Abstract
We have previously reported that therapeutic immunization by intramuscular injection of optimized plasmid DNAs encoding SIV antigens effectively induces immune responses able to reduce viremia in antiretroviral therapy (ART)-treated SIVmac251 infected Indian rhesus macaques. We subjected such therapeutically immunized macaques to a second round of therapeutic vaccination using a combination of plasmids expressing SIV genes and the IL-15/IL-15 receptor alpha as molecular adjuvant, which were delivered by the more efficacious in vivo constant-current electroporation. A very strong induction of antigen-specific responses to Gag, Env, Nef, and Pol, during ART (1.2-1.6% of SIV-specific T cells in the circulating T lymphocytes) was obtained with the improved vaccination method. Immunological responses were characterized by the production of IFN-γ, IL–2, and TNFα either alone, or in combination as double or triple cytokine positive multifunctional T cells. A significant induction of CD4+ T cell responses, mainly targeting Gag, Nef, and Pol, as well as of CD8+ T cells, mainly targeting Env, was found in both T cells with central memory and effector memory markers. After release from ART, the animals showed a virological benefit with a further ∼1 log reduction in viremia. Vaccination with plasmid DNAs has several advantages over other vaccine modalities, including the possibility for repeated administration, and was shown to induce potent, efficacious, and long-lasting recall immune responses. Therefore, these data support the concept of adding DNA vaccination to the HAART regimen to boost the HIV-specific immune responses.
Introduction
Although the introduction of highly active antiretroviral therapy (HAART) resulted in a remarkable decrease of AIDS related deaths, the current pharmacological regimens to treat HIV infection fail to eradicate the virus and are associated with several problems, including drug toxicity, and development of resistance with viral rebound. In this context, new strategies to control viral replication and to restore immune functions are needed. During the period of antiretroviral therapy (ART), due to efficient control of viral replication, the virus specific cellular immune responses in the periphery are strongly reduced. After interruption of ART treatment, there is a rapid increase of viremia within 10 days. The introduction of new therapeutic interventions that boost immune responses would be beneficial for the clinical management of HIV-infected individuals. Towards this goal, studies were designed to boost the immune system of SIV-infected macaques during ART using DNA immunization. It was previously observed that vaccination during ART using DNA plasmids (Lisziewicz et al., 2005; Lori et al., 2005; Fuller et al., 2006; Lisziewicz et al., 2007; von Gegerfelt et al., 2007; Halwani et al., 2008; Zur Megede et al., 2008), pox-virus vectors (Hel et al., 2000; Tryniszewska et al., 2002), antigen-pulsed dendritic cells (Lu et al., 2003) or peptide-pulsed blood (De Rose et al., 2008) in SIVmac251-infected macaques was able to evoke SIV-specific recall immune responses. After release from ART, variable results regarding virological benefit were reported from no control (Zur Megede et al., 2008), temporal control (Hel et al., 2000; Tryniszewska et al., 2002; Fuller et al., 2006), to long-lasting control (Lori et al., 2003; Lu et al., 2003; Lisziewicz et al., 2005; von Gegerfelt et al., 2007; De Rose et al., 2008). Using DNA only as vaccine, two reports showed successful immunological and virological benefit, which are intramuscular injection (von Gegerfelt et al., 2007) and topical administration of DNA-based Dermavir (Lori et al., 2003; Lisziewicz et al., 2005). Importantly, the ability to induce immune responses able to reduce viremia could therefore offer an opportunity to use vaccination as an additional component to antiretroviral therapy. The use of DNA only as vaccination method is a promising immunization strategy that has advantages (production, stability, repeated use) over other vaccination modalities. Therapeutic vaccination in humans against HIV-1 has given mixed results. Some studies have reported an immunological and sometimes also a virological benefit, whereas others did not (Rosenberg et al., 2000; Markowitz et al., 2002; Lu et al., 2003; Lu et al., 2004; Kinloch-De Loes et al., 2005; Levy et al., 2005; Tubiana et al., 2005; Andrieu and Lu, 2007; Hardy et al., 2007; Connolly et al., 2008; Pialoux et al., 2008; Wilson et al., 2008). Several studies suggest that vaccination during highly active antiretroviral therapy (HAART) induces HIV-specific recall responses. The efficacy of therapeutic vaccines may be variable, and it is hypothesized that more consistent immunological and virological benefits could be achieved by improving the vaccination approaches.
We focused on DNA vaccination, since previous data in macaques have been encouraging and demonstrated strong immunogenicity, long-term decrease of viral load and a survival benefit in the animals with robust response to the therapeutic vaccination (Lori et al., 2003; Lisziewicz et al., 2005; von Gegerfelt et al., 2007). Recent developments to improve DNA delivery include in vivo electroporation (Aihara and Miyazaki, 1998; Mathiesen, 1999; Rizzuto et al., 1999; Selby et al., 2000; Widera et al., 2000; Mir, 2001;Wang et al., 2004b; Prud'homme et al., 2006; Draghia-Akli et al., 2008), which showed to be more efficient than traditional intramuscular injection in SIV/HIV DNAs vaccinating rhesus macaques and induced significantly increased antigen-specific immunity (Selby et al., 2000; Otten et al., 2004; Otten et al., 2006; Luckay et al., 2007; Hirao et al., 2008; Rosati et al., 2008; Zur Megede et al., 2008).
In this study, we examined whether repeated immunotherapeutic vaccination is of further virological benefit. We used macaques animals previously immunized during ART with plasmid DNAs encoding SIV antigens (von Gegerfelt et al., 2007) and, after release from ART, showed a long-lasting partial control of viremia without any signs of progression towards immunodeficiency. Of the eight control animals that were ART-treated only without receiving vaccination (von Gegerfelt et al., 2007), none controlled viremia after ART release and they subsequently developed AIDS. In this report, we tested whether macaques that benefited from the first cycle of immunotherapy could further benefit from a 2nd round of immunotherapeutic immunization using the combination of improved plasmid DNAs and DNA delivery by electroporation. Here, we report, the induction of strong and potent increases in SIV-specific immune responses in the immunized macaques followed by a further significant virological benefit after release from ART.
Materials and Methods
Animals
The Indian rhesus macaques (Macaca mulatta) included in the study were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International. Screening for MHC alleles was performed by PCR (D. Watkins, Wisconsin Regional Primate Center). Monkey 538L was Mamu A*01 positive and B*17 negative, whereas the monkeys 920L and 965L were both negative for Mamu A*01 and B*17. These SIVmac251 infected animals were previously involved in a therapeutic SIV DNA vaccination study (von Gegerfelt et al., 2007). Approximately 3 years after release from ART, the animals were enrolled into the present study and subjected to a 2nd round of therapeutic vaccination. During this second antiretroviral treatment period (31 weeks), the animals received the following therapeutic regimen: 20 mg/kg ((R)-9-(2-phosphonylmethoxypropyl) adenine (PMPA), and 50 mg/kg FTC, both injected subcutaneously once daily, and 5 mg/kg Didanosine (ddI), injected intravenously once daily. After two weeks, ddI was discontinued, and the dose of PMPA was reduced to 10mg/kg/day.
DNA vectors
All plasmids used in the study contain the human CMV promoter without an intron, the bovine growth hormone polyadenylation site, and the kanamycin resistance gene, pCMV.kan (Rosati et al., 2005). The RNA/codon optimized genes (Schwartz et al., 1992a; Schwartz et al., 1992b; Nasioulas et al., 1994; Schneider et al., 1997) for gag, pol, and env were generated upon introduction of multiple silent point mutations not affecting the sequence of the encoded proteins. The animals were vaccinated with a mixture of DNAs producing secreted and intracellularly degraded variants of the SIV Gag generated by N-terminal fusion with either IP10-MCP3 (Biragyn et al., 1999) (MCP3-p39gag, 21S) or with a beta-catenin-derived peptide (aa 18-47) (Aberle et al., 1997) (CATEgagDX, 2S) as previously described (Rosati et al., 2005; von Gegerfelt et al., 2007). The authentic SIVmac239 Env protein sequence (native Env, 99S) and a fusion of Env to IP10-MCP3 replacing its native signal peptide with IP10-MCP3 (MCP3-Env, 73S) were used. The optimized pol with inactivating mutations in PRT, RT and INT was inserted into pCMVLAMP.kan between the human LAMP-1 luminal domain and the LAMP-1 transmembrane and cytoplasmic (TM/cyt) tail domain (Chikhlikar et al., 2004), generating the plasmid LAMPpol (103S). Plasmid LAMP-NTV (147S) expresses a LAMP-Nef-Tat-Vif fusion protein. LAMP targets the antigen to lysosomes and lysosome-like compartments, which in antigen presenting cells also contain MHC molecules and was shown to affect the trafficking and immunogenicity of HIV-1 gag (Valentin et al.; Marques et al., 2003; De Arruda et al., 2004; Chikhlikar et al., 2006). The rhesus macaque (rm) IL-15 plasmid (AG65) contains the optimized IL-15 DNA sequence expressing a stable mRNA encoding an IL-15 that has the native signal peptide replaced by the tPA signal and propeptide (Jalah et al., 2007). Plasmid rmIL15Rα (AG120) expresses the optimized rhesus IL-15 receptor alpha (Bergamaschi et al., 2007) chain.
Therapeutic immunization
Highly purified, endotoxin-free DNA plasmid preparations were produced using the Qiagen kit (Hilden, Germany). The 1 ml DNA mixture contained 100 μg of each SIV plasmid and 200 μg of the cytokine plasmids, respectively (a total of 1 mg of plasmid DNA). These DNAs were injected intramuscularly (0.5 ml per injection) at the left and right thighs using in vivo electroporation by the CELLECTRA® adaptive constant-current electroporator (VGX Pharmaceuticals, Inc., The Woodlands, TX).
Flow cytometric analysis
Flow cytometric analysis was performed as described (Rosati et al., 2008). To determine the number and phenotype of SIV-specific cells, isolated PBMCs were incubated at a density of 106 cells/ml in RPMI-1640 supplemented with 10% FBS, 100 U/ml penicillin/streptomycin and 2 mM L-glutamine, in the presence of pools of 15-aa peptides overlapping by 11 aa, derived from SIV Gag, Pol, Env, Nef, and Tat at a final concentration of 1 μg/ml for each peptide. Cells were treated overnight with monensin to prevent protein secretion, and cell surface staining was performed using the following antibody cocktail: CD3-APCCy7, CD4-PerCPCy5.5, CD45RA-PE, CD28-biotin and Streptavidin-APCCy5.5 (BD Pharmingen, San Jose, CA) and CD8-AF405 (Caltag, Carlsbad, CA). Cells were washed twice, fixed, and permeabilized with Cytofix/Cytoperm (BD Pharmingen). Staining for intracellular cytokine detection was performed using the following antibody mixture: IFN-γ-FITC, IL-2-APC and TNF-α-PECy7 (BD Pharmingen). In some experiments, surface staining was performed with a different antibody cocktail including CD95-FITC, CCR7-APC, CD3-APCCy7, CD4-PerCPCy5.5, CD45RA-PE, CD8-AF405, CD28-Biotin and Streptavidin-APCCy5.5, and the presence of antigen specific cells was monitored in permeabilized cells by intracellular staining with anti-IFN-PECy7 mAb. This second staining allows the comparison of memory cell populations defined by CD28/CD45RA or CD28/CD95 expression. After intracellular staining, the cells were washed twice and the samples were analyzed in a FacsAria or LSRII flow cytometers (BD Pharmingen). All data analysis was performed using the FlowJo platform (Tree Star, Inc., Ashland, OR).
Humoral immune responses
Antibody production against Gag and Env was measured in serial dilutions of plasma by separate ELISA assays. The plates were analyzed at the absorbance of 450 nm (A450). The negative cutoff value was twice the mean A450 values obtained with non-immune sera. All samples with A450 value higher than the cutoff were considered positive. The binding antibody titers are reported as the reciprocal of the highest positive dilution.
Viral load analysis
SIV RNA copy numbers were determined by a real-time nucleic acid sequence-based isothermal amplification (NASBA) assay using SIVmac251-specific primers (Romano et al., 2000) with a threshold of detection of 50 copies/ml.
Results
Study design
We used SIVmac251-infected Indian rhesus macaques (538L, 920L, 965L), which were previously subjected to one round of therapeutic vaccination (von Gegerfelt et al., 2007). During the previous study, the animals received three DNA immunizations, using intramuscular needle injection, which resulted in a strong virological benefit as demonstrated by decreased viral loads. These animals were kept and were monitored following the 1st cycle of therapeutic immunization (3.1, 3.7 and 3.8 years for monkey 920L, 965L and 538L, respectively), and their viral loads remained below the levels measured prior to treatment, demonstrating a long-term benefit of therapeutic DNA vaccination.
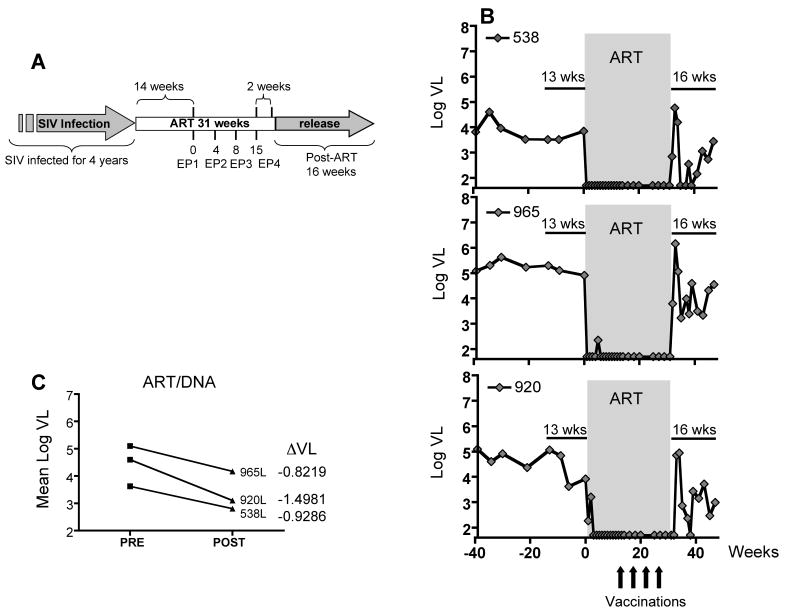
In the present study, we report data obtained after a 2nd round of therapeutic vaccination (ART/DNA) outlined in Fig. 1A. The animals were subjected to ART for 31 weeks using PMPA, FTC and ddI as described previously (von Gegerfelt et al., 2007). Starting at week 14 of ART (time point 0), the animals received 4 DNA vaccinations (week 0, 4, 8, and 15) by electroporation (EP) using the in vivo CELLECTRA® adaptive constant-current electroporator (VGX Pharmaceuticals, Inc.). Two weeks after EP4, ART treatment was terminated and the animals were monitored for another 16 wks (termination of the study).
FIG. 1.
Virological benefit from 2nd round of ART/DNA. (A) Study Outline. SIV-infected macaques were subjected to a 2nd round of ART/DNA after more than 3 years of infection. Previously, we reported the data obtained from the 1st cycle of ART/DNA (von Gegerfelt et al., 2007). After 3.1 to 3.8 years, the animals were subjected to a 2nd round of ART/DNA. The duration of the 2nd ART/DNA was 31 weeks and the animals were vaccinated by in vivo electroporation four times (EP1 to EP4) initiated at week 14 of ART. The animals were released from ART and monitored for another 16 weeks. (B) Viral loads of the three macaques during 2nd round of ART/DNA. Viral load data are shown for 39 weeks prior to ART (PRE), 31 weeks of ART/DNA and 16 weeks post release from ART (POST). (C) Mean virus loads and decrease in mean virus load (ΔVL) comparing 13 weeks PRE and 16 weeks POST treatment. The differences are statistically significant (paired T-test).
The SIV DNA mixture contained optimized expression vectors for different SIV antigens; antigen sequences were modified to alter their intracellular trafficking by fusions to either MCP-3, catenin (CATE) or human lysosomal membrane associated protein 1 (LAMP) (Rosati et al., 2005; von Gegerfelt et al., 2007; Rosati et al., 2008). The plasmid DNAs in vaccine mixture expressed Gag (MCP3gag, CATE-gag), Pol (LAMP-pol), Env (native and MCP3env), and the a Nef-Tat-Vif fusion to LAMP (LAMP-NTV) (Rosati et al., 2005; von Gegerfelt et al., 2007; Rosati et al., 2008). A combination of rhesus IL-15 and IL-15Rα expression plasmids (Bergamaschi et al., 2007; Jalah et al., 2007) was used as a molecular adjuvant. During the course of this study, the animals were monitored for the development of SIV-specific immune responses and changes in the viral loads.
Virological benefit of the 2nd therapeutic vaccination
Figure 1B shows the viral load data of the vaccinated macaques prior, during and after ART. During the period preceding the start of second ART (13 weeks), the animals showed mean viral loads ranging from 3.6 to 5.1 log10. All animals responded immediately to ART treatment, resulting in persistent control of viremia (<50 copies/ml plasma). After ART release, we observed a pattern of fluctuating viral loads as previously observed (von Gegerfelt et al., 2007). After the initial virus rebound, the viral load fluctuations were at levels lower than before ART/DNA, resulting in additional partial control of viremia. Importantly, the improved control of viremia was maintained during the 16 weeks of follow-up (Fig. 1B). At this point, the animals were enrolled in another study.
The mean viral loads during the 13 weeks before ART (PRE) and the 16 weeks after ART release (POST) were compared. Figure 1C shows the differential in viral loads (ΔVL) of the three animals as a result of the 2nd therapeutic vaccination. This analysis revealed a significant decrease (P=0.0356) in viremia with an average of ∼1 log10 in viral load reduction. A similar benefit in viremia reduction was found by comparing viral loads during 39 weeks PRE and 16 weeks POST ART. In conclusion, the reduction in plasma viral loads demonstrates that the 2nd round of therapeutic vaccination provides an additional virological benefit in these animals.
Increase of cellular immune responses
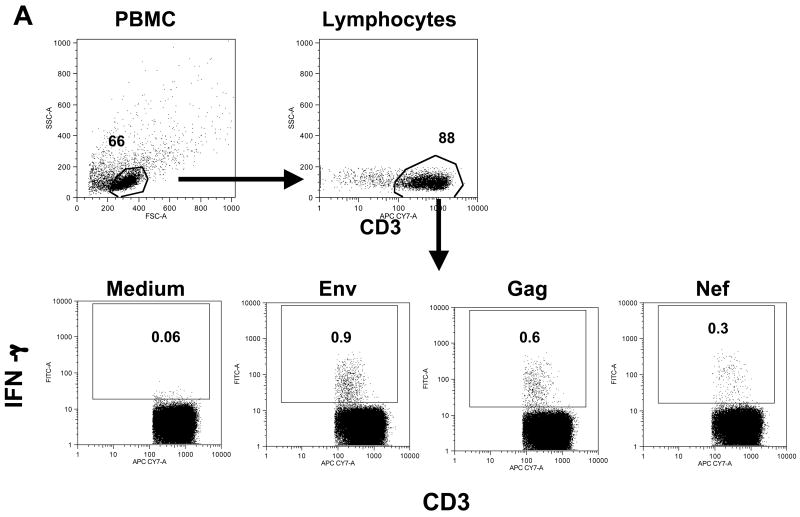
We studied the development of SIV-specific cellular immune responses in the peripheral blood from the immunized macaques using flow cytometric analysis. Figure 2A outlines the strategy used for the flow cytometric analysis to detect antigen-specific IFN-γ positive T cells using as example PBMC from animal 965L at 3 weeks post EP3. CD3+ T cells were identified within the main lymphocyte population, which was defined by forward and side scatter characteristics. The presence of SIV-specific T cells was determined by intracellular cytokine staining upon stimulation with peptide pools (15-aa peptides overlapping by 11 aa) or medium only, as negative control.
FIG. 2.
Increased SIV-specific IFN-γ producing T-cells in DNA immunized macaques during ART. (A) Identification of IFN-γ producing antigen-specific T cells by flow cytometry. PBMC stimulated with different peptide pools of SIV antigens were stained with a cocktail of cell surface antibodies as described in Material and Methods. The main lymphocyte population was gated based on forward and side scatter, and T cells were identified according to CD3 staining. Dot plots show the frequency of IFN-γ+ T cells upon stimulation with Env, Gag or Nef peptide pools. The background of the assay in the presence of medium alone is shown (data is from macaque 965L at week 3 post EP3). (B) The numbers of IFN-γ producing T cells of the three animals after stimulation with Env, Gag, Nef, Pol and Tat peptide pools, respectively, expressed per million circulating T lymphocytes are shown during ART/DNA and after release from ART.
Figure 2B shows the frequency of IFN-γ secreting T cells of the vaccinated animals determined upon stimulation with peptide pools spanning five of the six SIV proteins used for vaccination (Gag, Pol, Env, Nef, and Tat). At the day of the first vaccination, none of the animals showed detectable cellular immune responses, consistent with the absence of replicating SIV due to successful ART treatment. The first DNA vaccination by electroporation (EP1) immediately induced strong immune responses in all animals, at the range of ∼4,000-6,000 SIV-specific IFN-γ producing T cells/106 T cells. These high immune responses persisted for 4 weeks up to the day of EP2. After the 3rd vaccination, the SIV specific immune responses were further boosted to ∼12,000-16,000 IFN-γ producing T cells/106 T cells or 1.2-1.6% of SIV-specific T cells among the total circulating T lymphocyte population. After EP4, we only analyzed the 2-week post vaccination sample, since the animals were released from ART at this point of time. As observed for EP1, subsequent vaccinations not only boosted the recall of cellular immune responses, but these immune responses persisted.
We noted that individual animals responded to the different vaccine antigens to different extents. The recall immune responses in 538L were mainly targeted to Gag, whereas 965L had similar Env and Gag responses. In contrast, animal 920L had primarily an Env response and a very poor immune response to Gag (peak 790 and mean 460 IFN-γ+ T cells/106 T cells). The vaccination was unable to induce recall or de novo Gag-specific immune responses in this macaque. This observation suggests that the ability of 920L to respond to Gag may be exhausted, since this animal had shown higher Gag responses in the previous ART/DNA treatment (von Gegerfelt et al., 2007). All animals showed significant immune responses to the Nef peptide stimulation, while the responses to the Pol peptide pool were lower. We did not detect any significant immune responses to Tat and the responses to Vif were not analyzed.
To evaluate the efficacy of the DNA delivery by in vivo electroporation, we compared the responses in these animals to the data obtained previously after direct intramuscular DNA injection (von Gegerfelt et al., 2007). Immune responses after the 1st round of vaccination were measured by the ELIspot assay, therefore the comparison can only be approximate, comparing the cells producing IFN-γ per million (we could not repeat this measurements using intracellular cytokine staining because no frozen samples were available from the first DNA therapeutic vaccination). We noted that DNA vaccination via electroporation resulted in at least ∼10× higher levels of cellular immune responses compared to the direct IM injection. Therefore, in agreement with observations by us and others (Otten et al., 2004; Otten et al., 2006; Luckay et al., 2007; Hirao et al., 2008; Rosati et al., 2008; Zur Megede et al., 2008), vaccination with SIV/HIV DNA plasmids using electroporation is a potent method to achieve high immune responses in macaques.
After release from ART, we found a persistence of long-lasting SIV-specific cellular immune responses (∼4,000-14,000 IFN-γ+ T cells per million T cells) without any significant changes in the distribution of the antigens recognized by the T cells for at least 2 months (Fig. 2B). While the levels of cellular responses in animals 538L and 920L remain similar during ART and after release, the cellular immune responses in animal 965L decreased immediately after release from ART. We noted that the animals with higher levels of antigen-specific T cell responses showed lower mean viral loads, indicating control of viremia by the vaccine-elicited immune responses.
Characterization of T cell subsets induced by DNA vaccination
We next analyzed the phenotype of the antigen-specific IFN-γ producing T cells in more detail. The gating strategy is shown in Figs. 3A and 3B for animal 965L (3 weeks post EP3). Subsets of memory T cells were identified based on the staining with CD28 and CD45RA: CD3+CD45RA-CD28+ represents the population of central memory (CM) T cells and CD3+CD28- represents effector memory (EM) T cells (Fig. 3A). Cells with an EM phenotype were mainly CD8+ T cells (91% in macaque 965L), while the majority of CM cells were CD4+ T cells. These subsets were further examined for the presence of antigen-specific subpopulations as shown in the dot plots in Fig. 3B for the same animal (965L) and the data are summarized in Fig. 4 for all the animals.
FIG. 3.
Flow cytometric analysis of T cell subsets. (A) Identification of the subsets of CD4+ and CD8+ T cells. The T cells were divided in central memory (CM) and effector memory (EM) cells based on the pattern of CD28 and CD45RA expression: CD3+CD28+CD45RA- for CM T cells and CD3+CD28- for EM T cells. The two subsets of antigen experienced T cells were further divided in CD4+ and CD8+ populations. Cells with an EM phenotype were mainly CD8+ T cells (91% in macaque 965L), while the majority of CM cells were CD4+ T cells. (B) Identification of SIV-specific IFN-γ+ CM and EM T cells by flow cytometry. Dot plots show the presence of CD4+ and CD8+ T cells with CM and EM markers producing IFN-γ in the presence of Gag and Env SIV peptide pools or medium alone. Numbers inside the gates represent the percentage of IFN-γ+ T cells within the respective parent population. The data shown were obtained from the same animal 965L (3 weeks post EP3) as used in Fig. 3A.
FIG. 4.
Comparison of SIV-specific IFN-γ producing T cell subsets induced by immunization during ART. Frequency of SIV-specific CD4+ and CD8+ T cells with central memory (CM) or effector memory (EM) markers were determined as outlined in Fig. 4. Numbers indicate IFN-γ producing T cells after stimulation with Gag (A), Env (B), Nef (C), and Pol (D) peptide pools, expressed per million circulating T lymphocytes.
A second strategy to define CM and EM cells was also used, employing the CD28 and CD95 markers in parallel to the CD28 and CD45RA markers in samples stained with all three antibodies (Fig. 5). We also analyzed the frequency of the CD4+ and CD8+ T cells within each population. The comparison of the antigen-specific cells using either definition (CD3+CD45RA-CD28+ versus CD3+CD95+CD28+ for CM and CD3+CD28- versus CD3+CD95+CD28- for EM) showed no significant differences. Thus, both staining strategies give similar results for circulating T cells. These data indicate that either approach can be used to define CM and EM T cell subsets in macaque PBMC (Valentin et al.).
FIG. 5.
Flow cytometric analysis of SIV-specific memory T cell responses using different surface markers. This analysis shows that the frequency and phenotype of the antigen specific cells and of the memory T cell subsets are similar irrespective of the use of the combination of CD28 and CD45RA or CD28 and CD95 markers to define these T cell populations. Briefly, PBMC of macaque M538 (at 7 weeks post EP3) were analyzed by flow cytometry after peptide (env) stimulation and staining with monoclonal antibodies against different sets of markers as described in Materials and Methods. (A) The main lymphocyte population was gated based on forward and side scatter, and T cells were identified according to CD3 staining. These T cells were classified as effector memory (EM) and central memory (CM) cells based in the pattern of staining with CD28 and CD45RA (EM1 and CM1) or CD28 and CD95 (EM2 and CM2) (lower plots). (B) Phenotypic analysis of the antigen-specific (IFN-γ+) T cells. The frequency of Env-specific T cells was determined based on IFN-γ production. The cells were classified as EM1 and EM2 and CM1 and CM2 T cells according to the expression of either CD28 and CD45RA or CD28 and CD95, respectively, and the frequency of CD4+ and CD8+ T cells within each of these populations was determined. (C) Analysis of the frequency of IFN-γ+ Env-specific T cells, as well as the CD4+ and CD8+ distribution within the memory subsets as defined in panel A.
The responses against Gag (Fig. 4A) were dominated by CD4+ T cells with central memory phenotype, whereas the predominant T cell responses against Env (Fig. 4B) consisted of CD8+ T cells with effector phenotype. Low levels of CD8+ cells with CM phenotype could be detected in all three animals in response to both Gag and Env peptide stimulation. Similar to Gag, Nef responses (Fig. 4C) were predominantly produced by CD4+ T cells, which for 538L and 965L were almost exclusively cells with CM phenotype. The responses to Pol (Fig. 4D) were quantitatively the lowest and were predominantly CD4+ with CM phenotype in 965L and 920L, and a mixture of CD4+ and CD8+ with CM and EM phenotype in macaque 538L. In conclusion, vaccination by in vivo electroporation during ART potently induced SIV-specific recall immune responses in different T-cell subsets. The nature of the response was also influenced by the specific antigen, as shown by comparison of the Gag and Env responses (predominantly CM CD4 for Gag versus EM CD8 for Env).
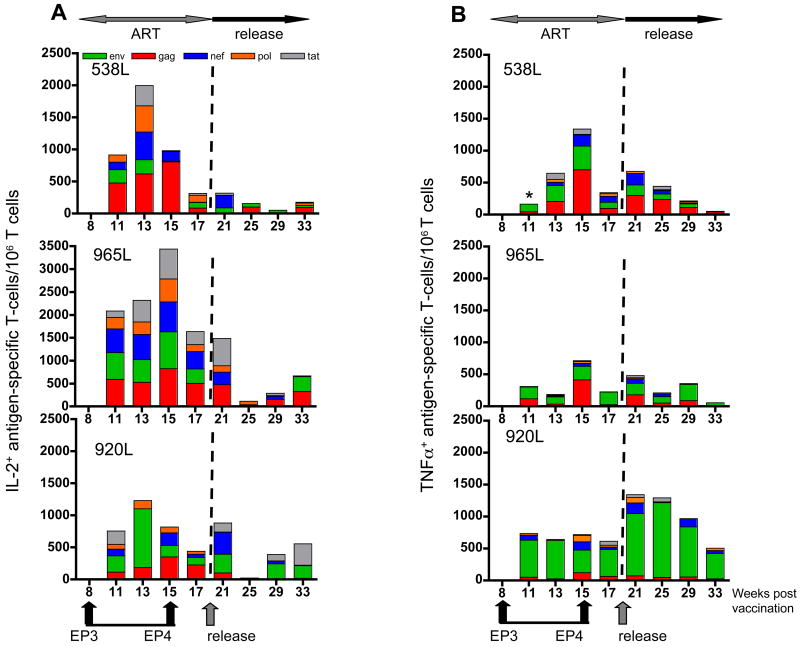
Vaccination by electroporation induces SIV-specific T cells producing IL-2 or TNFα
We further asked whether the antigen-specific T cells in these vaccinated animals produce other cytokines, such as IL-2 or TNFα (Fig. 6), in addition to IFN-γ production shown above (Figs. 2 through 5). Although we found high levels of IFN-γ-producing SIV-specific T cells after EP1 (Fig. 2B), we failed to detect SIV-specific T cells producing either IL-2 (Fig. 6A) or TNFα (Fig. 6B) until 3 weeks post EP3. From this time point on, a significant number of SIV-specific T cells producing IL-2 (Fig. 6A) and to a lesser extent TNFα (Fig. 6B) could be detected. We found these responses to be induced by all the peptide pools tested (Gag, Env, Pol, Nef, Tat). We observed that in comparison to IFN-γ (see Fig. 2B), the ratio of the responses to the individual antigens is changed for the IL-2 and TNFα producing cells, i.e. there are proportionally less responses to Gag and Env. As noted for the IFN-γ responses, animal 920L showed predominant Env-specific IL-2 and TNFα responses, further supporting the conclusion that this animal could no longer respond to Gag.
FIG. 6.
Induction of SIV-specific IL-2- and TNFα-producing T cells induced by DNA vaccination during ART. The analysis was performed using similar strategy as described for Fig. 3. IL-2 (A) and TNFα (B) producing T cells after stimulation with Env, Gag, Nef, Pol, and Tat peptide pools, respectively, were expressed per million circulating T lymphocytes. The ART and release periods are indicated. *, Tat-specific immune response was not determined.
Vaccination by electroporation induces multifunctional SIV-specific T cells
Having established that vaccination by in vivo electroporation induces SIV-specific T cells able to produce significant levels of IFN-γ, IL-2 or TNFα, we further analyzed the population of antigen-specific T cells producing 2 or more cytokines. First, we analyzed double positive cells secreting IFN-γ in combination with IL-2 (Fig. 7A) and with TNFα (Fig. 7B), respectively. We found dual cytokine producing T cells induced by the Gag, Env and Nef peptide pools, respectively, in all three animals. We noted that the levels of IFN-γ+IL-2+ cells were significantly higher, reflecting the respective higher single-positive levels. The dual cytokine producing T cells responded mainly to Gag, Env, and Nef, and no significant responses were induced upon stimulation by the Pol and Tat peptide pools. As observed for the single cytokine producing T cells, the nature of the responses varied among the animals with a major focus on Gag in animals 538L and 965L and on Env in animal 920L.
FIG. 7.
Induction of dual cytokine positive SIV-specific T cells in vaccinated animals. The dual IFN-γ plus IL-2 (A) and IFN-γ plus TNFα (B) producing SIV-specific (Gag, Env, Nef) T cells are indicated.
In addition to dual positive cells, DNA vaccination also induced SIV specific triple cytokine positive cells. Figure 8 shows a comparison of the levels of the double and triple cytokine producing SIV-specific (Gag, Env and Nef) T cells. For all animals, dual (IFN-γ plus IL-2; IFN-γ plus TNFα) as well as triple (IFN-γ, IL-2 and TNFα) positive cells were induced. Together, these findings indicate that vaccination by electroporation using the optimized DNA mixture is effective and potently induces SIV specific recall responses in T cells with multifunctional phenotypes. After ART release, the polyfunctional responses persisted in 2 of the 3 animals, but not in animal 965L that also had a significant drop of single IFN-γ responses (see Fig. 2B).
FIG. 8.
Induction of multifunctional SIV-specific T cells. A comparison of the levels of double positive (from Fig. 7; light grey IFNγ+IL-2+; dark grey and IFNγ+ TNFα+) and triple positive (IFNγ+ IL-2+ TNFα+; striped bar) total SIV specific T cells is shown.
Humoral immune responses
We also monitored the humoral immune responses before, during, and after release from ART/DNA. Figure 9 shows the reciprocal levels of binding antibody against Env (Fig. 9A) and Gag (Fig. 9B), respectively. Our results show that the humoral immune responses decline during ART and the vaccination period, resulting in a reduction of ∼0.5 log10 for Env (A) and ∼1-2 log10 for Gag (B). These data indicate that immunization with DNA only is insufficient to maintain or boost the humoral immune responses, and this is likely due to the relative low levels of protein produced upon vaccination. After release from ART, the animals showed anamnestic humoral immune responses to Gag and Env reaching the pre-ART levels as response to the replicating SIV after ART release. Therefore, DNA electroporation maintained the T helper type 1 nature of DNA vaccination.
FIG. 9.
Humoral immune responses during ART and DNA vaccination and the period after release from ART. The presence of binding antibodies to Env (gp120) (A) and to Gag p27 (B) was measured in plasma prior, during and after therapy.
Discussion
DNA based immunization is an attractive vaccination approach because its production is simple and cost effective, it can be repeatedly administered and it can be combined with other vaccine modalities and molecular adjuvants. Although several trials of DNA vaccination in humans have shown encouraging, though variable results (Mwau et al., 2004; Graham et al., 2006; Catanzaro et al., 2007; Eller et al., 2007; Tavel et al., 2007; Bansal et al., 2008; Gorse et al., 2008; Jaoko et al., 2008; Kutzler and Weiner, 2008; Wang et al., 2008; Wilson et al., 2008), it appears that naked DNA delivery and expression is inefficient in primates compared nonhuman primates and rodents, which is one key drawback for using DNA vaccination. Different strategies are being developed to improve the efficiency of DNA gene delivery include the combination of antigen expressing plasmids with vectors producing cytokines, or the use of DNA as prime in combination with recombinant virus or protein boost [(Hartikka et al., 2001; Fuller et al., 2002; Lori et al., 2003; Bertley et al., 2004; Wang et al., 2004a; Lisziewicz et al., 2005; Dale et al., 2006; Duerr et al., 2006; Girard et al., 2006; Hokey and Weiner, 2006; Liu et al., 2006; Lori et al., 2006; Lu, 2006; Mcmichael, 2006; Rodriguez-Chavez et al., 2006; Brave et al., 2007; Hinkula, 2007; Thorner and Barouch, 2007; Kutzler and Weiner, 2008; Manrique et al., 2008; Wang et al., 2008)]. The development of DNA delivery by in vivo electroporation is an important advance for DNA delivery (Aihara and Miyazaki, 1998; Mathiesen, 1999; Rizzuto et al., 1999; Selby et al., 2000; Widera et al., 2000; Mir, 2001; Wang et al., 2004b; Prud'homme et al., 2006; Draghia-Akli et al., 2008), and initial studies with DNAs producing HIV and SIV antigens have shown great improvement in gene expression as shown in this report and by others (Selby et al., 2000; Widera et al., 2000; Otten et al., 2004; Otten et al., 2006; Luckay et al., 2007; Halwani et al., 2008; Hirao et al., 2008; Rosati et al., 2008; Zur Megede et al., 2008).
Using DNA only as vaccination modality, we had previously demonstrated a significant virological benefit in a group of SIVmac251 infected animals (von Gegerfelt et al., 2007), resulting in ∼1 log10 drop in viremia. Importantly, the animals had maintained this reduced viral loads for more than 3 years. None of the control animals, which were subjected to ART treatment only without DNA vaccination, showed reduction of viral loads after release from ART (von Gegerfelt et al., 2007) and subsequently developed AIDS and were no longer available. Based on the success of the first therapeutic vaccination (von Gegerfelt et al., 2007), the current study was designed to examine the efficacy of a 2nd round of therapeutic immunization using macaques from that study. We decided to apply the 2nd therapeutic DNA vaccination by using the more potent in vivo electroporation as DNA delivery methodology, that further allowed detailed measurement of the development of SIV-specific immune responses.
We show that combination of optimized DNA vectors and in vivo electroporation induced high levels of SIV-specific cellular immune responses in the ART-treated SIV-infected animals. It is noteworthy that direct DNA intramuscular immunization into SIV-infected ART-treated rhesus macaques induced recall responses to all the antigens produced by the DNA mixture, but to a lower extent (von Gegerfelt et al., 2007; Halwani et al., 2008) when compared to the more effective in vivo electroporation, as shown in this report and by others (Widera et al., 2000; Otten et al., 2004; Otten et al., 2006; Luckay et al., 2007; Hirao et al., 2008; Rosati et al., 2008; Zur Megede et al., 2008). Thus, in vivo electroporation of plasmid DNAs producing SIV antigens induces higher and longer-lasting primary as well as recall immune responses in macaques. We typically observed that immune responses peaked at 4 weeks post immunization. The large increase in immune responses revealed up to 1.6% of SIV-specific IFN-γ-producing T cells in the blood. Importantly, the efficient immunization method led to the induction of high levels of both CD4+ and CD8+ antigen-specific T cells. A large proportion of these SIV antigen-specific cells had markers of effector memory. Although DNA vaccination resulted in both CD4+ and CD8+ T cell memory and effector cells, we noted that the type of antigen affected the responses: Gag, Pol and Nef induced mainly CD4+ T cell responses, whereas Env induced a higher CD8+ T cell response. Importantly, the vaccination effects were long-lasting and also led to significant development of multifunctional SIV-specific immune responses. These findings demonstrate the potency of DNA vaccination in inducing broad and diverse cellular immune responses. In contrast to the changes in the cellular immune responses, we observed no increase in the humoral immune responses. On the contrary, we found a rather significant decline for both Gag and Env binding antibody titers during ART/DNA vaccination, as reported during the 1st immunotherapy cycle (von Gegerfelt et al., 2007). Thus, DNA vaccination by in vivo electroporation in SIV-infected macaques continues to produce a polarized Th1 immune response. It is likely that the combination of DNA with a protein boost is necessary to activate higher antibody responses in chronically infected ART-treated animals.
Upon release from ART, the animals showed an initial virus rebound, followed by several viral load fluctuations, resulting in viral loads lower than before ART/DNA. We previously reported such fluctuations in virus levels that ultimately resulted in reduced viremia (von Gegerfelt et al., 2007). This manifestation is indicative of active immune control. Although the underlying reason for the fluctuation is not known, we anticipate that the initial virus rebound is eliminated by the immune system and a new homeostasis is achieved, usually after 2-3 fluctuations, resulting in reduced virus loads compared to pre-ART levels.
It is important to note that the animals involved in this study were infected by SIV for long periods of time. ART treatment only was reported to lead to complete or partial virus control after ART discontinuation uniquely in animals treated very early after infection (Tsai et al., 1998; Van Rompay et al., 1999; Emau et al., 2006), which is different than the experience with chronically infected macaques (Hel et al., 2000; Tryniszewska et al., 2002; Lisziewicz et al., 2005; Fuller et al., 2006; von Gegerfelt et al., 2007) and humans (Chun et al., 1999; Davey et al., 1999; Garcia et al., 1999; Neumann et al., 1999; Ortiz et al., 1999; Ortiz et al., 2001; Oxenius et al., 2002), where the virus rebounds rapidly to levels similar to those prior to ART. This may be a critical difference with a recently reported study (Zur Megede et al., 2008) in which the animals were treated with ART early after infection and DNA vaccination failed to show a virological benefit over the benefit achieved by ART only.
Important for the success of the immunotherapeutic vaccination is not only the quality of the DNA and the DNA delivery, but also the successful control of viremia during ART. Animals partially controlling viremia (median viral load of 4.9 log10) and not receiving ART during the DNA vaccination period did not show immunological or virologic benefit even with the more efficient EP DNA vaccination method. This observation is in agreement with previous studies (Hel et al., 2000; von Gegerfelt et al., 2007), which reported that animals that lost control of viremia due to development of drug resistance or non-adherence to drug treatment did not benefit from immunotherapy. Therefore, potent control of viremia using effective ART treatment is an essential part of the regimen in addition to the use of optimized DNA and efficient DNA delivery to induce high and long-lasting recall immune responses.
An important conclusion of our study is that rhesus macaques chronically infected with SIV benefited from a 2nd round of immunotherapy during ART. The combination of a cocktail of optimized SIV DNA plasmids and of efficient DNA delivery by in vivo electroporation was critical for achieving high levels of long-lasting recall immune responses. Furthermore DNA vaccination provided a virological benefit, since the therapeutically vaccinated macaques were able to further lower viremia upon release from ART. The 2nd round of immunotherapy demonstrated that an additional virological benefit can be obtained by repeated ART/DNA vaccination. In summary, our data provide support for a novel immunotherapeutic vaccination approach, which could be an addition to anti-retroviral drug therapy.
Acknowledgments
We thank D. Weiss, J. Treece, R. Pal, P. Markham and the staff at Advanced BioScience Laboratories, Kensington, MD for their expert help; N. Bischofsberger (Gilead Sciences, Inc.) for PMPA and FTC. We thank T. Jones for editorial assistance. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16:867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- Andrieu JM, Lu W. A dendritic cell-based vaccine for treating HIV infection: background and preliminary results. J Intern Med. 2007;261:123–131. doi: 10.1111/j.1365-2796.2006.01738.x. [DOI] [PubMed] [Google Scholar]
- Bansal A, Jackson B, West K, Wang S, Lu S, Kennedy JS, Goepfert PA. Multifunctional T-cell characteristics induced by a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine regimen given to healthy adults are dependent on the route and dose of administration. J Virol. 2008;82:6458–6469. doi: 10.1128/JVI.00068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, Zhang GM, Patel V, Felber BK, Pavlakis GN. Intracellular interaction of interleukin IL-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2007;283:4189–4199. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- Bertley FM, Kozlowski PA, Wang SW, Chappelle J, Patel J, Sonuyi O, Mazzara G, Montefiori D, Carville A, Mansfield KG, Aldovini A. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J Immunol. 2004;172:3745–3757. doi: 10.4049/jimmunol.172.6.3745. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253–258. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- Brave A, Ljungberg K, Wahren B, Liu MA. Vaccine delivery methods using viral vectors. Mol Pharm. 2007;4:18–32. doi: 10.1021/mp060098+. [DOI] [PubMed] [Google Scholar]
- Catanzaro AT, Roederer M, Koup RA, Bailer RT, Enama ME, Nason MC, Martin JE, Rucker S, Andrews CA, Gomez PL, Mascola JR, Nabel GJ, Graham BS. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25:4085–4092. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- Chikhlikar P, Barros De Arruda L, Agrawal S, Byrne B, Guggino W, August JT, Marques ET., Jr Inverted terminal repeat sequences of adeno-associated virus enhance the antibody and CD8(+) responses to a HIV-1 p55Gag/LAMP DNA vaccine chimera. Virology. 2004;323:220–232. doi: 10.1016/j.virol.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Chikhlikar P, De Arruda LB, Maciel M, Silvera P, Lewis MG, August JT, Marques ET. DNA encoding an HIV-1 Gag/human lysosome-associated membrane protein-1 chimera elicits a broad cellular and humoral immune response in Rhesus macaques. PLoS ONE. 2006;1:e135. doi: 10.1371/journal.pone.0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Davey RT, Jr, Engel D, Lane HC, Fauci AS. Re-emergence of HIV after stopping therapy. Nature. 1999;401:874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- Connolly NC, Whiteside TL, Wilson C, Kondragunta V, Rinaldo CR, Riddler SA. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) peptide-loaded dendritic cells is safe and induces immunogenicity in HIV-1-infected individuals. Clin Vaccine Immunol. 2008;15:284–292. doi: 10.1128/CVI.00221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CJ, Thomson S, De Rose R, Ranasinghe C, Medveczky CJ, Pamungkas J, Boyle DB, Ramshaw IA, Kent SJ. Prime-boost strategies in DNA vaccines. Methods Mol Med. 2006;127:171–197. doi: 10.1385/1-59745-168-1:171. [DOI] [PubMed] [Google Scholar]
- Davey RT, Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, Kovacs JA, Polis MA, Walker RE, Falloon J, Masur H, Gee D, Baseler M, Dimitrov DS, Fauci AS, Lane HC. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arruda LB, Chikhlikar PR, August JT, Marques ET. DNA vaccine encoding human immunodeficiency virus-1 Gag, targeted to the major histocompatibility complex II compartment by lysosomal-associated membrane protein, elicits enhanced long-term memory response. Immunology. 2004;112:126–133. doi: 10.1111/j.1365-2567.2004.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rose R, Fernandez CS, Smith MZ, Batten CJ, Alcantara S, Peut V, Rollman E, Loh L, Mason RD, Wilson K, Law MG, Handley AJ, Kent SJ. Control of viremia and prevention of AIDS following immunotherapy of SIV-infected macaques with peptide-pulsed blood. PLoS Pathog. 2008;4:e1000055. doi: 10.1371/journal.ppat.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draghia-Akli R, Khan AS, Brown PA, Pope MA, Wu L, Hirao L, Weiner DB. Parameters for DNA vaccination using adaptive constant-current electroporation in mouse and pig models. Vaccine. 2008;26:5230–5237. doi: 10.1016/j.vaccine.2008.03.071. [DOI] [PubMed] [Google Scholar]
- Duerr A, Wasserheit JN, Corey L. HIV vaccines: new frontiers in vaccine development. Clin Infect Dis. 2006;43:500–511. doi: 10.1086/505979. [DOI] [PubMed] [Google Scholar]
- Eller MA, Eller LA, Opollo MS, Ouma BJ, Oballah PO, Galley L, Karnasuta C, Kim SR, Robb ML, Michael NL, Kibuuka H, Wabwire-Mangen F, Graham BS, Birx DL, De Souza MS, Cox JH. Induction of HIV-specific functional immune responses by a multiclade HIV-1 DNA vaccine candidate in healthy Ugandans. Vaccine. 2007;25:7737–7742. doi: 10.1016/j.vaccine.2007.08.056. [DOI] [PubMed] [Google Scholar]
- Emau P, Jiang Y, Agy MB, Tian B, Bekele G, Tsai CC. Post-exposure prophylaxis for SIV revisited: animal model for HIV prevention. AIDS Res Ther. 2006;3:29. doi: 10.1186/1742-6405-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DH, Rajakumar PA, Wilson LA, Trichel AM, Fuller JT, Shipley T, Wu MS, Weis K, Rinaldo CR, Haynes JR, Murphey-Corb M. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J Virol. 2002;76:3309–3317. doi: 10.1128/JVI.76.7.3309-3317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DH, Rajakumar PA, Wu MS, Mcmahon CW, Shipley T, Fuller JT, Bazmi A, Trichel AM, Allen TM, Mothe B, Haynes JR, Watkins DI, Murphey-Corb M. DNA immunization in combination with effective antiretroviral drug therapy controls viral rebound and prevents simian AIDS after treatment is discontinued. Virology. 2006;348:200–215. doi: 10.1016/j.virol.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Garcia F, Plana M, Vidal C, Cruceta A, O'brien WA, Pantaleo G, Pumarola T, Gallart T, Miro JM, Gatell JM. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. Aids. 1999;13:F79–86. doi: 10.1097/00002030-199907300-00002. [DOI] [PubMed] [Google Scholar]
- Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: the human immunodeficiency virus (HIV) Vaccine. 2006;24:4062–4081. doi: 10.1016/j.vaccine.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Gorse GJ, Baden LR, Wecker M, Newman MJ, Ferrari G, Weinhold KJ, Livingston BD, Villafana TL, Li H, Noonan E, Russell ND. Safety and immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 2008;26:215–223. doi: 10.1016/j.vaccine.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Graham BS, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, Martin JE, Mccluskey MM, Chakrabarti BK, Lamoreaux L, Andrews CA, Gomez PL, Mascola JR, Nabel GJ. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J Infect Dis. 2006;194:1650–1660. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halwani R, Boyer JD, Yassine-Diab B, Haddad EK, Robinson TM, Kumar S, Parkinson R, Wu L, Sidhu MK, Phillipson-Weiner R, Pavlakis GN, Felber BK, Lewis MG, Shen A, Siliciano RF, Weiner DB, Sekaly RP. Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA + IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J Immunol. 2008;180:7969–7979. doi: 10.4049/jimmunol.180.12.7969. [DOI] [PubMed] [Google Scholar]
- Hardy GA, Imami N, Nelson MR, Sullivan AK, Moss R, Aasa-Chapman MM, Gazzard B, Gotch FM. A phase I, randomized study of combined IL-2 and therapeutic immunisation with antiretroviral therapy. J Immune Based Ther Vaccines. 2007;5:6. doi: 10.1186/1476-8518-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikka J, Bozoukova V, Ferrari M, Sukhu L, Enas J, Sawdey M, Wloch MK, Tonsky K, Norman J, Manthorpe M, Wheeler CJ. Vaxfectin enhances the humoral immune response to plasmid DNA-encoded antigens. Vaccine. 2001;19:1911–1923. doi: 10.1016/s0264-410x(00)00445-x. [DOI] [PubMed] [Google Scholar]
- Hel Z, Venzon D, Poudyal M, Tsai WP, Giuliani L, Woodward R, Chougnet C, Shearer G, Altman JD, Watkins D, Bischofberger N, Abimiku A, Markham P, Tartaglia J, Franchini G. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med. 2000;6:1140–1146. doi: 10.1038/80481. [DOI] [PubMed] [Google Scholar]
- Hinkula J. Clarification of how HIV-1 DNA and protein immunizations may be better used to obtain HIV-1-specific mucosal and systemic immunity. Expert Rev Vaccines. 2007;6:203–212. doi: 10.1586/14760584.6.2.203. [DOI] [PubMed] [Google Scholar]
- Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, Betts MR, Draghia-Akli R, Weiner DB. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26:3112–3120. doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokey DA, Weiner DB. DNA vaccines for HIV: challenges and opportunities. Springer Semin Immunopathol. 2006;28:267–279. doi: 10.1007/s00281-006-0046-z. [DOI] [PubMed] [Google Scholar]
- Jalah R, Rosati R, Kulkarni V, Patel V, Bergamaschi C, Valentin A, Zhang GM, Sidhu MK, Eldridge JH, Weiner DB, Pavlakis GN, Felber BK. Efficient systemic expression of bioactive IL-15 in mice upon delivery of optimized DNA expression plasmids. DNA Cell Biol. 2007;26:827–840. doi: 10.1089/dna.2007.0645. [DOI] [PubMed] [Google Scholar]
- Jaoko W, Nakwagala FN, Anzala O, Manyonyi GO, Birungi J, Nanvubya A, Bashir F, Bhatt K, Ogutu H, Wakasiaka S, Matu L, Waruingi W, Odada J, Oyaro M, Indangasi J, Ndinya-Achola J, Konde C, Mugisha E, Fast P, Schmidt C, Gilmour J, Tarragona T, Smith C, Barin B, Dally L, Johnson B, Muluubya A, Nielsen L, Hayes P, Boaz M, Hughes P, Hanke T, Mcmichael A, Bwayo J, Kaleebu P. Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine. 2008;26:2788–2795. doi: 10.1016/j.vaccine.2008.02.071. [DOI] [PubMed] [Google Scholar]
- Kinloch-De Loes S, Hoen B, Smith DE, Autran B, Lampe FC, Phillips AN, Goh LE, Andersson J, Tsoukas C, Sonnerborg A, Tambussi G, Girard PM, Bloch M, Battegay M, Carter N, El Habib R, Theofan G, Cooper DA, Perrin L. Impact of therapeutic immunization on HIV-1 viremia after discontinuation of antiretroviral therapy initiated during acute infection. J Infect Dis. 2005;192:607–617. doi: 10.1086/432002. [DOI] [PubMed] [Google Scholar]
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y, Gahery-Segard H, Durier C, Lascaux AS, Goujard C, Meiffredy V, Rouzioux C, Habib RE, Beumont-Mauviel M, Guillet JG, Delfraissy JF, Aboulker JP. Immunological and virological efficacy of a therapeutic immunization combined with interleukin-2 in chronically HIV-1 infected patients. Aids. 2005;19:279–286. [PubMed] [Google Scholar]
- Lisziewicz J, Calarota SA, Lori F. The potential of topical DNA vaccines adjuvanted by cytokines. Expert Opin Biol Ther. 2007;7:1563–1574. doi: 10.1517/14712598.7.10.1563. [DOI] [PubMed] [Google Scholar]
- Lisziewicz J, Trocio J, Xu J, Whitman L, Ryder A, Bakare N, Lewis MG, Wagner W, Pistorio A, Arya S, Lori F. Control of viral rebound through therapeutic immunization with DermaVir. Aids. 2005;19:35–43. doi: 10.1097/00002030-200501030-00004. [DOI] [PubMed] [Google Scholar]
- Liu MA, Wahren B, Karlsson Hedestam GB. DNA vaccines: recent developments and future possibilities. Hum Gene Ther. 2006;17:1051–1061. doi: 10.1089/hum.2006.17.1051. [DOI] [PubMed] [Google Scholar]
- Lori F, Kelly LM, Lisziewicz J. Immunological approaches for HIV therapy. Curr Drug Targets Infect Disord. 2003;3:171–178. doi: 10.2174/1568005033481204. [DOI] [PubMed] [Google Scholar]
- Lori F, Trocio J, Bakare N, Kelly LM, Lisziewicz J. DermaVir, a novel HIV immunisation technology. Vaccine. 2005;23:2030–2034. doi: 10.1016/j.vaccine.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Lori F, Weiner DB, Calarota SA, Kelly LM, Lisziewicz J. Cytokine-adjuvanted HIV-DNA vaccination strategies. Springer Semin Immunopathol. 2006;28:231–238. doi: 10.1007/s00281-006-0047-y. [DOI] [PubMed] [Google Scholar]
- Lu S. Combination DNA plus protein HIV vaccines. Springer Semin Immunopathol. 2006;28:255–265. doi: 10.1007/s00281-006-0028-1. [DOI] [PubMed] [Google Scholar]
- Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- Lu W, Wu X, Lu Y, Guo W, Andrieu JM. Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med. 2003;9:27–32. doi: 10.1038/nm806. [DOI] [PubMed] [Google Scholar]
- Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, Garcia-Hand D, Abdullah R, Braun R, Montefiori DC, Rosati M, Felber BK, Pavlakis GN, Mathiesen I, Israel ZR, Eldridge JH, Egan MA. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007;81:5257–5269. doi: 10.1128/JVI.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique M, Micewicz E, Kozlowski PA, Wang SW, Aurora D, Wilson RL, Ghebremichael M, Mazzara G, Montefiori D, Carville A, Mansfield KG, Aldovini A. DNA-MVA vaccine protection after X4 SHIV challenge in macaques correlates with day-of-challenge antiviral CD4+ cell-mediated immunity levels and postchallenge preservation of CD4+ T cell memory. AIDS Res Hum Retroviruses. 2008;24:505–519. doi: 10.1089/aid.2007.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz M, Jin X, Hurley A, Simon V, Ramratnam B, Louie M, Deschenes GR, Ramanathan M, Jr, Barsoum S, Vanderhoeven J, He T, Chung C, Murray J, Perelson AS, Zhang L, Ho DD. Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J Infect Dis. 2002;186:634–643. doi: 10.1086/342559. [DOI] [PubMed] [Google Scholar]
- Marques ET, Jr, Chikhlikar P, De Arruda LB, Leao IC, Lu Y, Wong J, Chen JS, Byrne B, August JT. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J Biol Chem. 2003;278:37926–37936. doi: 10.1074/jbc.M303336200. [DOI] [PubMed] [Google Scholar]
- Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 1999;6:508–514. doi: 10.1038/sj.gt.3300847. [DOI] [PubMed] [Google Scholar]
- Mcmichael AJ. HIV vaccines. Annu Rev Immunol. 2006;24:227–255. doi: 10.1146/annurev.immunol.24.021605.090605. [DOI] [PubMed] [Google Scholar]
- Mir LM. Therapeutic perspectives of in vivo cell electropermeabilization. Bioelectrochemistry. 2001;53:1–10. doi: 10.1016/s0302-4598(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Mwau M, Cebere I, Sutton J, Chikoti P, Winstone N, Wee EG, Beattie T, Chen YH, Dorrell L, Mcshane H, Schmidt C, Brooks M, Patel S, Roberts J, Conlon C, Rowland-Jones SL, Bwayo JJ, Mcmichael AJ, Hanke T. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J Gen Virol. 2004;85:911–919. doi: 10.1099/vir.0.19701-0. [DOI] [PubMed] [Google Scholar]
- Nasioulas G, Zolotukhin AS, Tabernero C, Solomin L, Cunningham CP, Pavlakis GN, Felber BK. Elements distinct from human immunodeficiency virus type 1 splice sites are responsible for the Rev dependence of env mRNA. J Virol. 1994;68:2986–2993. doi: 10.1128/jvi.68.5.2986-2993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann AU, Tubiana R, Calvez V, Robert C, Li TS, Agut H, Autran B, Katlama C. HIV-1 rebound during interruption of highly active antiretroviral therapy has no deleterious effect on reinitiated treatment. Comet Study Group Aids. 1999;13:677–683. doi: 10.1097/00002030-199904160-00008. [DOI] [PubMed] [Google Scholar]
- Ortiz GM, Nixon DF, Trkola A, Binley J, Jin X, Bonhoeffer S, Kuebler PJ, Donahoe SM, Demoitie MA, Kakimoto WM, Ketas T, Clas B, Heymann JJ, Zhang L, Cao Y, Hurley A, Moore JP, Ho DD, Markowitz M. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Invest. 1999;104:R13–18. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz GM, Wellons M, Brancato J, Vo HT, Zinn RL, Clarkson DE, Van Loon K, Bonhoeffer S, Miralles GD, Montefiori D, Bartlett JA, Nixon DF. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc Natl Acad Sci U S A. 2001;98:13288–13293. doi: 10.1073/pnas.221452198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten G, Schaefer M, Doe B, Liu H, Srivastava I, Zur Megede J, O'hagan D, Donnelly J, Widera G, Rabussay D, Lewis MG, Barnett S, Ulmer JB. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine. 2004;22:2489–2493. doi: 10.1016/j.vaccine.2003.11.073. [DOI] [PubMed] [Google Scholar]
- Otten GR, Schaefer M, Doe B, Liu H, Megede JZ, Donnelly J, Rabussay D, Barnett S, Ulmer JB. Potent immunogenicity of an HIV-1 gag-pol fusion DNA vaccine delivered by in vivo electroporation. Vaccine. 2006;24:4503–4509. doi: 10.1016/j.vaccine.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Oxenius A, Price DA, Gunthard HF, Dawson SJ, Fagard C, Perrin L, Fischer M, Weber R, Plana M, Garcia F, Hirschel B, Mclean A, Phillips RE. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc Natl Acad Sci U S A. 2002;99:13747–13752. doi: 10.1073/pnas.202372199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pialoux G, Quercia RP, Gahery H, Daniel N, Slama L, Girard PM, Bonnard P, Rozenbaum W, Schneider V, Salmon D, Guillet JG. Immunological responses and long-term treatment interruption after human immunodeficiency virus type 1 (HIV-1) lipopeptide immunization of HIV-1-infected patients: the LIPTHERA study. Clin Vaccine Immunol. 2008;15:562–568. doi: 10.1128/CVI.00165-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme GJ, Glinka Y, Khan AS, Draghia-Akli R. Electroporation-enhanced nonviral gene transfer for the prevention or treatment of immunological, endocrine and neoplastic diseases. Curr Gene Ther. 2006;6:243–273. doi: 10.2174/156652306776359504. [DOI] [PubMed] [Google Scholar]
- Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, Mathiesen I, Cortese R, Ciliberto G, Laufer R, La Monica N, Fattori E. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci U S A. 1999;96:6417–6422. doi: 10.1073/pnas.96.11.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Chavez IR, Allen M, Hill EL, Sheets RL, Pensiero M, Bradac JA, D'souza MP. Current advances and challenges in HIV-1 vaccines. Curr HIV/AIDS Rep. 2006;3:39–47. doi: 10.1007/s11904-006-0007-0. [DOI] [PubMed] [Google Scholar]
- Romano JW, Shurtliff RN, Dobratz E, Gibson A, Hickman K, Markham PD, Pal R. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J Virol Methods. 2000;86:61–70. doi: 10.1016/s0166-0934(99)00184-6. [DOI] [PubMed] [Google Scholar]
- Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, Alicea C, Weiss D, Treece J, Pal R, Markham P, Marques ETA, August JT, Khan A, Draghia-Akli R, Felber BK, Pavlakis GN. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26:5223–5229. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, Venzon D, Montefiori D, Markham P, Felber BK, Pavlakis GN. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J Virol. 2005;79:8480–8492. doi: 10.1128/JVI.79.13.8480-8492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES, Altfeld M, Poon SH, Phillips MN, Wilkes BM, Eldridge RL, Robbins GK, D'aquila RT, Goulder PJ, Walker BD. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol. 1997;71:4892–4903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber BK, Pavlakis GN. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol. 1992a;66:7176–7182. doi: 10.1128/jvi.66.12.7176-7182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Felber BK, Pavlakis GN. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol. 1992b;66:150–159. doi: 10.1128/jvi.66.1.150-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby M, Goldbeck C, Pertile T, Walsh R, Ulmer J. Enhancement of DNA vaccine potency by electroporation in vivo. J Biotechnol. 2000;83:147–152. doi: 10.1016/s0168-1656(00)00308-4. [DOI] [PubMed] [Google Scholar]
- Tavel JA, Martin JE, Kelly GG, Enama ME, Shen JM, Gomez PL, Andrews CA, Koup RA, Bailer RT, Stein JA, Roederer M, Nabel GJ, Graham BS. Safety and immunogenicity of a Gag-Pol candidate HIV-1 DNA vaccine administered by a needle-free device in HIV-1-seronegative subjects. J Acquir Immune Defic Syndr. 2007;44:601–605. doi: 10.1097/QAI.0b013e3180417cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner AR, Barouch DH. HIV-1 Vaccine Development: Progress and Prospects. Curr Infect Dis Rep. 2007;9:71–75. doi: 10.1007/s11908-007-0025-0. [DOI] [PubMed] [Google Scholar]
- Tryniszewska E, Nacsa J, Lewis MG, Silvera P, Montefiori D, Venzon D, Hel Z, Parks RW, Moniuszko M, Tartaglia J, Smith KA, Franchini G. Vaccination of macaques with long-standing SIVmac251 infection lowers the viral set point after cessation of antiretroviral therapy. J Immunol. 2002;169:5347–5357. doi: 10.4049/jimmunol.169.9.5347. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Emau P, Follis KE, Beck TW, Benveniste RE, Bischofberger N, Lifson JD, Morton WR. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubiana R, Carcelain G, Vray M, Gourlain K, Dalban C, Chermak A, Rabian C, Vittecoq D, Simon A, Bouvet E, El Habib R, Costagliola D, Calvez V, Autran B, Katlama C. Therapeutic immunization with a human immunodeficiency virus (HIV) type 1-recombinant canarypox vaccine in chronically HIV-infected patients: The Vacciter Study (ANRS 094) Vaccine. 2005;23:4292–4301. doi: 10.1016/j.vaccine.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Valentin A, Chikhlikar P, Patel V, Rosati M, Maciel M, Chang KH, Silvera P, Felber BK, Pavlakis GN, August JT, Marques ETA. Comparison of DNA vaccines producing HIV-1 gag and LAMP/gag chimera in rhesus macaques reveals antigen-specific T cell responses with distinct phenotypes. doi: 10.1016/j.vaccine.2009.05.093. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompay KK, Dailey PJ, Tarara RP, Canfield DR, Aguirre NL, Cherrington JM, Lamy PD, Bischofberger N, Pedersen NC, Marthas ML. Early short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine treatment favorably alters the subsequent disease course in simian immunodeficiency virus-infected newborn Rhesus macaques. J Virol. 1999;73:2947–2955. doi: 10.1128/jvi.73.4.2947-2955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gegerfelt AS, Rosati M, Alicea C, Valentin A, Roth P, Bear J, Franchini G, Albert PS, Bischofberger N, Boyer JD, Weiner DB, Markham P, Israel ZR, Eldridge JH, Pavlakis GN, Felber BK. Long-lasting decrease in viremia in macaques chronically infected with simian immunodeficiency virus SIVmac251 after therapeutic DNA immunization. J Virol. 2007;81:1972–1979. doi: 10.1128/JVI.01990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, Shen S, Green S, Rothman AL, Ennis FA, Arthos J, Pal R, Markham P, Lu S. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26:1098–1110. doi: 10.1016/j.vaccine.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Bertley FM, Kozlowski PA, Herrmann L, Manson K, Mazzara G, Piatak M, Johnson RP, Carville A, Mansfield K, Aldovini A. An SHIV DNA/MVA rectal vaccination in macaques provides systemic and mucosal virus-specific responses and protection against AIDS. AIDS Res Hum Retroviruses. 2004a;20:846–859. doi: 10.1089/0889222041725253. [DOI] [PubMed] [Google Scholar]
- Wang Z, Troilo PJ, Wang X, Griffiths TG, Pacchione SJ, Barnum AB, Harper LB, Pauley CJ, Niu Z, Denisova L, Follmer TT, Rizzuto G, Ciliberto G, Fattori E, Monica NL, Manam S, Ledwith BJ. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004b;11:711–721. doi: 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, Leung L, Otten GR, Thudium K, Selby MJ, Ulmer JB. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164:4635–4640. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- Wilson CC, Newman MJ, Livingston BD, Mawhinney S, Forster JE, Scott J, Schooley RT, Benson CA. Clinical phase 1 testing of the safety and immunogenicity of an epitope-based DNA vaccine in human immunodeficiency virus type 1-infected subjects receiving highly active antiretroviral therapy. Clin Vaccine Immunol. 2008;15:986–994. doi: 10.1128/CVI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Megede J, Sanders-Beer B, Silvera P, Golightly D, Bowlsbey A, Hebblewaite D, Sites D, Nieves-Duran L, Srivastava R, Otten GR, Rabussay D, Zhang L, Ulmer JB, Barnett SW, Donnelly JJ. A Therapeutic SIV DNA Vaccine Elicits T-Cell Immune Responses, but No Sustained Control of Viremia in SIVmac239-Infected Rhesus Macaques. AIDS Res Hum Retroviruses. 2008;24:1103–1116. doi: 10.1089/aid.2008.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]