Abstract
Synthetic oligodeoxynucleotides (ODN) containing unmethylated CpG motifs mimic the immunostimulatory activity of bacterial DNA. CpG ODN directly stimulate B cells and plasmacytoid dendritic cells (pDC), promote the production of Th1 and pro-inflammatory cytokines, and trigger the maturation/activation of professional antigen presenting cells. CpG ODN are finding use as vaccine adjuvants, where they increase the speed, magnitude and duration of vaccine-specific immune responses. For example, CpG ODN significantly prolong the protection induced by AVA (Anthrax Vaccine Adsorbed). Unexpectedly, a majority of animals immunized with CpG-adjuvanted AVA maintain resistance to anthrax infection even after their Ab titers decline to sub-protective levels. This survival is mediated by the de novo production of protective Abs by high affinity long-lived memory B cells. The immunostimulatory activity of CpG ODN was probed at the molecular level by microarray. Results show that a small group of ‘inducers’ rapidly up-regulated a large network genes following CpG treatment of mice. This stimulatory activity is quenched by ‘suppressors’ that down-regulate the expression of targeted genes, including most of the ‘inducers’. These findings shed light on the mechanism underlying CpG mediated immune activation and therapeutic activity.
Immunomodulatory properties of CpG ODN
Synthetic oligodeoxynucleotides (ODN) containing immunostimulatory “CpG motifs” interact with Toll-like receptor 9 to initiate an immunostimulatory cascade that culminates in the maturation, differentiation and/or proliferation of multiple cell types, including lymphocytes, dendritic cells, NK cells, monocytes and macrophages (Gursel et al, 2002; Hemmi et al, 2000; Hornung et al, 2002; Klinman et al, 1996; Stacey et al, 1998; Takeshita et al, 2001). Together, these secrete cytokines and chemokines that create a pro-inflammatory (IL-1, IL-6, IL-18 and TNF) and Th1-biased (IFNg and IL-12) immune milieu (Ballas et al, 1996; Halpern et al, 1996; Hemmi et al, 2000; Ishii et al, 2002; Klinman et al,1996; Krieg et al,1995; Takeshita et al, 2001). In humans, TLR9 is primarily present within human B cells and plasmacytoid DC, while in mice multiple cells of the myeloid lineage (including monocytes, macrophages and DC) express TLR 9 and directly respond to CpG stimulation (Bauer et al, 2001; Kadowaki et al, 2001; Krug et al, 2001).
USE OF CpG ODN AS VACCINE ADJUVANTS
Vaccine applications: CpG ODN improve the protective immunity induced by AVA
Anthrax Vaccine Adsorbed (AVA) is the sole vaccine licensed to prevent human anthrax in the US. AVA requires a series of 6 immunizations over 18 months to induce the production of neutralizing antibodies against the “protective antigen” (PA) of anthrax toxin (Pittman et al, 2001). Anthrax spores designed for aerosol delivery were released in the US by bioterrorists in 2001, causing morbidity, mortality, and widespread panic (Lane et al, 2001). That event underscored the need for a vaccine that induced protective immunity more rapidly than AVA and maintained protection without repeated boosts (Lane et al, 2001). One strategy to achieve these goals involves adding CpG ODN to AVA. The ability of CpG ODN to promote Th1 responses and induce the maturation and activation of professional antigen presenting cells suggested they might be useful vaccine adjuvants (Branda et al, 1996; Krieg et al,1998; Moldoveanu et al, 1998). Previous studies on this topic established that CpG ODN could both accelerate and magnify the immune response elicited by AVA (Klinman et al, 2007; Klinman et al, 2006; Xie et al, 2005).
As seen in Table I, adding CpG ODN to AVA increased the titer of serum neutralizing Ab of A/J mice by >10-fold (Xie et al, 2005). The survival of vaccinated mice following anthrax spore challenge was also significantly improved by immunizing with CpG adjuvanted AVA. In contrast, delaying the administration of CpG ODN until after AVA immunization yielded almost no booster effect, consistent with adjuvant activity requiring co-delivery with antigen (Table I). These findings were confirmed in studies of rhesus macaques, where co-administering CpG ODN with AVA induced a six-fold higher Ab response than AVA alone (Klinman et al, 2004). Serum from primates vaccinated with AVA plus CpG ODN transferred protection against anthrax spore challenge to murine recipients (Table I) (Klinman et al, 2004). A clinical trial examining the response of 69 normal healthy volunteers to 0.5 ml of AVA plus 1 mg of CpG ODN was conducted. Results from that study demonstrated that in humans, the inclusion of CpG ODN significantly accelerated the induction of protective immunity and increased serum IgG anti-PA titers by 9-fold when compared to AVA alone (p < .05) (Rynkiewicz et al, 2005).
Table I.
CpG ODN as adjuvant for AVA
| A) Response of vaccinated A/J mice | ||
|---|---|---|
| Treatment | % survival | TNA |
| None | 0 | 0 |
| AVA alone | 30 | 25 ± 7* |
| AVA plus CpG ODN (simultaneous) | 80* | 350 ± 48 |
| AVA plus CpG ODN (delayed) | 30 | 37 ± 6 |
| B) Response of vaccinated rhesus macaques | ||
| Treatment | % survival | TNA |
| None | 0 | 0 |
| AVA alone | 10 | 25 |
| AVA plus CpG ODN | 50* | 420 |
A) A/J mice were immunized once with 8 ul of AVA + 50 ug of CpG ODN either at the same time (simultaneous) or one day after (delayed) AVA delivery. Toxin neutralizing activity (TNA) was measured 3 weeks later. Mice were challenged with 60 LD50 of Sterne strain anthrax at 4 weeks. Data excerpted from (Klinman et al, 2006; Xie et al, 2005).
B) Rhesus macaques (5/group) were immunized with 0.5 ml of AVA ± 500 ug of CpGODN.
p <.05
Magnitude and duration of the IgG anti-PA response induced by CpG-adjuvanted AVA
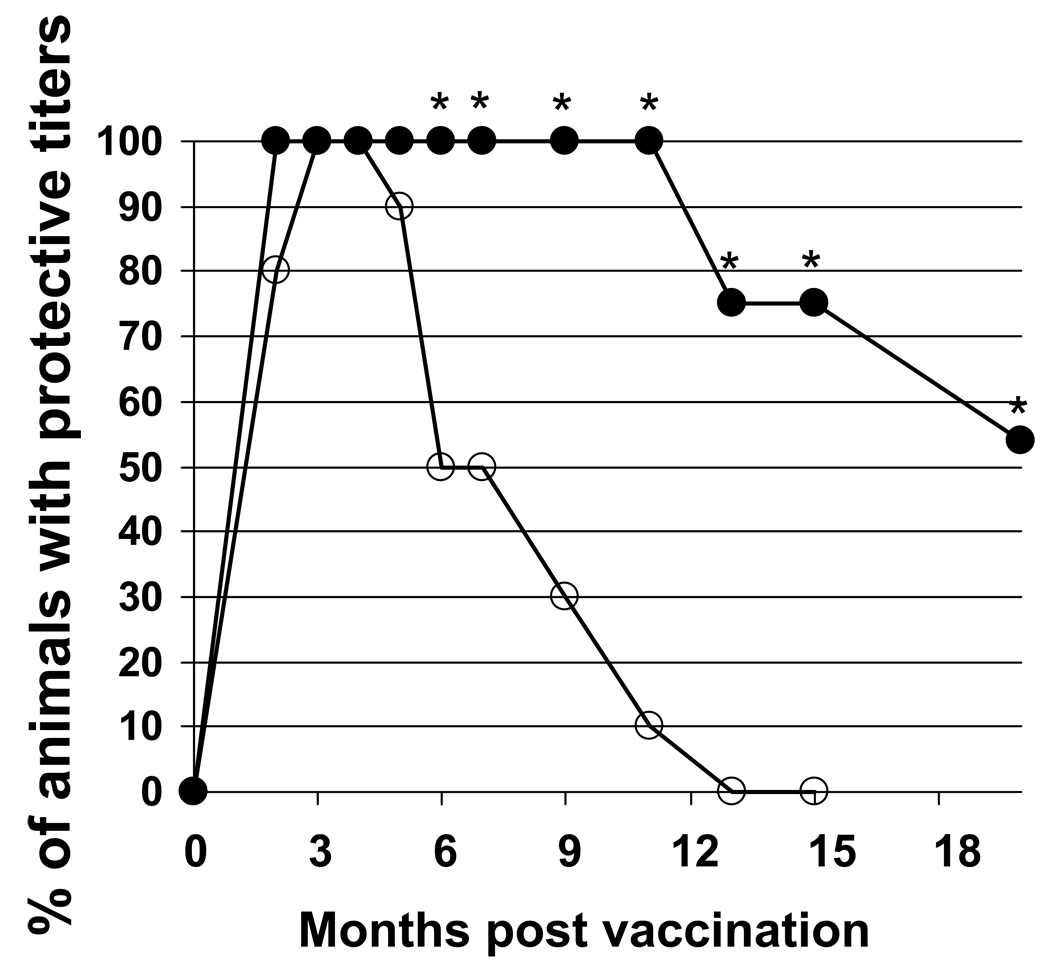
To evaluate the duration of the immune response induced by CpG-adjuvanted AVA, A/J mice were vaccinated and their serum IgG anti-PA titers monitored for nearly 2 years. IgG anti-PA titers persisted at significantly higher levels in the group vaccinated with CpG-adjuvanted AVA vs AVA alone for the duration of the study (p. <.01, Fig 1) (Tross et al, 2008). Survival following challenge correlated with serum IgG anti-PA titer. In A/J mice, a “protective titer” was identified above which animals were generally resistant to infection by high dose (100 LD50) spore challenge. As seen in Fig 1, every mouse immunized with CpG-adjuvanted vaccine maintained protective titers for at least one year, whereas titers fell below this level in half of the mice immunized with AVA alone within 6 months (p. <.001).
Figure 1. Persistence of protective Ab titers in mice immunized with AVA + CpG ODN.
A/J mice were immunized i.p. with 10 ul of AVA alone (○) or adjuvanted with 20 ug of CpG ODN (●). Serum IgG anti-PA titers were monitored individually for each of >30 mice/group over time. Data reflect the percent of animals in each group with anti-PA titers in the “protective range” (> 1:16,000) and represent the combined results of two similar but independent experiments.
*; p. <.05 vs AVA alone.
Memory B cells contribute to vaccine-induced protective immunity
A subset of mice was identified in challenge studies that survived infection despite having IgG anti-PA titers less than 1/10th the protective baseline. Almost all of these survivors had been immunized with CpG-adjuvanted AVA (16/17) rather than AVA alone (p <.01). Of interest, IgG anti-PA titers rose rapidly in the cohort of mice that survived infection (average increase 3.6 fold by day 3, Table II) but not in those that succumbed to infection (p. <.02)(Tross et al, 2008). These results suggest that the rapid production of IgG anti-PA Abs might protect hosts with initially low circulating anti-PA titers.
Table II.
Memory response of vaccinated mice
| A. Rapidity of memory B cell response | ||
|---|---|---|
| IgG anti-PA titer | ||
| Challenge outcome | Pre-challenge | 3 days post challenge |
| Survivors | 411 ± 118 | 1,492 ± 442 |
| Non-survivors | 360 ± 61 | 356 ± 82 |
| B. Analysis of memory B cells | ||
| Treatment | Frequency of PA-specific memory B cells/spleen |
% of B cells with high affinity |
| Unimmunized | 2 ± 2 | 0 |
| AVA alone | 10 ± 3 | 8 |
| AVA + CpG ODN | 45* ± 4 | 62* |
A) A/J mice were immunized i.p. with AVA ± CpG ODN. Those mice with IgG anti-PA titers measured >6 months post vaccination in the range of 1:100 – 1: 2,000 were challenged with 100 LD50 of Sterne strain anthrax spores. Serum Ab titers are shown for each animal one week before (pre) and 3 days after challenge (post). Note that anti-PA titers rose ≈3-fold in animals that survived (p. <.02), but did not change in animals that succumbed to infection. All of the surviving mice had been immunized with CpG adjuvanted AVA rather than AVA alone (p. <.01).
B) A/J mice were immunized with AVA ± CpG ODN. Fragment cultures were established from the spleens of mice after their Ab titers had fallen into the sub-protective range. These fragments were stimulated ex vivo with high (≥3×10−9 M) or low (≤10−11 M) concentrations of rPA. Results represent the frequency of IgG anti-PA secreting fragments (avg + SD) per spleen studied 3–6 days later. Data reflect the combined results from 6 independent experiments involving a total of 22 mice immunized with CpG-adjuvanted AVA, 17 immunized with AVA alone, and 8 naive mice.
p <.05, CpG adjuvanted AVA vs AVA alone.
The source of these Abs was probed. Findings indicate that 4-fold more PA-specific memory B cells were present in mice vaccinated with AVA ± CpG ODN vs AVA alone (p. <.05, Table II)(Tross et al, 2008). Moreover, the Ab produced by memory B cells from mice immunized with CpG-adjuvanted AVA were of considerably higher average affinity than those from mice vaccinated with AVA alone (p <.05, Table II). Kinetic studies showed that B cells from mice vaccinated with the CpG-adjuvanted vaccine responded to Ag exposure significantly more rapidly than those from mice immunized with AVA alone (p. <.01)(Tross et al, 2008). These results suggest that vaccination with CpG-adjuvanted AVA generates a significantly larger and higher affinity population of memory B cells than AVA alone.
REGULATION OF CpG-MEDIATED CHANGES IN GENE EXPRESSION
Regulatory networks underlie CpG-dependent gene activation in vivo
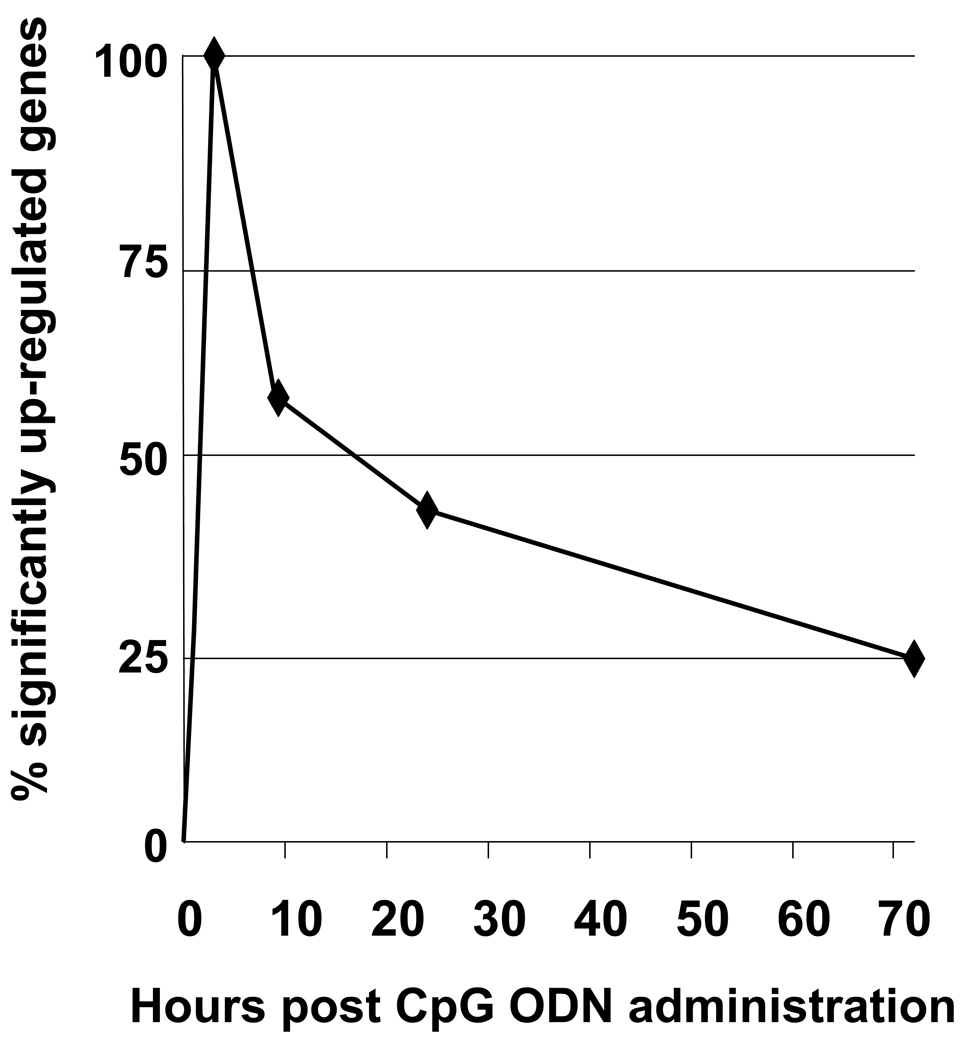
The scope of TLR9 dependent gene activation is incompletely understood. To examine this issue, normal BALB/c mice were treated with 400 µg of CpG ODN and changes in splenic mRNA levels monitored by microarray (Klaschik et al, 2008). Four animals/group were independently analyzed at each time point, and consistent changes in gene expression observed among similarly treated mice in independent experiments (R2 = 0.89 ± 0.04). A stringency cutoff of p <0.00001 was used to identify genes whose level of expression differed significantly from naive controls. Changes in gene expression were present at 30 min, peaked at 3 hr, and declined progressively thereafter (Fig. 2). These changes in mRNA expression were overwhelmingly CpG specific, as less than 0.4% of these genes were up-regulated in mice treated with control (non-CpG) ODN.
Figure 2. CpG ODN induce reproducible patterns of gene expression in vivo.
BALB/c mice were injected i.p. with 400 µg of CpG ODN. Gene expression in the spleen was monitored over time by microarray. Four biological replicates were independently analyzed at each time point. Up-regulated genes were identified by comparison to untreated mice using a stringency cut-off of p < 0.00001 and are displayed as % of maximum gene expression.
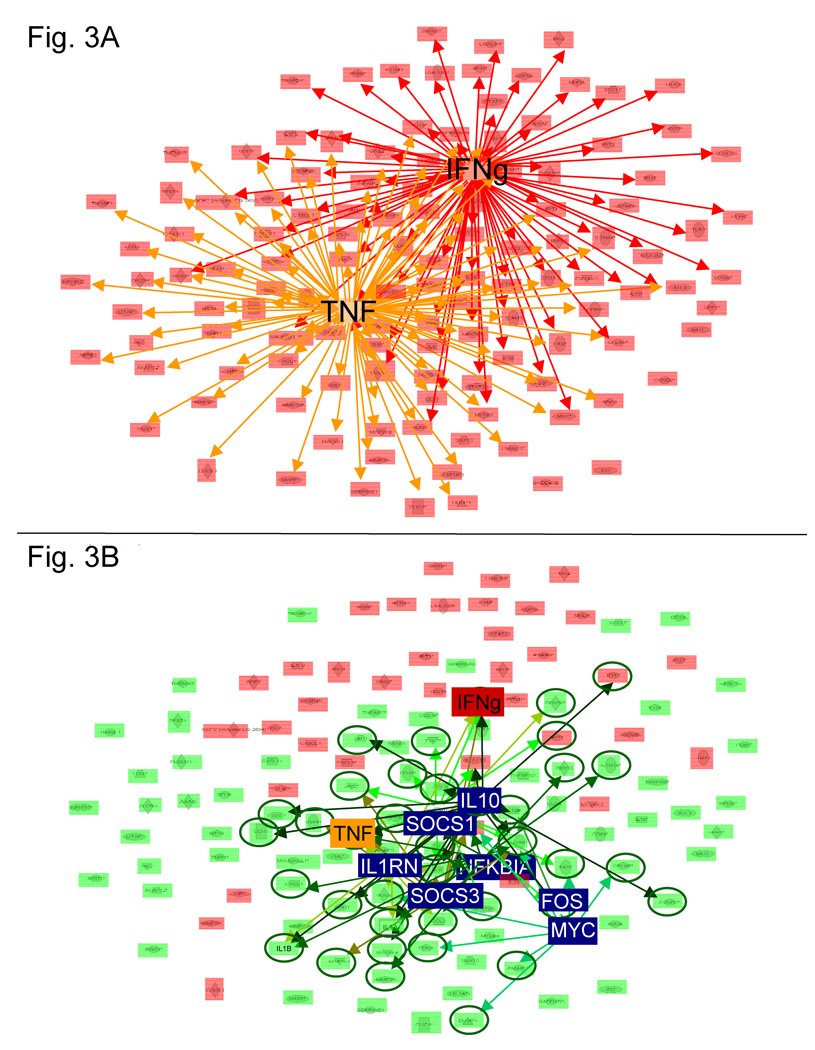
Ingenuity Pathway Analysis (IPA) of data from the peak of gene activation (3 h) showed that 90% of all evaluable genes were under the regulatory control of TNFa and/or IFNg (Fig 3A), and such regulators will hereafter be referred to as ‘inducers’.
Figure 3. Key networks contributing to the regulation of CpG-mediated gene expression.
All genes activated at 3 h (p <.00001) whose regulatory interactions could be mapped by IPA are shown. A) Network analysis identified two ‘inducers’ that each up-regulated >50% of all genes: IFNg (red network) and TNF (orange network). B) Genes that remain active through 24 h are highlighted in pink (p. <.00001) while those whose expression fell towards background are highlighted in green. This down-regulation is mediated by ‘supressors’, highlighted in blue. Green arrows identify genes down-regulated by ‘suppressors’. Note that 95% of the genes targeted by ‘suppressors’ are down-regulated (green circles) and that many of these ‘suppressors’ target the ‘inducer’ TNF.
Temporal pattern of gene regulation mediated by TLR9 engagement
Bioinformatic analysis showed that TNFa, IL1b, and IL1a acted as inducers of gene expression at 30 min and/or 1 hr (Table III)(Klaschik et al, 2008). These ‘inducers’ behaved similarly: the genes they regulated were stimulated rapidly but for only brief period (generally less than 24 hr). This differed from the pattern of gene activation attributable to IFNg, which became an ‘inducer’ at 3 hr post CpG stimulation and remained the dominant source of stimulation thereafter. Indeed, many of the genes triggered by IFNg were still up-regulated at 72 hr, by which time expression of most genes regulated by other inducers had waned (p < 0.001, Fig. 3A). Multiple inducers contributed to the up-regulation of genes shortly after CpG treatment (85% at 30 minutes, 75% at 1hr; 44% at 3 hr) whereas IFNG was frequently the sole inducer of genes that remained active at 24 – 72 hr (data not shown).
Table III.
Kinetics of CpG mediated gene up-regulation.
| Time (hr) | Regulatory genes at each time point (% of genes regulated) | ||
|---|---|---|---|
| 0.5 | TNF (54) | IL1B (62) | IL1A (85) |
| 1 | TNF (64) | IL1B (57) | |
| 3 | TNF (69) | IFNG (66) | |
IPA was used to identify ‘major inducers’ of gene activation (p<0.00001) from 0.5 – 3 hr after CpG ODN treatment of BALB/c mice. Results reflect the analysis of four biological replicates independently analyzed at each time point. Major inducers are defined as activating >50% of all evaluable genes up-regulated at a given time point.
Down-regulation of CpG-induced gene expression
The number of genes up-regulated by CpG ODN treatment peaked at 3 hr and then declined (Fig. 2). Bioinformatic analysis indicated that a small group of ‘suppressors’ contributed to this down-regulation. These ‘suppressors’ included IL10, MYC, NKKBIA SOCS1, SOCS3, IL1RN, and FOS (Fig. 3B) (Klaschik et al, 2007). Expression of virtually every gene (94%) targeted by these suppressors declined to background levels within 24 hr. These targets included the ‘inducers’ TNFa, IL1a and IL1b. Thus, the effect of ‘suppressors’ was magnified by their ability to inhibit the stimulatory networks whose expression relied upon these ‘inducers’. For example, the suppressors IL1RN, NFKBIA, SOCS1, SOCS3 and IL10 all targeted TNF, such that TNF mRNA levels declined 74% from 3 to 24 hr after CpG treatment. As the expression of TNF fell, 76% of the genes up-regulated by TNF also declined to background. These findings suggest that two factors influence the time-related decline in gene expression: 1) active suppression and 2) loss of induction.
DISCUSSION
DNA has multiple and complex effects on the immune system. CpG ODN trigger cells expressing TLR9 to initiate an immunostimulatory cascade culminating in the broad activation of the immune system and the production of Th1 and pro-inflammatory cytokines and chemokines (Ballas et al, 1996; Halpern et al, 1996; Hemmi et al, 2000; Ishii et al, 2002; Klinman et al, 1996; Krieg et al, 1995; Takeshita et al, 2001).
The ability of CpG ODN to trigger a strong innate immune response is being harnessed therapeutically to reduce host susceptibility to infection. Producing effective vaccines against conventional and biothreat pathogens is an important goal of such research. Towards that end, the utility of CpG ODN as an adjuvant for AVA was evaluated. Results show that CpG ODN both accelerate and magnify AVA induced immunity in mice, macaques and humans (Klinman et al, 2007; Klinman et al, 2006; Rynkiewicz et al, 2005; Xie et al, 2005). Moreover, the use of CpG ODN improve the persistence of protective immunity through two mechanisms: i) maintaining Ab titers in the protective range for longer periods and ii) generating a large and long-lived population of high affinity memory B cells that respond rapidly to challenge to protect animals whose Ab titers have declined (Tross et al, 2008).
There were important differences in the memory B cell response of mice vaccinated with CpG-adjuvanted AVA vs AVA alone. First, significantly more memory B cells were present in the spleens of mice vaccinated with CpG-adjuvanted vaccine (p. <.05, Table II). Second, these cells responded more rapidly to Ag stimulation, producing anti-PA Abs by day 3 post stimulation vs day 6 in mice vaccinated only with AVA. Finally, these B cells responded to lower concentrations of Ag, and produced Ab of higher affinity, that those from mice vaccinated with AVA alone (Table II)(Tross et al, 2008). These results are consistent with the in vivo observation that mice immunized with CpG-adjuvanted AVA responded rapidly to anthrax challenge by producing protective anti-PA Abs (Tross et al, 2008). The mechanism by which CpG ODN promote the induction of a long-lived high-affinity memory B cell response is under active investigation.
To better understand the mechanism by which CpG ODN activate immune-related genes, bioinformatic network analysis of microarray data was performed to identify the genes and regulatory networks triggered in vivo by CpG ODN (Klaschik et al, 2007; Klaschik et al, 2008). Results showed that a small group of ‘inducers’ was largely responsible for the patterned up-regulation of genes stimulated by CpG ODN administration. These ‘inducers’ included IL1a (30 min), IL1b (30 – 60 min), TNFa (1 – 3 hr) and IFNg (3 – 72 hr, Table III). Consistent with the known immunomodulatory properties of CpG ODN, the genes activated by these ‘inducers’ support the induction of a pro-inflammatory response. ‘Inducers’ frequently worked in tandem to control early gene expression.
CpG-dependent gene activation peaked at 3 hr and declined progressively thereafter. Two key mechanisms responsible for this decline in gene expression were identified. ‘Suppressors’ i) directly down-regulated the expression of targeted genes within 24 hr (Fig 3B) and ii) reduced the expression of ‘inducers’ (TNFa, IL1a and IL1b), thereby eroding the foundation upon which continued network stimulation relied (Klaschik et al, 2008). Based on these findings, we propose a model in which ‘inducers’ determine the order and magnitude with which specific genes are up-regulated following TLR9 engagement in vivo. The duration of gene activation reflects the persistence of the ‘inducer’ and whether the gene or it’s inducer are targeted by a suppressor. The magnitude of gene expression is increased when acted upon by multiple inducers and decreased when targeted by multiple suppressive mechanisms.
In summary, this work described studies designed to identify the regulatory pathways triggered by CpG ODN and the mechanisms by which immune genes are activated and then de-activated over time. This information is being harnessed to improve the ability of CpG ODN to act as vaccine adjuvants. As exemplified by the finding that CpG ODN improve the generation of high-affinity memory B cells, much remains to be learned about the impact of these agents on the immune system.
ACKNOWLEDGMENTS
The assertions herein are the private ones of the authors and are not to be construed as official or as reflecting the views of the NCI at large.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosure statement:
DMK is an inventor/co-inventor on several patents associated with CpG ODN. All rights to these patents are assigned to the US government.
REFERENCES
- Ballas ZD, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 1996;157:1840–1847. [PubMed] [Google Scholar]
- Bauer M, Redecke V, Ellwart JW, Sherer B, Kremer JP, Wagner H, Lipford GB. Bacterial CpG DNA triggers activation and maturation of human CD11c(−), CD123(+) dendritic cells. J. Immunol. 2001;166:5000–5007. doi: 10.4049/jimmunol.166.8.5000. [DOI] [PubMed] [Google Scholar]
- Branda RF, Moore AL, Lafayette AR, Mathews L, Hong R, Zon G, Brown T, McCormack JJ. Amplification of antibody production by phosphorothioate oligodeoxynucleotides. J. Lab. Clin. Med. 1996;128:329–338. doi: 10.1016/s0022-2143(96)90035-9. [DOI] [PubMed] [Google Scholar]
- Gursel M, Verthelyi D, Gursel I, Ishii KJ, Klinman DM. Differential and competitive activation of human immune cells by distinct classes of CpG oligodeoxynucleotides. J Leuko Biol. 2002;71:813–820. [PubMed] [Google Scholar]
- Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-gamma production by stimulation of IL-12 and tumor necrosis factor-alpha. Cell. Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- Ishii KJ, Takeshita F, Gursel I, Gursel M, Conover J, Nussenzweig A, Klinman DM. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J Exp. Med. 2002;196:269–274. doi: 10.1084/jem.20020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaschik S, Tross D, Klinman DM. Inductive and suppressive networks regulate TLR9-dependent gene expression in vivo. J Leuko Biol. 2008 doi: 10.1189/jlb.1008671. *** In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaschik S, Gursel I, Klinman DM. CpG-mediated changes in gene expression in murine spleen cells identified by microarray analysis. Mol. Immunol. 2007;44:1095–1104. doi: 10.1016/j.molimm.2006.07.283. [DOI] [PubMed] [Google Scholar]
- Klinman DM, Currie D, Lee G, Grippe V, Merkel T. Systemic but not mucosal immunity induced by AVA prevents inhalational anthrax. Microbes. Infect. 2007;9:1478–1483. doi: 10.1016/j.micinf.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman DM, xie H, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the licensed anthrax vaccine. Ann. N. Y. Acad. Sci. 2006;1082:137–150. doi: 10.1196/annals.1348.030. [DOI] [PubMed] [Google Scholar]
- Klinman DM, xie H, Little sF, Currie D, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine. 2004;22:2881–2886. doi: 10.1016/j.vaccine.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Klinman DM, Yi A, Beaucage SL, Conover J, Krieg AM. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNg. Proc. Natl. Acad. Sci. USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM. Antiinfective applications of toll-like receptor 9 agonists. Proc. Am. Thorac. Soc. 2007;4:289–294. doi: 10.1513/pats.200701-021AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM, Yi A, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–548. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lane HC, Montagne JL, Fauci AS. Bioterrorism: a clear and present danger. Nat. Med. 2001;7:1271–1273. doi: 10.1038/nm1201-1271. [DOI] [PubMed] [Google Scholar]
- McCluskie MJ, Davis HL. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 1998;161:4463–4466. [PubMed] [Google Scholar]
- Moldoveanu Z, Love-Homan L, Huang WQ, Krieg AM. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine. 1998;16:1216–1224. doi: 10.1016/s0264-410x(98)80122-9. [DOI] [PubMed] [Google Scholar]
- Pittman PR, Gibbs PH, Cannon TL, Friedlander AM. Anthrax vaccine: short-term safety experience in humans. Vaccine. 2001;20:972–978. doi: 10.1016/s0264-410x(01)00387-5. [DOI] [PubMed] [Google Scholar]
- Rynkiewicz D, Rathkopf M, Ransom J, Sim I, Giri L, Quinn J, Waytes T, Al-Adhami M, Johnson W, Nielsen C. ICAAC abstract. LB-25. 2005 [Google Scholar]
- Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116–2120. [PubMed] [Google Scholar]
- Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita F, Leifer CA, Gursel I, Ishii K, Takeshita S, Gursel M, Klinman DM. Cutting Edge: role of toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol. 2001;167:3555–3558. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- Tross D, Klinman DM. Effect of CpG oligonucleotides on vaccine-induced B cell memory. J. Immunol. 2008;181:5785–5790. doi: 10.4049/jimmunol.181.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Gursel I, Ivins BE, Singh M, O'Hagan DT, Ulmer JB, Klinman DM. CpG oligodeoxynucleotides adsorbed onto polylactide-co-glycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccine. Infect. Immun. 2005;73:828–833. doi: 10.1128/IAI.73.2.828-833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]