Abstract
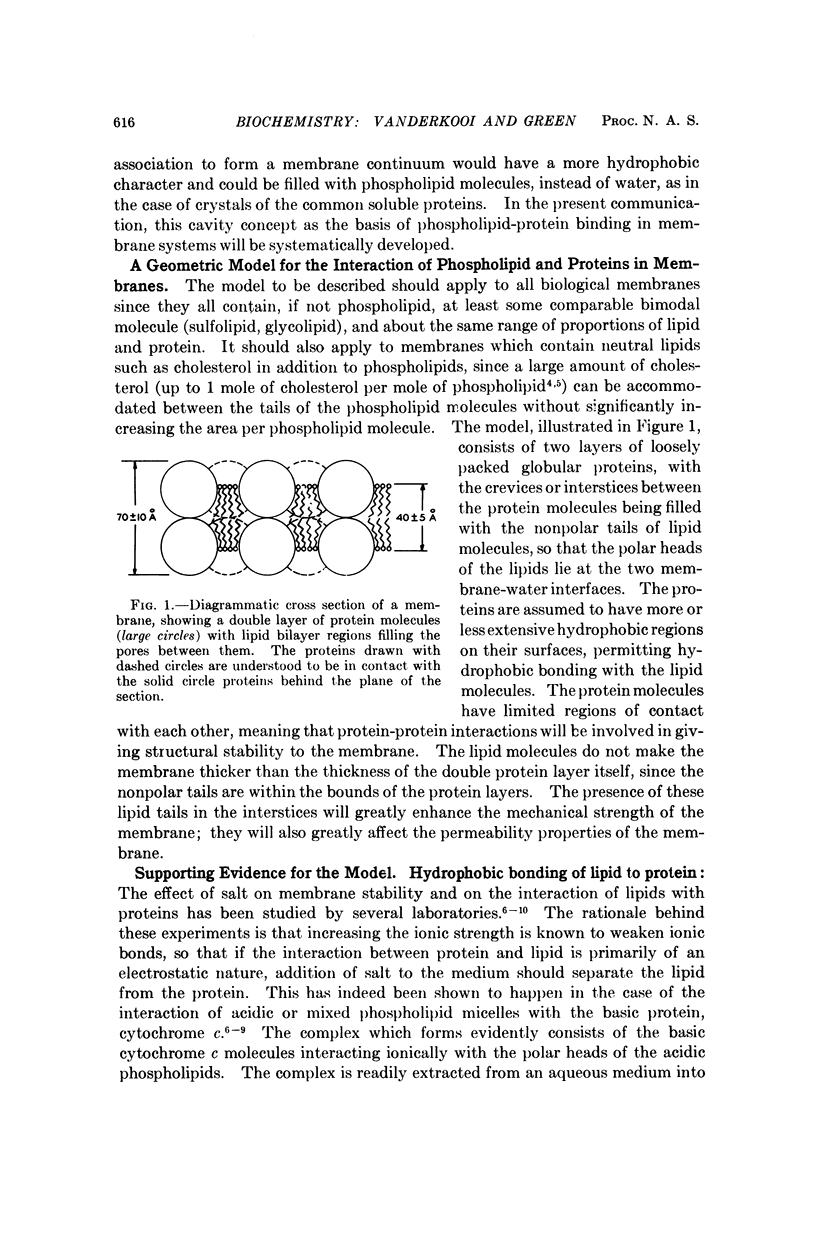
A geometric model for the arrangement of phospholipid and protein in biological membrane systems has been proposed. The essential principle underlying this model is that when membrane proteins polymerize, the points of contact between proteins are few, and cavities lined with predominantly nonpolar amino acids are formed. Phospholipid molecules become oriented with the fatty chains inserted into the cavities while the polar heads remain on the surface of the membrane. This orientation applies to both faces of the membrane continuum. All the lipid known to be present in membranes can be accommodated in this manner. The body of evidence supporting this model has been presented.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN A. D. HYDROGEN ION TITRATIONS OF INTACT AND DISSOLVED LIPOPROTEIN MEMBRANES. J Mol Biol. 1965 Jun;12:491–508. doi: 10.1016/s0022-2836(65)80272-8. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Mair G. A., North A. C., Phillips D. C., Sarma V. R. On the conformation of the hen egg-white lysozyme molecule. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):365–377. doi: 10.1098/rspb.1967.0034. [DOI] [PubMed] [Google Scholar]
- Blasie J. K., Worthington C. R. Planar liquid-like arrangement of photopigment molecules in frog retinal receptor disk membranes. J Mol Biol. 1969 Feb 14;39(3):417–439. doi: 10.1016/0022-2836(69)90136-3. [DOI] [PubMed] [Google Scholar]
- Bulgin J. J., Vinson L. J. The use of differential thermal analysis to study the bound water in stratum corneum membranes. Biochim Biophys Acta. 1967 Apr 25;136(3):551–560. doi: 10.1016/0304-4165(67)90013-x. [DOI] [PubMed] [Google Scholar]
- Crane F. L., Hall J. D. Binary membranes: a reinterpretation of membrane and myelin structure. Biochem Biophys Res Commun. 1969 Jul 7;36(1):174–178. doi: 10.1016/0006-291x(69)90665-2. [DOI] [PubMed] [Google Scholar]
- Cunningham W. P., Prezbindowski K., Crane F. L. The relation between structure and function in electron transport systems. II. Effect of solvent treatment on membrane structure. Biochim Biophys Acta. 1967 Sep 9;135(4):614–623. doi: 10.1016/0005-2736(67)90093-4. [DOI] [PubMed] [Google Scholar]
- DAS M. L., CRANE F. L. PROTEOLIPIDS. I. FORMATION OF PHOSPHOLIPID-CYTOCHROME C COMPLEXES. Biochemistry. 1964 May;3:696–700. doi: 10.1021/bi00893a017. [DOI] [PubMed] [Google Scholar]
- DAS M. L., HIRATSUKA H., MACHINIST J. M., CRANE F. L. The proteolipids of cytochrome c. Biochim Biophys Acta. 1962 Jul 2;60:433–434. doi: 10.1016/0006-3002(62)90427-4. [DOI] [PubMed] [Google Scholar]
- DE BERNARD L. Associations moléculaires entre les lipides. II. Lecithine et cholestérol. Bull Soc Chim Biol (Paris) 1958;40(1):161–170. [PubMed] [Google Scholar]
- Fleischer S., Fleischer B., Stoeckenius W. Fine structure of lipid-depleted mitochondria. J Cell Biol. 1967 Jan;32(1):193–208. doi: 10.1083/jcb.32.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., FLEISCHER S. THE ROLE OF LIPIDS IN MITOCHONDRIAL ELECTRON TRANSFER AND OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1963 Oct 22;70:554–582. doi: 10.1016/0006-3002(63)90793-5. [DOI] [PubMed] [Google Scholar]
- Gibson K. D., Scheraga H. A. Minimization of polypeptide energy. I. Preliminary structures of bovine pancreatic ribonuclease S-peptide. Proc Natl Acad Sci U S A. 1967 Aug;58(2):420–427. doi: 10.1073/pnas.58.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Perdue J. F. Membranes as expressions of repeating units. Proc Natl Acad Sci U S A. 1966 May;55(5):1295–1302. doi: 10.1073/pnas.55.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960 Feb;235:531–534. [PubMed] [Google Scholar]
- KENDREW J. C. Side-chain interactions in myoglobin. Brookhaven Symp Biol. 1962 Dec;15:216–228. [PubMed] [Google Scholar]
- Kimelberg H. K., Lee C. P. Binding and electron transfer to cytochrome c in artificial phospholipid membranes. Biochem Biophys Res Commun. 1969 Mar 31;34(6):784–790. doi: 10.1016/0006-291x(69)90248-4. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Structure of biological membranes. Science. 1966 Sep 23;153(3743):1491–1498. doi: 10.1126/science.153.3743.1491. [DOI] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Protein conformation in cell membrane preparations as studied by optical rotatory dispersion and circular dichroism. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1828–1835. doi: 10.1073/pnas.56.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Structure of membranes: reaction of red blood cell membranes with phospholipase C. Science. 1968 Feb 16;159(3816):738–739. doi: 10.1126/science.159.3816.738-a. [DOI] [PubMed] [Google Scholar]
- Lenaz G., Sechi A. M., Masotti L., Parenti Castelli G. Nonionic interaction between proteins and lipids in the mitochondrial membranes. Biochem Biophys Res Commun. 1969 Feb 21;34(4):392–397. doi: 10.1016/0006-291x(69)90394-5. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Lenaz G., Szarkowska L. Studies on the mechanims of oxidative phosphorylation. IX. Effect of cytochrome c on energy-linked processes. J Biol Chem. 1966 Nov 25;241(22):5251–5259. [PubMed] [Google Scholar]
- Margoliash E., Fitch W. M., Dickerson R. E. Molecular expression of evolutinary phenomena in the primary and tertiary structures of cytochrome c. Brookhaven Symp Biol. 1968 Jun;21(2):259–305. [PubMed] [Google Scholar]
- Matthews B. W., Sigler P. B., Henderson R., Blow D. M. Three-dimensional structure of tosyl-alpha-chymotrypsin. Nature. 1967 May 13;214(5089):652–656. doi: 10.1038/214652a0. [DOI] [PubMed] [Google Scholar]
- McConnell D. G., Tzagoloff A., MacLennan D. H., Green D. E. Studies on the electron transfer system. LXV. Formation of membranes by purified cytochrome oxidase. J Biol Chem. 1966 May 25;241(10):2373–2382. [PubMed] [Google Scholar]
- Napolitano L., Lebaron F., Scaletti J. Preservation of myelin lamellar structure in the absence of lipid. A correlated chemical and morphological study. J Cell Biol. 1967 Sep;34(3):817–826. doi: 10.1083/jcb.34.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steim J. M., Fleischer S. Aggregation-induced red shift of the Cotton effect of mitochondrial structural protein. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1292–1298. doi: 10.1073/pnas.58.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., MacLennan D. H. Studies of the electron-transfer system. LXIV. Role of phospholipid in cytochrome oxidase. Biochim Biophys Acta. 1965 Jun 22;99(3):476–485. doi: 10.1016/s0926-6593(65)80201-6. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Ji T. H. Distortions in circular dichroism patterns of particulate (or membranous) systems. Arch Biochem Biophys. 1968 Dec;128(3):802–807. doi: 10.1016/0003-9861(68)90088-x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Mednieks M., Bejnarowicz E. Optical rotation of mitochondrial membranes. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1043–1049. doi: 10.1073/pnas.57.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]



