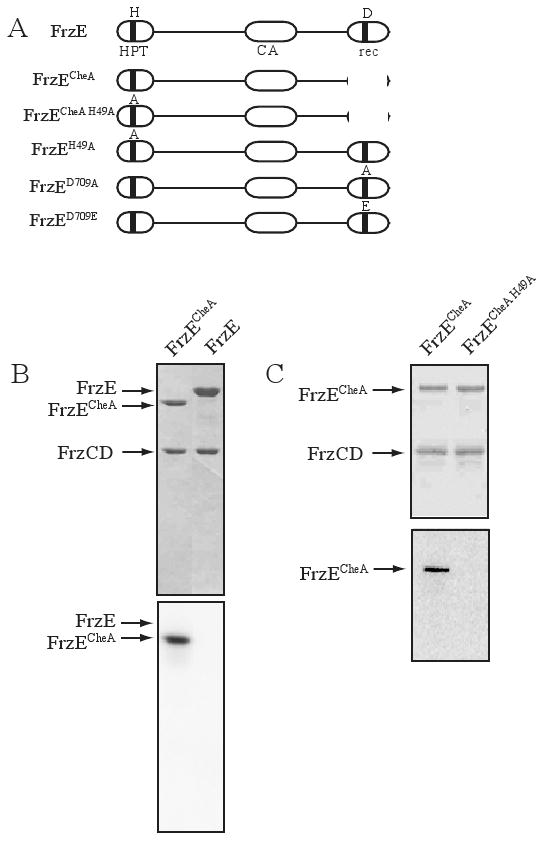
FrzECheA in vitro autophosphorylation. (A) A schematic of the domain structure of FrzE and mutants constructed. HPT refers to the histidine phospho-transfer domain and CA, refers to the catalytic domain. The conserved residues H-49 and D-709 are indicated. (B) A Coomassie stained gel above and a phosphorimage below of an SDS-PAGE gel containing FrzCD, FrzA, FrzECheA (lane1), FrzE (lane 2), and [γ-32P]-ATP. (C) A Coomassie stained gel above and a phosphorimage below of an SDS-PAGE gel containing FrzCD, FrzA, FrzECheA (lane1), FrzECheA H49A (lane2), and [γ-32P]-ATP. Note: FrzA is a small protein that has migrated off all gels in (B) and (C).
The FrzE receiver domain inhibits autophosphorylation of the FrzE kinase domain
In reviewing the results from Figure 1, we were concerned that hybrid CheA-CheY fusion proteins may not appear to phosphorylate in vitro if the CheA domain autophosphorylates slowly and the phosphoryl group is transferred rapidly to the CheY domain and is then hydrolyzed. This sequence of events has been shown to occur with RodK, a hybrid CheA-CheY protein from M. xanthus containing three CheY domains (Rasmussen et al., 2006). To address this possibility, we used an enzyme-coupled assay that indirectly reports autophosphorylation in real time. In this assay, when phosphorylation occurs, ATP is converted to ADP. The conversion of ADP to ATP is coupled to the conversion of phosphoenolpyruvate to pyruvate to lactate, by pyruvate kinase and lactate dehydrogenase respectively. As a byproduct, NADH is converted to NAD+. NADH absorbs strongly at 340 nm and the oxidation of NADH results in an absorbance decrease at 340 nm (Kiianitsa et al., 2002; Kreuzer and Jongeneel, 1983). Figure 2 shows that in the presence of full length FrzE or without the addition of enzyme, the absorbance at 340 nm remained unchanged with time. In the presence of FrzECheA, the absorbance steadily decreased with time, indicating the hydrolysis of ATP coupled to FrzECheA autophosphorylation. We can therefore rule out rapid hydrolysis as a reason for not observing a radiolabeled species of FrzE and conclude that full length FrzE does not autophosphorylate in vitro under our assay conditions.
Figure 2.
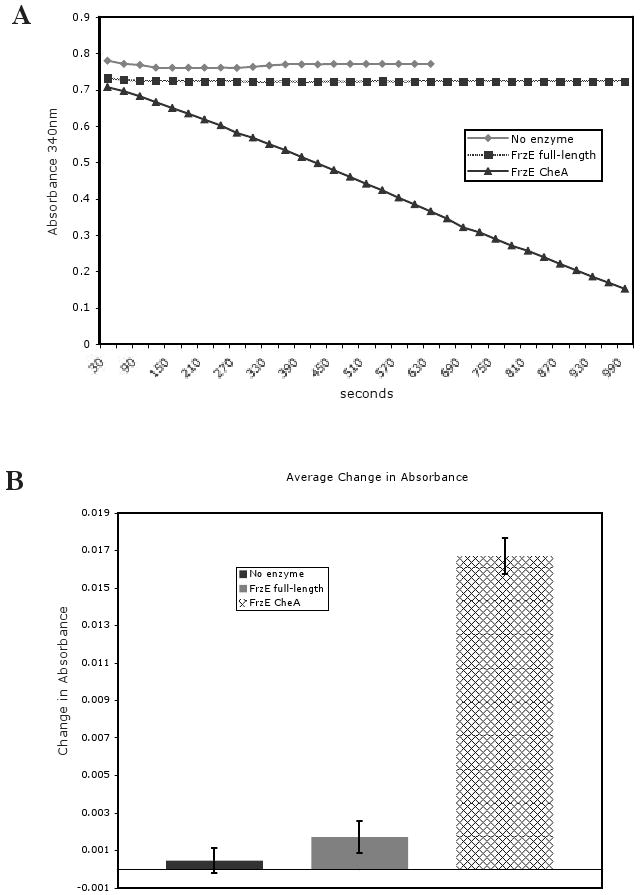
FrzE full-length does not autophosphorylate. (A) The plot shows the results of a single enzyme-coupled assay experiment. A decrease in absorbance indicates ATP hydrolysis. No enzyme refers to no FrzE protein present. (B) Statistics from multiple enzyme-coupled assay experiments. The bar graph indicates the average change in absorbance at 340 nm over 30 seconds. Error bars represent standard error of the mean.
If the CheY domain of FrzE inhibits autophosphorylation by the CheA domain of FrzE, we hypothesized that mutating the CheY receiver domain may relieve this inhibitory activity. We therefore constructed mutant forms of FrzE in which the conserved Asp-709 was changed to alanine, FrzED709A, or glutamate, FrzED709E. Alanine and glutamate substitutions should alter the charge and size of the original aspartate residue. Figure 3 shows that both mutant forms of FrzE, FrzED709A and FrzED709E, showed autophosphorylation in vitro. Notably, the autophosphorylation rates of the FrzECheA, FrzED709A, or FrzED709E did not differ significantly (data not shown), indicating that rate dependent Frz signaling is most likely not a significant factor in the regulation of autophosphorylation. These mutant forms of FrzE were also able to donate a phosphoryl group to FrzZ, the downstream target for FrzECheA phospho-transfer. These results show that the original aspartate containing form of the receiver domain of FrzE inhibits autophosphorylation of FrzECheA. Thus, the receiver domain of FrzE plays a regulatory role, such that the conformation of the receiver domain most likely controls the ability of FrzE to autophosphorylate and subsequently transfer a phosphoryl group to FrzZ.
Figure 3.
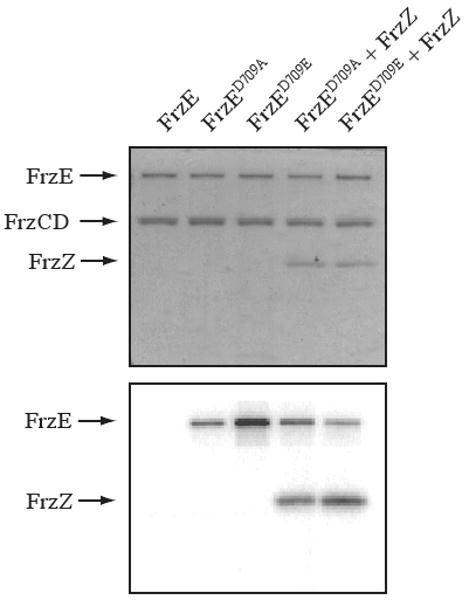
Mutating the putative phosphorylation site Asp-709 relieves the inhibition on the CheA domain of FrzE. (Above) A Coomassie stained SDS-PAGE gel containing [γ32P]-ATP. All lanes contain FrzCD and FrzA. In addition, lane 1 contains FrzE, lane 2 contains FrzED709A, lane 3 contains FrzED709E, lane 4 contains FrzED709A and FrzZ, and lane 5 contains FrzED709E and FrzZ. (Below) A phosphorimage of an identical gel as above.
The receiver domain of FrzE does not accept a phosphate from phospho-FrzECheA when added in trans
Since the receiver domain in full length FrzE inhibits autophosphorylation, we wanted to test whether the receiver domain could inhibit FrzECheA autophosphorylation when added separately, in trans. We therefore incubated FrzECheA, FrzCD, FrzA and [γ-32P]-ATP with increasing amounts of FrzECheY and measured FrzECheA autophosporylation. We observed no decrease in band intensity of phospho-FrzECheA as a result of FrzECheY addition (Fig. 4 and data not shown). Furthermore, although FrzECheA autophosphorylated in vitro, we did not observe a radioactive species at the FrzECheY migration point, indicating that there was no phosphotransfer to FrzECheY under these conditions even though the receiver domain of FrzE contains the conserved residues usually associated with phosphorylation. In contrast, phosphotransfer to FrzZ was observed, which served as a positive control (Fig. 4, lane 5). Thus, in trans, FrzECheY does not inhibit autophosphorylation of FrzECheA, nor does phosphotransfer occur to FrzECheY.
Figure 4.

The CheY domain of FrzE does not accept phosphate from phospho-FrzECheA. (Above) A Coomassie stained SDS-PAGE gel containing [γ32P]-ATP. All lanes contain FrzECheA, varying amounts of FrzECheY, FrzCD, and FrzA. In addition, lane 5 contains FrzZ. (Below) A phosphorimage of a gel identical to that above.
The receiver domain of FrzE accepts a phosphoryl group from phospho-FrzECheA
Although full length FrzE does not autophosphorylate in vitro and FrzE phosphotransfer does not occur between FrzECheA and FrzECheY, we were interested in investigating whether the receiver domain of FrzE can accept a phosphoryl group from phospho-FrzECheA. We therefore incubated FrzECheA with [γ-32P]-ATP for 10 min to allow autophosphorylation and then added FrzE to the reaction: after 1, 2, 4, and 10 min, samples were removed, the reaction stopped, and the samples analyzed. Figure 5 shows that FrzECheA was phosphorylated at 10 min, but that the level of radioactivity was greatly reduced when full length FrzE was added. Significantly, Figure 5 (lane 4) also shows a very faint band at the migration site of full length FrzE with increasing intensity at lanes 5 and 6 (see Fig. 5B). These results suggest that phospho-FrzECheA is donating a phosphoryl group to the receiver domain of FrzE and that it is subsequently hydrolyzed, which is why we do not see a strong signal at the FrzE migration point. It is also possible that FrzECheA can (trans-) heterophosphorylate FrzE at H-49. Autophosphorylation occurs in a homodimer of CheA histidine kinases such that monomer 1 phosphorylates monomer 2, thus heterophosphorylation occurs similarly in a heterodimer. As a control, to show that the phosphate was donated specifically by phospho-FrzECheA and not by the CheA domain of full length FrzE, and to eliminate the possibility of heterophosphorylation, we constructed a mutant form of FrzE in which the conserved His-49 was changed to alanine, FrzEH49A, and incubated this altered full length FrzE with FrzECheA. Figure 5 (lanes 7-10) shows that FrzEH49A behaved like FrzE and reduced FrzECheA phosphorylation, supporting our hypothesis that phospho-FrzECheA is donating a phosphoryl group to the receiver domain of FrzE and that it is subsequently hydrolyzed. It has been reported that aspartyl-phosphate bonds can be very labile with half-lives on receiver domains varying from seconds (Hess et al., 1988), to minutes (Goudreau et al., 1998; Makino et al., 1989; Weiss and Magasanik, 1988), to hours (Igo et al., 1989), so it is not surprising that little radioactive signal was captured in the receiver domain of FrzE.
Figure 5.

Phosphotransfer from phospho-FrzECheA to the receiver domain of FrzE. (A) Above, a Coomassie stained SDS-PAGE gel containing [γ32P]-ATP. All lanes contain FrzCD and FrzA. In addition, lane 1 contains FrzECheA incubated for 10 min., lane 2 FrzECheA, 20 min., lanes 3-6 contain phospho-FrzECheA and FrzE for 1min., 2 min., 4 min., and 10 min., lanes 7-10 contain phospho-FrzECheA and FrzEH49A for 1min., 2min., 4 min., and 10 min. Below, a phosphorimage of an identical gel as above. (B) Line graph of the relative intensity of full length FrzE (solid line) and FrzEH49A (dotted line) versus reaction time from lanes 3-10 of the phosphorimage in part A.
His-49 of FrzE is essential for Frz signaling
Since M. xanthus contains eight CheA homologues in chemosensory gene clusters as well as >100 additional sensor and hybrid histidine kinases (Goldman et al., 2006), it is plausible that in vivo FrzE could heterodimerize with another histidine kinase homologue and that this alternative histidine kinase could also play the role of a phosphodonor to FrzZ or other downstream receiver domain proteins. For example, Rhodobacter sphaeroides, which utilizes multiple motility systems and chemotaxis pathways, regulates downstream chemotaxis signaling via heterophosphorylation of multiple CheA proteins (Porter and Armitage, 2004). We hypothesized that if heterophosphorylation was occurring in vivo, the phenotype of a frzEH49A mutant would not display the same phenotype as a frz loss of function mutant such as ΔfrzE. However, if frzEH49A phenocopies the deletion mutant, then FrzE must be the sole phosphodonor to the Frz pathway. To explore these hypotheses, we introduced the frzEH49A mutation into M. xanthus at the endogenous locus. Figure 6A shows that cells containing the frzEH49A mutation produced a stable FrzEH49A protein, as determined from Western immunoblots. Figures 6B and C show that the frzEH49A mutant exhibited a phenotype identical to the ΔfrzE mutant: frizzy filaments during starvation conditions and decreased colony swarming in nutrient rich conditions. These results support the hypothesis that phosphorylation at H-49 of FrzE is required for Frz signaling.
Figure 6.

His-49 is necessary for Frz signaling in vivo. (A) Western blot of whole-cell extracts from wild-type, ΔfrzE, and frzEH49A cells incubated with anti FrzE serum. (B) Colony images of wild-type, ΔfrzE and frzEH49A in starvation conditions. The scale bar represents 0.2 cm. (C) Colony diameters were measured after three days on nutrient rich CYE plates containing either 0.3% agar (grey) or 1.5% agar (black). Error bars indicate standard error of the mean.
FrzECheY mutants retain normal coordination of A- and S- motility
Previous work showed that mutant forms of FrzECheY display opposing (hypo or hyper) reversal frequencies when cells move on surfaces that favor A- or S- motility (Inclan et al., 2007; Li et al., 2005). These experiments suggested that FrzECheY differentially controlled the two motility systems. However, since these experiments were performed under very different conditions (1.5% agar for A-motility versus 1% methyl cellulose on glass microscope slides for S-motility), we were concerned that the results may be subject to uncontrolled variables. Unfortunately, it is difficult to observe and follow reversal frequencies of A- and S- powered movement simultaneously with current techniques, especially since S-motility requires cell-cell contact and isolated S-motile cells are non-motile on agar surfaces (Kaiser and Crosby, 1983; Shi and Zusman, 1993). To circumvent this problem, we used A- and S- marker proteins as reporters of signaling to the two motility systems. The first reporter protein, FrzS, has been shown to be exclusively involved in S-motility (Ward et al., 2000). For example, during S-motility, fluorescently labeled FrzS shows dynamic pole-to pole localization such that the brighter focus of FrzS is found at the leading cell pole, but upon cell reversals, the brighter focus shifts to the new leading pole (Mignot et al., 2005). FrzS mutants defective in the N-terminal receiver domain do not localize normally (Mignot et al., 2007a). In a similar manner, the second reporter protein, AglZ, has been shown to be exclusively involved in A-motility (Yang, Bartle et al., 2004). Fluorescently labeled AglZ in moving cells is localized in an array of clusters, with the leading cell pole containing the brightest focus; upon cell reversals, when the lagging pole becomes the leading pole, the bright focus shifts to the new leading pole (Mignot et al., 2007b). Thus, the dynamics of AglZ localization are coordinated with reversals of the A-engine.
We obtained or constructed strains that contained FrzS labeled with green fluorescent protein (FrzS-GFP) or AglZ labeled with yellow fluorescent protein (AglZ-YFP) in wild type, frzEΔcheY, frzED709A, and frzED709E mutant backgrounds. The reporter frzS-GFP and aglZ-YFP alleles did not affect motility or colony phenotypes when compared to the parental strains (data not shown). Because of the availability of strains for this study, the frzEΔcheY strain was in the DZ2 wild-type background and the frzED709A, and frzED709E strains were in the DK1622 wild-type background. Previous work showed that the Frz phenotype is consistent in both of these backgrounds as these strains are very closely related (Bustamante et al., 2004; Li et al., 2005).
We observed FrzS-GFP and AglZ-YFP dynamics in moving cells in the wild-type, frzEΔcheY, frzED709A, and frzED709E backgrounds using fluorescence and time-lapse video microscopy. Table 1 shows that the dynamics of fluorescent protein localization in the mutants was similar to wild-type cells: in both cases, reversals and reporter protein fluorescence were coordinated. For example, FrzS-GFP dynamics showed nearly perfect correlation in all strains tested, such that when a cell reversed, the new leading pole contained a brighter focus of FrzS-GFP than the lagging pole. Similarly, the AglZ-YFP showed wild-type dynamics and 100% correlation in all strains tested; when cells reversed, the new leading pole contained a bright focus of AglZ-YFP. These results change the interpretation of previous experiments by Li et al. 2005 and suggest that FrzECheY does not independently control the two motility systems; the link between the Frz system and the two motility systems is most likely downstream of FrzE. In all of the FrzE mutants tested, the A- and S- motility systems were coordinated with cellular reversals.
Table 1.
| FrzS-GFP | wt (DZ2) | DZ2 frzEΔcheY | wt (Dk1622) | DK1622 frzED709A | DK1622 frzED709E |
|---|---|---|---|---|---|
| Bright leading pole | 88 | 88 | 84 | 100 | 82 |
| Dim leading pole | 0 | 1 | 0 | 0 | 0 |
| No asymmetry | 8 | 10 | 16 | 0 | 18 |
| Reversals + polar switch | 95 | 97 | 100 | 100 | 100 |
FrzS localization and dynamics are unaffected in the frzEΔcheY, frzED709A, and frzED709E strains. All numbers indicate a percentage of total cells analyzed.
Discussion
In this paper, we used an in vitro phosphorylation assay to explore the function of the CheY-like receiver domain of FrzE. Here, we show that the receiver domain of FrzE strongly inhibits autophosphorylation of the CheA domain, suggesting that this domain plays a major regulatory role in Frz signalling. The inhibition was alleviated when the putative phospho-accepting Asp-709 of FrzE was mutated to an alanine or glutamate. We do not know the mechanism for this inhibition but we speculate from the CheA molecular model from Baker et al. (2006) that the receiver domain occludes the HPT subdomain containing the phosphoaccepting His-49, thereby preventing it from interacting with the catalytic subdomain of CheA. We hypothesize that the mutants FrzED709E and FrzED709A alter the conformation of the CheY domain of FrzE in such a way that the His-49 residue is no longer occluded and can therefore be phosphorylated.
Other CheA-CheY hybrids, such as VirA in Agrobacterium tumefaciens, have been shown to function with an inhibitory CheY domain. However in the case of VirA, the inhibition is independent of the phosphoaccepting aspartate residue (Chang et al., 1996). These results differ from studies with receiver domains like NtrC, where the phospho-accepting Asp to Ala and Asp to Glu point mutations mimic the “inactive” and “active” states respectively (Klose et al., 1993), although these substitutions are not universally effective for all receiver domains (Porter et al., 2006; Smith et al., 2004). We anticipated that an “active” receiver domain might allow autophosphorylation of the CheA domain of FrzE, while an “inactive” receiver domain would not. In the case of FrzE, it appears that both the FrzED709A and FrzED709E mutations lock the CheY domain in a conformation that inactivates the receiver domain, since both mutations have the same effect on CheA autophosphorylation as deleting the entire receiver domain. From our in vitro data, one might expect that the frzED709A and frzED709E mutants should have the same phenotype in vivo, but this is not the case, as reported previously (Li et al., 2005). During development, frzEΔcheY and frzED709E mutants have the same phenotype; fruiting body development slightly altered from wild-type, with the appearance of looser mounds. Strikingly, the frzED709A mutant shows no fruiting body formation at all. Additionally, the reversal frequencies of these mutants differ, with the frzED709A mutant hyper-reversing while frzEΔcheY and frzED709E show varying reversal frequencies depending on the surface on which they are assayed (Li et al., 2005). The in vivo phenotypes suggest that the frzED709A is the only mutation that confers a constitutively “active” state, since the active state would result in hyper-reversals. However, inactivation of an inhibitory receiver domain might give the same phenotype. We speculate that the frzED709A mutant is able to interact with a downstream component (an unidentified protein or small molecule effector) enabling constitutive downstream signaling, while the frzED709E and frzEΔcheY mutants are unable to interact with this component even though they can autophosphorylate in vitro. It would be interesting to search for proteins that interact with FrzE, FrzED709A, and FrzED709E to verify this hypothesis.
Is the FrzE receiver domain phosphorylated? The FrzE receiver domain is very similar to other canonical receiver domains, and in our in vitro experiments, we observed phosphorylation when mixing phospho-FrzECheA with FrzE or FrzEH49A but not when mixing phospho-FrzECheA with FrzECheY. Thus, FrzECheY is most likely unable to accept a phosphoryl group when separated from the CheA domain. However, the receiver domain of FrzE is probably phosphorylated in vivo. How might this occur? In Figure 5, a faint band is visible at the FrzE migration point when FrzE is incubated with FrzECheA but is not as visible when FrzEH49A is incubated with FrzECheA. This faint band increases in intensity with increasing incubation time suggesting that FrzE is phosphorylated on the His-49 residue in addition to the Asp-709 residue. To explain these results, we suggest that once the receiver domain of FrzE is phosphorylated, autophosphorylation of FrzE on His-49 is possible and no longer inhibited. Once phosphorylated, the phosphoryl group on Asp-709 is lost but intra molecular phosphoryl transfer resumes from the kinase domain to the receiver domain of FrzE, followed by rapid hydrolysis. In vivo, another protein or a small molecule may interact with the receiver domain of FrzE to modulate the inhibitory state. For example, in the Arc two-component phosphorelay pathway, autophosphorylation levels are increased in the presence of certain anaerobic metabolites such as pyruvate and acetate in anoxic environments (Georgellis et al., 1999).
M. xanthus has many chemotaxis and two-component signal transduction pathways in its genome. How do they interact with each other, if at all? FrzE is a good candidate for interaction with other pathways, especially since the Frz system responds by changing the reversal frequency in response to multiple types of stimuli. It is possible that the FrzE interacting component referred to above may be another CheA protein that like FrzECheA, forms a heterodimer with FrzE, heterophosphorylates FrzE, and phospho-FrzE then proceeds to transfer a phosphoryl group to FrzZ. This cannot be ruled out from the mixing experiment in Figure 5 since the FrzE band observed when mixing FrzE with phospho-FrzECheA may have been a result of heterophosphorylation between FrzECheA and FrzE. Nonetheless, the frzEH49A mutant and a Frz loss of function mutant had the same phenotype, indicating that FrzE phosphorylation is necessary for Frz signaling. However, it did not rule out the possibility of heterophosphorylation in vivo, as reported in other organisms with multiple che operons (Porter and Armitage, 2004).
In this study, we were also concerned with the previously reported divergent effects of mutations in the receiver domain of FrzE on the A- and S- motility systems. M. xanthus requires a solid surface for motility and is non-motile in liquid media. Cell-cell contact is required for S-motility, except when cells are placed in 1% methylcellulose; A-motility does not require cell-cell contact but requires a firm surface and does not function well in methylcellulose. Thus, motility assays are often conducted in 1% methylcellulose when observing S-motility and 1.5% agar when observing A-motility since these conditions favor the respective motility system. Previous results showed that the frzEΔcheY mutant hyper-reverses on 1% methylcellulose but not on 1.5% agar (Inclan et al., 2007) and that the frzED709E mutant hyper-reverses on 1.5% agar but not on 1% methylcellulose (Li et al., 2005). If FrzE independently controlled the A- and S- motility systems, we would expect the two motility systems to be uncoupled in these FrzE mutants such that S-motility hyper-reverses while A-motility does not under the same condition. Since following reversal frequency under different conditions proved confusing, we decided to study the coupling between FrzECheY mutations and the two motility systems in the same conditions by using reporter proteins for S-motility (FrzS-GFP) and A-motility (AglZ-YFP). For example, in a frzEΔcheY mutant, if we followed the protein localization dynamics of FrzS in cells on 1.5% agar, we would expect that FrzS-GFP would hyper-reverse and the bright focus would not switch poles in concert with a respectively slower cellular reversal since the cell does not hyper-reverse on 1.5% agar; in contrast, AglZ-YFP localization dynamics would be expected to coordinate with a cellular reversal. However, if the two motility systems are coordinated in these mutants, we would expect the dynamics of both FrzS-GFP and AglZ-YFP would reverse coordinately with a cellular reversal. We made numerous movies of these FrzS-GFP and AglZ-YFP cells and found that in all cases, moving cells showed that the reporter markers localized normally as cells reversed, even in the frzEΔcheY and frzED709E mutant backgrounds. These results do not support the hypothesis that FrzE signals directly to the two different motility systems, as in a branched signaling pathway. There may indeed be branching in Frz signaling, however, we are suggesting that it does not occur at FrzE.
In conclusion, the results presented in this paper support the hypothesis that the receiver domain of FrzE plays a regulatory role in controlling cellular reversals in M. xanthus. Our results suggest that the receiver domain regulates the cellular reversal frequency by negatively regulating the CheA kinase domain of FrzE. Upon activation of the receiver domain, FrzE autophosphorylates at H-49, and phospho-FrzE transfers phosphoryl groups to the receiver domains of FrzZ. We also show the dynamics of motility system specific marker proteins AglZ and FrzS in FrzE mutants as cells move forward and reverse. Our studies indicate that the two motility systems are functionally coordinated and that any system specific branching to the pathway most likely occurs downstream of FrzE.
Materials and Methods
Strains and growth conditions
Bacterial strains and plasmids are listed in Table 1. All M. xanthus strains were cultured on CYE media (10 mM MOPS, pH 7.6, 1% (w/v) Casitone, 0.5% yeast extract and 4 mM MgSO4 (Campos et al., 1978)) with 1.5% agar and incubated at 32 °C in the dark. Liquid cultures were inoculated from plated cells and grown in CYE liquid while shaking at 250 rpm at 32 °C. For colony phenotypes, liquid cultures grown overnight were centrifuged at room temperature for 10 min at 8,000 × g in an SS34 rotor, resuspended to 2 × 109 cells/mL) in MMC liquid (10 mM MOPS pH 7.6, 4 mM MgSO4, 2 mM CaCl2), and 10 μL was plated on CYE media with 1.5% agar, or 0.3% agar for colony expansion measurements, (cells were resuspended to 4×109 cells/mL when plated on 0.3% agar) or CF media (0.015% Casitone, 0.2% sodium citrate, 0.1% sodium pyruvate, 0.02% (NH4)2SO4, 10 mM MOPS pH 7.6, 8 mM MgSO4, 1 mM KH2PO4 (Hagen et al., 1978)) with 1.5% agar for development. Photographs shown or colony diameters were captured and measured after 3 days of incubation at 32 °C. Error bars indicate standard error of the mean. Kanamycin was used at 100 μg/mL. E. coli strains were grown in LB media (Sambrook et al., 1989).
Gene replacements and fluorescent strains
Gene replacements were obtained by homologous recombination. Constructs were generated by overlap PCR containing approximately 750 bp upstream and downstream of the desired mutated region. Point mutants were constructed similarly, except the overlap PCR was performed with 40-mer primers containing the base substitution to change His-49 to Ala. FrzE gene replacements were confirmed by sequencing the PCR product amplified from the chromosome at the Berkeley sequencing facility. The resulting fragment was cloned into pBJ114 (Julien et al., 2000) using the EcoRI and HindIII restriction sites using standard methods (Sambrook et al., 1989), or BD-in fusion (Clontech). 1 μg of this plasmid was transformed into the appropriate strain and selected for kanamycin resistance. Transformants were grown in liquid culture overnight and then plated on a CYE-1.5% agar plate containing 2.5% galactose with 0.7% top agar also containing 2.5% galactose. Galactose resistant (and kanamycin sensitive) colonies were screened by performing PCR and sequencing the product, using either purified genomic DNA or whole cells as a template.
The FrzS-GFP allele was generated by electroporating pBJFG into the background strain of choice and the loop-in loop-out procedure was followed as stated above. The AglZ-YFP allele was generated similarly, except the second round of plating on galactose was eliminated. The AglZ-YFP strains are all kanamycin resistant.
Western blots
Western immunoblots were performed by growing cell cultures overnight, resuspending the culture to 8 × 109 cells/mL in SDS-PAGE loading buffer with DTT, sonicating briefly, and boiling for 10 min. 10 μL of whole-cell extract was loaded on a 10% SDS-PAGE gel and run at 150 V for approximately 1 h. Proteins were transferred to a nitrocellulose membrane using a wet transfer apparatus (Bio-Rad) and immunoblots were prepared using standard procedures (Sambrook et al., 1989); polyclonal anti-FrzE antibodies diluted to 1:1,000, and anti-rabbit Alexa Flour 680 secondary antibodies (Molecular Probes) were diluted to 1:10,000. The Odyssey imaging system was used to scan, image, and analyze the immunoblots (Li-cor Biosciences).
Protein purification and phosphotransfer assay
All FrzE mutants were amplified by PCR from genomic DNA from the corresponding M. xanthus mutant. PCR products were cloned into the pET28a (Invitrogen) EcoRI and HindIII restriction sites using BD-in fusion (Clontech). The constructs were transformed into Tuner cells (Novagen), grown to an OD550 in 1 L of LB media with kanamycin, induced with 0.2 mM of IPTG, grown overnight at 17 - 22 °C and harvested at 4 °C at 5,000 × g in a GS3 rotor. Pellets were washed with water, resuspended in 35 mL lysis buffer (20 mM NaH2PO4, 0.5 M NaCl, 40 mM imidazole pH 7.4, 2% mammalian protease inhibitor (Sigma), 1 mg/mL lysozyme (Sigma), sonicated with a Branson Sonifier, (duty cycle 50%, output 3, repeated 6×) and centrifuged at 4 °C at 24,000 × g in an SS34 rotor for 30 min. The lysate was filtered through a 0.22 μM filter and loaded on a HisTrap HP 5 mL column (GE Healthcare) and purified using an Akta FPLC (GE Healthcare). Purity was checked by running 2 μg of each fraction onto a 10% SDS-PAGE gel and stained with Coomassie brilliant blue (sigma). Immunoblot analysis was also performed using a monoclonal T7 antibody as well as a polyclonal FrzE antibody. FrzCD and FrzA were purified as described previously (Inclan et al., 2007). FrzECheY was purified under denaturing conditions as described in (Astling, 2003). Autolabeling and phosphotrasfer assays were performed at room temperature in 50 mM Tris pH 7.5, 0.5 mM EDTA, 1 mM DTT, 10% glycerol, 5 mM MgCl2, 5 mM MnCl2 and 50 mM KCl. The same molar ration of FrzECheA, FrzCD, FrzE and FrzE variants, and FrzA were used in all reactions, at1 μM or more. FrzCD, FrzE variants and FrzA were incubated with 50 μM ATP supplemented with 1 μCi of [γ-32P]-ATP for 10 min and aliquots were added to varying concentrations of FrzECheY and/or FrzZ to a final concentration of at least 1 μM for incubations times ranging from 30 s - 2 h. The 25 μL reaction was stopped by the addition of 4 × SDS-PAGE loading dye containing DTT and immediately after, 10 μL was loaded onto a 10% SDS- PAGE mini-gel (Biorad). Electrophoresis was performed at 150 volts for approximately one hour. Two identical gels were run simultaneously, one was subsequently stained with Coomassie brilliant blue and the other was dried with a gel dryer (Biorad) and exposed to a phosphor screen (Amersham). The phosphor screen was scanned on a Typhoon or Storm fluorescent gel scanner after approximately 12 h and analyzed with ImageQuant software. For quantitation, intensity of bands were scaled against a standard FrzECheA, 10 min, reaction band with an arbitrary value of “1”. Error bars indicate standard deviation after 3 rounds of experiments.
Enzyme-coupled assay
Each reaction was mixed to a final volume of 100 μL containing, 1 mM phosphoenolpyruvate, 0.2 mM nicotinamide adenine dinucleotide (NADH), 4 U of pyruvate kinase/L-lactate dehydrogenase (all from Sigma) and 0.2-2 mM ATP, 5-10 μM of candidate enzyme, (FrzE or variant of FrzE) and 1x of reaction buffer listed in the phosphorylation assay. Once all the reactants were mixed the reaction was immediately placed in a spectrophotometer (BioSpec-mini by Shimadzu) and the Absorbance was continuously measured at 340 nm and recorded every 30 s, after 30 s.
Fluorescent movies
The strain of interest was grown in liquid culture overnight and a 2-5 μL spot was placed on a thin layer of 1/2CTT (0.5% Bacto Casitone, 10 mM Tris, pH 7.6, 1 mM KH2PO4, 8 mM MgSO4) +1.5% agar on a glass slide. After approximately 15 min, a cover slip was placed on the spot and the cells were imaged using a deltavision microscope with a 100x objective. Time-lapse movies were taken with a total time of 10 or 15 min and a frame was captured every 30 s with pseudo-phased and using the FITC filter for imaging GFP or YFP filter for imaging YFP with an exposure time of 0.3 - 0.5 s and 1 s respectively. At least 50 cells were analyzed per strain.
Table 2.
| Strain or plasmid | Relevant genotype | Reference or source |
|---|---|---|
| M. xanthus strains | ||
| DZ2 | Wild type | (Campos and Zusman, 1975) |
| DK1622 | Wild type | (Wall et al., 1999) |
| TM9 | DZ2 AglZ-YFP | (Mignot et al., 2007b) |
| TM3 | DZ2 FrzS-GFP | (Mignot et al., 2005) |
| DZ4621 | DK1622 AglZ-YFP | This study |
| DZ4622 | DK1622 FrzS-GFP | This study |
| DZ4623 | DZ2 frzEΔcheY AglZ-YFP | This study |
| DZ4624 | DZ2 frzEΔcheY FrzS-GFP | This study |
| DZ4625 | DK1622 frzED709A AglZ-YFP | This study |
| DZ4626 | DK1622 frzED709A FrzS-GFP | This study |
| DZ4627 | DK1622 frzED709E AglZ-YFP | This study |
| DZ4628 | DK1622 frzED709E FrzS-GFP | This study |
| DZ4629 | DZ2 frzEH49A | This study |
| SW901 | DK1622 frzED709A | (Li et al., 2005) |
| SW902 | DK1622 frzED709E | (Li et al., 2005) |
| E. coli strains | ||
| DH10B | Host for cloning | Invitrogen |
| tuner | Host for overexpression of FrzE constructs | Novagen |
| Plasmids | ||
| pBJ114 | Used to create gene replacements galKS, kanR | (Julien et al., 2000) |
| pBJFG | Used to generate FrzS-GFP | (Mignot et al., 2005) |
| pBJAglZY | Used to generate AglZ-YFP | (Mignot et al., 2007b) |
| pet28a | Used to create overexpression constructs kanR | Novagen |
| pYFI103 | pet28a with frzEcheA for overexpression | (Inclan et al., 2007) |
| pYFI108 | pet28a with frzEcheAH49A for overexpression | This study |
| pYFI109 | pet28a with frzEH49A for overexpression | This study |
| pDPA-145 | frzECheY for overexpression | (Astling, 2003) |
| pYFI104 | pet28a with frzZ for overexpression | (Inclan et al., 2007) |
| pYFI110 | pet28a with frzED709A for overexpression | This study |
| pYFI111 | pet28a with frzED709E for overexpression | This study |
| pYFI112 | pet28a with frzE for overexpression | This study |
| pDPA-24 | pRSET with frzCD for overexpression | (Inclan et al., 2007) |
| pDPA-143 | pRSET with frzA for overexpression | (Inclan et al., 2007) |
Acknowledgments
We are grateful to John P. Merlie for purifying FrzECheY. We are grateful to members of the Zusman laboratory for helpful discussions and especially to Emilia Mauriello, Ansley Scott, Tâm Mignot, and Hera Vlamakis for critical review of the manuscript. This research was supported by a grant from the National Institute of Health to DRZ (GM20509).
References
- Astling DP. Molecular and Cell Biology. University of California; Berkeley: 2003. Novel regulatory mechanisms of a chemotaxis pathway in the gliding bacterium Myxococcus xanthus. [Google Scholar]
- Baker MD, Wolanin PM, Stock JB. Signal transduction in bacterial chemotaxis. Bioessays. 2006;28:9–22. doi: 10.1002/bies.20343. [DOI] [PubMed] [Google Scholar]
- Blackhart BD, Zusman DR. “Frizzy” genes of Myxococcus xanthus are involved in control of frequency of reversal of gliding motility. Proc Natl Acad Sci U S A. 1985;82:8767–8770. doi: 10.1073/pnas.82.24.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante VH, Martinez-Flores I, Vlamakis HC, Zusman DR. Analysis of the Frz signal transduction system of Myxococcus xanthus shows the importance of the conserved C-terminal region of the cytoplasmic chemoreceptor FrzCD in sensing signals. Mol Microbiol. 2004;53:1501–1513. doi: 10.1111/j.1365-2958.2004.04221.x. [DOI] [PubMed] [Google Scholar]
- Campos JM, Geisselsoder J, Zusman DR. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- Campos JM, Zusman DR. Regulation of development in Myxococcus xanthus: effect of 3′:5′-cyclic AMP, ADP, and nutrition. Proc Natl Acad Sci U S A. 1975;72:518–522. doi: 10.1073/pnas.72.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Zhu J, Winans SC. Pleiotropic phenotypes caused by genetic ablation of the receiver module of the Agrobacterium tumefaciens VirA protein. J Bacteriol. 1996;178:4710–4716. doi: 10.1128/jb.178.15.4710-4716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgellis D, Kwon O, Lin EC. Amplification of signaling activity of the arc two-component system of Escherichia coli by anaerobic metabolites. An in vitro study with different protein modules. J Biol Chem. 1999;274:35950–35954. doi: 10.1074/jbc.274.50.35950. [DOI] [PubMed] [Google Scholar]
- Goldman BS, Nierman WC, Kaiser D, Slater SC, Durkin AS, Eisen JA, Ronning CM, Barbazuk WB, Blanchard M, Field C, Halling C, Hinkle G, Iartchuk O, Kim HS, Mackenzie C, Madupu R, Miller N, Shvartsbeyn A, Sullivan SA, Vaudin M, Wiegand R, Kaplan HB. Evolution of sensory complexity recorded in a myxobacterial genome. Proc Natl Acad Sci U S A. 2006;103:15200–15205. doi: 10.1073/pnas.0607335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudreau PN, Lee PJ, Stock AM. Stabilization of the phospho-aspartyl residue in a two-component signal transduction system in Thermotoga maritima. Biochemistry. 1998;37:14575–14584. doi: 10.1021/bi980869i. [DOI] [PubMed] [Google Scholar]
- Hagen DC, Bretscher AP, Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978;64:284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Hess JF, Oosawa K, Kaplan N, Simon MI. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988;53:79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): genes controlling movement of single cells. Mol General Genet. 1979a;171:167–176. [Google Scholar]
- Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol Gen Genet. 1979b;171:177–191. [Google Scholar]
- Igo MM, Ninfa AJ, Stock JB, Silhavy TJ. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989;3:1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- Inclan YF, Vlamakis HC, Zusman DR. FrzZ, a dual CheY-like response regulator, functions as an output for the Frz chemosensory pathway of Myxococcus xanthus. Mol Microbiol. 2007;65:90–102. doi: 10.1111/j.1365-2958.2007.05774.x. [DOI] [PubMed] [Google Scholar]
- Julien B, Kaiser AD, Garza A. Spatial control of cell differentiation in Myxococcus xanthus. Proc Natl Acad Sci U S A. 2000;97:9098–9103. doi: 10.1073/pnas.97.16.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser AD, Crosby C. Cell movement and its coordination in swarms of Myxococcus xanthus. Cell Motility. 1983;3:227–245. [Google Scholar]
- Kearns DB, Shimkets LJ. Chemotaxis in a gliding bacterium. Proc Natl Acad Sci U S A. 1998;95:11957–11962. doi: 10.1073/pnas.95.20.11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiianitsa K, Solinger JA, Heyer WD. Rad54 protein exerts diverse modes of ATPase activity on duplex DNA partially and fully covered with Rad51 protein. J Biol Chem. 2002;277:46205–46215. doi: 10.1074/jbc.M207967200. [DOI] [PubMed] [Google Scholar]
- Klose KE, Weiss DS, Kustu S. Glutamate at the site of phosphorylation of nitrogen-regulatory protein NTRC mimics aspartyl-phosphate and activates the protein. J Mol Biol. 1993;232:67–78. doi: 10.1006/jmbi.1993.1370. [DOI] [PubMed] [Google Scholar]
- Kreuzer KN, Jongeneel CV. Escherichia coli phage T4 topoisomerase. Methods Enzymol. 1983;100:144–160. doi: 10.1016/0076-6879(83)00051-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Bustamante VH, Lux R, Zusman D, Shi W. Divergent regulatory pathways control A and S motility in Myxococcus xanthus through FrzE, a CheA-CheY fusion protein. J Bacteriol. 2005;187:1716–1723. doi: 10.1128/JB.187.5.1716-1723.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sun H, Ma X, Lu A, Lux R, Zusman D, Shi W. Extracellular polysaccharides mediate pilus retraction during social motility of Myxococcus xanthus. Proc Natl Acad Sci U S A. 2003;100:5443–5448. doi: 10.1073/pnas.0836639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino K, Shinagawa H, Amemura M, Kawamoto T, Yamada M, Nakata A. Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J Mol Biol. 1989;210:551–559. doi: 10.1016/0022-2836(89)90131-9. [DOI] [PubMed] [Google Scholar]
- Mauriello EM, Zusman DR. Polarity of motility systems in Myxococcus xanthus. Curr Opin Microbiol. 2007;10:624–629. doi: 10.1016/j.mib.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MJ, Kohler T, Zusman DR. Methylation of FrzCD, a methyl-accepting taxis protein of Myxococcus xanthus, is correlated with factors affecting cell behavior. J Bacteriol. 1992;174:4246–4257. doi: 10.1128/jb.174.13.4246-4257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MJ, Zusman DR. FrzCD, a methyl-accepting taxis protein from Myxococcus xanthus, shows modulated methylation during fruiting body formation. J Bacteriol. 1993;175:4936–4940. doi: 10.1128/jb.175.15.4936-4940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot T. The elusive engine in Myxococcus xanthus gliding motility. Cell Mol Life Sci. 2007 doi: 10.1007/s00018-007-7176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot T, Merlie JP, Jr, Zusman DR. Regulated pole-to-pole oscillations of a bacterial gliding motility protein. Science. 2005;310:855–857. doi: 10.1126/science.1119052. [DOI] [PubMed] [Google Scholar]
- Mignot T, Merlie JP, Jr, Zusman DR. Two localization motifs mediate polar residence of FrzS during cell movement and reversals of Myxococcus xanthus. Mol Microbiol. 2007a;65:363–372. doi: 10.1111/j.1365-2958.2007.05789.x. [DOI] [PubMed] [Google Scholar]
- Mignot T, Shaevitz JW, Hartzell PL, Zusman DR. Evidence that focal adhesion complexes power bacterial gliding motility. Science. 2007b;315:853–856. doi: 10.1126/science.1137223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SL, Armitage JP. Chemotaxis in Rhodobacter sphaeroides requires an atypical histidine protein kinase. J Biol Chem. 2004;279:54573–54580. doi: 10.1074/jbc.M408855200. [DOI] [PubMed] [Google Scholar]
- Porter SL, Wadhams GH, Martin AC, Byles ED, Lancaster DE, Armitage JP. The CheYs of Rhodobacter sphaeroides. J Biol Chem. 2006;281:32694–32704. doi: 10.1074/jbc.M606016200. [DOI] [PubMed] [Google Scholar]
- Rasmussen AA, Wegener-Feldbrugge S, Porter SL, Armitage JP, Sogaard-Andersen L. Four signalling domains in the hybrid histidine protein kinase RodK of Myxococcus xanthus are required for activity. Mol Microbiol. 2006;60:525–534. doi: 10.1111/j.1365-2958.2006.05118.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsc EF, Maniatis T. Molecular Cloning: a Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Shi W, Ngok FK, Zusman DR. Cell density regulates cellular reversal frequency in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1996;93:4142–4146. doi: 10.1073/pnas.93.9.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Zusman DR. The two motility systems of Myxococcus xanthus show different selective advantages on various surfaces. Proc Natl Acad Sci U S A. 1993;90:3378–3382. doi: 10.1073/pnas.90.8.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerker JM, Berg HC. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci U S A. 2001;98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliusarenko O, Zusman DR, Oster G. The motors powering A-motility in Myxococcus xanthus are distributed along the cell body. J Bacteriol. 2007;189:7920–7921. doi: 10.1128/JB.00923-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JG, Latiolais JA, Guanga GP, Pennington JD, Silversmith RE, Bourret RB. A search for amino acid substitutions that universally activate response regulators. Mol Microbiol. 2004;51:887–901. doi: 10.1046/j.1365-2958.2003.03882.x. [DOI] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Wall D, Kolenbrander PE, Kaiser D. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pilus biogenesis, social motility, and development. J Bacteriol. 1999;181:24–33. doi: 10.1128/jb.181.1.24-33.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MJ, Lew H, Zusman DR. Social motility in Myxococcus xanthus requires FrzS, a protein with an extensive coiled-coil domain. Mol Microbiol. 2000;37:1357–1371. doi: 10.1046/j.1365-2958.2000.02079.x. [DOI] [PubMed] [Google Scholar]
- Weiss V, Magasanik B. Phosphorylation of nitrogen regulator I (NRI) of Escherichia coli. Proc Natl Acad Sci U S A. 1988;85:8919–8923. doi: 10.1073/pnas.85.23.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth C, Hoiczyk E, Kaiser D, Oster G. How myxobacteria glide. Curr Biol. 2002;12:369–377. doi: 10.1016/s0960-9822(02)00716-9. [DOI] [PubMed] [Google Scholar]
- Zusman DR. “Frizzy” mutants: a new class of aggregation-defective developmental mutants of Myxococcus xanthus. J Bacteriol. 1982;150:1430–1437. doi: 10.1128/jb.150.3.1430-1437.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman DR, Scott AE, Yang Z, Kirby JR. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat Rev Microbiol. 2007;5:862–872. doi: 10.1038/nrmicro1770. [DOI] [PubMed] [Google Scholar]


