Abstract
Metabolic syndrome is characterized by cardiometabolic risk factors that include obesity, insulin resistance, hypertension and dyslipidemia. Oxidative stress is known to play a major role in the pathogenesis of metabolic syndrome. The objective of this study was to examine the effectiveness of hydrogen rich water (1.5–2 L/day) in an open label, 8-week study on 20 subjects with potential metabolic syndrome. Hydrogen rich water was produced, by placing a metallic magnesium stick into drinking water (hydrogen concentration; 0.55–0.65 mM), by the following chemical reaction; Mg + 2H2O → Mg (OH)2 + H2. The consumption of hydrogen rich water for 8 weeks resulted in a 39% increase (p<0.05) in antioxidant enzyme superoxide dismutase (SOD) and a 43% decrease (p<0.05) in thiobarbituric acid reactive substances (TBARS) in urine. Further, subjects demonstrated an 8% increase in high density lipoprotein (HDL)-cholesterol and a 13% decrease in total cholesterol/HDL-cholesterol from baseline to week 4. There was no change in fasting glucose levels during the 8 week study. In conclusion, drinking hydrogen rich water represents a potentially novel therapeutic and preventive strategy for metabolic syndrome. The portable magnesium stick was a safe, easy and effective method of delivering hydrogen rich water for daily consumption by participants in the study.
Keywords: hydrogen, drinking water, magnesium, oxidative stress, metabolic syndrome
Introduction
Metabolic syndrome is characterized by a constellation of metabolic and anthropometric abnormalities, which include excess weight, hyperglycemia, hypertension, low concentration of high density lipoprotein (HDL) cholesterol and hypertriglyceridemia [1–3]. Metabolic disease remains a serious concerns in the United States and people with metabolic syndrome are at increased risk of developing cardiovascular disease and type II diabetes [3, 4].
Free radicals and other reactive oxygen species (ROS) are derived either from normal essential metabolic processes in the human body or from external sources such as exposure to X-rays, ozone, cigarette smoking, air pollutants and industrial chemicals [5]. Disturbance of the balance between production of oxygen free radicals (or some other radical species) and activity of the antioxidant system of protection causes oxidative stress [6]. Recent evidence implicated oxidative stress in the pathogenesis of metabolic syndrome [1, 2, 7]. Oxidative stress and nutritional changes also contribute to the aging process and to many age-related diseases and may affect cardiovascular function by either involving the long-term development of atherosclerosis or causing immediate damage during a heart attack or stroke [8]. Typically, ROS reacts with lipids causing lipid peroxidation leading to oxidative destruction of unsaturated fatty acids and damage of cell membranes with indirect damage to other cell constituents [9]. Therefore mitigating oxidative stress may have a significant impact for people in pre-metabolic syndrome status.
Hydrogen has been identified as having therapeutic antioxidant properties by selectively reducing cytotoxic ROS in tissues [10, 11]. As hydrogen is a gaseous molecule, inhaled hydrogen might be an easy delivery strategy. Although it is safe at a concentration lower than its threshold of 4.6% in air, the translational applicability of inhaled hydrogen gas is limited to medical care facilities as it is an inflammable gas and cannot be realistically and safely administered [12]. Oral intake of liquid containing hydrogen represents a novel and easily translatable method of delivery of hydrogen gas. Previous animal studies have linked daily consumption of hydrogen rich water, generated by bubbling or direct contact with hydrogen gas, with reduced atherosclerosis in apolipoprotein E knockout mice [13], alleviated cisplatin-induced nephrotoxicity [14], improved vitamin C deficiency-induced brain injury [15] and prevented chronic allograft nephropathy after renal transplantation [16]. In addition, the beneficial effects of consuming hydrogen rich water in the prevention of adult onset diabetes and insulin resistance has been reported in a human study [17].
We hypothesized that oral intake of hydrogen rich water generated via a magnesium stick may reduce oxidative stress in human subjects with potential metabolic syndrome. As metabolic syndrome is a disease closely associated with lifestyle-related habits, oral intake of hydrogen on a daily basis via drinking water may be ideal, for people without complicating or changing their life style. The administration of hydrogen rich water via a portable magnesium stick was considered to be a safe and feasible method of delivery and was investigated in an open label study, on subjects with potential metabolic syndrome.
Materials and Methods
Subjects and Design
This study was an open label pilot study conducted at a single site with an 8 week treatment period. Twenty subjects ≥40 years, males (n = 10) and females (n = 10) were enrolled from existing patient databases or by advertisement. In order to qualify, subjects were required to have one or more of the following conditions: body mass index (BMI) between 25.0 and 34.9 kg/m2, waist circumference of ≥100 cm for males and ≥88 cm for females, pre-hypertension (diastolic blood pressure of 80–89 mmHg and systolic blood pressure of 139 mmHg or lower), pre-diabetes (fasting plasma glucose from 5.2 to 6.9 mmol/L), total cholesterol >5.18 mmol/L and/or low density lipoprotein (LDL) >2.59 mmol/L. At screening, subjects provided written informed consent and, inclusion and exclusion criteria, medical history and prior use of concomitant medications were reviewed.
Subjects were required to be weight stable (for 3 months prior to study) and those subjects that were smokers were encouraged not to change their smoking habits. Subjects were required to discontinue other natural health products three weeks prior to randomization and during the study and to maintain their current level of physical activity and dietary habits during the course of the study. Subjects were excluded from participating if they were pregnant, breastfeeding, or planning to become pregnant, had uncontrolled hypertension, or history of diagnosed disease or condition including diabetes (Type I or II), cardiovascular disease, cancer, renal and/or liver disease, history of psychiatric disorder or drug/alcohol abuse, used prescription or over the counter products for vasodilation, erectile dysfunction, weight loss, and/or hypercholesterolemia, use of anticoagulants or had participated in a clinical research trial within 30 days prior to randomization.
This study was conducted at KGK Synergize, Inc., London ON, Canada. The study was conducted in accordance with Good Clinical Practice Guidelines and the ethical principles of the Declaration of Helsinki (2000). The study protocol and materials were approved by the Institutional Review Board Services (Aurora, Ontario), and all subjects gave written informed consent prior to participation.
Investigational products (Production of hydrogen water)
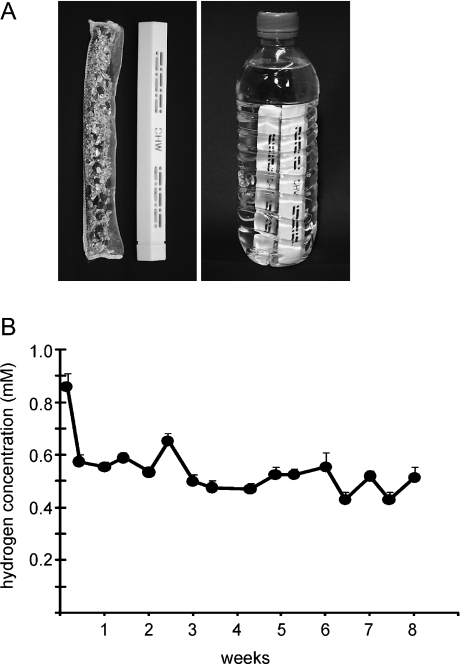
A plastic shelled product consisting of metallic magnesium (99.9% pure) and natural stones in the polypropylene containers combined with ceramics (Doctor SUISOSUI®, Friendear, Tokyo, Japan) was used to produce hydrogen. The product was capable of generating hydrogen when placed in drinking water by the following chemical reaction; Mg + 2H2O → Mg (OH)2 + H2 (Fig. 1). Hydrogen water sticks were dispensed at baseline and week 4 and used sticks were collected at week 4 and week 8 and compliance calculated.
Fig. 1.
A. Magnesium stick and the methods to generate hydrogen water in 500 ml bottles of drinking water. A plastic shelled product consisting of metallic magnesium (99.9% pure) and natural stones in the polypropylene containers combined with ceramics (Doctor SUISOSUI®, Friendear, Tokyo, Japan) was used to produce hydrogen. The product was capable of generating hydrogen when placed in drinking water by the following chemical reaction; Mg + 2H2O → Mg (OH)2 + H2. B. Hydrogen concentrations in the water bottle (n = 3). The hydrogen concentration in a water bottle was maintained between 0.55 and 0.65 mM over an 8 week period.
In a retrospective study conducted at the University of Pittsburgh, in a setting similar to the study procedures followed in the current study, the hydrogen concentration in a water bottle was sequentially monitored using a hydrogen needle sensor (DHS-001, ABLE, Tokyo, Japan). It was determined that the hydrogen concentration was maintained between 0.55 and 0.65 mM and pH between 7.9 and 8.1 over a 12 to 36 h period. When monitored twice a day at weekly intervals for 4 weeks, it was further documented, that the magnesium stick maintained the hydrogen concentration in the water bottles for the desired length of the study. The concentration of magnesium and calcium in the water were also measured using a standard test method (ASTM D511-09, ASTM International, West Conshohocken, PA, conducted at University of Pittsburgh) and found to be <1.0 mg/L and <1.0 mg/L, respectively.
Study protocols (dose and mode of administration)
Subjects were provided with 500 ml bottles of drinking water and instructed to place two magnesium sticks in each of five bottles of water at the end of each day in preparation for consumption the following day. Participants were asked to drink 300–400 ml from bottle one, each morning, one hour before breakfast; 300–400 ml from bottle two, one hour before lunch; 300–400 ml from bottle three, two hours after lunch; 300–400 ml from bottle four, one hour before supper; and 300–400 ml from bottle five, one-half hour before bedtime as per instructions provided in the informed consent form. Subjects were instructed to reuse the magnesium sticks by transferring the sticks to a new bottle of water after use. In summary, subjects were expected to consume 300–400 ml of hydrogen rich water 5 times/day for a total minimum consumption of 1500 ml (1.5 L) to a maximum consumption of 2000 ml (2.0 L).
Assessment of health and physiological parameters
The study included 4 clinic visits, which occurred at screening, baseline, week 4 and week 8. At baseline, week 4 and week 8, blood pressure, heart rate, waist circumference and concomitant therapies were assessed, weight measurements were recorded and fasting peripheral blood was collected to determine glucose and lipid profile. Serum chemistry and hematology were repeated at week 4 and week 8 and first morning void urine samples from two consecutive days were pooled for urinalysis at baseline, week 4 and week 8. A treatment diary was dispensed at baseline and week 4 and included forms to record daily product use, changes in concomitant therapies and adverse events and was returned and reviewed at week 4 and week 8. Adverse events were reviewed at week 4 and week 8.
Analysis of oxidative stress markers
Laboratory tests for routine health markers such as complete blood count (CBC), creatinine, aspartate aminotransferase (AST), alanine transaminase (ALT), gamma glutamyl transferase (GGT), bilirubin, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides and fasting glucose were conducted using standardized procedures at Life Labs Medical Laboratory Services in London, ON. Concentration of 8-hydroxy-2'-deoxyguanosine (8-OHdG) was analyzed by enzyme immunoassay (EIA) (Caymen Chemical, Ann Arbor, MI, Cat. #589320), 8-isoprostane by EIA (Caymen Chemical, Cat. #516351.1), superoxide dismutase (SOD) by enzyme colorimetric assay (Caymen Chemical, Cat. #706002) and thiobarbituric acid reactive substances (TBARS) was analyzed spectrophotometrically using TBARS ASSAY (Caymen Chemical, Cat. #10009055).
Statistical analysis
As this was a pilot study, no formal sample size calculation was performed. Repeated measures analysis of variance (ANOVA) was used to compare pre- and post-treatment measurements of effectiveness and general health markers. Probability values less than 0.05 were considered to be statistically significant. The change from baseline to week 4, and week 8 were compared using Tukey’s multiple comparisons test for 8-OHdG, 8-isoprostane, TBARS, and SOD, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides and fasting glucose. Adverse events were summarized descriptively using frequencies, and categorizing by intensity and body system. A post hoc sub analysis was also conducted on female and male subjects to determine any differences in response according to gender and on subjects who were current smokers for effectiveness. SAS version 9.1 was used to perform the statistical analysis.
Results
Subject characteristics
All subjects completed treatment with mean compliance of 98.7 ± 3.3 and were included in the analysis. The baseline demographics of subjects are presented in Table 1. Subjects enrolled in the study included those who were pre-hypertensive (n = 3), pre-diabetic (n = 7), and had total cholesterol >5.18 mmol/L (n = 12), LDL-c >2.59 mmol/L (n = 17), BMI 25–34.9 (n = 10), and/or smokers (n = 4). All subjects showed mean normal clinical levels of baseline biometric parameters, clinical chemistry and hematology.
Table 1.
Characteristics of subjects Biometrics, Lipid panel and clinical chemistry for all subjects and by gender at baseline
| Variable | All (n = 20) | Female (n = 10) | Male (n = 10) |
|---|---|---|---|
| Age (years) | *50.8 ± 9.6 | 50.0 ± 9.7 | 51.5 ± 10.0 |
| Gender-Female | **10/20 (50.0) | 10/10 (100.0) | 0/10 (0.0) |
| Gender-Male | 10/20 (50.0) | 0/10 (0.0) | 10/10 (100.0) |
| Mean Systolic BP (mmHg) | *114.4 ± 9.5 | 110.8 ± 10.8 | 117.9 ± 6.7 |
| Mean Diastolic BP (mmHg) | 72.2 ± 7.5 | 69.8 ± 7.2 | 74.5 ± 7.4 |
| Mean Heart Rate (bpm) | 69.1 ± 6.9 | 70.0 ± 6.1 | 68.2 ± 7.8 |
| Height (cm) | 171.9 ± 7.7 | 167.3 ± 5.9 | 176.5 ± 6.5 |
| Weight (kg) | 84.6 ± 17.7 | 80.3 ± 19.1 | 88.8 ± 16.1 |
| Waist Circumference (cm) | 97.8 ± 11.5 | 94.2 ± 10.1 | 101.5 ± 12.3 |
| BMI (kg/m2) | 28.6 ± 5.8 | 28.7 ± 6.9 | 28.5 ± 4.7 |
| Fasting Glucose (mmol/L) | 4.9 ± 0.5 | 4.6 ± 0.6 | 4.9 ± 0.4 |
| Alcohol Use | |||
| Daily | **2/20 (10.0) | 0/10 (0.0) | 2/20 (10.0) |
| Occasional | 14/20 (70.0) | 6/10 (60.0) | 8/10 (30.0) |
| Weekly | 4/20 (20.0) | 4/10 (40.0) | 0/10 (0.0) |
| Tobacco Use | |||
| Current | 4/20 (20.0) | 1/10 (10.0) | 3/10 (30) |
| Former | 7/20 (35.0 ) | 3/10 (30.0) | 4/10 (40.0) |
| None | 9/20 (45.0) | 6/10 (60.0) | 3/10 (30.0) |
* Mean ± SD, **f/n (%) = Number of subjects/Total Number of subjects (percent). BP, indicates blood pressure; BMI, body mass index.
The proportion of male smokers was greater (n = 3) than that of the female smokers (n = 1) however all subjects were occasional smokers. The compliance with respect to reporting the number of cigarettes smoked in all the visits was 100%. In two subjects the number of cigarettes smoked remained the same throughout the visits (10 and 20).
Oxidative stress biomarkers
Oxidative stress is a well-recognized mechanism playing an important role in pathological conditions seen in metabolic syndrome [1]. The effect of hydrogen rich water on markers of oxidative stress is presented in Table 2. TBARS are a marker of lipid peroxidation which is indicative of malondialdehyde formation and lipid damage and is a well-established method for screening and monitoring lipid peroxidation [18]. The concentration of urinary TBARS decreased significantly (p<0.05) from baseline to week 4 and week 8. Subjects demonstrated a significant increase (p<0.05) in SOD from baseline to week 8. Subjects demonstrated increasing trends in 8-isoprostane from baseline to week 4 and week 8. When a post hoc sub analysis by gender was conducted, male subjects demonstrated a significant decrease in urinary TBARS from baseline to week 8 and a significant increase (p<0.05) in SOD from baseline to week 8. During oxidative damage to DNA, damaged products are usually eliminated by repair enzymes and detected as nucleoside derivatives. Urinary 8-OHdG is one adduct of this reaction and has been proposed as a sensitive biomarker of oxidative DNA damage and repair [19]. In subjects who were current smokers, there was a trend toward a decrease in urinary 8-OHdG and TBARS levels from baseline to week 4 and week 8. Subjects demonstrated increasing trends in SOD from baseline to week 8 and 8-isoprostane from baseline to week 4 and week 8. Urinary 8-OHdG, 8-isoprostane, TBARS and SOD were higher in subjects who were current smokers (data not shown).
Table 2.
Urinary oxidative stress markers and by gender at baseline and after 4 and 8 weeks of treatment with hydrogen rich water.
| All (n = 20) | Female (n = 10) | Male (n = 10) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD (Difference between means) | (95% CI) | Mean ± SD (Difference between means) | (95% CI) | Mean ± SD (Difference between means) | (95% CI) | |
| Urine 8-OHdG (ng/mg creatinine) | ||||||
| Baseline (Week 0) | 31.8 ± 16.8 | 34.6 ± 16.5 | 28.9 ± 17.5 | |||
| Week 4 | 31.7 ± 10.8 | 33.4 ± 11.6 | 30.0 ± 10.3 | |||
| (−0.0) | (−7.9, 7.8) | (−1.2) | (−13.4, 11.0) | (1.1) | (−10.2, 12.5) | |
| Week 8 | 31.1 ± 12.9 | 31.1 ± 16.3 | 31.0 ± 9.2 | |||
| (−0.7) | (−8.5, 7.1) | (−3.6) | (−15.8, 8.7) | (2.2) | (−9.2, 13.5) | |
| 8-Isoprostane (ng/mmol creatinine) | ||||||
| Baseline (Week 0) | 122.9 ± 33.9 | 125.9 ± 29.5 | 120.0 ± 39.2 | |||
| Week 4 | 130.0 ± 43.1 | 122.8 ± 38.4 | 137.2 ± 48.2 | |||
| (7.1) | (−17.7, 31.8) | (−3.1) | (−34.6, 28.5) | (17.2) | (−24.8, 59.2) | |
| Week 8 | 140.3 ± 32.8 | 138.2 ± 20.8 | 142.4 ± 42.8 | |||
| (17.4) | (−7.4, 42.2) | (12.3) | (−19.2, 43.8) | (22.5) | (−19.5, 64.5) | |
| TBARS (µmol/g creatinine) | ||||||
| Baseline (Week 0) | 7.7 ± 5.2 | 8.4 ± 5.9 | 7.1 ± 4.5 | |||
| Week 4 | 5.0 ± 3.8 | 5.7 ± 4.6 | 4.3 ± 2.9 | |||
| (−2.7) | (−4.9, −0.6)* | (−2.6) | (−5.7, 0.4) | (−2.8) | (−6.2, 0.6) | |
| Week 8 | 4.5 ± 2.9 | 5.4 ± 3.4 | 3.6 ± 2.0 | |||
| (−3.3) | (−5.4, −1.1)* | (−3.0) | (−6.1, 0.1) | (−3.5) | (−6.9, −0.2)* | |
| SOD (U/mmol creatinine) | ||||||
| Baseline (Week 0) | 122.1 ± 106.4 | 155.9 ± 122.3 | 88.3 ± 80.2 | |||
| Week 4 | 129.8 ± 62.3 | 153.6 ± 61.3 | 106.1 ± 56.3 | |||
| (7.8) | (−25.3, 40.8) | (−2.3) | (−59.6, 55.0) | (17.8) | (−22.4, 58.0) | |
| Week 8 | 169.7 ± 94.1 | 208.2 ± 106.2 | 131.3 ± 64.3 | |||
| (47.7) | (14.6, 80.7)* | (52.3) | (−5.0, 109.6) | (43.0) | (2.8, 83.2)* | |
* denotes statistically significant differences (p<0.05), 95% confidence intervals about the mean difference between baseline and week 4 and baseline and week 8 were obtained via Tukey’s multiple comparisons test. ** Change in urinary oxidative stress markers from baseline to week 4. † Change in urinary oxidative stress markers from baseline to week 8 of treatment. 8-OHdG, indicates 8-hydroxy-2'-deoxyguanosine; TBARS, thiobarbituric acid; SOD, superoxide dismutase.
Lipid profile and fasting glucose
Subjects demonstrated a significant increase in HDL-cholesterol from baseline to week 4 and week 8 and a decrease in total cholesterol/HDL ratio from baseline to week 4 (Table 3). Post hoc sub analysis by gender demonstrated that female subjects had a significant increase (p<0.05) in HDL-cholesterol from baseline to week 4, and a significant decrease (p<0.05) in LDL-cholesterol and total cholesterol/HDL-cholesterol ratio from baseline to week 4. There were no changes in HDL cholesterol, cholesterol/HDL ratio and triglycerides from baseline to week 8. Male subjects demonstrated a significant increase in HDL-cholesterol from baseline to week 4 and week 8 and significant decrease in total cholesterol/HDL ratio from baseline to week 4 (p<0.05) (Table 3).
Table 3.
Blood lipid profile of all subjects and by gender at baseline and after 4 and 8 weeks of treatment with hydrogen rich water.
| All (n = 20) | Female (n = 10) | Male (n = 10) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD (Difference between means) | (95% CI) | Mean ± SD (Difference between means) | (95% CI) | Mean ± SD (Difference between means) | (95% CI) | |
| Total Cholesterol (mmol/L) | ||||||
| Baseline (Week 0) | 5.3 ± 1.1 | 5.6 ± 1.4 | 5.1 ± 0.7 | |||
| Week 4 | 5.3 ± 0.9 | 5.4 ± 1.1 | 5.3 ± 0.6 | |||
| †(−0.0) | (−0.3, 0.2) | (−0.2) | (−0.6, 0.2) | (0.2) | (−0.1, 0.5) | |
| Week 8 | 5.5 ± 1.0 | 5.8 ± 1.2 | 5.3 ± 0.7 | |||
| ††(0.2) | (−0.1, 0.5) | (0.2) | (−0.2, 0.6) | (0.2) | (−0.1, 0.5) | |
| LDL Cholesterol (mmol/L) | ||||||
| Baseline (Week 0) | 3.4 ± 0.9** | 3.6 ± 1.1 | 3.2 ± 0.5*** | |||
| Week 4 | 3.2 ± 0.8 | 3.2 ± 1.0 | 3.2 ± 0.4 | |||
| (−0.2) | (−0.4, 0.0) | (−0.4) | (−0.8, −0.0)* | (−0.0) | (−0.3, 0.3) | |
| Week 8 | 3.5 ± 0.8 | 3.7 ± 1.0 | 3.3 ± 0.6 | |||
| (0.1) | (−0.1, 0.4) | (0.1) | (−0.3, 0.5) | (0.1) | (−0.2, 0.4) | |
| HDL Cholesterol (mmol/L) | ||||||
| Baseline (Week 0) | 1.2 ± 0.3 | 1.4 ± 0.2 | 1.1 ± 0.3 | |||
| Week 4 | 1.4 ± 0.4 | 1.5 ± 0.3 | 1.3 ± 0.4 | |||
| (0.2) | (0.1, 0.3)* | (0.2) | (0.1, 0.3)* | (0.2) | (0.1, 0.3)* | |
| Week 8 | 1.3 ± 0.3 | 1.4 ± 0.3 | 1.2 ± 0.3 | |||
| (0.1) | (0.0, 0.2)* | (0.1) | (−0.0, 0.2) | (0.1) | (0.0, 0.2)* | |
| Cholesterol/HDL Ratio | ||||||
| Baseline (Week 0) | 4.5 ± 1.4 | 4.1 ± 1.1 | 5.0 ± 1.5 | |||
| Week 4 | 3.9 ± 1.1 | 3.6 ± 0.9 | 4.3 ± 1.2 | |||
| (−0.6) | (−0.9, −0.4)* | (−0.6) | (−0.9, −0.2)* | (−0.7) | (−1.0, −0.4)* | |
| Week 8 | 4.4 ± 1.3 | 4.1 ± 1.1 | 4.7 ± 1.4 | |||
| (−0.2) | (−0.4, 0.1) | (0.0) | (−0.3, 0.3) | (−0.3) | (−0.7, 0.0) | |
| Triglycerides (mmol/L) | ||||||
| Baseline (Week 0) | 1.6 ± 1.1 | 1.3 ± 0.8 | 1.9 ± 1.4 | |||
| Week 4 | 1.5 ± 0.9 | 1.3 ± 0.8 | 1.7 ± 1.0 | |||
| (−0.1) | (−0.3, 0.2) | (0.1) | (−0.2, 0.3) | (−0.2) | (−0.7, 0.4) | |
| Week 8 | 1.5 ± 0.8 | 1.3 ± 0.7 | 1.6 ± 0.9 | |||
| (−0.1) | (−0.4, 0.2) | (0.1) | (−0.2, 0.3) | (−0.3) | (−0.8, 0.3) | |
* denotes statistically significant differences (p<0.05), 95% confidence intervals about the mean difference between baseline and week 4 and baseline and week 8 were obtained via Tukey’s multiple comparisons test. **N = 19, ***N = 9. †Change in lipid profile of subjects from baseline to week 4. ††Change in lipid profile of subjects from baseline to week 8. LDL, indicates low density lipoprotein; HDL, high density lipoprotein.
The effects of hydrogen rich water on the lipid profile in subjects who were current smokers demonstrated that there was a significant decrease in the total cholesterol/HDL ratio from baseline to week 4 (data not shown) and a significant increase in HDL from baseline to week 4 (p<0.05).
Results showed that there were no statistical differences from baseline to week 8 for fasting glucose in participants after consumption of hydrogen rich water (data not shown).
Biometric parameters, clinical chemistry and hematology
There were no significant differences in blood pressure, heart rate, weight and BMI assessed at any time point (data not shown). Analysis of clinical chemistry parameters demonstrated that ALT and creatinine were significantly decreased (p<0.05) from baseline to week 4 and week 8 in all subjects (Table 4). Further analysis demonstrated that 80% of subjects (9 females and 7 males) had a decrease in ALT from baseline to week 8 and 95% of subjects (10 females and 9 males) had a decrease in creatinine from baseline to week 8.
Table 4.
Clinical Chemistry of all subjects and by gender at screening and after 4 and 8 weeks of treatment with hydrogen rich water.
| All (n = 20) | Female (n = 10) | Male (n = 10) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD (Difference between means) | (95% CI) | Mean ± SD (Difference between means) | (95% CI) | Mean ± SD (Difference between means) | (95% CI) | |
| AST (U/L) | ||||||
| Week 0 | 26.3 ± 6.8 | 24.1 ± 5.9 | 28.5 ± 7.3 | |||
| Week 4 | 21.9 ± 6.2 | 19.7 ± 6.0 | 24.0 ± 5.9 | |||
| **(−4.5) | (−10.3, 1.4) | (−4.4) | (−7.8, −1.0)* | (−4.5) | (−16.4, 7.4) | |
| Week 8 | 23.6 ± 12.3 | 19.2 ± 4.6 | 28.0 ± 15.9 | |||
| †(−2.7) | (−8.5, 3.1) | (−4.9) | (−8.3, −1.5)* | (−0.5) | (−12.4, 11.4) | |
| ALT (U/L) | ||||||
| Week 0 | 32.2 ± 11.0 | 28.4 ± 9.1 | 35.9 ± 11.8 | |||
| Week 4 | 24.7 ± 9.8 | 21.3 ± 10.9 | 28.1 ± 7.7 | |||
| (−7.5) | (−11.9, −3.0)* | (−7.1) | (−11.8, −2.4)* | (−7.8) | (−16.1, 0.5) | |
| Week 8 | 26.1 ± 10.2 | 21.2 ± 7.0 | 30.9 ± 10.9 | |||
| (−6.1) | (−10.6, −1.6)* | (−7.2) | (−11.9, −2.5)* | (−5.0) | (−13.3, 3.3) | |
| GGT (U/L) | ||||||
| Week 0 | 25.7 ± 14.0 | 24.8 ± 16.3 | 26.5 ± 12.1 | |||
| Week 4 | 30.0 ± 16.2 | 25.9 ± 13.4 | 34.0 ± 18.4 | |||
| (4.3) | (−0.6, 9.2) | (1.1) | (−3.2, 5.4) | (7.5) | (−1.7, 16.7) | |
| Week 8 | 31.8 ± 20.3 | 28.0 ± 19.6 | 35.5 ± 21.4 | |||
| (6.1) | (1.2, 11.0)* | (3.2) | (−1.1, 7.5) | (9.0) | (−0.2, 18.2) | |
| Total Bilirubin (umol/L) | ||||||
| Week 0 | 8.1 ± 3.6 | 6.7 ± 3.1 | 9.4 ± 3.7 | |||
| Week 4 | 10.4 ± 4.5 | 9.5 ± 3.8 | 11.2 ± 5.1 | |||
| (2.3) | (0.8, 3.8)* | (2.8) | (0.9, 4.7)* | (1.8) | (−0.8, 4.4) | |
| Week 8 | 10.1 ± 3.9 | 8.8 ± 3.6 | 11.3 ± 3.9 | |||
| (2) | (0.5, 3.5)* | (2.1) | (0.2, 4.0)* | (1.9) | (−0.7, 4.5) | |
| Creatinine (umol/L) | ||||||
| Week 0 | 83.6 ± 14.1 | 76.2 ± 11.8 | 90.9 ± 12.6 | |||
| Week 4 | 76.3 ± 12.0 | 68.4 ± 8.7 | 84.2 ± 9.5 | |||
| (−7.3) | (−11.2, −3.3)* | (−7.8) | (−12.8, −2.8)* | (−6.7) | (−13.4, −0.0)* | |
| Week 8 | 73.8 ± 14.3 | 64.7 ± 9.4 | 82.9 ± 12.6 | |||
| (−9.8) | (−13.7, −5.8)* | (−11.5) | (−16.5, −6.5)* | (−8.0) | (−14.7, −1.3)* | |
| eGFR (mL/min/1.73 m2) | ||||||
| Week 0 | 86.2 ± 14.3 | 83.2 ± 15.6 | 89.2 ± 12.9 | |||
| Week 4 | 82.7 ± 12.3 | 80.9 ± 14.7 | 84.5 ± 9.9 | |||
| (−3.5) | (−9.1, 2.1) | (−2.3) | (−9.6, 5.0) | (−4.7) | (−13.9, 4.5) | |
| Week 8 | 85.7 ± 15.5 | 86.1 ± 13.3 | 85.3 ± 18.3 | |||
| (−0.5) | (−6.1, 5.1) | (2.9) | (−4.4, 10.2) | (−3.9) | (−13.1, 5.3) | |
* denotes statistically significant differences (p<0.05), 95% confidence intervals about the mean difference between baseline and week 4 and baseline and week 8 were obtained via Tukey’s multiple comparisons test. **Change in clinical chemistry parameters of subjects from baseline to week 4, †Change in clinical chemistry parameters of subjects from baseline to week 8. AST, indicates aspartate aminotransferase; ALT, alanine transaminase; GGT, gamma glutamyl transferase; eGFR, estimated glomerular filtration rate.
The decrease in ALT was significant (p<0.05) from baseline to week 4 and week 8 in female subjects but not male subjects. Creatinine was significantly decreased (p<0.05) in both genders from baseline to week 4 and week 8. Significant increases were demonstrated from baseline to week 4 and week 8 for bilirubin in subjects on hydrogen rich water. Eight females and seven males demonstrated an increase in bilirubin from baseline to week 8. This increase was significant in female subjects, but not in male subjects. GGT was significantly increased (p<0.05) from baseline to week 8 with 85% of all subjects demonstrating an increase during this period (8 females and 9 males). Ninety percent of all subjects demonstrated a decrease in AST from baseline to week 8 (9 females and 9 males). This decrease was significant from baseline to weeks 4 and week 8 in female subjects but not in male subjects. The mean values for these parameters were within the normal acceptable reference range for male and female subjects. In subjects who were current smokers, significant increases were demonstrated from baseline to week 8 for bilirubin and this increase was within normal acceptable reference ranges (data not shown).
Adverse events
A total of 28 adverse events were experienced by 13 of the 20 subjects (65.0%) enrolled in the study. Overall, 6 adverse events, experienced by 4 subjects (20.0%) were assessed by the investigator as having a possible relationship to the test article. These adverse events included loose stools (3 subjects), increase in frequency of bowel movement (1 subject) heartburn (1 subject), and headache (1 subject). These adverse events having “possible” relationship to the test article were classified as mild in intensity. There were no serious adverse events which occurred during the study.
Discussion
In this study, we demonstrated that drinking hydrogen rich water increased urinary anti-oxidant enzyme SOD, an endogenous defensive system against ROS-induced cellular injury, associated with reduction of oxidative stress markers, in subjects with metabolic syndrome [7]. SOD plays an important role in the antioxidant defense system against superoxide anion (O2−) generated in vivo and is involved in defense against many diseases [20–22]. Our data demonstrated that subjects consuming hydrogen rich water for 8 weeks showed significantly increased SOD levels from baseline to week 8, suggesting that hydrogen rich water is capable of inducing SOD activity. Although the detailed mechanisms are undefined an increase in SOD levels correlated with decreasing trends in 8-OHdG levels, and thus supported our hypothesis that oxidative stress is reduced by consuming hydrogen rich water.
Oxidative modification of LDL in the arterial wall plays a key role in the pathogenesis of atherosclerosis [2]. A high level of HDL-cholesterol is reported to protect against cardiovascular disease, and low HDL-cholesterol levels (less than 40 mg/dL) increase the risk of heart disease [23]. Results of the current study demonstrated a significant increase in HDL-cholesterol leading to a significant decrease in total cholesterol/HDL ratio by week 4. Decreasing trends were also seen for LDL-cholesterol from baseline to week 4, and triglycerides from baseline to week 8. Though there was an increase in total cholesterol and LDL-cholesterol in subjects consuming hydrogen rich water from baseline until week 8, these values were not clinically significant and were still within a normal acceptable range. The increasing trends may possibly be associated with higher saturated fat consumption, individual food habits and physical activity of subjects. It is possible that the hypolipidemic effect of hydrogen rich water may be due to its ability to prevent lipid peroxidation, as demonstrated by the significant decrease in TBARS, resulting in lower total cholesterol/ HDL ratio, triglycerides and an increase in HDL-cholesterol. Although an improvement of lipid and glucose metabolism after supplementation with hydrogen rich water have been observed in patients with type II diabetes [17], our results showed that there were no statistical differences in fasting glucose in pre-diabetic participants from baseline to week 8. These results are supported by a previous study where hydrogen water was found to lower the blood glucose level of participants with abnormally high blood glucose levels and did not induce a reduction of a normal blood glucose level [17].
GGT is an enzyme widely distributed in the human body, especially in the kidney and liver [24]. The results of the present study demonstrated that there was a significant increase in GGT (p<0.05) within group from baseline to week 8. However this increase was still within the normal acceptable clinical range for these values for both females and males.
Previous studies have showed that there is a positive association between dietary factors and GGT levels [25]. Alcohol and meat consumption are reported to increase GGT levels in a dose dependant manner. However as food records were not maintained in this study we were unable to confirm that the increases in the GGT levels were related to these factors. As the other liver markers such as AST and ALT were not impacted it is possible to suggest that hydrogen water did not have a negative effect on liver function. In this study we found that AST decreased from baseline to week 4 and week 8 in both female and male subjects and these decreases attained significance in the female subjects. The levels of ALT decreased significantly from week 4 to week 8 and in the subgroup analysis this significance was also seen in the female subjects.
Taken together it is possible to suggest that the increases in GGT may reflect changes associated with food intake and alcohol consumption of the participants. The values for GGT remained within an acceptable clinical range for this parameter.
Interestingly, subjects demonstrated a significant increase in total bilirubin from baseline to week 4 and week 8. These increases remained within normal clinically acceptable range. Serum ALT and AST decreased with hydrogen rich water consumption and the elevation of bilirubin levels seen in this study may be a specific effect afforded by hydrogen. Schwertner et al. previously reported that there was a significant inverse correlation between bilirubin concentration and the prevalence of cardiovascular disease and lower serum bilirubin concentrations were correlated with the presence of ischemic heart disease [26]. Madhavan et al. showed that plasma bilirubin concentration is positively correlated with HDL-cholesterol and confirms the results demonstrated in our study [27]. Thus, the elevations of serum bilirubin levels, below toxic levels, are likely to be protective for cardiovascular disease.
The exact mechanisms involved in bilirubin elevation in the subjects treated with hydrogen rich water are not fully understood, however, the antioxidant effects of hydrogen may not be the sole explanation for this increase and other as yet undefined mechanisms may be involved, such as a role in signaling pathways or perhaps other physiological functions. There is a possibility that the higher bilirubin levels are associated with the degradation of heme by heme oxygenase into equimolar quantities of biliverdin (bilirubin) and carbon monoxide (CO), while the central iron is released [28]. The induction of heme oxygenase (HO-1), which is the rate-limiting enzyme, catalyzes the degradation of heme [29]. Further studies are required to determine if hydrogen can induce HO-1. As our hematological data was not altered by hydrogen water consumption and as the elevations in serum bilirubin remained within the normal acceptable range, it is not likely that hemolysis contributed to the increase of serum bilirubin levels.
Mean values of all hematological parameters were within normal clinically acceptable ranges. Biometric parameters assessed as a measure of safety remained unchanged during the 8 week period of the study. Results also showed that there were no changes in blood pressure, BMI and weight in subjects after consuming hydrogen rich water for 8 weeks.
A sub analysis was conducted on subjects who were smokers as previous documentation has established that smokers are likely to have more oxidative stress [30] and thus may show a greater benefit from an antioxidant intervention. Subjects who smoked demonstrated a decrease in urinary creatinine, urinary 8-OHdG and TBARS with hydrogen rich water. Further subjects who smoked demonstrated increasing trends in SOD from baseline to week 8 and 8-isoprostane from baseline to week 4 and week 8, and higher urinary 8-OHdG, 8-isoprostane, TBARS and SOD. There was a statistically significant and a clinically important decrease in total cholesterol/HDL ratio from baseline to week 4 and a statistically significant increase in HDL from baseline to week 4. These results demonstrated that oxidative stress was perhaps impacted more significantly in subjects who smoked.
In conclusion, consumption of hydrogen rich water generated via a magnesium stick demonstrated improvement in the levels of oxidative stress markers associated with metabolic syndrome and boosted the body’s antioxidant activity. Hydrogen rich water represents a potentially novel therapeutic and preventive strategy for the treatment of metabolic syndrome. This method of delivery was advantageous as magnesium sticks are portable and proved to be an easy and safe administration of hydrogen rich water for daily consumption.
Acknowledgments
We would like to thank the volunteers of this study for their willingness and diligence in complying with the protocol. This study was managed by KGK Synergize Inc. London, ON, Canada, under the supervision of the medical directors, David Crowley, MD and Dale Wilson, MD. Statistical guidance and analysis was provided by Larry Stitt, Assistant Director of the Biostatistical Support Unit, University of Western Ontario, London, ON, Canada. We acknowledge the technical contribution of Joshua Baisley and thank Sonya Barss for overseeing the conduct of the study. This study was supported by Friendear Inc. (Decoupage Minami Aoyama 4F, 5-10-13 Minami Aoyama, Minato-ku, Tokyo 107-0062, Japan).
Abbreviations
- 8-isoprostane
15-F2t-15-isoprostane (8-isoprostane F2α)
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- ALT
alanine transaminase
- ANOVA
analysis of variance
- AST
aspartate aminotransferase
- BMI
body mass index
- DNA
deoxyribonucleic acid
- GCP
Good Clinical Practices
- GGT
gamma glutamyl transferase
- HDL
high density lipoproteins
- LDL
low density lipoprotein
- IRB
institutional review board
- MCH
mean corpuscular hemoglobin
- Mg
Magnesium metal
- Mg (OH)2
Magnesium hydroxide
- ROS
reactive oxygen species
- SD
standard deviation
- SOD
superoxide dismutase
- TBARS
Thiobarbituric Acid Reactive Substances
- eGFR
estimated glomerular filtration rate
- HO-1
heme oxygenase
References
- 1.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holvoet P., Lee D.H., Steffes M., Gross M., Jacobs D.R.Jr. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299:2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 4.Grundy S.M., Brewer H.B. Jr., Cleeman J.I., Smith S.C.Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 5.Dean R.T., Fu S., Stocker R., Davies M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997;324:1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halliwell B., Gutteridge J.M., Cross C.E. Free radicals, antioxidants, and human disease: where are we now? J. Lab. Clin. Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 7.Ford E.S., Mokdad A.H., Giles W.H., Brown D.W. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003;52:2346–2352. doi: 10.2337/diabetes.52.9.2346. [DOI] [PubMed] [Google Scholar]
- 8.Villeponteau B., Cockrell R., Feng J. Nutraceutical interventions may delay aging and the age-related diseases. Exp. Gerontol. 2000;35:1405–1417. doi: 10.1016/s0531-5565(00)00182-0. [DOI] [PubMed] [Google Scholar]
- 9.Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am. J. Med. 1991;91:14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 10.Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., Katsura K., Katayama Y., Asoh S., Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 11.Buchholz B.M., Kaczorowski D.J., Sugimoto R., Yang R., Wang Y., Billiar T.R., McCurry K.R., Bauer A.J., Nakao A. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am. J. Transplant. 2008;8:2015–2024. doi: 10.1111/j.1600-6143.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 12.N.A.S.A., author Safety Standard for Hydrogen and Hydrogen Systems. National Aeronautics and Space Administration. 2005 [Google Scholar]
- 13.Ohsawa I., Nishimaki K., Yamagata K., Ishikawa M., Ohta S. Consumption of hydrogen water prevents atherosclerosis in apolipoprotein E knockout mice. Biochem. Biophys. Res. Commun. 2008;377:1195–1198. doi: 10.1016/j.bbrc.2008.10.156. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima-Kamimura N., Mori T., Ohsawa I., Asoh S., Ohta S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer. Chemother. Pharmacol. 2009;64:753–761. doi: 10.1007/s00280-008-0924-2. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y., Kajiyama S., Amano A., Kondo Y., Sasaki T., Handa S., Takahashi R., Fukui M., Hasegawa G., Nakamura N., Fujinawa H., Mori T., Ohta M., Obayashi H., Maruyama N., Ishigami A. Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem. Biophys. Res. Commun. 2008;375:346–350. doi: 10.1016/j.bbrc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Cardinal J.S., Zhan J., Wang Y., Sugimoto R., Tsung A., McCurry K.R., Billiar T.R., Nakao A. Oral administration of hydrogen water prevents chronic allograft nephropathy in renal transplantation. Kidney Int. 2009 doi: 10.1038/ki.2009.421. in press. [DOI] [PubMed] [Google Scholar]
- 17.Kajiyama S., Hasegawa G., Asano M., Hosoda H., Fukui M., Nakamura N., Kitawaki J., Imai S., Nakano K., Ohta M., Adachi T., Obayashi H., Yoshikawa T. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008;28:137–143. doi: 10.1016/j.nutres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Yagi K. Simple procedure for specific assay of lipid hydroperoxides in serum or plasma. Methods. Mol. Biol. 1998;108:107–110. doi: 10.1385/0-89603-472-0:107. [DOI] [PubMed] [Google Scholar]
- 19.Loft S., Fischer-Nielsen A., Jeding I.B., Vistisen K., Poulsen H.E. 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. J. Toxicol. Environ. Health. 1993;40:391–404. doi: 10.1080/15287399309531806. [DOI] [PubMed] [Google Scholar]
- 20.Kinnula V.L., Crapo J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care. Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 21.Noor R., Mittal S., Iqbal J. Superoxide dismutase—applications and relevance to human diseases. Med. Sci. Monit. 2002;8:RA210–215. [PubMed] [Google Scholar]
- 22.Fukai T., Folz R.J., Landmesser U., Harrison D.G. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc. Res. 2002;55:239–249. doi: 10.1016/s0008-6363(02)00328-0. [DOI] [PubMed] [Google Scholar]
- 23.Batiste M.C., Schaefer E.J. Diagnosis and management of lipoprotein abnormalities. Nutr. Clin. Care. 2002;5:115–123. doi: 10.1046/j.1523-5408.2002.t01-1-00005.x. [DOI] [PubMed] [Google Scholar]
- 24.Whitfield J.B. Gamma glutamyl transferase. Crit. Rev. Clin. Lab. Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 25.Lee D.H., Blomhoff R., Jacobs D.R.Jr. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic. Res. 2004;38:535–539. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 26.Schwertner H.A., Jackson W.G., Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin. Chem. 1994;40:18–23. [PubMed] [Google Scholar]
- 27.Madhavan M., Wattigney W.A., Srinivasan S.R., Berenson G.S. Serum bilirubin distribution and its relation to cardiovascular risk in children and young adults. Atherosclerosis. 1997;131:107–113. doi: 10.1016/s0021-9150(97)06088-7. [DOI] [PubMed] [Google Scholar]
- 28.Tenhunen R., Marver H.S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. U.S.A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otterbein L.E., Soares M.P., Yamashita K., Bach F.H. Heme oxygenase-1: unleashing the protective properties of heme. Trends. Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 30.Dilsiz N., Olcucu A., Cay M., Naziroglu M., Cobanoglu D. Protective effects of selenium, vitamin C and vitamin E against oxidative stress of cigarette smoke in rats. Cell Biochem. Funct. 1999;17:1–7. doi: 10.1002/(SICI)1099-0844(199903)17:1<1::AID-CBF800>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]