Abstract
Recently, genetic alterations activating the receptor tyrosine kinase KIT have been demonstrated in some types of melanoma. KIT mutations can be successfully targeted by approved drugs in other cancers. Emerging evidence suggests that melanomas with KIT activation may also respond to KIT inhibitors that are already in clinical use. If confirmed in ongoing clinical trials, this experience would underscore the importance to recognize the biological diversity among melanomas, representing a first decisive step towards individualized and mechanism-based treatment of melanoma.
KIT is a member of the type III transmembrane receptor tyrosine kinase (RTK) family, which are comprised of five extracellular immunoglobulin domains, a single transmembrane region, an inhibitory cytoplasmic juxtamembrane domain, and a split cytoplasmic kinase domain separated by a kinase insert segment (Yarden et al., 1987). Under physiologic conditions, binding of the KIT ligand, stem cell factor (SCF), to the extracellular domain of the receptor results in receptor dimerization, activation of the intracellular tyrosine kinase domain through autophosphorylation of specific tyrosine residues, and receptor activation (Lev et al., 1992). The downstream signal transduction pathways includes the mitogen activated protein kinase- (MAPK), phosphatidylinositol 3’-kinase- (PI3K), and Janus kinase/signal transducers and activators of transcription- (JAK/STAT) pathways. The intracellular signalling through KIT plays a critical role in the development of several mammalian cell types, including melanocytes, hematopoietic progenitor cells, mast cells, primordial germ cells, and interstitial cells of Cajal (Nishikawa et al., 1991; Galli et al., 1995). Although it has been extensively demonstrated that KIT signalling is essential for melanocyte development, its precise role in migration, survival, proliferation, and differentiation remains incompletely understood. KIT function is important for the survival of melanoblasts as melanocyte precursor with loss of function mutations in KIT never disperse and ultimately disappear (Wehrle-Haller and Weston, 1995). In the mouse, survival of melanogenic subpopulations of neural crest cells depends on SCF for a critical period, which begins after the second day of dispersal, and lasts about 4 days, ending about the time that melanocytes terminally differentiate, as indicated by the presence of functional melanosomes (Morrison-Graham and Weston, 1993). A role for KIT in the dorsoventral migration of melanocytes is indicated by the fact that loss of function mutations in the KIT pathway lead to patterned pigmentation phenotypes such as white midline spotting in animals and piebaldism in humans (Giebel and Spritz, 1991). Specifically, KIT activation has been shown to be transiently required in the dorsal dermatome before the onset of trunk neural crest dispersal on the lateral pathway (Wehrle-Haller and Weston, 1995). Alexeev and Yoon (2006) have shown that activation of KIT receptor is primarily responsible for stimulation of migration rather than proliferation of melanocytes and found that KIT activation results in morphological changes of melanocytes such as spindle-shaped bodies and reduced number of dendrites (Alexeev and Yoon, 2006). KIT activation in melanocytes results in rapid increase in actin stress fiber formation and elevated melanocyte migration on fibronectin (Scott et al., 1996), indicating a role for KIT in the reorganization of the cytoskeleton and higher migratory properties of melanocytes. When melanocytes with activating KIT mutations are transplanted into the dorsal skin of albino mice, cells demonstrate a distinctive migration pattern from the injected sites to the upper dermis and dermal-epidermal border, toward the follicular and/or interfollicular keratinocytes (Kunisada et al., 1998).
Despite the clear evidence that KIT activation is linked to phenotypes such as migration and survival also found in cancer cells, its role as an oncogene melanoma did not immediately become clear. Early studies of KIT in melanoma lesions found its expression frequently down-regulated during the progression from early to advanced lesions (Lassam and Bickford, 1992; Natali et al., 1992, Montone et al., 1997) and functional studies even demonstrated that KIT had anti-proliferative and anti-metastatic properties in some settings (Huang et al., 1996, Zakut et al., 1993). When expressed in melanoma cell lines without KIT expression, KIT induced cell cycle arrest and apoptosis (Huang et al., 1996). Also, albeit constitutive activation of KIT in primary melanocytes resulted in increased migration it also decreased proliferation (Alexeev and Yoon, 2006). These observations have led to the view that KIT may rather act as tumor suppressor in melanoma and that in order to acquire proliferative advantage and escape from the epidermal boundaries, malignant melanocytes need to lose KIT expression.
Our attention to KIT as a potential melanoma oncogene was first attracted by a narrow sub-centromeric amplification on chromosome 4q that we observed in acral melanoma, and which appeared to include the KIT locus (Bastian et al., 1998). However, the method of chromosome-based comparative genomic hybridization used at the time did not permit the resolution to sufficiently narrow the affected genomic region and the published data on tumor-suppressive effects of KIT at the time appeared to be inconsistent with KIT as a compelling driver gene within the amplicon. Our interest in KIT rose again, when we observed a striking field effect in acral melanomas, in which we found single basal melanocytes in the basilar non-lesional epidermis adjacent to acral melanomas that shared similar chromosomal aberrations with melanoma (Bastian et al., 2000). The extent of these field cells was substantial, reaching up to 2 cm in some cases, suggesting that the melanoma cells were highly migratory, but at least during this early phase of progression still under homeostatic control, as they were not increased in number and still equidistantly spaced just as normal melanocytes (North et al., 2008). When BRAF was discovered by the Sanger Center as a melanoma oncogene, we carried out follow-up studies in a larger series of primary melanomas and found that BRAF mutations were infrequent in melanomas mucosal, acral and CSD-melanomas (Maldonado et al., 2003), i.e. melanoma types that typically show a lentiginous growth pattern characterized by single melanocytes distributed along the basilar epidermis. The relative dearth of BRAF mutations in these three categories of melanoma raised the question which oncogene was activated in these melanoma types. The common feature of a lentiginous growth pattern in the three types, along with a marked field effect in acral melanoma raised the possibility that a gene inducing melanocytes migration may be involved. A larger study using array CGH provided us with a refined boundary of the amplicon on chromosome 4q and confirmed that KIT was included in the region. It was also remarkable that the amplification was virtually exclusive with mutations in BRAF or NRAS (Curtin et al., 2006). As KIT was known to induce melanocyte migration and because of its functional overlap with BRAF and NRAS, KIT rose to the top of list of candidate genes within the region. When we sequenced KIT, we found recurrent mutations in KIT in mucosal (21%), acral (11%) and CSD-melanomas (17%), but not in non-CSD melanomas, in which BRAF mutations predominate. At the time of publication of our results, others had already described occasional mutations of KIT in a few cases of melanomas. Went et al. (2004) performed immunohistochemistry for KIT protein and found expression in 36% (14 of 39). One of the cases with positive expression showed a mutation in exon 11 (L576P). Another study analyzed 100 metastases and demonstrated expression in 29 cases (29%), two of which had mutations, also in exon 11 (L576P) (Willmore-Payne et al., 2005). In our study we also found that in addition to KIT mutations there were copy number increases of the KIT locus in the same three melanoma categories. Mostly, mutations and copy number increases were mutually exclusive. However, mutations at codon K642E were frequently accompanied by amplifications of the mutated allele. This mutation has also been reported in the germline of patients with familial gastrointestinal stromal tumors indicating that it may be a weak gain-of-function allele that is tolerable in the germline (Isozaki et al., 2000), but needs to be increased in gene dosage by amplification to be fully oncogenic. A recent larger follow-up study has confirmed the recurrent mutations in acral and mucosal melanomas but did not distinguish between CSD and non-CSD melanomas (Beadling et al., 2008). Table 1 summarizes the mutations reported in KIT to date and their relative distribution across melanoma subtypes.
Table 1.
KIT mutations in melanomas subtypes
| References | Mucosal melanomas | Acral melanomas | CSD-melanomas |
|---|---|---|---|
| Ashida et al., 2009 | 0/6 (0%) | 2/22 (9%) | ND |
| Rivera et al., 2008 | 4/18 (22.2%) | ND | ND |
| Curtin et al., 2006 | 8/38 (21%) | 3/28 (11%) | 3/18 (16.7%) |
| Satzger et al., 2008 | 6/37 (16%) | ND | ND |
| Antonescu et al., 2007 | 3/20 (15%) | ND | ND |
| Beadling et al., 2008 | 7/45 (15.6%) | 3/13 (23%) |
1/58(1.7%) cutaneous melanomas* |
| Carvajal et al., 2009 | 12/45 (27%) | 5/22 (23%) | 0/13 (0%) |
study did not sub-classify melanomas on sun-exposed skin.
ND: not determined
We like to note that the finding of known oncogenic mutations in KIT in melanoma subsets is not necessarily contradictory to the findings of tumor suppressive effects of KIT reported earlier. In fact, the discrepancies between these results and previous reports in the literature (Lassam and Bickford, 1992; Natali et al., 1992, Montone et al., 1997) could be reconciled by the fact that those studies were carried out in melanoma cell lines, which are usually derived from the melanoma subtype characterized by its occurrence on intermittently exposed skin (non-CSD). Together with the dearth of KIT mutations or copy number increases of KIT in non-CSD melanoma the diverging findings may therefore indicate different roles of KIT signalling in these melanoma types and further support the notion that these melanoma types are biologically distinct.
KIT as a therapeutic target in GIST
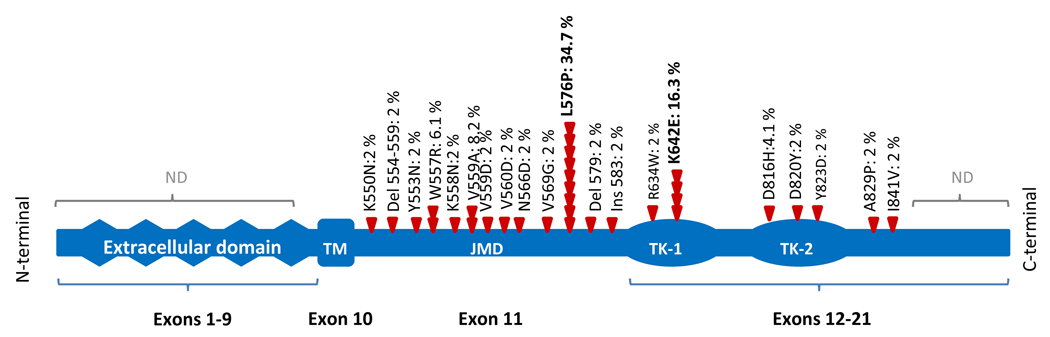
The clinical importance of KIT mutations in melanoma relates to the fact that approved drugs are available for inhibition of its kinase activity and are already used successfully in other cancers. The mutation spectrum of KIT seen in melanoma partially overlaps with that seen in GIST, where most mutations also occur in exon 11. This exon encodes the juxtamembrane domain (Figure 1), which has an α-helical conformation and provides a critical auto-inhibitory function, which is disrupted by mutations that change the amino-acid sequence (Wardelmann et al., 2007). The spectrum of exon 11 mutations in melanoma and GIST (65–70%) consists of point mutations as well as of in-frame deletions and insertions, which have been shown to promote KIT dimerization in the absence of SCF and release the receptor from its auto-inhibited conformation, resulting in constitutive activation (Hornick and Fletcher, 2007, Debiec-Rychter et al., 2006). GISTs with exon 11 mutations respond well to imatinib, a competitive inhibitor of BCR-ABL, ARG (ABL-related-gene), KIT, PDGFRA, and PDGFRB tyrosine kinases (Heinrich et al., 2003, Heinrich et al., 2006). Overall, more than 80% of patients with advanced GIST experience an objective clinical benefit showing partial response or stable disease to imatinib therapy (Demetri et al., 2002, Hornick and Fletcher, 2007). The median response duration exceeds 2 years, and patients with GIST diagnosed with specific mutations expect to live for median of about 5 years compared with only a year in the pre-imatinib era (Guo et al., 2007). However, responses to imatinib depend on the functional domain affected (Heinrich et al., 2003). 61.3–83.5% of GIST patients with exon 11 mutations show partial responses and 8.2–31.9% show stable disease in response to imatinib (Debiec-Rychter et al., 2006, Heinrich et al., 2003). By contrast, mutations in exon 9 of KIT (10–15%), which encodes the fifth extracellular immunoglobulin-like loop, are less responsive, albeit this limitation can be partially overcome by a high-dose regimen (400mg twice daily) (Debiec-Rychter et al., 2006). Different from melanoma, KIT mutations located in exon 13 (encoding the tyrosine kinase 1) and 17 (encoding the tyrosine kinase 2) occur only in a minority of GISTs (<2% and <1%, respectively) (Debiec-Rychter et al., 2006). Because of the relative infrequency in GIST, the therapeutic experience with imatinib with these types of mutations is less extensive, although responses have been reported (Hornick and Fletcher, 2007). About 10–20% of GIST patients exhibit primary resistance to imatinib, mostly due to mutations in the receptor that are not covered by imatinib or mutations in other, yet to be discovered genes (Heinrich et al., 2003, Debiec-Rychter et al., 2006).
Figure 1.
KIT as a therapeutic target in melanoma
To date, only anecdotal reports of melanoma patients with KIT mutations are available, but these are so encouraging that the therapeutic successes in GIST may translate to melanomas with mutations. Two patients mucosal melanomas, harbouring mutations in exon 11 and exon 13 respectively, have demonstrated dramatic responses to imatinib (Hodi et al., 2008, Lutzky et al., 2008), showing near complete responses.
The importance of proper patient selection is highlighted by the negative experience with imatinib in prior trials with unselected melanoma patients. Three phase II trials of imatinib in metastatic melanomas were carried out before the discovery of KIT mutations in melanoma and have proven mostly disappointing (Wyman et al., 2006, Kim et al., 2008a, Ugurel et al., 2005). In retrospect, one of the trials found a near complete response of a patient with metastatic acral melanoma 12 weeks of treatment (Kim et al., 2008a), consistent with the finding of genetic alterations of KIT in acral melanomas. Together, these results have renewed the enthusiasm for KIT inhibitors and indicate the promise of treatments targeted against specific genetic alterations in melanoma. The results to date are particularly encouraging, as they occur in acral and mucosal melanomas, melanoma categories, which are characterized by a high degree of chromosomal instability with numerous amplifications (Curtin et al., 2005). The observation that responses seem to occur despite the presence of numerous other genetic alterations are indicative that melanomas with KIT mutations are “addicted” to KIT activation. The concept of oncogene addition, first described by Weinstein (2000), posits a critical dependency on an activated oncoprotein. One proposed mechanism includes differential attenuation rates of the pro-survival and pro-apoptotic signals downstream of many activated kinases. Upon acute inhibition, the pro-survival signals attenuate faster than the pro-apoptotic signals, resulting in cell death (Sharma et al., 2006, Sharma et al., 2007).
Lessons from the use of kinase inhibitors in other cancer types
While the experience in treating melanoma patients with inhibitors of KIT is just emerging, several lessons can be learned from the use of RTK inhibitors in other cancers.
10–60% of non-small cell lung cancers (NSCLC) have amplification or activating mutations of the epidermal growth factor receptor (EGFR), and 10–30% has mutations in KRAS (Bonomi et al., 2007, Ahrendt et al. 2001, Eberhard et al., 2005). Both activate the MAPK and PI3K/AKT pathways, similar as KIT. The experience in lung cancer indicates that if the pathways are activated at the level of EGFR, targeted agents against the receptor are effective (Eberhard et al., 2005). By contrast, if the pathways are activated at the level of RAS, i.e. downstream of the receptor, no responses to anti-EGFR therapy can be expected. Other than in melanoma, in which RAS mutations occur almost exclusively in NRAS, KRAS mutations are found in NSCLC. More recent studies suggest that patients with KRAS mutations not only fail to benefit from erlotinib, but may experience decreased survival and “time to progression” (Eberhard et al., 2005, Bonomi et al., 2007). However, the picture is confounded by the fact that cancers with EGFR activation have a better prognosis than those with RAS activation independent of treatment (Kim et al., 2008b).
Interestingly, co-administration of targeted agents with conventional chemotherapy appears to be contra-productive in lung cancers with EGFR mutations and can even defeat the effects of targeted agent (Sharma et al., 2006, Sharma et al., 2007). This is because chemotherapeutic agents may promote cell cycle arrest and therefore suppress apoptosis triggered by acute inactivation of an oncogenic kinase achieved through targeted therapy. This mechanism has potentially contributed to the disappointing results observed when EGFR kinase inhibitors were administered together with conventional chemotherapy drugs in non-small-cell lung cancer (Sharma et al., 2006). While it is not clear whether the same would apply to melanomas treated with KIT inhibitors, these observations at least raise caution for combination approaches with conventional chemotherapy.
Biomarkers of response to KIT inhibitors in melanoma
It is likely that the pattern of mutually exclusive mutations in EGFR and KRAS in NSCLC is analogous to that in melanoma. In melanoma, KIT, NRAS, BRAF, and GNAQ mutations occur in virtually exclusive patterns, indicating that they provide partially redundant functions. Based on these assumptions it seems unlikely that melanoma patients with BRAF, NRAS, or GNAQ mutations will respond to treatments directed at KIT. The limited data in melanoma to date indicate that the experience in GIST may translate to melanoma: cases with mutations will respond to drugs active against the specific oncoprotein. While KIT mutations are frequently accompanied by overexpression of KIT protein, overexpression alone appears not to be sufficiently specific, as clinical trials using expression alone as a marker have not been able to identify responders. In GIST, CD117 expression and KIT genotype do not always correlate (Medeiros et al., 2004). In melanoma, KIT-mutant cases can lack detectable expression of CD117, so that a negative CD117 stain cannot be reliably used to rule out a mutation (Beadling et al., 2008, Rivera et al., 2008, Curtin et al., 2006). While Curtin et al. (2006) demonstrated KIT expression in most of their initially negative cases when a 10-fold higher concentration of the antibody was employed; false positive staining at such antibody concentration has been reported (Hornick and Fletcher, 2002). Important questions that need to be answered in ongoing and future trials with KIT inhibitors include the nature of indicators of response, mutations and possibly copy number increases are good candidates, but other biomarkers may emerge.
Monitoring response to therapy
While the anecdotal reports of responses of melanomas to KIT inhibitors such as imatinib have been dramatic and easy to detect, more subtle responses may occur in other patients. In GIST, metabolic responses seen on positron emission tomography (PET) using fluorine-18-fluorodeoxyglucose (18FDG) can precede significant decrease in tumor size on computed tomography (CT) by weeks or months and can be seen as an early response indicator (Hofman et al., 2007). Conversely, lack of metabolic response on FDG-PET indicates primary resistance to the drug and re-emergency of metabolic activity within tumor sites following a period of therapeutic response indicates secondary resistance to the drug (Van Den Abbeele, 2008). Ongoing clinical trials in melanoma will provide information, which methods are likely to assess and monitor responses under therapy with KIT inhibitors.
Resistance to therapy
Despite the remarkable initial response to imatinib therapy in several cancers, resistance frequently occurs during treatment. This secondary or acquired resistance has to be distinguished from primary resistance, which can be caused by mutations in portions of the gene that are not inhibited by the drug or the presence of the presence of additional genetic factors. In GIST, acquired resistance to imatinib typically occurs through second site mutations in KIT, which bypass the inhibitory effects of the drug either by interfering with drug binding or by activating a different portion of the receptor. The fact that resistance occurs at the level of KIT and not by additional mutations in downstream components or other signaling pathways are the most stunning illustration of the specificity of oncogene addiction and underscore the unique role of KIT as a therapeutic target in these tumors. In GIST, the second site mutation occur without exception on the same allele as the primary mutation and affect either the first or the second kinase domains (exons 13, 14, and 17), leading to an imatinib-resistant KIT oncoprotein (Heinrich et al., 2006). In some cases, multiple secondary KIT mutations can be detected in addition to the primary mutation (Heinrich et al., 2006). It is likely that imatinib-resistant KIT-mutant sub-clones already exist at low levels in untreated GISTs and undergo positive selection during therapy rather than representing de novo arising mutations under therapy (Fletcher and Rubin, 2007). Exposure to imatinib thus may drive selection of double KIT-mutant imatinib-resistant clones. In GIST, two of the most common recurrent secondary mutations are V654A and T670I (Prenen et al., 2006).
Therapeutics options for patients with GIST who progress on imatinib include dose escalation or treatment with alternative tyrosine–kinase inhibitors. Sunitinib, an approved drug with demonstrated efficacy against KIT is specifically effective in GISTs with exon 9 mutations, and also has good inhibitory activity against the V654A and T670I mutations in KIT (Prenen et al., 2006). It also is an orally active drug that inhibits multiple receptor tyrosine kinases, including PDGFRA, vascular endothelial growth factor receptor FLT3 and KIT (Sakamoto, 2004). Similar to imatinib, the efficacy of sunitinib for the treatment of imatinib-resistant GISTs depends on the type of mutation (Prenen et al., 2006) and the emergence of resistant clones with second site mutations has been described as well, primarily in exon 17 (Liegl et al., 2008).
Additional KIT inhibitors that are already in clinical use include nilotinib, sorafenib and dasatinib (Guo et al., 2007). Nilotinib (AMN107), a multitargeted kinase inhibitor selectively inhibits both wild type and KIT mutants in exon 11 (V560del) and (V560G) and exon 13 (K642E), is active against KIT double mutants involving exon 11 and exons 13 or 17 (Gou et al., 2007, Weisberg et al., 2005, Verstovsek et al., 2006). In contrast, nilotinib does not appear to be effective against the KIT exon 14 (T670I) mutants. Dasatinib is an ATP-competitor that inhibits both wild type and KIT with juxtamembrane domain mutations (V559D and V560G) more potently than imatinib (Schittenhelm et al., 2006). It is also active against the double mutant KIT (V560G/D816V), as well as exon 13 or exon 17 single or double mutants, while it has no effect on KIT mutant (T670I) oncoprotein (Schittenhelm et al., 2006). Sorafenib (BAY 43-9006), initially developed as an inhibitor of the serine-threonine kinase RAF, has also activity against KIT as well as vascular endothelial growth factor receptor-2, vascular endothelial growth factor receptor-3, and PDGFRB (Guo et al., 2007). Although sorafenib has been shown to inhibit KIT in in vitro assays (Wilhelm et al., 2004), including the T670I KIT mutation as a single or double mutant Ba/F3 cell lines (Guo et al., 2007), the clinical activity in patients with various types of KIT mutations have not been fully investigated.
Concurrent genetic alterations in addition to mutation or amplification of KIT may also modify the tumor’s response to therapy. One alterations that is frequently found in acral and CSD melanomas is amplification of cyclin D1, which acts downstream of KIT at the junction to the cell cycle entry checkpoint (Sauter et al., 2002, Curtin et al., 2005). Cyclin D1 is amplified in about 40% of acral melanomas and extra copies are also found in about up to 50% of CSD melanomas. Based on these observations, we have previously suggested that increased copies of cyclin D1 may blunt the response to upstream inhibition (Curtin et al., 2005), and this has recently been confirmed for melanoma cells with BRAF mutations (Smalley et al., 2008).
Considerations of when to initiate treatment
The phenomenon of secondary resistance due to the presence of second-site mutant cells at the time of therapy initiation strongly argues that treatment should be initiated at low tumor burden to maximize the changes of success. The smaller the number of tumor cells at therapy initiation, the smaller are the chances of the presence of a genetic variant that carries resistant mutations. Another factor supporting treatment at earlier stages is the observations that melanoma patients with KIT mutations that responded initially but later relapse in the brain, while extra-cranial disease, remains controlled (unpublished observation). This pattern has been also observed in preclinical mouse models of chronic myeloid leukemia (CML) with development of central nervous involvement under imatinib, while systemic disease was fully controlled (Wolff et al., 2003). Similar experiences have been made in patients with CML (Pertzer et al., 2002) and GIST (Hughes et al., 2004) where cerebral relapses are observed, while systemic disease progression is controlled by imatinib. This indicates that the central nervous system represents a sanctuary site for imatinib. While other KIT inhibitors such as dasatinib appear to have better penetration of the blood-brain barrier (Porkka et al., 2008), their efficacy of treating manifest disease in the brain needs to be demonstrated. Even if brain metastases occur under therapy it may be important to maintain treatment to control disease progression outside of the brain, while cerebral metastases are addressed surgically or radiotherapeutically (Hughes et al., 2004).
Conclusion
In summary, the emerging data on KIT inhibitor therapy in melanoma indicates that these drugs will be an effective therapy for selected patients. If confirmed, this would represent a paradigm-shifting entry into an era of personalized medicine for melanoma patients, where therapy can be tailored to mechanistically relevant changes in the cancer cells. This development raises hope that melanomas with mutations in other oncogenes also may become treatable, once effective drugs against them are found.
References
- Ahrendt SA, Decker PA, Alawi EA, Zhu YrYR, Sanchez-Cespedes M, Yang SC, et al. Cigarette smoking is strongly associated with mutation of the k-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–1530. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Alexeev V, Yoon K. Distinctive role of the KIT receptor tyrosine kinase signalling in mammalian melanocytes. J Invest Dermatol. 2006;126:1102–1110. doi: 10.1038/sj.jid.5700125. [DOI] [PubMed] [Google Scholar]
- Antonescu CR, Busam KJ, Francone TD, Wong GC, Guo T, Agaram NP, et al. L576P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int J Cancer. 2007;121:257–264. doi: 10.1002/ijc.22681. [DOI] [PubMed] [Google Scholar]
- Ashida A, Takata M, Murata H, Kido K, Saida T. Pathological activation of KIT in metastatic tumors of acral and mucosal melanomas. Int J Cancer. 2009;124:862–868. doi: 10.1002/ijc.24048. [DOI] [PubMed] [Google Scholar]
- Bastian BC, LeBoit PE, Hamm H, Brocker EB, Pinkel D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hibridization. Cancer Res. 1998;58:2170–2175. [PubMed] [Google Scholar]
- Bastian BC, Kashani-Sabet M, Hamm H, Godfrey T, Moore DH, 2nd, Bröcker EB, et al. Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Res. 2000;60:1968–1973. [PubMed] [Google Scholar]
- Beadling C, Jacobson-Dunlop E, Hodi FS, Le C, Warrick A, Patterson J, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14:6821–6828. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- Bonomi PD, Buckingham L, Coon J. Selecting patients for treatment with epidermal growth factor tyrosine kinase inhibitors. Clin Cancer Res. 2007;13(Suppl 15):4606–4612. doi: 10.1158/1078-0432.CCR-07-0332. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in Distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kaeshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and k-ras are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- Fletcher JA, Rubin BP. KIT mutations in GIST. Curr Opin Genet Dev. 2007;17:3–7. doi: 10.1016/j.gde.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Galli Sj, Tsai M, Wershil BK, Tam SY, Costa JJ. Regulation of mouse and human mast cell development, survival and function by stem cell factor, the ligand for the KIT receptor. Int Arch Allergy Immunol. 1995;107:51–53. doi: 10.1159/000236928. [DOI] [PubMed] [Google Scholar]
- Giebel LB, Spritz RA. Mutation of the KIT (mast/stem cell growth factor receptor) protooncogene in human piebaldism. Proc Natl Acad Sci USA. 1991;88:8696–8699. doi: 10.1073/pnas.88.19.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Agaram NP, Wong GC, Hom G, D’Adamo D, Maki RG, et al. Sorafenib inhibits the imatinib-resistant KITT670<I gatekeeper mutation in gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:4874–4881. doi: 10.1158/1078-0432.CCR-07-0484. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764–4774. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- Hodi FS, Friedlander P, Corless CL, Heinrich MC, Mac Rae S, Kruse A, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26:2046–2051. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- Hofman MS, Constantinidou A, Acland K, Healy C, Harries M, O’Doherty M, et al. Assessing response to chemotherapy in metastatic melanoma with FDG PET: Early experience. Nucl Med Commun. 2007;28:902–906. doi: 10.1097/MNM.0b013e3282f1b97b. [DOI] [PubMed] [Google Scholar]
- Hornick JL, Fletcher CD. Immunohistochemical staining for KIT (CD117) is soft tissue sarcomas is very limited in distribution. Am J Clin Pathol. 2002;117:188–193. doi: 10.1309/LX9U-F7P0-UWDH-8Y6R. [DOI] [PubMed] [Google Scholar]
- Hornick JL, Fletcher CDM. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007;38:679–687. doi: 10.1016/j.humpath.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Huang S, Luca M, Gutman M, Mc Conkey DJ, Langley KE, Lyman SD, et al. Enforced c-KIT expression renders highly metastatic human melanoma cells susceptible to stem cell factor-induced apoptosis and inhibits their tumorgenic and metastatis potential. Oncogene. 1996;13:2339–2347. [PubMed] [Google Scholar]
- Hughes B, Yip D, Goldstein D, Waring P, Beshay V, Chong G. Cerebral relapse of metastatic gastrointestinal stromaml tumor during treatment with imatinib mesylate: case report. BMC Cancer. 2004;4:74–81. doi: 10.1186/1471-2407-4-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isozaki K, Terris B, Belghiti J, Schiffmann S, Hirota S, Vanderwinden JM. Germline-activating mutation in the kinase domain of KIT gene in familial gastrointestinal stromal tumors. Am J Pathol. 2000;157:1581–1585. doi: 10.1016/S0002-9440(10)64795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KB, Eton O, Davis DW, Frazier ML, McConkey DJ, Diwan AH, et al. Phase II trial of imatinib mesylate in patients with metastatic melanoma. Br J Cancer. 2008 a;99:734–740. doi: 10.1038/sj.bjc.6604482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YT, Kim TY, Lee DS, Park SJ, Park JY, Seo SJ, et al. Molecular changes of epidermal growth factor receptor (EGFR) and KRAS and their impact on the clinical outcomes in surgically resected adenocarcinoma of the lung. Lung Cancer. 2008 b;59:111–118. doi: 10.1016/j.lungcan.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kunisada T, Yoshida H, Yamazaki H, Miyamoto A, Hemmi H, Nishimura E, et al. Transgene expression of steel fator in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915–2923. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- Lassam N, Bickford S. Loss of KIT expression in cultured melanoma cells. Oncogene. 1992;7:51–56. [PubMed] [Google Scholar]
- Lev S, Yarden Y, Givol D. Dimerization and activation of the kit receptor by monovalent and bivalent binding of the stem cell factor. J Biol Chem. 1992;267:15970–15977. [PubMed] [Google Scholar]
- Liegl B, Kepten I, Le C, Zhu M, Demetri GD, Heinrich MC, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216:64–74. doi: 10.1002/path. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzky J, Bauer J, Bastian BC. Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res. 2008;21:492–493. doi: 10.1111/j.1755-148X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, et al. Determinants of BRAF Mutations in Primary Melanomas. J Natl Cancer Inst. 2003;95:1878–1880. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004;28:889–894. doi: 10.1097/00000478-200407000-00007. [DOI] [PubMed] [Google Scholar]
- Montone KT, van Belle P, Elenitsas R, Elder DE. Proto-oncogene KIT expresión in malignant melanoma: protein loss with tumor progresión. Mod Pathol. 1997;10:939–944. [PubMed] [Google Scholar]
- Morrison-Graham K, Weston JA. Transient steel factor dependence by neural crest derived melanocytes precursors. Dev Biol. 1993;159:346–352. doi: 10.1006/dbio.1993.1246. [DOI] [PubMed] [Google Scholar]
- Natali PG, Nicotra MR, Winkler AB, Cavaliere R, Bigotti A, Ullrich A. Progression of human cutaneous melanoma is associated with loss of expression of KIT proto-oncogene receptor. Int J Cancer. 1992;52:197–201. doi: 10.1002/ijc.2910520207. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S, Kunisada T, et al. In utero manipulation of coat color formation by a monoclonal anti-KIT antibody: two distinct waves of KIT dependency during melanocyte development. EMBO J. 1991;10:2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North JP, Kageshita T, Pinkel D, LeBoit PE, Bastian BC. Distribution and significance of occult intraepidermal tumor cells surrounding primary melanoma. J Invest Dermatol. 2008;128:2024–2030. doi: 10.1038/jid.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzer AL, Gunsilius E, Hayes M, Stockhammer G, Duba HC, Schneller F, et al. Low concentrations of STI571 in the cerebrospinal fluid: a case report. Br J Haematol. 2002;117:623–625. doi: 10.1046/j.1365-2141.2002.03523.x. [DOI] [PubMed] [Google Scholar]
- Porkka K, Koskenvesa P, Lundán T, Rimpiläinen J, Mustjoki S, Smykla R, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112:1005–1012. doi: 10.1182/blood-2008-02-140665. [DOI] [PubMed] [Google Scholar]
- Prenen H, Cools J, Mentens N, Folens C, Sciot R, Schöffski P, et al. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin Cancer. 2006;12:2622–2627. doi: 10.1158/1078-0432.CCR-05-2275. [DOI] [PubMed] [Google Scholar]
- Rivera RS, Nagatsuka H, Gunduz M, Cengiz B, Gunduz E, Siar C, et al. C-kit protein expression correlated with activating mutations in KIT gene in oral mucosal melanoma. Virchows Arch. 2008;452:27–32. doi: 10.1007/s00428-007-0524-2. [DOI] [PubMed] [Google Scholar]
- Sakamoto KM. SU11248 Sugen. Curr Opin Investig Drugs. 2004;5:1329–1339. [PubMed] [Google Scholar]
- Satzger I, Schaefer T, Kuettler U, Broecker V, Voelker B, Ostertag H, et al. Analysis of KIT mutation in human mucosal melanomas. Br J Cancer. 2008;99:2065–2069. doi: 10.1038/sj.bjc.6604791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter ER, Yeo UC, von Stemm A, Zhu W, Litwin S, Tichansky DS, et al. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res. 2002;62:3200–3206. [PubMed] [Google Scholar]
- Schittenhelm MM, Shiraga S, Schroeder A, Corbin AS, Griffith D, Lee FY, et al. Dasatinib (BMS-354825), a dual SRC/Abl kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- Scott G, Liang H, Luthra D. Stem cell factor regulates the melanocyte cytoskeleton. Pigment Cell Res. 1996;9:134–141. doi: 10.1111/j.1600-0749.1996.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Gajowniczek P, Way IP, Lee DY, Jiang J, Yuza Y, et al. A common signalling cascade may underlie “addiction” to the Src, BRC-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10:425–435. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KS, Lioni M, Dalla Palma M, Xiao M, Desai B, Egyhazi S, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008;7:2876–2883. doi: 10.1158/1535-7163.MCT-08-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurel S, Hildenbrand R, Zimpfer A, La Rosée P, Paschka P, Sucker A, et al. Lack of clinical efficacy of imatinib in metastatic melanoma. Br J Cancer. 2005;92:1398–1405. doi: 10.1038/sj.bjc.6602529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Abbeele AD. The lessons of GIST-PET and PET/CT: a new paradigm for imaging. The oncologist. 2008;13 suppl2:8–13. doi: 10.1634/theoncologist.13-S2-8. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Akin C, Manshouri T, Quintás-Cardama A, Huynh L, Manley P, et al. Effects of AMN107, a novel aminopyrimidine tyrosine kinase inhibitor, on human mast cells bearing wild-type or mutated codon 816 KIT. Leuk Res. 2006;30:1365–1370. doi: 10.1016/j.leukres.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Wardelmann E, Buttner, Merkelbach-Bruse S, Schildhaus HU. Mutation analysis of gastrointestinal stromal tumors: increasing significance for risk assessment and effective targeted therapy. Virchows Arch. 2007;451:743–749. doi: 10.1007/s00428-007-0473-9. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Weston JA. Soluble and cell bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121:731–742. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- Weinstein IB. Addiction to oncogenes-The Achilles heel of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Manley PW, Breitenstein W, Brüggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Went PT, Dirnhofer S, Bundi M, Mirlacher M, Schraml P, Mangialaio S, et al. Prevalence of KIT expression in human tumors. J Clin Oncol. 2004;22:4514–4520. doi: 10.1200/JCO.2004.10.125. [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in yumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- Willmore-Payne C, Holden JA, Tripp S, Layfield LJ. Human malignant melanoma: detection of BRAF and KIT activating mutations by high-resolution amplicon melting analysis. Hum Pathol. 2005;36:486–493. doi: 10.1016/j.humpath.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Wolff NC, Richardson JA, Egorin M, Ilaria RL., Jr The CNS is a sanctuaryfor leukemic cells in mice receiving imatinib mesylate for Bcr/Abl-induced leukemia. Blood. 2003;101:5010–5013. doi: 10.1182/blood-2002-10-3059. [DOI] [PubMed] [Google Scholar]
- Wyman K, Atkins MB, Prieto V, Eton O, McDermott DF, Hubbard F, et al. Multicenter phase II trial of imatinib mesylate in metastatic melanoma: significant toxicity with no clinical efficacy. Cancer. 2006;106:2005–2011. doi: 10.1002/cncr.21834. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, et al. Human proto-oncogen KIT: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut R, Perlis R, Eliyahu S, Yarden Y, Givol D, Lyman SD, et al. KIT ligand (mast cell growth factor) inhibits the growth of KIT-expressing melanoma cells. Oncogene. 1993;8:2221–2229. [PubMed] [Google Scholar]