Abstract
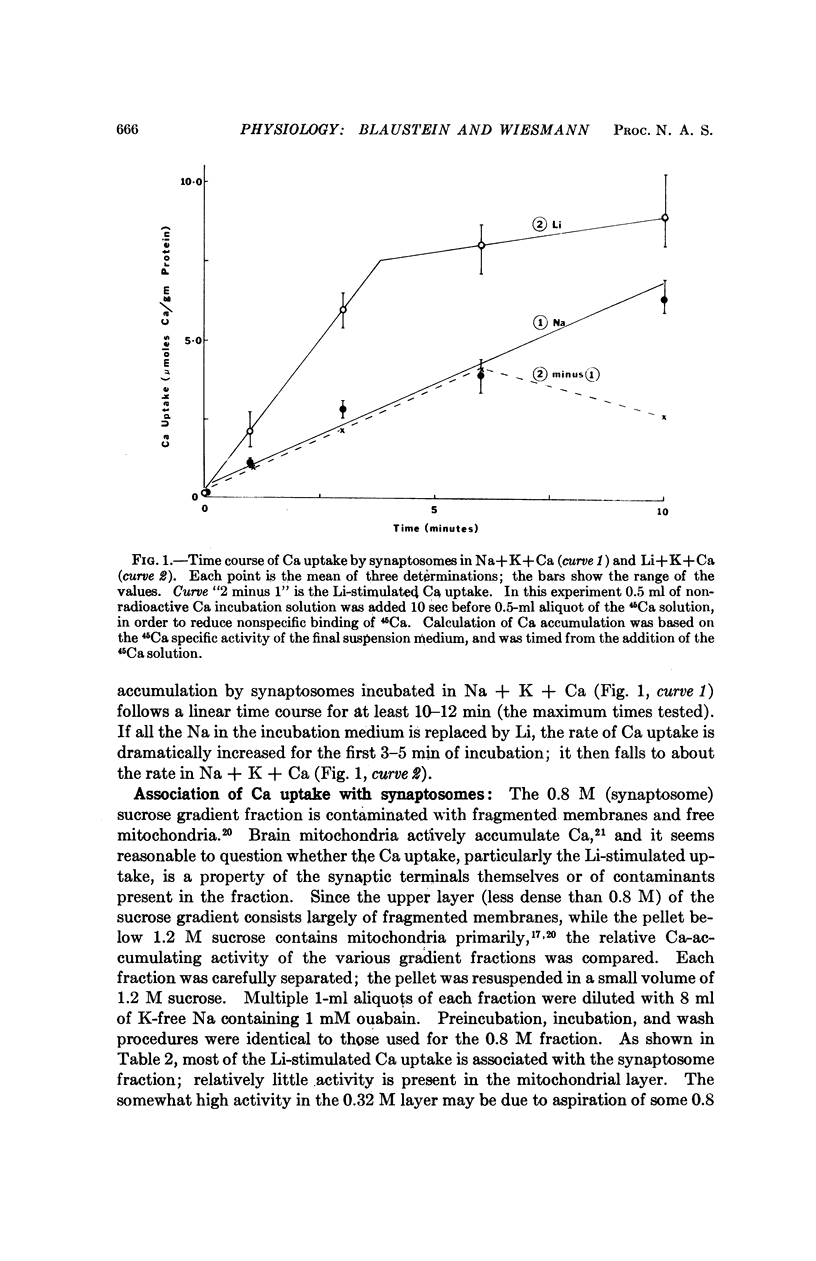
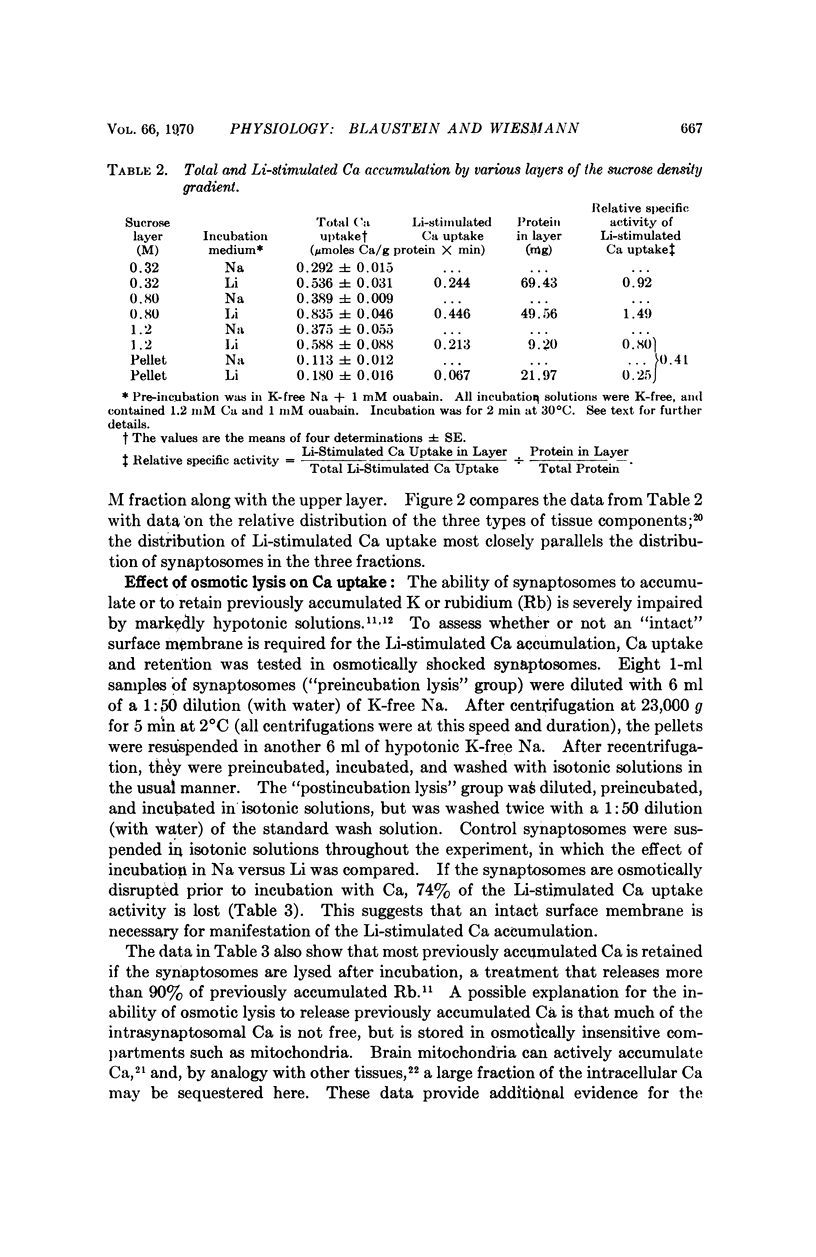
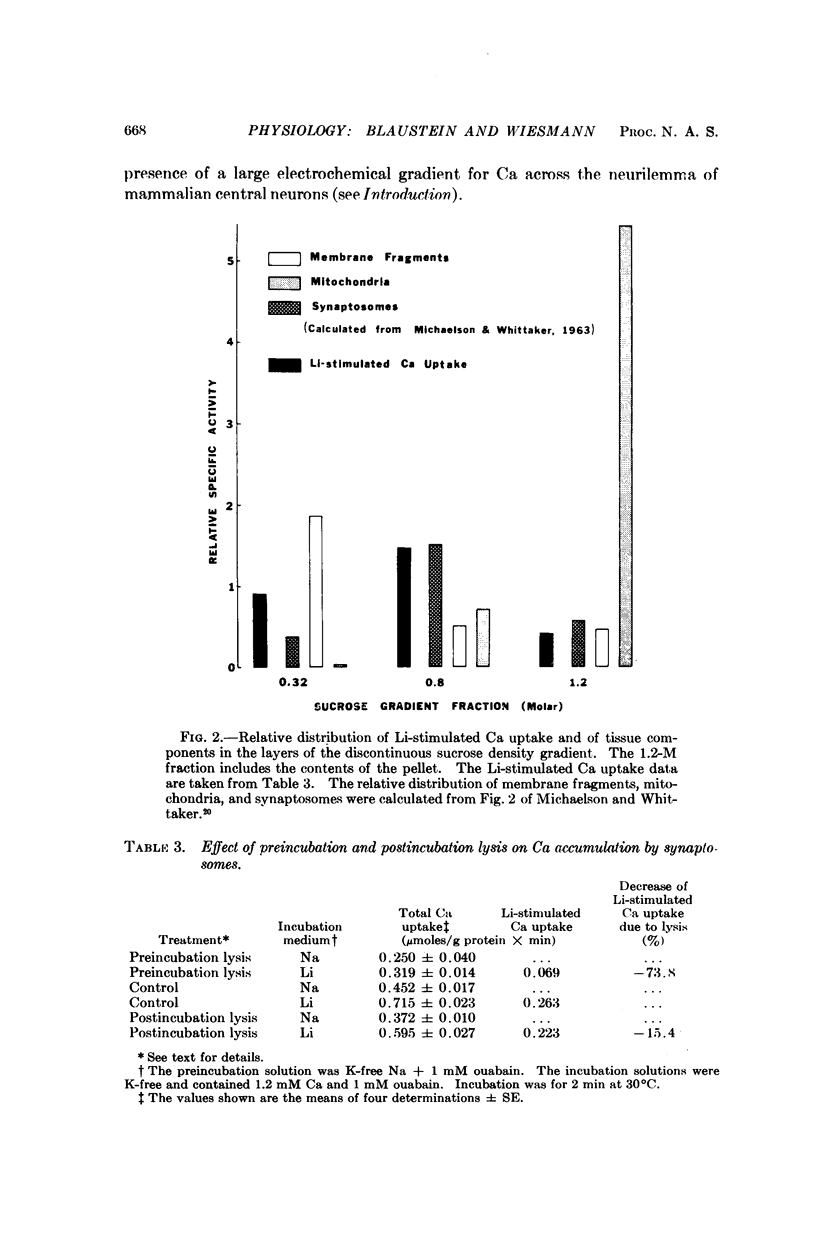
Calcium accumulation has been studied in isolated presynaptic nerve terminals from rat brain. Calcium influx is increased when external sodium is replaced by lithium or choline, or when the internal sodium concentration is increased by treatment with ouabain. Calcium efflux is reduced when external sodium is replaced by lithium. The lithium-stimulated calcium accumulation appears to be associated with the nerve terminals, and not with mitochondria or membrane fragments. It is dependent upon an osmotically sensitive surface membrane. The results are consistent with the hypothesis that calcium is actively extruded from mammalian central neurons by a mechanism which exchanges calcium for sodium, and which derives its energy from the sodium concentration gradient.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. In vivo effect of uncoupling agents on the incorporation of calcium and strontium into mitochondria and other subcellular fractions of rat liver. J Gen Physiol. 1967 Aug;50(7):1849–1864. doi: 10.1085/jgp.50.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Goldring S. Contribution to steady potential shifts of slow depolarization in cells presumed to be glia. Electroencephalogr Clin Neurophysiol. 1970 Feb;28(2):109–118. doi: 10.1016/0013-4694(70)90178-1. [DOI] [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. L. Cortical intracellular potentials and their responses to strychnine. J Neurophysiol. 1959 Jul;22(4):436–450. doi: 10.1152/jn.1959.22.4.436. [DOI] [PubMed] [Google Scholar]
- LOLLEY R. N. THE CALCIUM CONTENT OF ISOLATED CEREBRAL TISSUES AND THEIR STEADY-STATE EXCHANGE OF CALCIUM. J Neurochem. 1963 Sep;10:665–676. doi: 10.1111/j.1471-4159.1963.tb08938.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ling C. M., Abdel-Latif A. A. Studies on sodium transport in rat brain nerve-ending particles. J Neurochem. 1968 Aug;15(8):721–729. doi: 10.1111/j.1471-4159.1968.tb10316.x. [DOI] [PubMed] [Google Scholar]
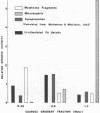
- MICHAELSON I. A., WHITTAKER V. P. The subcellular localization of 5-hydroxytryptamine in guinea pig brain. Biochem Pharmacol. 1963 Feb;12:203–211. doi: 10.1016/0006-2952(63)90185-0. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M. The osmotically sensitive potassium and sodium compartments of synaptosomes. Biochem J. 1967 Jul;104(1):148–157. doi: 10.1042/bj1040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUPE J. Comparaison entre fractions calciques de liquides céphalo-rachidiens et de sérums normaux chez l'homme. C R Seances Soc Biol Fil. 1957;151(2):318–320. [PubMed] [Google Scholar]
- PHILLIPS C. G. Intracellular records from Betz cells in the cat. Q J Exp Physiol Cogn Med Sci. 1956 Jan;41(1):58–69. doi: 10.1113/expphysiol.1956.sp001163. [DOI] [PubMed] [Google Scholar]
- Tower D. B. Ouabain and the distribution of calcium and magnesium in cerebral tissues in vitro. Exp Brain Res. 1968;6(4):273–283. doi: 10.1007/BF00233179. [DOI] [PubMed] [Google Scholar]