Abstract
Background
Immune challenge impacts behavior in many species. In mammals, this adaptive behavior is often manifested as an increase in sleep. Sleep has therefore been proposed to benefit the host by enhancing immune function and thereby overcome the challenge. To facilitate genetic studies on the relationship between sleep and immune function, we characterized the effect of the immune response on sleep in Drosophila melanogaster. Behavioral features of sleep as well as the innate immune response signaling pathways are well characterized in flies and are highly conserved in mammals.
Results
An immune response induced by infection with Gram-negative bacteria or by aseptic injury increased sleep in flies. The increase in sleep occurred during the morning hours after treatment and the magnitude of the effect was dependent on the time-of-day of inoculation or injury such that night-time treatment had a stronger effect than that during the daytime. This pattern persisted in constant darkness, indicating a role of the circadian clock. Mutants of the circadian clock gene, period, eliminated the increase in sleep observed in the morning, but instead showed enhanced sleep immediately after injury or infection.
Null mutants of the Nuclear Factor κB (NFκB) Relish, which is central to the innate immune response, do not increase sleep in response to injury or infection at any time of day. Instead, they maintain a normal sleep pattern until they die. Expression of a full-length Relish transgene in the fat bodies of Relish mutants restored the morning increase in sleep during an immune response. Fat bodies are a major site of immune signalling in flies and have a key role in host defense.
Conclusions
These data demonstrate that an immune response increases sleep in flies in a manner that is gated by the circadian clock and that requires the NFκB Relish. These findings support a role of sleep in a recovery process and demonstrate a conserved feature of the Drosophila model of sleep.
Background
Sleepiness and excess sleep are commonly experienced with infectious illness and other diseases, such as cancer [1]. Sleep has been proposed to be an adaptive response to immune challenge, and to thereby have a role in sustaining a robust immune system [2,3]. The immune system and sleep are indeed tightly linked in mammals, as components of the innate immune response, particularly proinflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor α (TNFα), promote sleep likely through their actions in hypothalamic nuclei. Exogenous application of these compounds increases sleep in mammals, depending on the dose, time of day and the site of injection [4]. In addition, loss of either the IL-1 Type I [5] or the 55 kDa TNF [6] receptor in mice reduces baseline (spontaneous, undisturbed) sleep during the light hours or during the dark hours, respectively. Although some of the molecular components of the mammalian immune system have a well defined role in sleep, we lack a full understanding of how these two physiological processes interact. Determining how sleep is involved in the immune response will have important implications for understanding its putative role in a recovery process as well as for human health.
Drosophila melanogaster has proven to be a powerful model for studying sleep. Flies exhibit all of the behavioural features of a sleep-like state [7,8]. One example of these features is that keeping flies awake for an extended period results in a compensatory sleep response, which indicates that flies have a need for this behaviour and that it is controlled by a homeostatic mechanism. Key to understanding a function of sleep is to determine its relationship with other physiological processes. Recent studies have indeed demonstrated that sleep in Drosophila is involved in aging [9], learning [10-13], and immune responses [14].
The first indication that sleep and the immune response are linked in Drosophila was the observation that many immune related genes increase mRNA expression after sleep deprivation [14-16]. The innate immune response in Drosophila is highly conserved with that in mammals, as they express central immune signalling components with corresponding functions. For example, the NFκB protein Relish, which is most similar to NFκB2 or p100 in humans [17], is central to the immune deficiency (Imd) pathway in flies, and is sensitive to injury and infection with Gram-negative bacteria [18]. In our previous report, we confirmed that Relish gene expression increases with short term sleep deprivation [14], which is consistent with observations that NFκB activity increases in the brain of mouse or rat [19-22] and human blood mononuclear cells [23] after sleep deprivation. We also demonstrated that flies deficient in, but not lacking, Relish expression exhibit decreased baseline, or spontaneous undisturbed sleep [14].
To further explore the association between sleep and the immune response, we determined the effect of infection with Gram-negative bacteria and aseptic injury on sleep in Drosophila. We show that the immune response triggered by both infection and injury promotes sleep in flies and that this effect is controlled by the circadian clock. We also show that the NFκB Relish has a strong role in promoting sleep during the immune response and that its expression in fat body is required for its effect on sleep. Together, these data suggest a role of sleep in a recovery process during immune challenge and demonstrate a behavioral feature of Drosophila sleep that is shared with that in mammals.
Results and Discussion
Infection and injury promote sleep
We monitored sleep in Canton Special (CS) flies during an immune response that was triggered either by infection (inf) with E. Coli or by aseptic injury (inj; see methods). A handled control group (HC) was subjected to the same handling such that they were removed from the activity monitors and CO2 anesthetized for the same duration, but they were not infected or injured. Flies were generally monitored for three baseline days and received treatment on the fourth day at one of four different time points in a 12: 12 light: dark (LD) cycle: ZT 0, ZT 6, ZT 12 or ZT 18 (ZT = zeitgeber time, where ZT 0-12 = lights on, and ZT 12-24 = lights off). Sleep was monitored for three days after treatment.
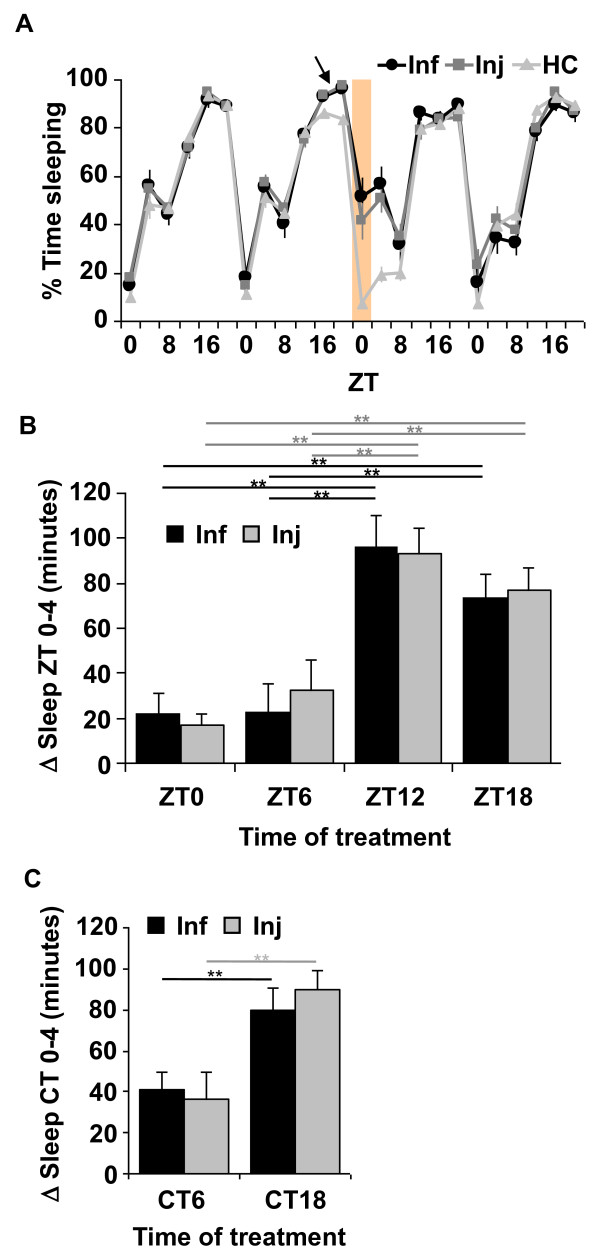
Significant increases in sleep were observed in both injured and infected groups relative to HC in the morning hours after treatment. Specifically, the most robust and consistent increase in sleep was from ZT 0-4 (shaded area in Figure 1A), but occasionally lasted up to 8 h (from ZT 0-8). A representative experiment is illustrated in Figure 1A, where flies were treated at ZT 18, and a significant increase in sleep was observed the following morning (shaded area and Additional File 1: Table S1; p < 5 × 10-7) in both infected and injured groups. Treatment at other times of day also produced increases in sleep the morning after treatment, from ZT 0-4, but with variable effects (Additional File 1: Table S1). In particular, treatment during night time hours produced stronger effects than those in the day time.
Figure 1.
Infection and injury promote sleep from ZT 0-4 after treatment in wild-type CS flies. (A) Representative results from infection (Inf) with E. coli and aseptic injury (Inj) at ZT 18 (arrow). Mean ± SEM percent time sleeping is plotted for 4 consecutive days in 4 hr increments. Shaded area indicates the increase in sleep from ZT 0-4 after treatment. [n = 12 for Inf; n = 15 for Inj; and n = 16 for handled control group (HC).] (B) Mean ± SEM net changes in sleep are reported from ZT 0-4 the morning after treatment at each of four time points (ZT 0, 6, 12 or 18). p < 0.0001, ANOVA; ** p < 0.01, Tukey's post-hoc comparisons within infected or injured groups. n = 26-61 flies per group. (C) Net changes in sleep from circadian time (CT) 0-4, the subjective morning after treatment in constant darkness, at CT 6 or CT 18. ** p < 0.01 with student's t-test within infected or injured groups. n = 28-32 flies per group. Values for mean net changes in sleep in (B) and (C) and all other subsequent figures, except where otherwise indicated, are normalized to those in handled control groups (see Methods).
We further analyzed the effect of time-of-day of infection and injury on sleep by comparing the net changes in sleep that occurred in the morning from ZT 0-4, after treatment at one of four time points across the day (Figure 1B). To correct for effects of handling, net change in sleep was determined in each fly by calculating the difference in time sleeping in minutes from ZT 0-4 before and after treatment and normalizing to the change in sleep observed between the corresponding time points in the handled control group (see Methods for details). Analysis of variance (ANOVA) revealed a significant effect of time-of-day of treatment in both injured and infected groups (p < 0.0001). Post-hoc comparisons indicated that mean change in sleep from ZT 0-4 that was induced by infection or injury at night, ZT 12 and ZT 18, was significantly larger than sleep induced by that in the daytime, ZT 0 and ZT 6 (Figure 1B).
To ensure that the daily oscillation of the sleep increase associated with the immune response was not an effect of a 12:12 LD cycle, CS flies were infected or injured on the third day in constant darkness (DD). Similar to the effect during LD, both infected and injured flies showed an increase in sleep in the subjective morning hours from CT 0-4 (CT = circadian time) after treatment. The increase in sleep was significantly higher when flies were treated during subjective nighttime (CT 18) than during subjective daytime (CT 6) in both infected and injured groups (Figure 1C).
To exclude the possibility that the increase in sleep was due to an effect on the flies' ability to move by debilitating injury due to the injection, we calculated the average number of activity counts per waking minute (activity rate) for flies treated at ZT 18. We focused on treatment at ZT 18, because this time point produced highly robust effects on sleep as well as on survival rate in response to infection with pathogenic bacteria [24]. If the increase in sleep was due to an effect on the flies' ability to move by injury, we would expect a decrease in activity rate. Instead, waking activity rates were unchanged in the infected group, and higher in injured groups as compared to HC (p < 0.05 for ANOVA; p < 0.05 for post-hoc comparison in injured and HC group; Additional File 2: Figure S1), indicating that the increase in sleep is not simply a reflection of lack of movement associated with injury.
In summary, an immune response triggered by infection or injury promotes sleep. The increase in sleep is restricted to the morning hours after treatment, and is dependent on the time of day of treatment such that night time treatment produced stronger effects than those in the daytime. This pattern persists in constant darkness, which strongly suggests a role of the circadian clock in regulating this response.
The observation that night-time infection produces a stronger effect on sleep than that in the daytime correlates with a previous observation that infection with pathogenic Gram-negative bacteria at night-time produced a better survival outcome than that in the daytime [24]. Whether the increased sleep experienced by the host contributes to survival during pathogenic infection will be an interesting topic for future study. Interestingly, an earlier study reported a disruption of circadian locomotor rhythm and sleep in flies after infection with Gram-positive pathogenic strains of bacteria [25], which contrasts with our current findings. One possible explanation for this is the different species of bacteria used in each of the studies. The pattern of sleep alteration associated with bacterial infection in mammals is also dependent on the bacterial species and route of infection [26]. Nonetheless, Shirasu-Hiza and colleagues [25] also proposed that in this case, the disruption of circadian rhythms and sleep was a contributing factor to the pathogenesis of the infection, thus further supporting a beneficial role of sleep in host defense.
Both infection and injury increase NFκB reporter activity
Sleep is a complex process that is sensitive to many different manipulations. It is therefore not surprising that injury and infection produce effects on sleep that are indistinguishable. To ensure the effect was not restricted to E. coli, flies were also infected with a mutated Gram-negative bacterial line P. aeruginosa (plcS) which has pathogenic ability [27]. A similar increase in sleep was observed in the morning from ZT 0-4 after nighttime treatment at ZT 18 in both infected and injured groups (p < 5 × 10-5; Additional File 3: Figure S2A).
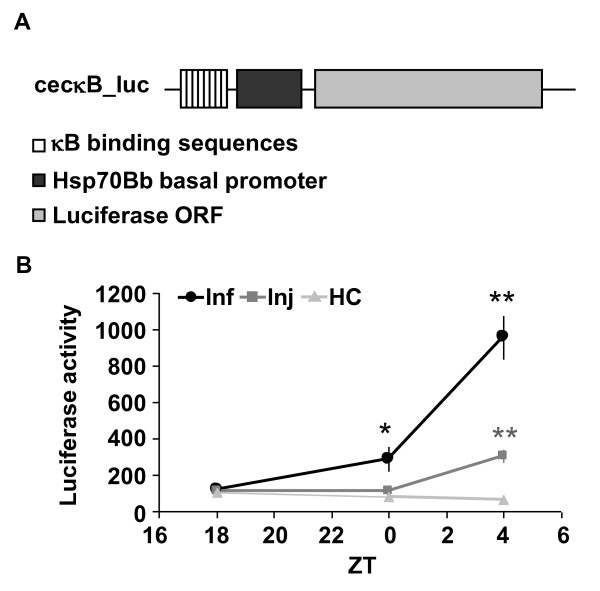
Both infection and injury trigger equivalent effects on a cellular immune response [28]. Infection with Gram-negative bacteria, in particular, activates the NFκB Relish, which is central to the Imd pathway. Injury also induces Relish-dependent gene expression, but to a lesser extent than infection [29]. To confirm this finding, we measured NFκB dependent luciferase activity in living flies which contain an NFκB response element upstream of a luciferase reporter gene (κB-luc; Figure 2A). Both infection with E. coli and injury at ZT 18 produced significant increases in κB-luc activity by the next morning, at ZT 4, relative to baseline (Figure 2B; ANOVA, p < .0001; Tukey's post-hoc comparison, p < .01 for both Inf and Inj groups). No significant change in reporter activity was detected in the HC group (ANOVA, p < .22). κB-luc was increased by approximately 5 fold relative to HC in injured flies, and 15 fold in infected flies. κB-luc activity was also sensitive to infection at ZT 18 with P. aeruginosa. Large increases in κB-luc activity were detected at ZT 4, 10 hours after the infection (p < .01, Tukey's post-hoc comparison, relative to baseline at ZT 18), and were further increased at ZT 8 (Additional File 3: Figure S2B). The delay in onset of κB-luc activity is consistent with a previous finding that P. aeruginosa infection delayed the onset of expression of antimicrobial peptides [30].
Figure 2.
Infection and injury increase κB-luc reporter activity in living flies. (A) The plasmid used for generation of κB-luc flies. Plasmid contained 8 copies of κB binding sequences, a heat shock protein (Hsp) 70 Bb basal promoter and a luciferase open reading frame (ORF). (B) Mean ± SEM luciferase activity (arbitrary units) was measured at indicated ZT times when κB-luc flies were treated at ZT 18. **p < 0.0001 and *p < 0.01, student's t-test compared to HC at corresponding time points. n = 32 flies for each group.
These results demonstrate that while infection and injury produce similar effects on sleep, they are clearly distinguishable at a molecular level. The increase in κB-luc activity by injury is comparable to that induced by a short term sleep deprivation [14], and both are sufficient to promote sleep (Figure 1, and [14,31]). The observation that infection produces a sustained increase in κB-luc activity (Additional File 3: Figure S2B) suggests that a component other than NFκB limits the duration and timing of sleep during immune challenge.
Sleep during the immune response is disrupted in a clock mutant
To understand why the increase in sleep during an immune response was restricted to the morning hours, we further examined a role of the circadian clock in this process. We measured sleep during the immune response in flies which contain a mutation in the clock gene, period (per01 [32]). period is an integral component of the biological clock in insects and vertebrate species [33]. Sleep-wake activity in per01 loss of function mutants fluctuates in LD cycles, but is completely arrhythmic in constant darkness [34].
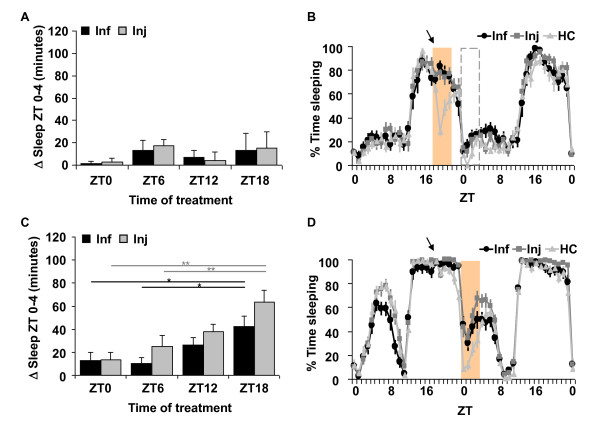
Sleep in the morning from ZT 0-4 after infection with E. coli or aseptic injury in per01 flies was not significantly affected as compared to HC regardless of the time-of-day of treatment (Additional File 1: Table S1 and Figure 3A; ANOVA p > .5 for both Inf and Inj groups). Instead, we found that sleep increased immediately after treatment, with the most robust effects occurring within the first four hours (Figure 3B, and Additional File 4: Figure S3A). The immediate increase in sleep in the infected group was significant as compared to HC (p < 0.01 for all time points tested; n = 14-16 flies each group) and no significant oscillation across time points was detected (ANOVA, p = 0.83). Sleep also increased immediately after injury, but increases were lower when flies were treated during the night time (Additional File 4: Figure S3A; ANOVA p < .0001). Figure 3B illustrates an experiment performed in per01 mutants treated at night-time, ZT 18. Sleep after manipulation at this time was clearly decreased in the HC group. An earlier study reported that LD cycles mask locomotor behaviour that is otherwise arrhythmic in these flies [34]. The sharp decrease in sleep in the control group is thus attributed to the light exposure that was necessary for administering the injections in the treatment groups. Nonetheless, the flies subjected to infection or injury slept more despite the exposure to light. Wild type CS flies and per01;;per flies, discussed below, did not show this sensitivity to light exposure (compare Figure 3B with Figures 1A and 3D). Handling of per01 flies during the daytime period, at ZT 0 and 6, did not produce any changes in sleep in the control group, while sleep in flies subjected to infection or injury at these times produced significant increases in sleep that occurred immediately after the treatment (Additional File 4: Figure S3A).
Figure 3.
Sleep during the immune response is gated by the circadian clock. (A) Mean ± SEM net changes in sleep in per01 flies from ZT 0-4 the morning after treatment at each of four time points (ZT 0, 6, 12 or 18). n = 13-16 flies per group. (B) Results from treatment at ZT 18 (arrow) in per01 flies. Mean ± SEM percent time sleeping is plotted for 2 consecutive days in 1 hr increments. Shaded area indicates the increase in sleep immediately after treatment from ZT 18-22. Dashed box area highlights ZT 0-4 time and corresponds to results reported in (A); n = 14 for Inf, n = 16 for Inj and n = 13 for HC. (C) Mean ± SEM net changes in sleep in per01;;per flies from ZT 0-4 after treatment at each of four time points (ZT 0, 6, 12 or 18). p < 0.0005 and p < 0.01 for ANOVA in Inj and Inf groups, respectively. ** p < 0.01 and * p < 0.05, Tukey's post-hoc comparisons. n = 22-45 flies per group. (D) Representative results from treatment at ZT 18 (arrow) in per01;; per flies. Data are plotted as described in (B). Shaded area indicates the increase in sleep from ZT 0-4 after treatment. n = 14 for Inf, n = 13 for Inj and n = 15 for HC.
To confirm the role of period in the sleep promoting effect, we examined sleep behaviour during the immune response in per01 flies expressing a full-length genomic construct of the period gene (per01;;per), as previously described [35]. Locomotor activity rhythms are restored in per01 flies carrying this transgene [35,36]. As compared to HC groups, per01;;per flies showed significant increases in sleep in the morning from ZT 0-4 after injury at ZT 6, ZT 12, and ZT 18 (Additional File 1: Table S1). Infection with E. coli also produced a significant increase in morning sleep relative to HC, but only when flies were infected at ZT 18 (p < .05). In both infected and injured groups, the time-of-day effect on the morning increase in sleep was restored in the per01;;per flies (ANOVA p < 0.0005 for injured, and p < 0.01 for infected groups). Night-time treatment produced stronger effects on morning sleep than that in the daytime (Figure 3C and 3D). In contrast to per01 mutants, the immediate effect on sleep occurred only when per01;;per flies were treated at ZT 0 (p < 0.05 for Inf; p < 0.01 for Inj, Additional File 4: Figure S3B). Interestingly, CS flies also exhibited a strong immediate effect on sleep after infection (101 ± 7 minutes, p < 1 × 10-7) and injury (111 ± 5 minutes, p < 5 × 10-7) at ZT 0, but not at ZT 6 or at ZT 18. Treatment at ZT 12 produced weaker effects on sleep from ZT 12-16 with an increase of 53 ± 8 minutes (p < 0.05) in injured flies. Infected flies also increased sleep at this time, but the increase fell short of significance (p < .07).
Together, these data demonstrate a role of the circadian clock in an adaptive behavioural response to bacterial infection and injury. The effect on sleep is gated by the clock, such that the response is restricted to early morning hours. Thus the clock permits a response when flies are subjected to immune challenge at the onset of this period (ZT 0) or at earlier times during the night before (ZT 12 and ZT 18). However, this permissive gate is 'closed' shortly after the morning period, and flies do not increase sleep when challenged at ZT 6. As discussed above, this gating pattern persists in constant dark conditions (Figure 1C) and is abolished in per01 mutants, which lack a functional clock. These findings correlate with those of an earlier study which reported that night-time infection of flies with pathogenic bacteria produced better survival rates than that in the daytime [24]. Furthermore, per01 flies were more susceptible to infection. Whether this is due to a disrupted sleep response or to other aspects of host defense is an interesting topic for future study.
Relish is required for the sleep promoting effect of the immune response
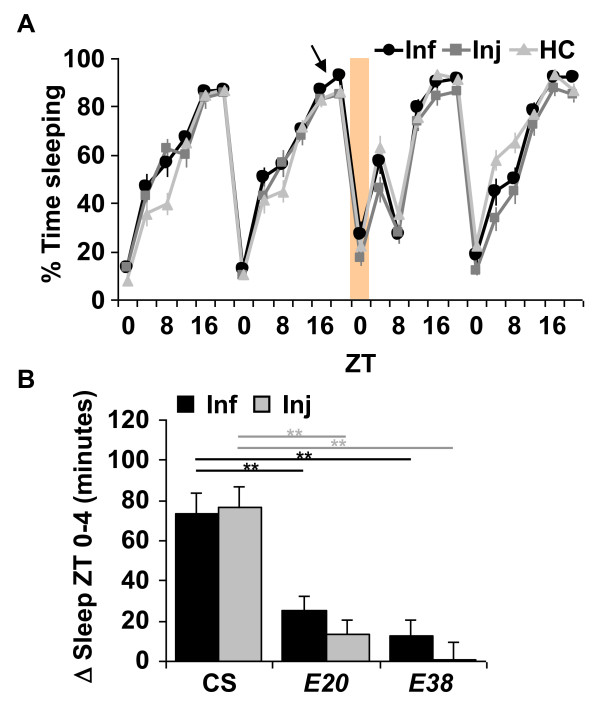
We next determined a role of the NFκB Relish in sleep that is triggered by an immune response. We focused on Relish because our previous study indicated that expression of its mRNA was very strongly affected by sleep deprivation, and that heterozygous mutants had reduced baseline, or spontaneous sleep [14]. Furthermore, as discussed above, Relish dependent gene expression is increased with both infection and injury (Figure 2B and [29]). Homozygous RelishE20 (E20) null mutants are severely immunocompromised and die within a few days after infection with non-pathogenic bacteria such as E. coli [37]. E20 homozygous flies in a CS background [14] were subjected to infection with E. coli or aseptic injury, as described above. Flies infected at ZT 18 (Figure 4A) and at ZT 6 (Additional File 1: Table S1) did not increase sleep as compared to HC and instead, continued to maintain a relatively normal sleep pattern until they died. Sleep from ZT 0-4 in both infected and injured E20 flies showed no significant change as compared to HC (p > 0.1) but showed significantly reduced net changes in sleep as compared to CS flies (Figure 4B). To ensure the differences between wild-type and E20 flies was not attributable to handling, we compared sleep in handled control groups from ZT 0-4 during baseline (the morning before treatment) with sleep during the morning after treatment. No significant change in sleep was detected (data not shown; p > 0.5 for paired t-test comparisons within groups as well as t-test comparisons between CS and E20). Together, these observations indicate that the NFκB Relish is required for the increase in sleep during the immune response.
Figure 4.
NFκB Relish null mutants abolish the increase in sleep from ZT 0-4 after infection or aseptic injury. (A) Representative results from treatment in E20 flies at ZT 18 (arrow). Data are plotted as described in Figure 1A. n = 14 for Inf, n = 16 for Inj and n = 16 for HC. (B) Mean ± SEM net changes in sleep from ZT 0-4 in CS, E20 and E38 flies that were treated at ZT 18. p < 0.0001, ANOVA in both Inf and Inj groups. ** p < 0.01; Tukey's post-hoc comparisons. n = 29-62 flies per group.
We next performed studies to confirm that this phenotype was attributable to a lesion in the Relish gene. First, another Relish null allele, RelishE38 (E38) [37], was examined. Similar to the E20 flies, E38 mutants did not increase sleep in response to infection with E. coli or to injury (p > 0.4; Additional File 1: Table S1). The net change in sleep during the immune response was also significantly smaller as compared to CS flies (Figure 4B).
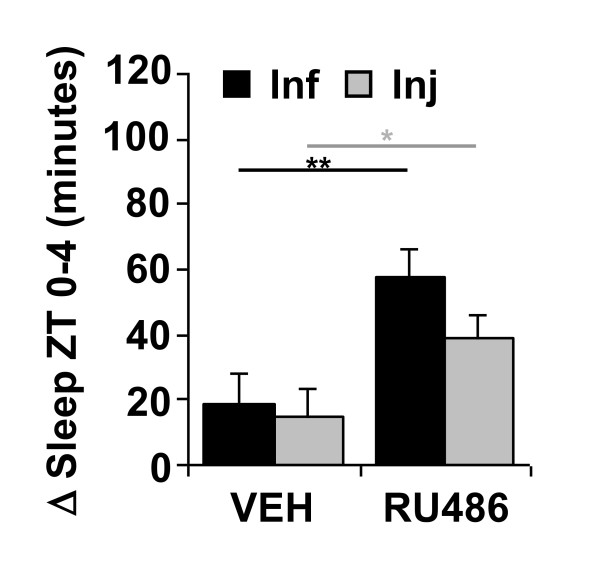
We next determined whether expressing a UAS-Relish transgene in fat bodies in E20 mutants could restore the increase in sleep during the immune response. Fat bodies are masses of adipose tissue located throughout the fly and are a major site of immune signaling and metabolism [18]. To circumvent the effects of genetic background, we used the RU486-dependent Gal4 driver S1106 [38], which expresses specifically in fat body. S1106-Gal4/UAS-Rel; E20 flies that were chronically fed RU486 showed a significant increase in sleep from ZT 0-4 after treatment at ZT 18 as compared to siblings of the same genotype that were fed a vehicle control (Figure 5; student's t-test, p < 0.005 for Inf and p < 0.05 for Inj). To determine whether the effect of Relish on sleep was specific to fat bodies, we used a pan-neuronal RU486-dependent Gal4 driver, elav-GeneSwitch (elavGS) [39] to express UAS-Rel in the central nervous system. Neither the RU486-fed nor vehicle control fed elav-GS/UAS-Rel; E20 flies showed a significant increase in morning sleep after infection (p > 0.1) or injury (p > 0.08) at ZT 18 (Additional File 5: Figure S4). Thus UAS-Rel restores sleep during an immune response in E20 mutants when expressed in fat bodies, but not in neurons.
Figure 5.
Expression of Relish in fat bodies restores sleep induced by infection or injury in E20 mutants. Mean ± SEM net changes in sleep from ZT 0-4 in S1106-Gal4/UAS-Rel; E20 flies. Flies were chronically exposed to vehicle (VEH) or RU486 and treated at ZT 18. ** p < 0.005 and *p < 0.05, t-test; n = 33-61 flies per group.
These data demonstrate that Relish is necessary for the sleep promoting effect of the immune response and that its expression in fat body is sufficient for this effect. In our previous study, we reported that Relish also functioned from fat body to modulate baseline or spontaneous undisturbed sleep such that sleep was reduced in heterozygous Relish null mutants, but not affected in Relish homozygous mutants [14]. We now report that Relish homozygous mutants virtually abolish a sleep promoting effect of the immune response. Together these observations indicate that sleep induced by infection or injury is different from baseline sleep and that each is controlled by different mechanisms. Others have proposed that baseline and rebound sleep, which is sleep after prolonged wakefulness or sleep deprivation, are also controlled by different mechanisms [10,40]. For example, Hyperkinetic and shaker mutations strongly reduce daily sleep levels, but do not alter responses to sleep deprivation [10,41]. In contrast, a recent study described a hypomorph of the sleepless gene that minimally affected baseline sleep but had severe effects on sleep rebound [40]. Mutants of the clock gene, cycle, also reduced baseline sleep [42], but produced sex dimorphic effects on sleep rebound [42,43]. Thus the results described here indicate that the predominant role of Relish in sleep is during a recovery period after immune challenge.
As discussed earlier, injury and infection produce distinct effects on κB-luc activity such that the effect of infection increases reporter activity to a much greater extent (Figure 2B and Additional File 3: Figure S2B). Given the role of Relish in sleep induced by immune challenge, one may expect that the impact of infection on sleep would be greater than that during aseptic injury. This is clearly not the case, which suggests that a component other than NFκB limits the duration of the morning sleep response. One possibility is that the pressure to be awake at this time of day outweighs the pressure to sleep. However, the duration of this response was unchanged in per01 mutants. In particular, daytime infection or injury in these flies produced an immediate increase in sleep that lasted approximately four hours, which is similar to the duration of the morning increase in sleep seen in wild type flies. This observation suggests that the circadian clock determines when the response occurs, but does not determine the duration of the effect on sleep. We propose that while Relish is necessary for inducing sleep during an immune response, a separate mechanism is involved in limiting the duration of sleep during a recovery period. This mechanism may involve neural circuits in the brain, which are known to regulate sleep (reviewed in [44]). Alternatively, negative regulatory factors that act directly on Relish target genes are also potential candidates that may be involved in this process [45].
Studies in mammals have shown that blocking IL-1 or TNFα cytokines also prevent increased sleep during immune challenge (reviewed in [46]), which is consistent with our current findings. NFκB p50 knockout mice also show reduced sleep responses to infection with influenza virus, but had enhanced responses to immune challenge with bacterial lipopolysaccharide [47]. Although Relish has similarity with p50, it is possible that different NFκB family members in both mammals and flies will have distinct roles in baseline sleep and in sleep induced by infection or injury.
Conclusions
The observation that the immune response promotes sleep in Drosophila demonstrates an additional feature of insect sleep that is shared with that in mammals. The effect of infection and injury on sleep is gated by the circadian clock, such that the effect is restricted to the morning after treatment and is affected by the time-of-day of the treatment. Night-time injury or infection with Gram-negative bacteria produced stronger effects than equivalent manipulations in the daytime. This pattern is disrupted in the per01 clock mutant, which responds by increasing sleep immediately after infection or injury. These findings correlate with the observation that survival rates were lower when flies were infected with Gram-negative pathogenic bacteria in the daytime than at nighttime [24]. We have also demonstrated that Relish is required for the sleep promoting effect of the immune response, and that its expression in fat body is sufficient for this process. Together, these findings suggest that adaptive sleep during an immune response involves an integration of signals from the peripheral immune system and the circadian clock. Further genetic dissection of this process will better our understanding of a function of sleep in recovery from immune challenge or injury.
Methods
Fly stocks
Flies were grown on standard agar, corn meal, malt extract, and soy flour medium with 1.84 mg/L tegosept. RelishE20 (E20) mutants were isogenized to a CS background as described previously [14]. Briefly, the ebony marker was removed by recombination, and offspring were backcrossed into the CS background for at least four generations. The presence of the E20 mutation was confirmed using PCR. κB-luc transgenic flies were generated as described below. w;S1106;RelE20, w;elav-GS;RelE20, w1118, per01 and w1118, per01;; per flies [35] were provided by Dr. Isaac Edery, Rutgers University. Other strains used were CS, RelishE38(E38) and UAS-Rel;RelE20/TM3, sb.
Behavioral assays
Sleep was measured as described previously [14]. All experiments were performed in females. Flies 1-3 days in age were loaded into glass activity tubes containing 5% sucrose and 2% agar medium, maintained in 12:12 light: dark cycles at 25°C, and activity was measured using the Trikinetics DAM2 system (Waltham, MA). For experiments in which flies were treated with drug, food contained 2% sucrose and 2% agar. Activity counts were collected every minute, and sleep defined as activity counts of zero for a minimum of 5 consecutive minutes [31]. Sleep parameters were analyzed using custom Matlab based software, Insomniac2, generously provided by Dr. Lesley Ashmore, University of Pennsylvania.
For induction of mifepristone (RU486; Sigma) dependent Gal4 drivers [38,39], 25 μM RU486 was added to standard fly food medium for chronic exposure throughout development. RU486 was diluted from a 10 mM stock in 80% ethanol. Equivalent dilutions were made with 80% ethanol for the vehicle control groups. Adult flies were exposed to 500 μM RU486, or equivalent control vehicle dilution, in sucrose/agar medium used in the activity tubes for behavioural experiments.
Infection and Injury
Infections were conducted by injecting flies with E. coli or P. aeruginosa using small glass pipettes (tip diameter ~50 μm). Bacteria were grown to saturating concentrations (OD600 = 0.5 - 1.0) in LB medium containing 50 μg/ml ampicillin (for E. coli) or 50 μg/ml gentamycin (for P. aeruginosa) and then diluted in phosphate buffered saline (PBS) and food coloring solution. The final concentrations for bacteria were OD600 of 0.1 for E. coli and OD600 of 1 × 10-4 for P. aeruginosa. A second group of flies (injured groups, or Inj) was subjected to injections of dilutions of LB broth (with indicated antibiotics) in PBS/food coloring solution. A third group (handled control, or HC) was subjected to the same handling, but not injected.
For treatment in both LD and DD cycles, activity monitors containing flies were removed from incubators. Individual flies were then removed from each glass activity tube, placed onto a CO2 pad for anesthetization, and were infected or injured. Handled control flies were CO2 anesthetized for the same duration as the infected and injured groups. Flies that were treated during night-time hours or in DD were typically exposed to a 1 hour light pulse. The time of treatment in constant dark conditions corresponded to times when light pulses produce minimal phase shifts in locomotor activity rhythms [48].
Luciferase reporter assay
Transgenic flies (κB-luc) that express a luciferase reporter (cecκB-luc) were generated as follows: The cecκB-luc reporter plasmid was constructed by inserting into a pGL3 basic luciferase vector (Promega), 8 direct repeats of NFκB binding sequences (5'-ATCGGGGATTTTTGCAGAGAAAA-3') derived from the cecropin A1 promoter region [49] followed by a Drosophila heat shock protein (Hsp70Bb) basal promoter. The cecκB-luc construct was then subcloned into a pCaSpeR4 backbone. Transgenic flies were then generated in a w1118 background (BestGene Inc., CA).
κB-luc flies 1-3 days in age maintained in 12:12 light: dark cycles were loaded into vials containing 5% sucrose, 2% agar medium and entrained for another 2 days. 2 days later flies were transferred individually to a 96-well plate containing 2 mM luciferin (the substrate of luciferase; Gold Biotechnology Inc.), 2% sucrose and 1% agar medium. Flies were then subjected to infection with E. coli or injury, as described above, at ZT 18 the next day. Luciferase activity in living flies was measured immediately before treatment at ZT 18 and after treatment (ZT 0 and ZT 4) with a Fusion Universal Microplate Analyzer (Packard). A control group was subjected to CO2 anesthesia for same duration as the other groups, but was not injured or infected.
A previous study demonstrated that a similar construct was sensitive to the NFκBs Dif and Dorsal in an insect cell culture system [49]. We determined whether the κB-luc reporter was also sensitive to Relish activity in living flies. κB-luc was expressed in RelishE20 homozygous mutants and in E20/TM3, Sb heterozygous siblings. Flies were infected with E. coli at night- time, ZT 18, and luciferase reporter activity was measured at ZT 18 prior to infection, ZT 0, and ZT 4, as described above. A significant induction of κB-luc was detected in both groups of flies (p < .0001 ANOVA) such that κB-luc activity was significantly higher at ZT 4 as compared to baseline at ZT 18 and ZT 0 (p < 0.01, Tukey's post-hoc comparisons; see Additional File 6: Figure S5). However, the signal from E20/E20 homozygous flies was significantly lower than the heterozygous siblings at baseline, ZT 18 (p < .0001; student's t-test), and values from the E20/E20 flies at ZT 4 did not differ significantly from the baseline values of the E20/TM3sb flies (p < .066). Although we detected an induction of κB-luc during infection in the E20/E20 flies, the level of induction did not surpass the baseline values in the control siblings. These data indicate that the κB-luc signal is highly sensitive to Relish activity during infection with E. coli.
Statistical analysis
To analyze changes in sleep during the immune response, a statistical algorithm was developed in collaboration with Dr. Minge Xie and Chingray Yu in the Department of Statistics, Rutgers University, to make t-test comparisons between the amount of sleep at a given time points for treated and handled control groups. This algorithm is based on the simple formula:
where I = infected or injured group, T0 = sleep time from ZT 0-4 before treatment, T1 = sleep time from ZT 0-4 post-treatment, C = handled control and nC = number of flies in the corresponding control group. This is a conservative approach that corrects for arbitrary differences in baseline sleep that may occur between conditions and is the basis for calculating a t-statistic for comparing sleep before and after treatment. The net changes in sleep after treatment were determined by formula [i]. t-tests were performed using custom software (available upon request) written in R version 2.3.1, downloaded from http://www.r-project.org/. Using this approach, sleep during the immune response was initially analyzed using a range of increments (ZT 0-1, 0-2, 0-4, 0-6 and 0-8). We found that bacterial infection or aseptic injury consistently produced the most robust effect on sleep from ZT 0-4. Values reported in Additional File 1: Table S1 and in Figures 1 and 3 for treatments in the daytime, ZT 0 and ZT 6, correspond to the ZT 0-4 time point 24 h and 18 h, respectively, after treatment. The immediate response at ZT 0-4 elicited by treatment at ZT 0 is discussed in the main text and is reported in Additional File 4: Figure S3.
Multi-group comparisons between mean net changes in sleep were performed by one-way ANOVA http://statpages.org/ followed by Tukey's post-hoc comparison through online software from the Department of Obstetrics and Gynecology, The Chinese University of Hong Kong http://department.obg.cuhk.edu.hk/researchsupport/statstesthome.asp. For the luciferase reporter assays, within group comparisons using ANOVA were performed to determine whether the signal was affected after treatment in each group (Inf, Inj, or HC). Comparisons were also made at each time point using student's t-test to determine differences from the handled control group.
Authors' contributions
T-HK and DHP performed and analyzed experiments to characterize sleep during the immune response in both wild type and mutant flies. T-HK generated the κB-luc construct, performed experiments in κB-luc flies, and determined the effects of UAS-Relish on restoring sleep in Relish mutants. ZB performed experiments conducted in DD and analyzed the effect of P. aeruginosa on sleep. JAW designed and supervised experiments. T-HK and JAW wrote the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Table S1. Sleep from ZT 0-4 following infection or injury at indicated times. Mean ± SEM values for minutes sleep during the 4 hour morning period following treatment. HC = handled control, Inj = Injury, and Inf = Infection with E. coli. p values are derived from t-test comparisons between the change in sleep before (BL = baseline) and after (PT = post-treatment) treatment with that from the corresponding HC group (using formula [i], see Methods). For flies treated at ZT 0, the morning ZT 0-4 time is the day 24 h after infection or injury. See text for discussion of immediate effects of infection and injury on sleep.
Figure S1. Infection and injury do not affect locomotor ability of flies. Activity counts per waking minute (activity rate) of infected, injured and HC groups during the morning after treatment at ZT 18. *p < 0.05, Tukey's post-hoc comparison; n = 42-61 flies per group.
Figure S2. Infection with P. aeruginosa promotes morning sleep similar to injury. (A) Representative experiment in CS flies that were infected with P. aeruginosa (Inf) or injured (Inj) at ZT 18 (arrow). Data are plotted as described in Figure 1A. n = 10 for Inf, n = 14 for Inj and n = 13 for HC. (B) Mean ± SEM luciferase activity (arbitrary units) was measured at indicated ZT times when κB-luc flies were infected with P. aeruginosa or injured at ZT 18. ***p < 0.0005, **p < 0.005 and *p < 0.05, student's t-test compared to HC at corresponding time points. n = 28-48 flies for each group.
Figure S3.per01 flies increase sleep immediately after infection with E. coli or aseptic injury. (A) per01 flies were treated at indicated time points, and mean ± SEM net changes in sleep are plotted for the 4 h period immediately after treatment. ANOVA, p < 0.0001 in Inj group. ** p < 0.01 and * p < 0.05, Tukey's post-hoc comparison. n = 13-16 flies per group. (B) Mean ± SEM net changes in sleep in per01;; per flies for the 4 h period immediately after treatment at each of four indicated time points. ANOVA, p < 0.0001 in both Inf and Inj groups.** p < 0.01 and * p < 0.05, Tukey's post-hoc comparison. n = 22-45 flies per group.
Figure S4. Expression of Relish in neurons does not restore sleep during infection or injury in E20 mutants. Mean ± SEM net changes in sleep from ZT 0-4 in elavGS/UAS-Rel; E20 flies. Flies were chronically fed vehicle (VEH) or RU486 and treated at ZT 18. n = 16-48 flies per group.
Figure S5.κB-luc reporter activity is sensitive to NFκB Relish. Mean ± SEM luciferase activity (arbitrary units) was measured at indicated ZT times when flies were infected with E. coli at ZT 18. Infected flies included κB-luc expressed in siblings carrying one copy of RelE20 (κB-luc;;RelE20/TM3, Sb; n = 16) or two copies of RelE20 (κB-luc;;RelE20; n = 32). **p < 0.0001, t-test comparison between two genotypes at indicated time points.
Contributor Information
Tzu-Hsing Kuo, Email: kuo@cabm.rutgers.edu.
Douglas H Pike, Email: pike@cabm.rutgers.edu.
Zahra Beizaeipour, Email: zbeiza@eden.rutgers.edu.
Julie A Williams, Email: jwilliams@cabm.rutgers.edu.
Acknowledgements
This work was supported by start-up funds, the UMDNJ Foundation, and a Young Investigator award from NARSAD to JAW. We thank Dr Minge Xie and Chingray Yu in the Department of Statistics, Rutgers University for development of statistical software, Dr Lesley Ashmore for sleep analysis software, and Dr Isaac Edery and members of his laboratory for fly stocks.
References
- Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116(6):1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Preston BT, Capellini I, McNamara P, Barton RA, Nunn CL. Parasite resistance and the adaptive significance of sleep. BMC Evol Biol. 2009;9:7. doi: 10.1186/1471-2148-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp MR. Sleeping to fuel the immune system: mammalian sleep and resistance to parasites. BMC Evol Biol. 2009;9:8. doi: 10.1186/1471-2148-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obal F Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol. 1998;274(3 Pt 2):R655–660. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFalpha treatment. J Neurosci. 1997;17(15):5949–5955. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25(1):129–138. doi: 10.1016/S0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287(5459):1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Koh K, Evans JM, Hendricks JC, Sehgal A. A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci USA. 2006;103:13843–13847. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Huber R, Tononi G, Cirelli C. Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J Neurosci. 2007;27(20):5384–5393. doi: 10.1523/JNEUROSCI.0108-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324(5923):109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18(15):1110–1117. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324(5923):105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in Drosophila: A role for the NFκB Relish. Sleep. 2007;30(4):389–400. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JE, Rizzo W, Shockley KR, Raizen DM, Naidoo N, Mackiewicz M, Churchill GA, Pack AI. Multiple mechanisms limit the duration of wakefulness in Drosophila brain. Physiol Genomics. 2006;27(3):337–350. doi: 10.1152/physiolgenomics.00030.2006. [DOI] [PubMed] [Google Scholar]
- Cirelli C, LaVaute TM, Tononi G. Sleep and wakefulness modulate gene expression in Drosophila. J Neurochem. 2005;94(5):1411–1419. doi: 10.1111/j.1471-4159.2005.03291.x. [DOI] [PubMed] [Google Scholar]
- Minakhina S, Steward R. Nuclear factor-kappa B pathways in Drosophila. Oncogene. 2006;25(51):6749–6757. doi: 10.1038/sj.onc.1209940. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Ramesh V, Thatte HS, McCarley RW, Basheer R. Adenosine and sleep deprivation promote NF-kappaB nuclear translocation in cholinergic basal forebrain. J Neurochem. 2007;100(5):1351–1363. doi: 10.1111/j.1471-4159.2006.04314.x. [DOI] [PubMed] [Google Scholar]
- Brandt JA, Churchill L, Rehman A, Ellis G, Memet S, Israel A, Krueger JM. Sleep deprivation increases the activation of nuclear factor kappa B in lateral hypothalamic cells. Brain Res. 2004;1004(1-2):91–97. doi: 10.1016/j.brainres.2003.11.079. [DOI] [PubMed] [Google Scholar]
- Basheer R, Rainnie DG, Porkka-Heiskanen T, Ramesh V, McCarley RW. Adenosine, prolonged wakefulness, and A1-activated NF-kappaB DNA binding in the basal forebrain of the rat. Neuroscience. 2001;104(3):731–739. doi: 10.1016/S0306-4522(01)00111-7. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gardi J, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB-like activity increases in murine cerebral cortex after sleep deprivation. Am J Physiol. 1999;276(6 Pt 2):R1812–1818. doi: 10.1152/ajpregu.1999.276.6.R1812. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64(6):538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Edery I. Circadian Regulation in the Ability of Drosophila to Combat Pathogenic Infections. Curr Biol. 2008;18(3):195–199. doi: 10.1016/j.cub.2007.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu-Hiza MM, Dionne MS, Pham LN, Ayres JS, Schneider DS. Interactions between circadian rhythm and immunity in Drosophila melanogaster. Current Biology. 2007;17(10):R353–R355. doi: 10.1016/j.cub.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Opp MR, Toth LA. Neural-immune interactions in the regulation of sleep. Front Biosci. 2003;8:d768–779. doi: 10.2741/1061. [DOI] [PubMed] [Google Scholar]
- Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268(5219):1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- Markus R, Kurucz E, Rus F, Ando I. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett. 2005;101(1):108–111. doi: 10.1016/j.imlet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003;5(3):441–450. doi: 10.1016/S1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Apidianakis Y, Mindrinos MN, Xiao W, Lau GW, Baldini RL, Davis RW, Rahme LG. Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc Natl Acad Sci USA. 2005;102(7):2573–2578. doi: 10.1073/pnas.0409588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27(4):628–639. doi: 10.1093/sleep/27.4.628. [DOI] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417(6886):329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- Wheeler DA, Hamblen-Coyle MJ, Dushay MS, Hall JC. Behavior in Light-Dark Cycles of Drosophila Mutants That Are Arrhythmic, Blind, or Both. J Biol Rhythms. 1993;8(1):67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- Kim EY, Ko HW, Yu W, Hardin PE, Edery I. A DOUBLETIME Kinase Binding Domain on the Drosophila PERIOD Protein Is Essential for Its Hyperphosphorylation, Transcriptional Repression, and Circadian Clock Function. Mol Cell Biol. 2007;27(13):5014–5028. doi: 10.1128/MCB.02339-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JC, Vanselow JT, Kramer A, Edery I. The phospho-occupancy of an atypical SLIMB-binding site on PERIOD that is phosphorylated by DOUBLETIME controls the pace of the clock. Genes Dev. 2008;22(13):1758–1772. doi: 10.1101/gad.1682708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, Hultmark D. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell. 1999;4(5):827–837. doi: 10.1016/S1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2001;98(22):12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci USA. 2001;98(22):12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321(5887):372–376. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434(7037):1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Lu S, Kume K, Yin JC, Yang Z, Sehgal A. Gender dimorphism in the role of cycle (BMAL1) in rest, rest regulation, and longevity in Drosophila melanogaster. J Biol Rhythms. 2003;18(1):12–25. doi: 10.1177/0748730402239673. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417(6886):287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- Harbison ST, Mackay TF, Anholt RR. Understanding the neurogenetics of sleep: progress from Drosophila. Trends Genet. 2009;25(6):262–269. doi: 10.1016/j.tig.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim LK, Choi UY, Cho HS, Lee JS, Lee WB, Kim J, Jeong K, Shim J, Kim-Ha J, Kim YJ. Down-Regulation of NF-kappaB Target Genes by the AP-1 and STAT Complex during the Innate Immune Response in Drosophila. PLoS Biol. 2007;5(9):e238. doi: 10.1371/journal.pbio.0050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10(3):199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri KA, Ramkumar V, Trammell RA, Toth LA. Spontaneous, homeostatic, and inflammation-induced sleep in NF-{kappa}B p50 knockout mice. 10.1152/ajpregu.00262.2006. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1516–1526. doi: 10.1152/ajpregu.00262.2006. [DOI] [PubMed] [Google Scholar]
- Suri V, Qian Z, Hall JC, Rosbash M. Evidence that the TIM light response is relevant to light-induced phase shifts in Drosophila melanogaster. Neuron. 1998;21(1):225–234. doi: 10.1016/S0896-6273(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Gross I, Georgel P, Kappler C, Reichhart J, Hoffmann J. Drosophila immunity: a comparative analysis of the Rel proteins dorsal and Dif in the induction of the genes encoding diptericin and cecropin. Nucl Acids Res. 1996;24(7):1238–1245. doi: 10.1093/nar/24.7.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sleep from ZT 0-4 following infection or injury at indicated times. Mean ± SEM values for minutes sleep during the 4 hour morning period following treatment. HC = handled control, Inj = Injury, and Inf = Infection with E. coli. p values are derived from t-test comparisons between the change in sleep before (BL = baseline) and after (PT = post-treatment) treatment with that from the corresponding HC group (using formula [i], see Methods). For flies treated at ZT 0, the morning ZT 0-4 time is the day 24 h after infection or injury. See text for discussion of immediate effects of infection and injury on sleep.
Figure S1. Infection and injury do not affect locomotor ability of flies. Activity counts per waking minute (activity rate) of infected, injured and HC groups during the morning after treatment at ZT 18. *p < 0.05, Tukey's post-hoc comparison; n = 42-61 flies per group.
Figure S2. Infection with P. aeruginosa promotes morning sleep similar to injury. (A) Representative experiment in CS flies that were infected with P. aeruginosa (Inf) or injured (Inj) at ZT 18 (arrow). Data are plotted as described in Figure 1A. n = 10 for Inf, n = 14 for Inj and n = 13 for HC. (B) Mean ± SEM luciferase activity (arbitrary units) was measured at indicated ZT times when κB-luc flies were infected with P. aeruginosa or injured at ZT 18. ***p < 0.0005, **p < 0.005 and *p < 0.05, student's t-test compared to HC at corresponding time points. n = 28-48 flies for each group.
Figure S3.per01 flies increase sleep immediately after infection with E. coli or aseptic injury. (A) per01 flies were treated at indicated time points, and mean ± SEM net changes in sleep are plotted for the 4 h period immediately after treatment. ANOVA, p < 0.0001 in Inj group. ** p < 0.01 and * p < 0.05, Tukey's post-hoc comparison. n = 13-16 flies per group. (B) Mean ± SEM net changes in sleep in per01;; per flies for the 4 h period immediately after treatment at each of four indicated time points. ANOVA, p < 0.0001 in both Inf and Inj groups.** p < 0.01 and * p < 0.05, Tukey's post-hoc comparison. n = 22-45 flies per group.
Figure S4. Expression of Relish in neurons does not restore sleep during infection or injury in E20 mutants. Mean ± SEM net changes in sleep from ZT 0-4 in elavGS/UAS-Rel; E20 flies. Flies were chronically fed vehicle (VEH) or RU486 and treated at ZT 18. n = 16-48 flies per group.
Figure S5.κB-luc reporter activity is sensitive to NFκB Relish. Mean ± SEM luciferase activity (arbitrary units) was measured at indicated ZT times when flies were infected with E. coli at ZT 18. Infected flies included κB-luc expressed in siblings carrying one copy of RelE20 (κB-luc;;RelE20/TM3, Sb; n = 16) or two copies of RelE20 (κB-luc;;RelE20; n = 32). **p < 0.0001, t-test comparison between two genotypes at indicated time points.