Abstract
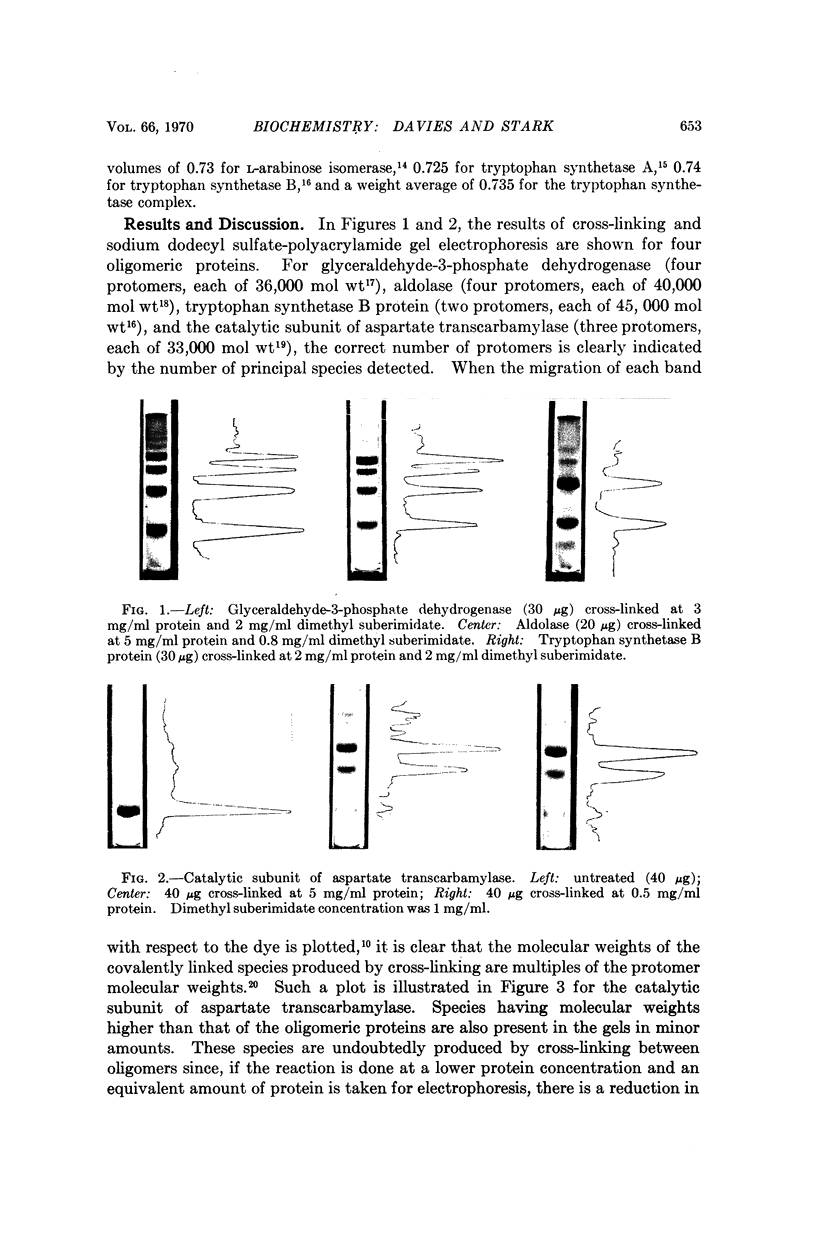
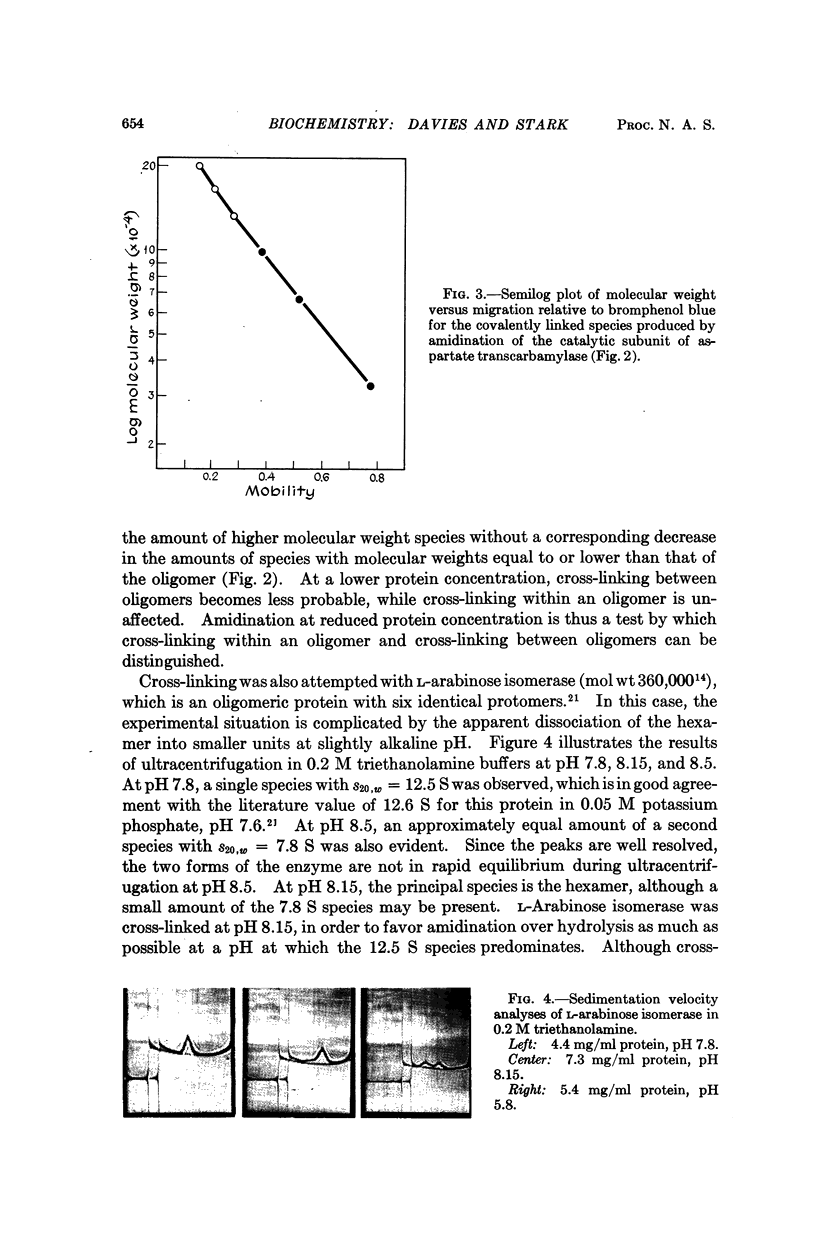
Amidination of aldolase, glyceraldehyde-3-phosphate dehydrogenase, tryptophan synthetase B protein, L-arabinose isomerase, and the catalytic subunit of E. coli aspartate transcarbamylase with the bifunctional reagent dimethyl suberimidate produces cross-linked proteins, with reaction predominating within oligomers. Disc electrophoresis of a modified protein on polyacrylamide gel in the presence of sodium dodecyl sulfate resolves a set of species with molecular weights equal to integral multiples of the protomer molecular weight. For oligomers composed of identical protomers, the number of principal species observed is identical to the number of protomers in the oligomer. Application of the method to two proteins composed of dissimilar protomers, native aspartate transcarbamylase and tryptophan synthetase α2β2 complex of E. coli, revealed differences in the reactivities of the different kinds of protomer within each oligomer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castellino F. J., Barker R. Examination of the dissociation of multichain proteins in guanidine hydrochloride by membrane osmometry. Biochemistry. 1968 Jun;7(6):2207–2217. doi: 10.1021/bi00846a025. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Association of the alpha and beta-2 subunits of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):980–990. [PubMed] [Google Scholar]
- Davidson B. E., Sajgò M., Noller H. F., Harris J. I. Amino-acid sequence of glyceraldehyde 3-phosphate dehydrogenase from lobster muscle. Nature. 1967 Dec 23;216(5121):1181–1185. doi: 10.1038/2161181a0. [DOI] [PubMed] [Google Scholar]
- Dutton A., Adams M., Singer S. J. Bifunctional imidoesters as cross-linking reagents. Biochem Biophys Res Commun. 1966 Jun 13;23(5):730–739. doi: 10.1016/0006-291x(66)90462-1. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Guest J. R., Drapeau G. R., Carlton B. C., Yanofsky C. The amino acid sequence of the A protein (alpha subunit) of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1967 Nov 25;242(22):5442–5446. [PubMed] [Google Scholar]
- HENNING U., HELINSKI D. R., CHAO F. C., YANOFSKY C. The A protein of the tryptophan synthetase of Escherichia coli. Purification, crystallization, and composition studies. J Biol Chem. 1962 May;237:1523–1530. [PubMed] [Google Scholar]
- Hartman F. C., Wold F. Cross-linking of bovine pancreatic ribonuclease A with dimethyl adipimidate. Biochemistry. 1967 Aug;6(8):2439–2448. doi: 10.1021/bi00860a021. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Kida S., Crawford I. P. Subunit structure of the B component of Escherichia coli tryptophan synthetase. Biochemistry. 1969 Mar;8(3):989–997. doi: 10.1021/bi00831a032. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Schmid D., Knof S. Monodispersity and quaternary structure of glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Mar;7(3):919–926. doi: 10.1021/bi00843a006. [DOI] [PubMed] [Google Scholar]
- Patrick J. W., Lee N. Purification and properties of an L-arabinose isomerase from Escherichia coli. J Biol Chem. 1968 Aug 25;243(16):4312–4318. [PubMed] [Google Scholar]
- Patrick J. W., Lee N. Subunit structure of L-arabinose isomerase from Escherichia coli. J Biol Chem. 1969 Aug 25;244(16):4277–4283. [PubMed] [Google Scholar]
- Penhoet E., Kochman M., Valentine R., Rutter W. J. The subunit structure of mammalian fructose diphosphate aldolase. Biochemistry. 1967 Sep;6(9):2940–2949. doi: 10.1021/bi00861a039. [DOI] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]