Abstract
Objectives:
Only a handful of studies have investigated the nature, functional significance, and course of white matter abnormalities associated with mild traumatic brain injury (mTBI) during the semi-acute stage of injury. The present study used diffusion tensor imaging (DTI) to investigate white matter integrity and compared the accuracy of traditional anatomic scans, neuropsychological testing, and DTI for objectively classifying mTBI patients from controls.
Methods:
Twenty-two patients with semi-acute mTBI (mean = 12 days postinjury), 21 matched healthy controls, and a larger sample (n = 32) of healthy controls were studied with an extensive imaging and clinical battery. A subset of participants was examined longitudinally 3–5 months after their initial visit.
Results:
mTBI patients did not differ from controls on clinical imaging scans or neuropsychological performance, although effect sizes were consistent with literature values. In contrast, mTBI patients demonstrated significantly greater fractional anisotropy as a result of reduced radial diffusivity in the corpus callosum and several left hemisphere tracts. DTI measures were more accurate than traditional clinical measures in classifying patients from controls. Longitudinal data provided preliminary evidence of partial normalization of DTI values in several white matter tracts.
Conclusions:
Current findings of white matter abnormalities suggest that cytotoxic edema may be present during the semi-acute phase of mild traumatic brain injury (mTBI). Initial mechanical damage to axons disrupts ionic homeostasis and the ratio of intracellular and extracellular water, primarily affecting diffusion perpendicular to axons. Diffusion tensor imaging measurement may have utility for objectively classifying mTBI, and may serve as a potential biomarker of recovery.
GLOSSARY
- ADC
= apparent diffusion coefficient;
- CC
= corpus callosum;
- CCI
= cortical impact injury model;
- CR
= corona radiata;
- DTI
= diffusion tensor imaging;
- EC
= external capsule;
- FA
= fractional anisotropy;
- FPI
= fluid percussion injury model;
- HC
= healthy controls;
- IC
= internal capsule;
- JHU
= Johns Hopkins University;
- MANCOVA
= multivariate analysis of covariance;
- mTBI
= mild traumatic brain injury;
- RD
= radial diffusivity;
- ROI
= region of interest;
- SCR
= superior corona radiata;
- SLF
= superior longitudinal fasciculus;
- UF
= uncinate fasciculus.
Complex cognitive processes such as attention, executive functions, and memory depend on intact white matter tracts among frontal, parietal, and medial temporal lobes,1 which are likely disrupted following mild traumatic brain injury (mTBI). Histologic evidence of white matter changes have been observed in both human autopsy2,3 and animal4 studies of mTBI. Although traditional neuroimaging sequences (i.e., T1- and T2-weighted imaging) are typically insensitive to these putative white matter changes, diffusion tensor imaging (DTI) is capable of measuring white matter pathology with histologic correlates in animal models of injury.5
The majority of human mTBI studies have been cross-sectional in nature, examining selected patients (i.e., those with persistent complaints) during the chronic (e.g., after several months or years) injury phase.6–9 This can be problematic as the majority (80%–95%) of mTBI patients fully recover from their injuries within 6 months.10,11 An initial DTI study on 5 unselected patients (i.e., all eligible patients) reported reduced fractional anisotropy (FA) in the corpus callosum (CC), internal capsule (IC), and external capsule (EC) within 24 hours of injury.12 More recent studies focusing on unselected patients in semi-acute phase of injury have reported mixed findings, with 2 adult studies reporting reduced FA13,14 whereas other adolescent15 and adult16 studies have reported increased FA. Inglese et al.13 reported reduced FA in the CC and IC at approximately 5 years postinjury in an adult sample, with no significant FA differences between chronic and semi-acutely injured patients, suggesting limited recovery. Another study examining mTBI patients longitudinally (2 out of 5 patients studied) reported evidence of partial FA normalization at 1 month.12
Additionally, few studies have examined potential differences in axial diffusivity or radial diffusivity (RD) following mTBI in either selected or unselected populations.12,15,17 The distinction between axial diffusivity and RD is critical given that FA is determined from these measurements, and each is putatively associated with different pathologies. Specifically, animal models of retinal ischemia suggest that axial diffusivity corresponds to axonal pathology whereas RD measures myelin pathology.18 Mouse models of TBI indicate that axonal pathology (reduced axial diffusivity) is more pronounced in the acute phase of injury, followed by both pseudonormalization of axial diffusivity values and increased involvement of demyelinating processes (RD) and edema.19
The present study examined FA, axial diffusivity, and RD prospectively in an unselected sample of mTBI patients. Based on previous clinical studies, we predicted that FA and axial diffusivity would be reduced in the CC, IC, superior longitudinal fasciculus (SLF), uncinate fasciculus (UF), and corona radiata (CR) in mTBI patients compared to controls in the semi-acute phase of injury (21 days postinjury) with increased findings in terms of myelin integrity (RD) during the more chronic injury stages.
METHODS
Participants.
Twenty-two patients (recruited from the University Emergency Department) with mTBI and 21 sex-, age-, and education-matched controls participated in an ongoing study. DTI data from an independent sample of healthy controls (HC) were also collected.
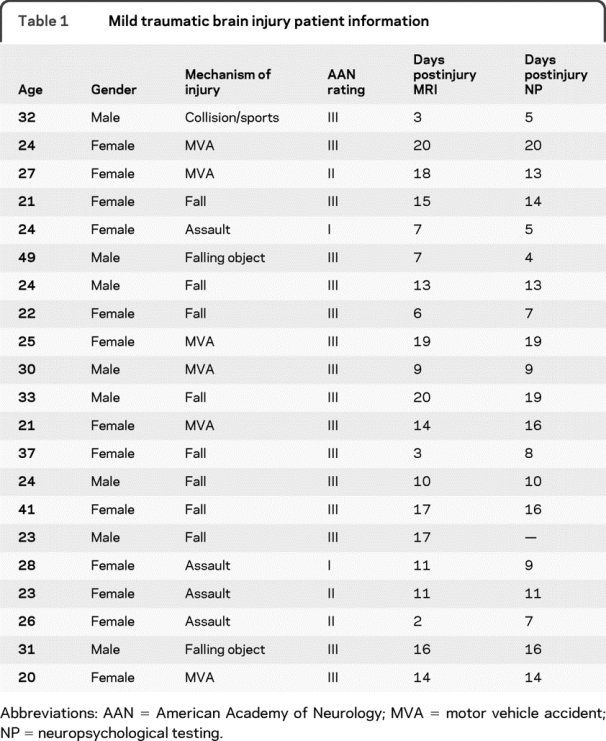
All patients experienced a closed head injury resulting in an alteration in mental status (see table 1) and were evaluated within 21 days of injury (clinical examination = 11.75 ± 4.97 days postinjury; imaging examination = 12.50 ± 5.40 days postinjury). The majority (85%) of patients completed the imaging and clinical protocols within 3 days of each other. Inclusion criteria for the mTBI group were based on the American Congress of Rehabilitation Medicine (Glasgow Coma Score of 13–15, loss of consciousness <30 minutes, posttraumatic amnesia <24 hours). mTBI participants and controls were excluded if there was a positive history of neurologic disease, psychiatric disturbance, additional closed head injuries with more than 5 minutes loss of consciousness or any head injury within the last year, learning disorder, attention deficit hyperactivity disorder, or a history of substance or alcohol abuse.
Table 1 Mild traumatic brain injury patient information
Standard protocol approvals, registrations, and patient consents.
Informed consent was obtained from all participants according to institutional guidelines at the University of New Mexico.
Clinical assessment.
Similar to previous studies,20 composite indices were calculated for attention, working memory, processing speed, executive function, memory, and emotional status based on participants' mean t score in each of the domains (appendix e-1 on the Neurology® Web site at www.neurology.org). Somatic and cognitive complaints were also assessed along with estimates of overall premorbid cognitive functioning and effort (appendix e-1).
MRI and analyses.
T1, T2, and DTI images were collected on a 3-Tesla Siemens Trio scanner (appendix e-1). The AFNI software package21 was used to process and analyze DTI data (appendix e-1). Region of interest (ROI) analyses were conducted on the genu, splenium, and body of the CC, as well as the SLF, the CR, the superior corona radiata (SCR), the UF, and the IC for both hemispheres based on the Johns Hopkins University (JHU) white matter atlas.22 Scalar means (axial diffusivity, RD, and FA) were calculated for each ROI, as were measures of interhemispheric variability between homologous left and right ROI (right ROI − left ROI)/([right ROI + left ROI]/2) to investigate increased asymmetry as a marker of injury. Multivariate analyses were used whenever possible to reduce the number of multiple comparisons. Effect sizes (Cohen's d) are also reported as a measure of clinical significance.23
RESULTS
Neuropsychological and clinical measures.
A compilation of all major neuropsychological and clinical indices is presented in table 2. Results indicated an increase in emotional (t1,38 = −3.11; p < 0.05; mTBI > HC), cognitive (t1,38 = −4.20; p < 0.001), and somatic (t1,38 = −3.62; p < 0.005) complaints for mTBI patients compared to controls. Estimates of premorbid intellectual functioning were lower in mTBI patients (t1,37 = 2.09; p < 0.05) despite educational matching.
Table 2 Demographic and clinical measures for visit 1
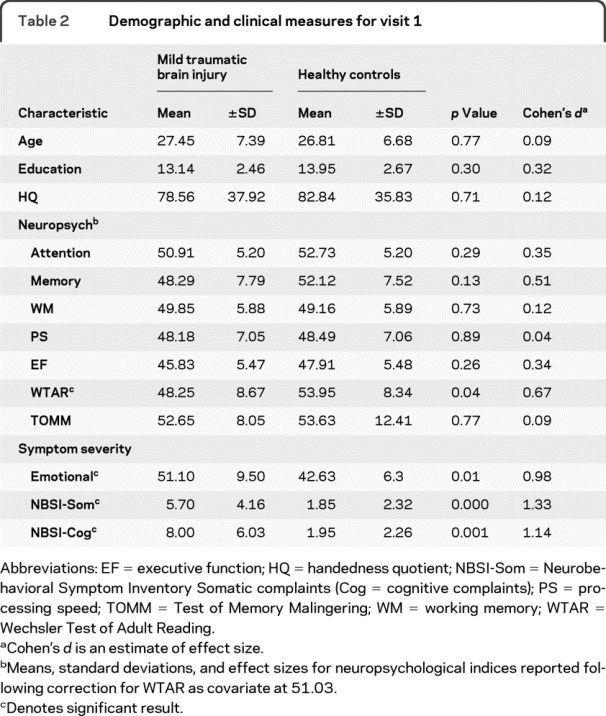
A multivariate analysis of covariance (MANCOVA) examining differences in neuropsychological testing using premorbid intelligence as a covariate was not significant for group differences. However, effect sizes (table 2) in the domains of attention, executive functioning, and memory were of similar magnitude to those reported in recent meta-analyses on cognitive deficits in mTBI.
Structural imaging data.
Anatomic images were limited to T1- and T2-weighted images. These were found to be free of pathology for both groups of subjects by a board-certified neuroradiologist (i.e., all mTBI patients were classified as being noncomplicated).
ROI analyses.
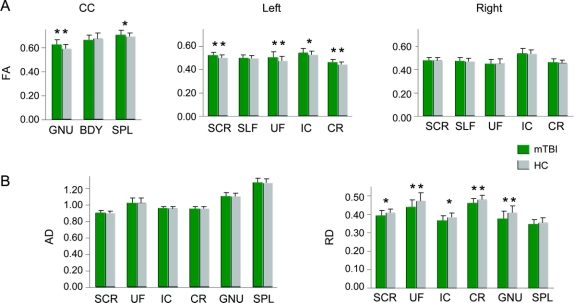
Three MANCOVAs were conducted to examine group differences (mTBI patients vs matched controls) in FA values within the corpus callosum and left and right hemisphere ROI (figure 1A) with estimates of premorbid intellectual functioning as a covariate. Results indicated a multivariate effect of group for both the CC (F3,36 = 3.81; p < 0.05) and the left (F5,34 = 2.70; p < 0.05) but not right (p > 0.10) hemisphere. Follow-up univariate tests indicated that mTBI patients had higher FA within the genu (F1,38 = 7.52; p < 0.01, d = −0.91), left SCR (F1,38 = 5.54; p < 0.05, d = −0.77), left CR (F1,38 = 5.47; p < 0.05, d = −0.74), and left UF (F1,38 = 6.67; p < 0.05, d = −0.84). Trends were observed for the left IC (F1,38 = 3.69; p = 0.062, d = −0.62) and the splenium (F1,38 = 2.95; p = 0.094, d = −0.53) with mTBI patients again exhibiting higher FA values than HC (see figure e-1 for normalized FA histograms).
Figure 1 Fractional anisotropy (FA) values from all regions of interest (ROI)
This figure presents the mean FA values from all ROI for the mild traumatic brain injury patients (mTBI; green bars) and healthy controls (HC; gray bars) for visit 1 (A) corrected for differences in premorbid intelligence estimates. ROI included the genu (GNU), body (BDY), and splenium (SPL) of the corpus callosum (CC), the superior corona radiata (SCR), the superior longitudinal fasciculus (SLF), the uncinate fasciculus (UF), the corona radiata (CR), and the internal capsule (IC). Significant effects are denoted with double asterisks, statistical trends with a single asterisk. (B) Axial diffusivity (AD) and radial diffusivity (RD) measurements for mTBI patients and HC for regions exhibiting statistical differences in FA. For the y-axis, the units of FA are dimensionless, whereas axial diffusivity and RD are equivalent to mm2/s.
HC were then compared with a larger normative sample. However, there were no multivariate effects of group for all multivariate analyses of variance (p > 0.10), suggesting that our control group was statistically similar to the larger normative sample in terms of FA.
Next, we compared axial diffusivity and RD values for the 6 ROI that exhibited significant or trend differences in FA using one-way analyses of covariance (figure 1B). There were no significant differences between patients and controls in terms of axial diffusivity. In contrast, RD was lower in mTBI patients within the genu (F1,38 = 5.09; p < 0.05, d = 0.74), the left UF (F1,38 = 5.67; p < 0.05, d = 0.77), and the left CR (F1,38 = 4.42; p < 0.05, d = 0.66), with trends present in the left SCR (F1,38 = 3.58; p = 0.06, d = 0.59) and left IC (F1,38 =3.99; p = 0.053, d = 0.66). Histograms for the normalized RD data are presented in figure e-2.
Finally, a MANCOVA (figure 2) comparing variability in FA measurements between right and left hemisphere homologue ROI (SFL, IC, UF, SCR, and CR) revealed a group effect (F5,34 = 4.53; p < 0.005), with univariate tests indicating increased variability in patients compared to controls for the SCR (F1,38 = 15.06; p < 0.001, d = −1.21), with a trend for the UF (F1,38 = 3.82, p = 0.058; d = −0.63).
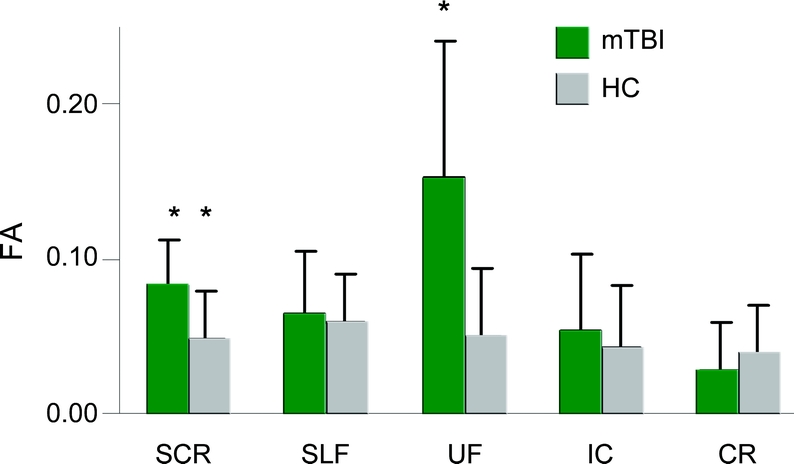
Figure 2 Variability in mean fractional anisotropy (FA) between right and left hemisphere regions of interest (ROI)
A measurement of variability in mean FA values between right and left hemisphere homologue ROI for the mild traumatic brain injury patients (mTBI; green bars) and healthy controls (HC; gray bars) corrected for differences in premorbid intelligence estimates. ROI included the superior longitudinal fasciculus (SLF), the uncinate fasciculus (UF), the corona radiata (CR), and the internal capsule (IC). Significant effects are denoted with double asterisks, statistical trends with a single asterisk.
DTI and clinical measures.
Hierarchical multiple regressions were performed on the 6 clinical measures with the largest effect sizes (attention, memory, executive functions, cognitive complaints, somatic complaints, and emotional complaints) using FA from the CC and right and left hemisphere ROI as the independent variables and premorbid intelligence as a covariate. Although premorbid intelligence accounted for significant variance in terms of both attentional and executive functioning, only FA levels in the right hemisphere (F2,18 = 6.84; p < 0.01) predicted variance in attentional deficits (positive relationship) for the mTBI group.
Next we determined which of our objective measures of deficits (FA or neuropsychological testing) would more accurately classify mTBI patients and HC using binary logistical regression. Estimates of premorbid intelligence were entered into both models as it discriminated (Wald = 4.16; p < 0.05) between HC (65% accuracy) and mTBI patients (66.7%) at slightly above chance levels. Traditional neuropsychological measures (attention, memory, and executive function) did not significantly improve classification accuracy in the first model (HC = 60%; mTBI = 71.4%). In contrast, results from the second model indicated that both the left (Wald = 7.73; p < 0.05) and right (Wald = 5.66; p < 0.05) hemisphere FA indices improved classification accuracy (HC = 70%; mTBI = 81%), with a trend being noted for the CC (Wald = 3.59; p = 0.059). A support vector machine analysis with the leave-one-out methodology confirmed the generality (HC = 65%; mTBI = 81%) of the classification findings.
Visit 2 data.
To date, 10 out of 17 (59%) eligible mTBI patients and 15 out of 16 (94%) eligible HC participants have returned for their 3- to 5-month follow-up visit (see appendix e-1). Intraclass correlation coefficient values for FA were highly reliable (all ROI = 0.65 < r >.93; p < 0.01) in the HC sample; however, reliability of homologue measures was much more variable (SCR r14 = 0.64, p < 0.01; SLF r14 = 0.81, p < 0.001; UF r14 = 0.22, p > 0.10; CR r14 = 0.71, p < 0.01; IC r14 = −0.26, p > 0.10).
There were no significant differences for all clinical measures for mTBI patients who returned and those who did not. Additionally, there were no significant differences in FA values between the 2 groups across the 3 sets of ROI (CC, right and left hemisphere).
Change scores in clinical measures were calculated (visit 2 − visit 1 data) for those measurements that were most suggestive (i.e., based on significance or effect sizes) of group differences at visit 1 (attention, memory, executive functions, emotional distress, somatic and cognitive complaints) using premorbid intelligence as a covariate. Although there were no significant group effects, effect sizes suggested that memory scores improved (d = −0.52) and cognitive complaints decreased (d = 0.79) for the returning mTBI group compared to their matched controls at visit 2.
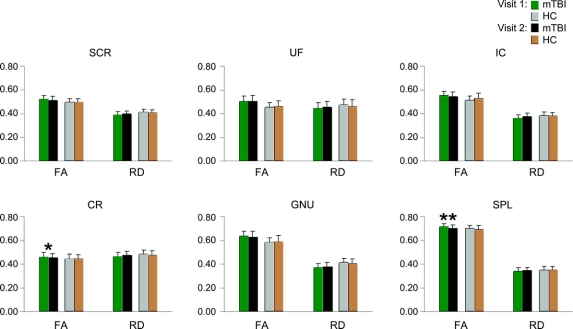
Differences in visit 1 and 2 FA and RD measurements were compared separately across the 2 groups with paired samples t tests to maximize power (see figure 3). Tests were again limited to those ROI that exhibited significant or trend differences in mean FA and RD (genu, splenium, left SCR, left IC, left UF, and left CR) at visit 1. In HC, there were no significant differences for either FA or RD across the 2 visits. In contrast, partial normalization (i.e., decrease) in FA values was evident in the splenium (t8 = 4.17, p < 0.005) and CR (t8 = 1.89, p = 0.09) at visit 2 for mTBI patients. Although none of the RD effects reached statistical levels of significance, visual examination of the data suggests that RD differences may have partially normalized at visit 2 as well.
Figure 3 Fractional anisotropy (FA) and radial diffusivity (RD) values at both visits
Mean FA and RD for the mild traumatic brain injury patients (mTBI; green bars = visit 1, black bars = visit 2) and healthy controls (HC; gray bars = visit 1, brown bars = visit 2). Analyses were limited to ROI that displayed significant effects at visit 1, and included the left superior corona radiata (SCR), the left uncinate fasciculus (UF), the left internal capsule (IC), the left corona radiata (CR), the genu (GNU), and the splenium (SPL). For the y-axis, the units of FA are dimensionless, whereas RD is equivalent to mm2/s.
DISCUSSION
The types of abnormalities seen in human neuropathology studies of mTBI2,3 are poorly revealed by neuroimaging techniques, limiting detection of potential white matter pathologies, and prediction of cognitive impairment and functional outcome.24 Hence, conventional imaging modalities cannot provide an objective measure of injury for the difficult differential diagnoses that most clinicians face when confronted with mTBI patients.10 Contrary to our initial hypothesis, mTBI patients demonstrated increased FA and reduced RD within the genu and several left hemisphere white matter tracts compared to age- and education-matched controls during the semi-acute phase of injury.
Animal research indicates that there are several morphologic changes, metabolic processes, and inflammatory responses that follow mTBI.25,26 Therefore, a definitive mechanistic explanation for current results is challenging at best given the many constraints of an in vivo human clinical imaging study. With this caveat in mind, perhaps the 2 most plausible explanations for the current and previous15,16 observations of increased diffusion anisotropy following mTBI are cytotoxic edema or changes in water content within the myelin sheath. The mechanical forces of mTBI typically result in the stretching of axons and related supporting structures such as oligodendrocytes,27 altering the function of gated ion channels and resulting in an increase in intracellular water and a decrease in extracellular water.28 The decrease in extracellular water leads to a decrease in diffusivity perpendicular to the axon (second and third eigenvalues; RD), secondary to more tightly compacted axons and potential differences in the tortuosity of intracellular and extracellular water.28,29 Modeling studies suggest that even small departures from the normal distribution of intracellular and extracellular water can lead to dramatic changes in perpendicular diffusion coefficients.30
A central role for cytotoxic edema is also partially supported by animal models of both ischemic stroke and TBI, in which perilesional white matter shows increased FA in the first 3 hours of stroke followed by a reduction in FA and RD from 4 to 120 hours postinjury.25,26,31 Of note, the timeline from these animal models suggests that reduced rather than increased FA should be observed at days to weeks postinjury. However, cytotoxic edema may follow a somewhat more prolonged course in human TBI than in the animal models of TBI, peaking between 24 and 48 hours postinjury and persisting for days postinjury.32,33 An alternative explanation for our findings is that mTBI decreases water content in the myelin sheaths rather than in extracellular space. Although myelin only accounts for approximately 13% of total water in white matter compartments, a reduction in this percentage would theoretically also decrease diffusivity perpendicular to the axon.30
At a more basic level, there may be qualitative differences in neuropathologic processes among appropriately diagnosed mTBI patients as illustrated by a recent study34 comparing the fluid percussion (FPI) and cortical impact (CCI) injury models. Injured animals from both groups differed from shams in terms of T2 values and apparent diffusion coefficients (ADC), but in opposite directions. The FPI injury, which might be a better model for mTBI injuries caused by motor vehicle accidents, showed decreased T2 and ADC, while the CCI injury, perhaps a better model for falls or assaults, showed increased ADC and elevated T2. Both groups showed evidence of increased immunoreactivity.
Magnetic resonance spectroscopy also captures unique information about white matter pathology that may elucidate potential mechanisms of pathology. Increased creatine-phosphocreatine concentrations in supraventricular white matter and in the splenium have been observed in mTBI, perhaps related to an increased need for energy resources (ATP) for repair.35 Though such metabolic derangements may follow a different recovery course than DTI abnormalities,36 they likely represent an important component of the suite of pathologic processes. A tentative hypothesis linking the 2 imaging modalities suggests that disruption of ionic homeostasis causes increased intracellular water, simultaneously reducing RD and increasing ATP demand so as to upregulate membrane pumps and restore ionic homeostasis.
Current results also suggest that DTI results are more accurate in objectively classifying mTBI patients from carefully matched HC. Although limited in nature, our anatomic protocol was completely insensitive (e.g., all mTBI and HC scans were interpreted as trauma-free) to the putative underlying pathology following trauma. Second, although our mTBI patients exhibited cognitive deficits on several neuropsychological domains (attention, memory, and executive functioning) that were consistent in magnitude with previous meta-analyses,37 these deficits did not substantially improve classification accuracy even though neuropsychological testing has traditionally served as the gold standard for differential diagnosis.10,11 In contrast, classification accuracy improved to 75% with data derived from DTI images. Future studies should examine the classification accuracy of DTI and neuropsychological measures in orthopedically injured patients or similar populations38 to better control for nonspecific effects of trauma.
Similarly, longitudinal studies with larger samples spanning the acute to chronic time frame are also needed to chart the evolving nature of mTBI, which has been documented in studies employing animal models.19,39 FA measurements appear to be relatively stable over month-long intervals in HC, rendering it an ideal mechanism for monitoring potential changes associated with recovery of function. Our preliminary longitudinal data suggest a partial normalization of FA (i.e., a decrease toward levels observed in HC) within several ROI in our mTBI group. Although others have examined more severely injured populations,40 we examined longitudinal DTI changes in mTBI. Consistent with patients' self-report of continued cognitive and somatic symptoms at visit 2, not all of our ROI demonstrated significant changes as a function of time, suggesting that a more extensive postimaging interval may be necessary to track recovery.
ACKNOWLEDGMENT
The authors thank Diana South and Cathy Smith for assistance with data collection; Rex Jung, PhD, for contribution of normative sample data; Reyaad Hayek, MD, for review of anatomic images; and Gayle Pohl and her students for contributions to help fund this study.
DISCLOSURE
Dr. Mayer has received/receives research support from the NIH (1 P20 RR021938-01 [Co-I, Project PI], R21NS064464 [PI], R24HD050836 [PI], Clinical LRP [PI], 1 R03 DA022435-01A1 [PI], and R03 DA022435-01A1 [PI]). J. Ling and M.V. Mannell report no disclosures. Dr. Gasparovic has received/receives research support from the NIH (R21NS064464 [Co-I], 1R01 NS052305-01 [Co-I], R01-NS35708-04 [Co-I], 1R21AA017313-01 [Co-I], and NCRR 1P20RR021938-01 [Co-I]). Dr. Phillips reports no disclosures. Dr. Doezema receives research support from the NIH (R21NS064464 [Sub-contract PI]). Dr. Reichard reports no disclosures. Dr. Yeo serves on the editorial advisory boards of Laterality and Frontiers in Neuroscience.
Supplementary Material
Address correspondence and reprint requests to Dr. Andrew Mayer, The Mind Research Network, Pete & Nancy Domenici Hall, 1101 Yale Blvd. NE, Albuquerque, NM 87106 amayer@mrn.org
Editorial, page 626
Supplemental data at www.neurology.org
e-Pub ahead of print on January 20, 2010, at www.neurology.org.
Study funding: Supported by the Department of Energy [DE-FG02-99ER62764 to The Mind Research Network] and the National Institutes of Health [R24-HD050836 and R21-NS064464-01A1 to A.M.].
Disclosure: Author disclosures are provided at the end of the article.
Received July 24, 2009. Accepted November 5, 2009.
REFERENCES
- 1.Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- 2.Bigler ED. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J Int Neuropsychol Soc 2004;10:794–806. [DOI] [PubMed] [Google Scholar]
- 3.Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet 1994;344:1055–1056. [DOI] [PubMed] [Google Scholar]
- 4.Dikranian K, Cohen R, Mac DC, et al. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp Neurol 2008;211:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol 2007;205:116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell MH, Ulug AM, Zhang L, et al. Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study. J Magn Reson Imaging 2006;24:537–542. [DOI] [PubMed] [Google Scholar]
- 7.Lipton ML, Gellella E, Lo C, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J Neurotrauma 2008;25:1335–1342. [DOI] [PubMed] [Google Scholar]
- 8.Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol 2008;29:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM. Diffusion anisotropy changes in the brains of professional boxers. AJNR Am J Neuroradiol 2006;27:2000–2004. [PMC free article] [PubMed] [Google Scholar]
- 10.Belanger HG, Vanderploeg RD, Curtiss G, Warden DL. Recent neuroimaging techniques in mild traumatic brain injury. J Neuropsychiatry Clin Neurosci 2007;19:5–20. [DOI] [PubMed] [Google Scholar]
- 11.Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc 2008;14:1–22. [DOI] [PubMed] [Google Scholar]
- 12.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- 13.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg 2005;103:298–303. [DOI] [PubMed] [Google Scholar]
- 14.Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj 2008;22:115–122. [DOI] [PubMed] [Google Scholar]
- 15.Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 2008;70:948–955. [DOI] [PubMed] [Google Scholar]
- 16.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007;24:1447–1459. [DOI] [PubMed] [Google Scholar]
- 17.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Rev Neurol 2007;130:2508–2519. [DOI] [PubMed] [Google Scholar]
- 18.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003;20:1714–1722. [DOI] [PubMed] [Google Scholar]
- 19.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci 2007;27:11869–11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer AR, Mannell MV, Ling J, et al. Auditory orienting and inhibition of return in mild traumatic brain injury: a FMRI study. Hum Brain Mapp Epub 2009. [DOI] [PMC free article] [PubMed]
- 21.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 22.Mori S, van Zijl PC. Human white matter atlas. Am J Psychiatry 2007;164:1005. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 24.Hughes DG, Jackson A, Mason DL, Berry E, Hollis S, Yates DW. Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology 2004;46:550–558. [DOI] [PubMed] [Google Scholar]
- 25.Albensi BC, Knoblach SM, Chew BG, O'Reilly MP, Faden AI, Pekar JJ. Diffusion and high resolution MRI of traumatic brain injury in rats: time course and correlation with histology. Exp Neurol 2000;162:61–72. [DOI] [PubMed] [Google Scholar]
- 26.Van Putten HP, Bouwhuis MG, Muizelaar JP, Lyeth BG, Berman RF. Diffusion-weighted imaging of edema following traumatic brain injury in rats: effects of secondary hypoxia. J Neurotrauma 2005;22:857–872. [DOI] [PubMed] [Google Scholar]
- 27.Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil 2005;20:76–94. [DOI] [PubMed] [Google Scholar]
- 28.Rosenblum WI. Cytotoxic edema: monitoring its magnitude and contribution to brain swelling. J Neuropathol Exp Neurol 2007;66:771–778. [DOI] [PubMed] [Google Scholar]
- 29.Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury: a review. NMR Biomed 2002;15:561–569. [DOI] [PubMed] [Google Scholar]
- 30.Peled S. New perspectives on the sources of white matter DTI signal. IEEE Trans Med Imaging 2007;26:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Zijden JP, van der TA, van der MK, Dijkhuizen RM. Longitudinal in vivo MRI of alterations in perilesional tissue after transient ischemic stroke in rats. Exp Neurol 2008;212:207–212. [DOI] [PubMed] [Google Scholar]
- 32.Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus 2007;22:E1. [DOI] [PubMed] [Google Scholar]
- 33.Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neurosci 2004;129:1021–1029. [DOI] [PubMed] [Google Scholar]
- 34.Obenaus A, Robbins M, Blanco G, et al. Multi-modal magnetic resonance imaging alterations in two rat models of mild neurotrauma. J Neurotrauma 2007;24:1147–1160. [DOI] [PubMed] [Google Scholar]
- 35.Gasparovic C, Yeo R, Mannell M, et al. Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: a 1H magnetic resonance spectroscopy study. J Neurotrauma Epub 2009. [DOI] [PMC free article] [PubMed]
- 36.Vagnozzi R, Signoretti S, Tavazzi B, et al. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes: part III. Neurosurgery 2008;62:1286–1295. [DOI] [PubMed] [Google Scholar]
- 37.Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc 2005;11:345–357. [DOI] [PubMed] [Google Scholar]
- 38.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med 2008;358:453– 463. [DOI] [PubMed] [Google Scholar]
- 39.Immonen RJ, Kharatishvili I, Niskanen JP, Grohn H, Pitkanen A, Grohn OH. Distinct MRI pattern in lesional and perilesional area after traumatic brain injury in rat: 11 months follow-up. Exp Neurol 2009;215:29–40. [DOI] [PubMed] [Google Scholar]
- 40.Kumar R, Husain M, Gupta RK, et al. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma 2009;26:481–495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.