Abstract
Previous studies in Drosophila have demonstrated that whether flies fight like males or females can be switched by selectively manipulating genes of the sex determination hierarchy in male and female nervous systems. Here we extend these studies by demonstrating that changing the sex of cholinergic neurons in male fruit fly nervous systems via expression of the transformer gene increases the levels of aggression shown by the flies without altering the way the flies fight. Transformer manipulation in this way does not change phototaxis, geotaxis, locomotion or odor avoidance of the mutant males compared to controls. Cholinergic neurons must be feminized via this route during the late larval/early pupal stages of development to show the enhanced aggression phenotype. Other investigators have shown that this is the same time period during which sexually dimorphic patterns of behavior are specified in flies. Neurons that co-express fruitless and choline acetyl transferase are found in varying numbers within different clusters of fruitless-expressing neurons: together they make up approximately 10% of the pool of fruitless-expressing neurons in the brain and nerve cord.
Keywords: Drosophila, aggression, cholinergic neurons, transformer, feminization
Introduction
Aggression between male Drosophila was first described by Sturtevant in 1915 in studies concerned with sexual selection.1 These were followed several decades later by further studies of aggression by Jacobs,2 Dow and von Schilcher,3 and most thoroughly by Hoffmann in 1987.4-6 Despite this early literature, research on aggression in fruit flies did not attract much attention until recently when a renaissance of interest was triggered in using laboratory-reared species of Drosophila for studies of aggression.7-10 This was fueled by papers demonstrating agonistic interactions between pairs of fruit flies in simplified fight arenas.7-10
Aggression is a complex behavior defined by competitive interactions over resources between pairs of animals. Its execution combines intrinsic factors such as the individual's aggressiveness and extrinsic factors concerned with whether a resource is worth fighting for and what the chances are of acquiring the resource in competition with an opponent. In genetic studies with Drosophila that have searched for high and low aggression phenotypes,9,10 authors are searching for factors that deal with aggressiveness. Two groups of investigators report that multiple genes are implicated in aggression,9,10 which may not be surprising for a complex behavior like aggression.
In previous studies we showed that male and female fruit flies use sexually dimorphic patterns of behavior during fights in same sex pairings and that males but not females form stable hierarchical relationships.11 We subsequently showed that by manipulating either the transformer (tra) or fruitless (fru) genes of the sex determination hierarchy in flies, we could change the way flies fight so that males fight using female patterns of aggression and females fight using male patterns.12,13 By feminizing individual FruM/octopamine-containing neurons in an otherwise male nervous system, we could influence male behavioral choice between courtship and aggression.14 Here we continue the manipulation of subgroups of neurons in Drosophila nervous systems and show that by changing the sex of cholinergic neurons to female in an otherwise male nervous system we significantly enhance aggression in dyadic pairings of male fruit flies.
Results
Gal4 screen
To search for neurons important in aggression, we performed an initial screen of 60 Gal4 lines that expressed the yeast transcription factor in various sub-groups of central nervous system neurons (see Supplementary Material, Table S2, reviewed in ref. 13). These were crossed with UAS-tra flies and the male progeny of these crosses were examined in multi-well plates for abnormal levels or patterns of aggression (Fig. S1). In one of these crosses we noted males showing unusually high levels of boxing and/or other high level patterns of aggression. This turned out to be the progeny of the cha-Gal4 driver and the UAS-tra line and this driver is reported to express the yeast transcription factor in large numbers of cholinergic neurons in fly brains.15
Secondary screens and characterization of the behavior
Before proceeding to a secondary screen in our standard fight chambers, we checked that this result was not due to genetic differences in the backgrounds of the flies we were using. This was done by testing cha-Gal4 and UAS-tra parent lines that had been out-crossed to Canton-S flies. The progeny of the experimental cross showed highly significant increases in the numbers of lunges seen (Fig. 1A). To demonstrate that this was due to feminizing cholinergic neurons specifically and not to genetic background differences relating to the parent lines, we used constitutively expressed tub-Gal-80ts to turn Tra-expression on or off at different times during development in the progeny. Flies raised at the permissive temperature showed no enhanced aggression (Fig. 1B, column 1), while flies raised at the restrictive temperature demonstrated an increase in lunging (Fig. 1B, column 2). If flies were raised at the restrictive and switched to permissive temperatures during the wandering larval stage, the enhanced aggression phenotype was not seen. In the converse experimental situation, (raised at permissive, switched to restrictive temperature), enhanced aggression was observed (Fig. 1B, columns 3 and 4). Control parent lines showed no enhanced aggression relating to temperature. Thus by using this approach, we found that Tra expression was required during the late larval/early pupal stage. This same time window of Tra expression has been demonstrated by others to be important for the expression of sexually dimorphic patterns of courtship behavior.16
Figure 1.
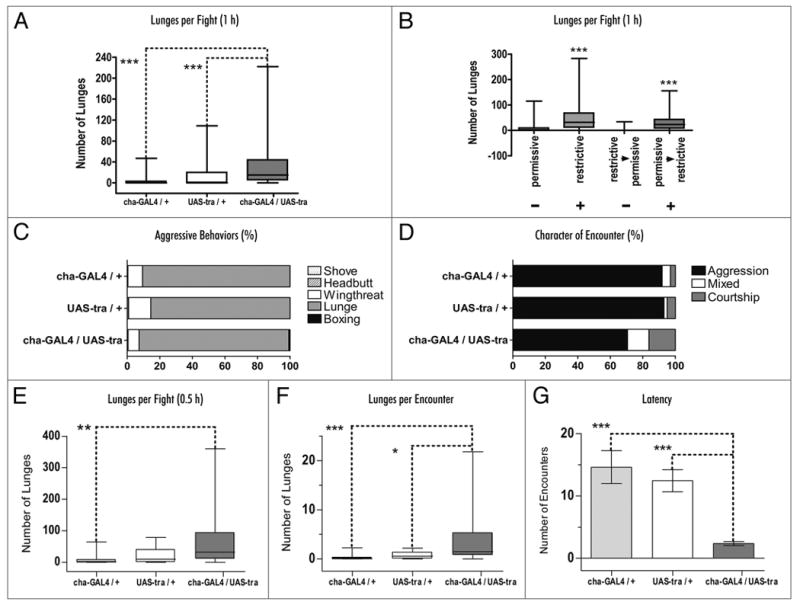
Enhanced aggression is seen in male flies with feminized cholinergic neurons. (A) Significant increases are seen in the numbers of lunges in male flies with feminized cholinergic neurons (cha-Gal4/UAS-tra, n = 35) when compared to the two parental lines (cha-Gal4/+, n = 34 and UAS-tra/+, n = 35) (B) The enhanced aggression seen in male flies with feminized cholinergic neurons is dependent on the developmental stage. Flies carrying the temperature sensitive Gal80 (w; cha-GAL4/UAS-tra;tub-Gal80ts/+) were raised at 19°C (permissive) or 30°C (restrictive). Enhanced aggression was seen in flies reared continuously at the restrictive temperature (n = 34). Temperature shifts were performed at the late third instar stage to search for a critical period for feminization. Flies that were feminized during the embryonic and larval stages (restrictive to permissive) showed no enhanced aggression (n = 34). When cholinergic neurons were feminized during the late larval/early pupal stages, however, significant increases were seen in the numbers of lunges (n = 35). (C) Males with feminized cholinergic neurons showed the typical male aggressive behavioral patterns, lunging and boxing rather than the female patterns, shove and head butt (n = 20). (D) Males showed higher levels of male-male courtship behavior than the parental controls. (E–G) Male flies with feminized cholinergic neurons (experimental flies) showed high levels of aggression earlier than control parent lines in standard fighting arenas. Both the numbers of lunges per fight (E) and the lunges per encounter (F) were significantly higher in the experimental line (cha-Gal4/UAS-Tra) compared to both parental lines. The latency to the first lunge (G) also was shorter in the experimental flies (n = 20). (*p < 0.05; **p < 0.01; ***p < 0.001, analyzed by Kruskus-Wallis ANOVA and Dunn's Multiple Comparison Test).
Further analysis in the standard fighting chambers
We next examined the patterns of fighting behavior in our normal fight chambers7,17 to ask whether the enhanced aggression phenotype still was observable. Since feminizing the entire fly central nervous system yields males that court other males and that fight like females12,13 we first asked whether flies fought like males or females and whether they showed high levels of male:male courtship. To address these issues we examined five sexually dimorphic patterns of male and female aggressive behavior (shove and head butt for female behavior, wing threat, lunge and boxing for male behavior) and scored the interactions between flies (encounters) as courtship, aggression or both in control parent lines and in their experimental progeny (Fig. 1C and D). The results showed that all three groups of flies fought like males, therefore shove and head butt patterns of behavior are rarely seen (<1%), but the experimental line spent much more time courting males than controls. The total number of aggressive encounters (i.e., meetings between flies that result in any type of fighting pattern) in fights between pairs of experimental flies (∼420 encounters) is similar to those seen in both parental control lines (UAS-Tra controls ∼450 encounters and Cha-Gal4 controls ∼390 encounters). However, when we considered only high intensity aggressive behaviors like lunging and boxing, the results were different. Looking at lunging alone, a component of aggression that is characteristic of male aggression and is the determinant of the outcome of a fight, we observed significant increases in the experimental line compared to the parents (Fig. 1E–G). In examining the numbers of lunges per 30 min fight (Fig. 1E), the numbers of lunges per encounter (Fig. 1F), and the latency to the first lunge (Fig. 1G), the experimental flies were significantly different from the parent lines. In contrast, the parent lines did not differ from each other. This suggests that the fighting intensity and the latency to reach higher intensity levels during fights are enhanced in the experimental lines, and that general activities like the numbers of interactions remain unchanged.
Control experiments
While we believe that the observed changes in lunging behavior reflect enhanced aggression shown by the male flies with feminized cholinergic neurons, there remains the possibility that they could result from changes in locomotion or general levels of activity of the flies as well. We therefore carried out a series of control experiments testing phototaxis, geotaxis, locomotion and odor avoidance. In all cases, we observed no differences between the two parent control lines and the experimental flies (Fig. 2A–D).
Figure 2.
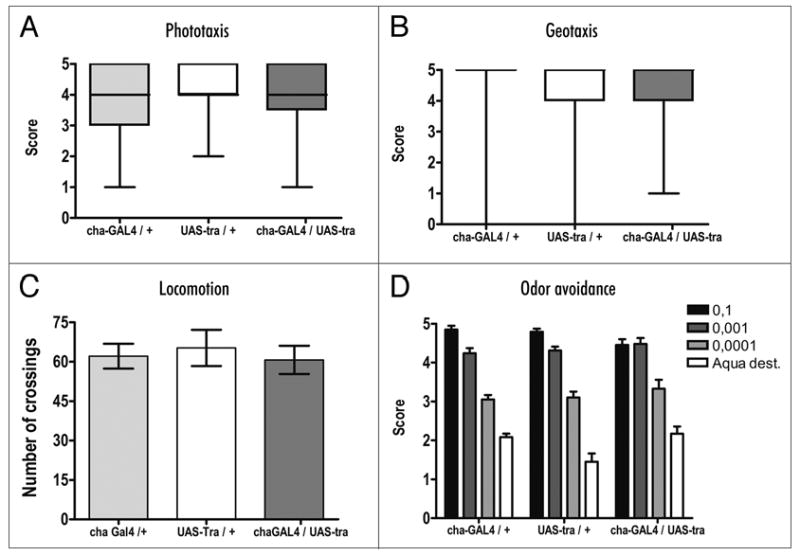
Male flies with feminized cholinergic neurons showed no abnormalities in other behaviors. (A–D) No significant differences were seen in (A) phototaxis, (B) geotaxis, (C) locomotion and (D) odor avoidance between controls (cha-Gal4/+ and UAS-tra/+) and experimental flies (cha-Gal4/UAS-tra) (n = 30). Data was analyzed by Kruskus-Wallis ANOVA for (A and B) and ANOVA for (C and D).
Cholinergic neurons in Drosophila brains
In Drosophila species, most sensory neurons and many central neurons reportedly are cholinergic.15 Thus the phenotype we generate by feminizing cha-Gal4 driven cholinergic neurons using Tra expression in an otherwise male nervous system could be due to changing the sex of either central or peripheral cholinergic neurons. Accordingly, large numbers of neurons might be involved. If these changes are taking place only within neurons in male flies that express the male forms of fruitless (FruM) and/or DsxM, however, the numbers of candidate neurons might be drastically reduced.18-20 Since there is little information about the numbers of neurons within the central nervous system proper that show co-localization of FruM and choline acetylase (CA), we examined this next. For this purpose, we used the cha-Gal4 driver line in crosses with a UAS-redstinger line to label the nuclei of cholinergic pool neurons and combined that with immunostaining for FruM (Fig. 3). While the cha-Gal4 driver line might not label all peripheral and central cholinergic neurons, this line does produce the behavioral phenotype. Hence the patterns of labelling should be informative. Only about 10% of the FruM-expressing neurons in the brain and nerve cord showed co-expression of FruM and CA (Table S1) using this approach. Moreover, great variability was seen in the percentages of cholinergic neurons found within different FruM clusters. For example, the mAL cluster contains 35 FruM-expressing neurons but only two are cholinergic by these criteria. The mAL cluster is interesting in that it: (i) has been shown to be sexually dimorphic in terms of the numbers of FruM-expressing neurons and in the arbors of processes in male and female brains;21 and (ii) also has been shown to be important in whether flies fight like males or females.14 In contrast, a much higher percentage of the FruM-expressing neurons in the subesophageal ganglion (a major gustatory receiving area of the brain) are stained using the cha-Gal4 driver (33 FruM positive, 15 cha-Gal4 labeled; 44% cholinergic).
Figure 3.
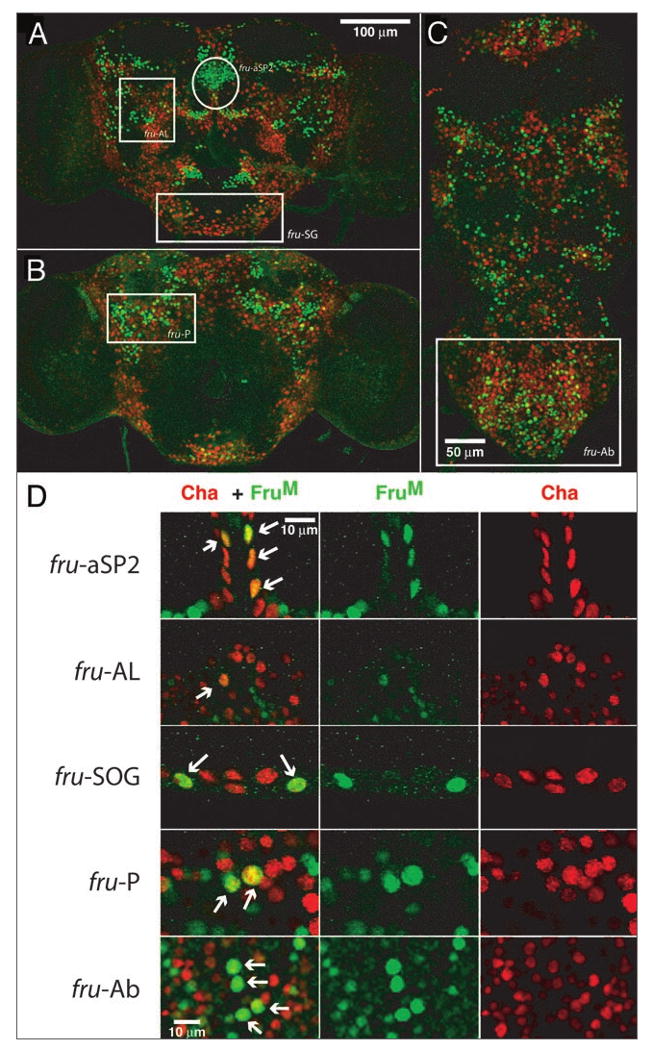
Co-localization of FruM and cha-Gal4 driven positive neurons. Anterior (A) and posterior (B) brain and ventral nerve cord (C) of cha-GAL4/UAS-RedStinger males stained with FruM (green). (D) Higher magnification of selected clusters highlighted in (A–C) (aSP2-anterior region of the superior protocerebrum; SG-subesophageal ganglion; AL-broadly scattered cells above the antennal lobe; P-broadly scattered in posterior region, ventral to calyx; Ab-abdominal ganglion region). Arrows show examples of neurons showing co-localization of FruM and cha-Gal4 labeling. See Table S1 for complete counts.
Discussion
The results of this paper demonstrate that the intensity of aggression can be altered without changing the ways that flies fight (like males or females) by feminizing cholinergic neurons in an otherwise male nervous system. Controls demonstrate that this change in aggression is not due to changes in sensory responsiveness to the cues that normally activate geotaxis, phototaxis or responses to odors. Nor does it reflect a general enhancement of mobility. Instead the mutant flies respond more rapidly to the presence of an opponent and use higher intensity aggression patterns like lunging more frequently in fights. In fights between pairs of wild type female flies, retaliation is commonly seen and females generally do not establish hierarchical relationships.11 If the effects of feminization of the cholinergic neurons in male flies leads to enhanced retaliation by opponents or a reduced ability to form hierarchical relationships, either effect could cause escalation in fights, thereby leading to increased aggression.
Altering levels of aggression without altering how flies fight adds a new dimension to our earlier studies demonstrating that whether flies fight like males or females can be changed by manipulation of the fruitless gene of the sex determination cascade.12 Whether the changes in intensity we observe relate to the aggressiveness of individual flies, however, still cannot be definitively established, since in these studies we too measure levels of aggression during paired agonistic interactions. Despite this, the results raise interesting questions relating to aggression. One question asks how feminization of a single category of neurons in a male nervous system can trigger changes in aggression. More generally, we can ask whether differences exist in the physiological properties of closely related or even identical neurons in male and female brains? Previous studies from our laboratory14 have offered hints that differences exist between male and female versions of the same neurons in fly brains. In those studies, we showed that by feminizing octopaminergic neurons in an otherwise male brain, we altered the behavioral choice between courtship and aggression during a particular segment of the fight ritual. A particularly nice example of sexual dimorphism in the morphology of FruM-expressing neurons in the brains of fruit flies is seen in work from the Yamamoto laboratory on neurons within the mAL cluster.21 Only small numbers of these neurons survive to adulthood in female flies. Of those that do, different arbors of dendritic processes are seen within the SOG, when comparing male and female versions of similar or identical neurons.
Here we show that an aggression-related behavioral change is triggered when we feminize cholinergic neurons in male nervous systems via Tra expression. Thus far we have not identified which of the large numbers of cholinergic neurons found in fly nervous systems are involved. The behavioral changes might relate to inappropriate sensory input arriving in the CNS or to changes within the CNS circuits themselves. If the behavioral differences are due to alterations in the restricted pool of cholinergic neurons in flies that co-express FruM, however, it should be possible to develop fly lines that will selectively identify, target and allow experimental manipulation of those neurons. Finally, while further work will be required to elaborate methods that measure the aggressiveness of individual flies, and to separate that from the aggression triggered by flies competing over resources with other flies, it is of interest that large changes in the intensity of aggression during agonistic interactions can be introduced by changing the sex of a single sub-population of neurons within male fly nervous systems.
Materials and Methods
Fly stocks and rearing conditions
Flies were reared on standard cornmeal medium at 25°C in a 12 hr:12 hr light:dark cycle. The wild-type Canton-S (FBst0000001), w;;tub-Gal80ts/TM2 (FBst0007017), cha-Gal4 (FBst0006798), UAS-tra (FBst0004590) and w;;UAS-red stinger (FBst0008547) lines, and the remainder of the 60 lines tested in the initial screens (for full list see Supporting Information, Table S2, ref. 13) were obtained from the Bloomington Stock Center (Bloomington, IN). Both cha-Gal4 and the UAS-tra lines were out-crossed to Canton-S by standard methods to eliminate the white mutation before using in any behavioral assays.
Aggression assays: screening and multi-well assays
Details of the preliminary screens of the 60 Gal4 lines are presented in our previous studies.13 Abnormally high level of aggression were seen only in male flies with feminized cholinergic neurons. For the multi-well assays, two 4- to 5-day-old, socially naive male flies of the parental lines (controls) or of the progeny of the cross (experimental line) were lightly anesthetized and introduced into each well of the Multiwell™ 12-well plate (Falcon 353225), which contained 3 ml of standard fly food (Fig. S1). On the following day, starting one hour after lights on, plates were videotaped from above, and “lunges” were scored during a one hour examination period.
Aggression assays: standard fight chamber assays
Flies were isolated as pupae in 16 × 100 mm glass vials containing 2 ml of food medium and were kept for 5–6 days after emerging as adults before pairing for fights. The aggressive patterns of behavior seen during fights were scored as described previously.7,11 Pairs of male flies of the same genotype were inserted simultaneously into the chamber. All fights were started 1–1.5 hours after lights-on. Each fight was videotaped for 1.5 hours with a Sony Digital 8 Handycam. To avoid bias on analyses, tapes were coded and analyzed blinded.
Temperature shift trials
For the temperature shift experiments, w;cha-Gal4;tub-Gal80ts flies were generated by standard crosses between w;;tub-Gal80ts/TM2 (FBst0007017) and cha-Gal4 (FBst0006798). The resulting lines were crossed to w;UAS-tra flies to generate the experimental line w;cha-Gal4/UAS-tra;tub-Gal80ts/+. Embryos were collected from grape juice plates and incubated either at the permissive (19°C) or restrictive (30°C) temperatures. In some experiments flies were reared at one temperature and then switched to the other at the start of the 3rd instar (wandering) larval stage (details in Fig. 1 legend). In these cases, the aggression assays were performed in Multiwell™ plates as described above.
Additional behavioral tests
The following tests were performed with flies that had been collected on the day of eclosion and used in experiments at 5–6 days of age.
Phototaxis
Phototaxis was measured using a maze that presents the flies with five consecutive light/dark choices. This maze was modified from the one originally described by Hadler.22 A moveable plexiglass face plate covered the maze and one arm of each choice point was covered with black paint. The maze was placed vertically on a table top and the face plate was illuminated with a fluorescent light source (F20T12). Flies were inserted one at a time into an open channel at the base of the maze and scored from 0–5 depending on their exit point from the maze, 5 points equaled five consecutive light choices.
Geotaxis
The same maze and procedure was used to test for geotaxis by turning the maze by 90° and sliding the moveable face plate so as to eliminate the black paint covering one of the choice points.
Locomotion
Locomotor activity was measured as described in Kulkarni and Hall.23 Single flies were inserted into twelve small cylindrical chambers (13 mm diameter × 10 mm height) with a black line painted at the midpoint. These were videotaped and fly movement in each chamber was scored separately. The numbers of times a fly crossed the painted line were counted for 5 minutes after an initial acclimation period of 5 minutes.
Odor avoidance
Flies were tested for odor avoidance using an assay described by Anholt et al.24 We tested groups of five flies 10 times for each concentration and each genotype. Benzaldehyde (concentration >99%, purchased from Sigma-Aldrich, St. Louis, Missouri) was used as odorant.
Immunocytochemistry
To visualize putative cholinergic neurons, the cha-Gal4 driver line was crossed to the UAS-RedStinger line. Brains and ventral nerve cords of 1- to 3-day-old adult male flies were dissected and fixed in 4% paraformaldehyde at 4°C in 1× phosphate buffered saline (EMD™ #6505 137 mM NaCL, 2.7 mM KCl, 10 mM Phosphate Buffer) for 20 minutes, then blocked in 5% normal goat serum, 0.1% Triton-X-100 (Mallinckrodt) in PBS (PBT) for 30 minutes. Tissues were incubated with FruM anti-serum (kindly supplied by Dickson laboratory) diluted to 1:2,000 overnight at 4°C. After washing in PBT 4 × 20 minutes at 25°C, tissues were incubated with 1:200 goat anti-rabbit:Alexa Fluor 488 (Molecular Probes A11034, Eugene, OR) for 2 hours at 25°C, then washed in PBT for 1 hour and mounted in Vectorshield on a slide. Samples were imaged with an Olympus Fluoview FV1000 confocal microscope. Confocal Z-stacks were acquired with a UAPO 20× or 60× water-immersion objective. Automatic colocalization analyses and 3-D object counting were performed with the algorithms available in Imaris. The accuracy of the final counts was verified by manual inspection of tissues.
Supplementary Material
Acknowledgments
We thank current and former members of the Kravitz Lab for helpful discussions and comments. We thank Barry Dickson for the supply of FruM antibodies. Funding was provided by grants from NIGMS (GM-067645; GM-074675) and from NSF (IDS-075165) to Adelaine K.W. Leung.
Footnotes
References
- 1.Sturtevant AH. Experiments on sex recognition and the problem of sexual selection in Drosophila. J Anim Behav. 1915;5:351–66. [Google Scholar]
- 2.Jacobs ME. Influence of light on mating of Drosophila melanogaster. Ecology. 1960;41:182–8. [Google Scholar]
- 3.Dow MA, von Schilcher F. Aggression and mating success in Drosophila melanogaster. Nature. 1975;254:511–2. doi: 10.1038/254511a0. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann AA. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and D. simulans. Anim Behav. 1987;35:807–18. [Google Scholar]
- 5.Hoffmann AA. Territorial encounters between Drosophila males of different sizes. Anim Behav. 1987;35:1899–901. [Google Scholar]
- 6.Hoffmann AA. The Influence of Age and Experience with Conspecifics on Territorial Behavior in Drosophila melanogaster. Jounal of Insect Behavior. 1990;3:1–12. [Google Scholar]
- 7.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Nat Acad Sci. 2002;99:5664–8. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, Heisenberg M. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–67. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 9.Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nature Genet. 2006;38:1023–31. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- 10.Edwards AC, Rollmann SM, Morgan TJ, Mackay TF. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2006;2:1386–95. doi: 10.1371/journal.pgen.0020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Nat Acad Sci. 2004;101:12342–7. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. Fruitless regulates aggression and dominance in Drosophila. Nature Neurosci. 2006;9:1469–71. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- 13.Chan YB, Kravitz EA. Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc Nat Acad Sci. 2007;104:19577–82. doi: 10.1073/pnas.0709803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Certel SJ, Savella MG, Schlegel DCF, Kravitz EA. Modulation of Drosophila male behavioral choice. Proc Nat Acad Sci. 2007;104:4706–11. doi: 10.1073/pnas.0700328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvaterra PM, Kitamoto T. Drosophila cholinergic neurons and processes visualized with Gal4/UAS-GFP. Brain Res Gene Expr Patterns. 2001;1:73–82. doi: 10.1016/s1567-133x(01)00011-4. [DOI] [PubMed] [Google Scholar]
- 16.Arthur BI, Jr, Jallon JM, Caflisch B, Choffat Y, Nöthiger R. Sexual behaviour in Drosophila is irreversibly programmed during a critical period. Curr Biol. 1998;8:1187–90. doi: 10.1016/s0960-9822(07)00491-5. [DOI] [PubMed] [Google Scholar]
- 17.Mundiyanapurath S, Certel S, Kravitz EA. Studying aggression in Drosophila (fruit flies) Journal of Visualized Experiments (JoVE) 2007. 2007;(2nd) doi: 10.3791/155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 19.Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–94. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–29. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 21.Kimura KI, Manabu O, Tatsunori T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–33. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- 22.Hadler NM. Genetic influence on phototaxis in Drosophila melanogaster. Biol Bull. 1964;126:264–73. doi: 10.1093/genetics/50.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni SJ, Hall JC. Behavioral and cytogenetic analysis of the cacophony courtship song mutant and interacting genetic variants in Drosophila melanogaster. Genetics. 1987;115:461–75. doi: 10.1093/genetics/115.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anholt RR, Lyman RF, MacKay TF. Effects of single P-element insertions on olfactory behavior in Drosophila melanogaster. Genetics. 1996;143:293–301. doi: 10.1093/genetics/143.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


