Abstract
Oxidative stress is thought to play a role in the development of insulin resistance. In order to elucidate the molecular effect of oxidative stress on liver insulin signaling, we analyzed the effect of paraquat (1,1-dimethyl-4,4-dipyridynium; PQ)-derived oxidative stress on the expression of insulin-dependent genes and activation of liver insulin signaling pathway. Incubation of primary cultured rat hepatocytes with 2 mM PQ for 6 h impaired the suppressive effect of insulin on insulin-like growth factor-binding protein-1 (IGFBP-1) gene expression, but did not influence glucose-6-phosphatase gene expression. Insulin-dependent phosphorylation or activation of insulin receptor, insulin receptor substrate-1 and -2, phosphatidylinositol 3-kinase, Akt and forkhead in rhabdomyosarcoma were not affected by PQ pre-treatment. In contrast, PQ treatment impaired insulin-dependent phosphorylation of mammalian target of rapamycin (mTOR). These results indicate that PQ-induced oxidative stress impairs insulin-dependent mTOR activation and that this impairment probably causes inhibition of insulin-dependent repression of IGFBP-1 expression.
Keywords: insulin, paraquat, IGFBP-1, mTOR, hepatocyte
Introduction
Oxidative stress has been suggested to play an important role in the development and progression of diabetes. Increased levels of oxidative products have been detected in diabetic animals and humans [1–3], and intake of antioxidants improves insulin resistance in vivo [4, 5]. Furthermore, induction of oxidative stress by inhibiting glutathione synthesis in rats has been reported to induce insulin resistance [6], and overexpression of antioxidative enzymes in mice reduces insulin resistance induced by adipocytokines [7].
After insulin binds to its specific cell surface receptor, insulin receptor (IR) tyrosine kinase is activated, causing autophosphorylation of IR. Fully activated IR tyrosine kinase phosphorylates intracellular substrates such as insulin receptor substrate (IRS)-1 and -2. Tyrosine phosphorylated IRSs are recognized by SH2-domain containing signaling molecules, resulting in activation of downstream signaling pathways including the phosphatidylinositol 3-kinase (PI 3-kinase) pathway. When this pathway is activated, one of the downstream Ser/Thr kinases, Akt/PKB, is phosphorylated at Ser 473 and Thr308, resulting in its activation. Akt/PKB phosphorylates various substrates, for example the transcription factor forkhead in rhabdomyosarcoma (FKHR), which is phosphorylated at Ser 249, Ser 256, and Ser 319, inducing its nuclear export and loss of transcription regulatory activities to target genes. Another important downstream Ser/Thr kinase of the PI 3-kinase pathway is mammalian target of rapamycin (mTOR), which is activated by phosphorylation of Ser 2448; activated mTOR plays important roles in the induction of protein synthesis.
The molecular effects of oxidative stress on cellular insulin signaling have been investigated mainly using adipocytes or muscle cells treated with hydrogen peroxide (H2O2). The results of those studies have indicated that treatment of cells with H2O2 inhibits insulin-induced IR phosphorylation followed by reduced PI 3-kinase activation and glucose uptake [8–10]. Further investigations have shown that H2O2 or buthionine sulfoximine, an inhibitor of glutathione synthesis, depresses insulin-induced phosphorylation of IRS-1 and activation of PI 3-kinase in low-density microsome fractions in 3T3-L1 adipocytes and L6 myotube cells [11, 12]. Taken together, these studies indicate that oxidative stress may cause impairment of insulin signal transduction by inhibiting phosphorylation or activation of upstream insulin signaling molecules in adipose tissue or muscle. However, since the effect of oxidative stress on liver insulin signaling has not been well investigated, the effect of reactive oxygen species (ROS) on hepatic insulin action was examined in this study.
In order to examine the effect of ROS, we used paraquat, 1,1-dimethyl-4,4-dipyridynium (PQ) as a radical generator, which effectively inhibits hepatic insulin action as well as H2O2, as we previously reported [13]. PQ is known to generate paraquat radicals by reduced nichotinamide adenine dinucleotide phosphate reductase, which is reoxidized by molecular oxygen and produces superoxide radicals [14–16]. Because of this mechanism of radical generation, PQ is regarded as an intracellular radical generator and has been widely used as an experimental ROS generator [17–21].
In this study, we measured the expression of insulin-like growth factor-binding protein-1 (IGFBP-1) and glucose-6-phosphatase (G6Pase) genes as indicators of insulin action, because the expression of these genes is repressed by insulin in liver or hepatocytes [22]. IGFBP-1 inhibits the growth-promoting effect of insulin-like growth factors (IGFs) by binding to IGFs [23], and G6Pase is a rate-limiting enzyme of hepatic gluconeogenesis. Increased expression of these genes in animals has been shown to produce insulin resistance or metabolic profiles similar to type 2 diabetes [24, 25]. Although both IGFBP-1 and G6Pase genes are regulated by insulin via transcription factor FKHR, which binds to the insulin response element in their promoter region [26–29], the existence of distinct mechanisms has been also reported [29–34]. In particular, IGFBP-1 gene expression is regulated by the mTOR-dependent pathway that is distinct from the regulation of the G6Pase gene [32, 33]. In this study, we first measured the effect of PQ treatment on the expression of IGFBP-1 and G6Pase genes, and further analyzed insulin-induced phosphorylation of IR, IRS-1 and -2, Akt, mTOR, and FKHR, and insulin-induced PI 3-kinase activation in order to investigate the molecular effect of PQ on insulin action in liver.
Materials and Methods
Materials
Williams’ medium E (WE) and Hanks’ balanced-salt solution (HBSS) were purchased from SIGMA (St. Louis, MO); newborn bovine serum (NBS) was from JRH Bio-science (Lenexa, KS); penicillin G, streptomycin sulfate, and kanamycin were from SIGMA; collagen-coated dishes were from Asahi Techno Glass Co. (Tokyo, Japan); collagen (Cellmatrix Type I) and collagenase (Collagenase S-1) were from Nitta Gelatin Inc. (Osaka, Japan); glycogen (glycogen type II from Oyster) was from SIGMA; and trypsin inhibitor was from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Anti IRS-1, anti-IRS-2, anti-phosphotyrosine (p-Tyr), anti-FKHR, and secondary antibodies for immunoblotting were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Anti-phospho-Akt (Ser 473), anti-phospho-mTOR (Ser 2448), and anti-phopho-FKHR (Ser 256) were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Anti-IRS-1 and anti-IRS-2 antibodies for immunoprecipitation were prepared according to the method described previously [35]. [γ-32P] ATP (6,000 Ci/mmol) was purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). Other chemicals were of reagent grade available commercially.
Primary cultures of rat liver parenchymal cells
Primary cultured rat hepatocytes were isolated from livers of male Wistar strain rats (body weight 150–300 g, Japan Laboratory Animal, Tokyo, Japan) by perfusion with collagenase as described previously [36]. Parenchymal cells were inoculated on collagen-coated dishes at densities of 1.4 × 106 cells/60 mm dish or 4.2 × 106 cells/100 mm dish and cultured for 2 h in WE with 10% (w/v) NBS, 10 nM insulin, and 1 µM dexamethasone at 37°C in a humidified incubator with an atmosphere of 95% air/5% CO2. Cells for the trypan blue exclusion assay were inoculated on collagen-coated cover glass at the density described above. All media contained penicillin (0.1 U/ml), streptomycin (0.1 µg/ml), and kanamycin (30 µg/ml). Unattached cells were removed by aspiration 3 h after inoculation, and attached cells were incubated further for 20–24 h in the same medium. The medium was then discarded and the cells were washed three times with HBSS before treatment with various concentrations of PQ in WE containing 0.1% (w/v) bovine serum albumin for an additional period.
Trypan blue exclusion assay
Cell viability was assessed by adding 0.03% (w/v) trypan blue to the cells and counting dead cells under a light microscope.
Lactate dehydrogenase (LDH) activity assay
Cell culture medium (50–200 µl) was added to 2 ml of 50 mM phosphate buffer (pH 7.5) containing 0.62 mM lithium pyruvate and 0.18 mM nichotinamide adenine dinucleotide that had been pre-warmed at 35°C, and the decreasing rate of absorbance at 340 nm was measured. LDH activity (unit/ml) was defined as 1 unit = 1720 × (decrease in absorbance at 340 nm for 2 min).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was prepared from the cultured cells as described previously [37]. cDNA synthesis and polymerase chain reaction (PCR) were performed as described previously [13]. PCR with graded amounts of control template were performed at the same time, and a reaction cycle that gave quantitative amplification of the control template was adopted for each reaction. PCR primers used were as follows: G6Pase sense, 5'-GGTTACTTCTACTCTCGCTATG-3'; G6Pase antisense, 5'-GCTGGCAAAGGGTGTAGTGT-3'; IGFBP-1 sense, 5'-TCCTGATCCTCCTGTCCTT-3'; IGFBP-1 antisense, 5'-TATAGAGTTCCCGTTGG-3'; β-actin sense, 5'-ACCCACACTGTGCCCATCTA-3'; β-actin antisense, 5'-CGTCACACTTCATGATG-3'. The results were quantified using the public domain NIH Image program.
Immunoblotting
Cells were lysed at 0°C in 0.25 ml of Tris/Triton lysis buffer [50 mM Tris-HCl pH 7.4, 1% (v/v) triton X-100, 1 mM ethylenediaminetetraacetic acid (EDTA), 150 mM NaCl, 1 mM NaF, 10% (v/v) glycerol] containing 10 mg/ml p-nitrophenyl phosphate (PNPP), 10 mM Na3VO4, 20 µg/ml aprotinin, 20 µg/ml phenylmethylsulfonyl fluoride (PMSF), 10 µg/ml leupeptin, and 5 µg/ml pepstatin, and then centrifuged. A fraction of each sample that contained the same amount of protein was subjected to SDS-PAGE and the proteins on the gel were transferred to a polyvinylidene fluoride membrane (Hybond-P, Amersham Bioscience, Tokyo, Japan) and immunoblotted with a monoclonal antibody (1:100 for anti-phosphotyrosine antibody) or polyclonal antibody (1:500 for anti-IRS-1 antibody, 1:500 for anti-IRS-2 antibody, 1:100 for anti-phospho-Akt antibody, 1:100 for anti-phospho-mTOR antibody, and 1:100 for anti-FKHR antibody). The blots were then probed for 1 h with anti-mouse IgG (1:3,000), anti-rabbit IgG (1:3,000), or anti-goat IgG (1:3,000) conjugated with horseradish peroxidase. Proteins were detected using an ECL kit, according to the manufacturer’s directions (Amersham ECL kit or ECL Plus kit; Amersham Life Sciences Co.), and the results were quantified using the public domain NIH Image program.
Immunoprecipitation
Immunoprecipitation was performed by incubating cell lysate (2.5 mg protein) with anti-IRS-1 or anti-IRS-2 antibody (20 µl) bound to protein G sepharose beads for 1.5 h at 4°C. After centrifugation, precipitant was washed three times with Tris/Triton lysis buffer and boiled for 3 min with 1x Laemmli’s sample buffer to elute immunoprecipitated proteins.
PI 3-kinase activity assay
Cells were lysed at 0°C in 0.25 ml of Tris/Triton lysis buffer containing 10 mg/ml PNPP, 10 mM Na3VO4, 20 µg/ml aprotinin, 20 µg/ml PMSF, 10 µg/ml leupeptin, and 5 µg/ml pepstatin. The lysate was centrifuged at 3,000 × g for 5 min at 4°C. The supernatant (500 µg protein) was incubated with anti-IRS-1 antibody (10 µl) or anti-IRS-2 antibody (10 µl) overnight at 4°C. Ten microliters of Protein A-Sepharose (30%, v/v) was then added and the incubation was continued for 2 h. The immunoprecipitates were collected by centrifugation at 4°C, washed once with Tris/Triton lysis buffer, and then with LiCl buffer (100 mM Tris-HCl pH 7.5, 500 mM LiCl), distilled water, TNE buffer (20 mM Tris-HCl pH 7.5, 100 mM NaCl, and 1 mM EDTA), and finally with reaction buffer (20 mM Tris-HCl pH 7.5, 100 mM NaCl, and 0.5 mM EDTA). The PI 3-kinase assay was carried out as described previously [38], with slight modifications. Briefly, the assay was initiated by preincubation of immunocomplexes in 40 µl of assay mixture I (39.5 µl of reaction buffer with 0.5 µl of phosphatidylinositol) at 25°C for 5 min, to which was added 10 µl of assay mixture II [100 mM MgCl2, 5 mM dithiothreitol, 0.15 mM, ATP 1% (v/v) [γ-32P] ATP (4 µCi/mmol)] and further incubated for 30 min. After incubation, One hundred microliters of chloroform/methanol/HCl (10:20:1) was added to the reaction mixture to stop the reaction. The mixture was centrifuged at 20,100 × g for 5 min at 4°C to extract lipid, spotted on a silica gel plate, and developed with chloroform/methanol/NH4OH/water (43:38:6:6). 32P radioactivity incorporated into phosphatidylinositol was detected by autoradiography as PI 3-kinase activity. Each data point represents the mean of three replicate dishes.
Statistical analysis
Each treatment effect was analyzed by Student’s t test, and differences were considered significant at p<0.05. Statistical analysis was performed on three samples from identical preparations of cells and the reproducibility of results was confirmed by using at least two different preparations.
Results
PQ-induced oxidative stress in primary cultured rat hepatocytes
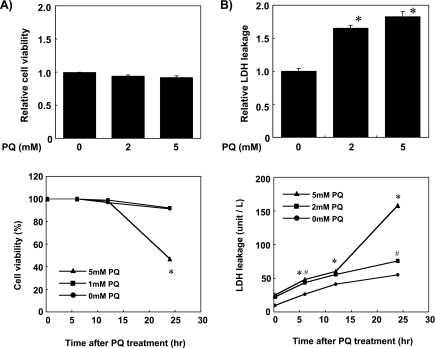
The effect of PQ-induced oxidative stress on cell viability was evaluated by trypan blue exclusion assay. Incubating cells with 1 mM or 5 mM PQ within 12 h did not affect cell viability (Fig. 1A), while incubation with 5 mM PQ for 24 h decreased cell viability to 50%. Damage of the plasma membrane, estimated by LDH activity in the cell culture medium, was increased slightly until 12 h after PQ treatment and increased remarkably by incubating cells with 5 mM PQ for 24 h (Fig. 1B).
Fig. 1.
PQ treatment induced oxidative stress in primary cultured rat hepatocytes. (A) The viability of cells treated with 0, 1, or 5 mM PQ for 15 min, 6 h, 12 h and 24 h was estimated by trypan blue exclusion assay. (B) Plasma membrane damage of the cells treated with 0, 2, or 5 mM PQ for 15 min, 6 h, 12 h and 24 h was estimated by measuring LDH activity in the cell culture medium. Values shown are means ± SEM of three dishes, and expressed as relative values to cells without PQ treatment (PQ 0 mM). *, # Significantly different from cells without PQ treatment (p<0.05).
Effect of PQ on insulin-dependent gene expression
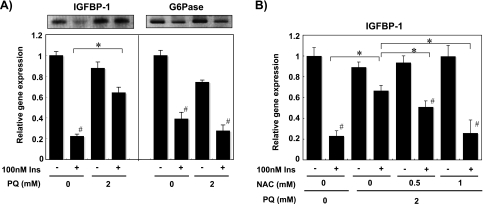
Because insulin is known to reduce hepatic IGFBP-1 and G6Pase gene expression predominantly [39–42], we analyzed the effect of PQ on the mRNA content of these genes. Treatment of the cells with 100 nM insulin for 6 h decreased the mRNA level of these genes to 20–40% (Fig. 2A). The suppressive effect of insulin on IGFBP-1 gene expression was impaired by 2 mM PQ treatment while that on G6Pase gene expression was not inhibited (Fig. 2A). Co-incubation with 1 mM N-acetyl-L-cysteine, an antioxidant, almost completely blocked the effect of PQ on insulin-dependent repression of IGFBP-1 gene expression (Fig. 2B).
Fig. 2.
PQ-derived oxidative stress impairs insulin regulation of the IGFBP-1 mRNA level in primary cultured rat hepatocytes. (A) Effect of PQ on insulin regulation of IGFBP-1 and G6Pase mRNA level was measured with cells treated with/without 2 mM PQ, 100 nM insulin for 6 h. Total RNA was prepared from the cells and the mRNA contents of IGFBP-1 and G6Pase were estimated by RT-PCR. Values shown are relative mRNA levels compared to cells without insulin and PQ treatment (Ins-, PQ 0 mM) and expressed as means ± SEM of three dishes. (B) Effect of NAC on PQ treatment was examined with cells treated with/without 2 mM PQ, 100 nM insulin and antioxidant NAC simultaneously for 6 h. Values shown are relative IGFBP-1 mRNA levels compared to the cells without PQ, NAC, and insulin treatment (Ins-, NAC 0 mM, PQ 0 mM) and expressed as means ± SEM of three dishes. #Significantly different from cells without insulin treatment (p<0.05). *Significantly different between the two groups by PQ or NAC treatment (p<0.05).
Effect of PQ on insulin-dependent phosphorylation of IR, IRS-1, and IRS-2
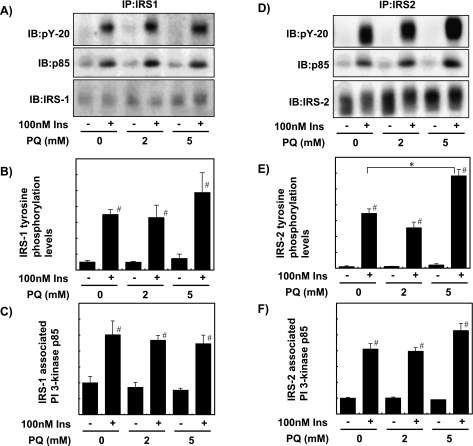
We next examined the effect of PQ on insulin signaling at the early stage. Treatment of the cells with 100 nM insulin for 1 min increased tyrosine phosphorylation of IR, IRS-1, and IRS-2, and the amount of PI 3-kinase p85 subunit associated with IRS. These effects of insulin were not affected by pretreatment of the cells with 2 mM or 5 mM PQ for 6 h (Fig. 3A–F). The amounts of IRS-1 and IRS-2 were not significantly changed (Fig. 3A and D).
Fig. 3.
PQ treatment did not affect insulin-dependent phosphorylation of IRSs and association of PI 3-kinase with IRS. (A, D) Phosphorylation of IRSs and PI 3-kinase association was estimated with primary cultured rat hepatocytes pre-treated with 0, 2, or 5 mM PQ for 6 h and then treated with/without 100 nM insulin for 1 min. After the protein lysate of the cells was immunoprecipitated with anti-IRS antibody, the IRS phosphorylation level, IRS-associated PI 3-kinase p85 subunit level, and IRS protein level were analyzed by immunoblotting with anti-phosphotyrosine (PY-20), anti-p85 and anti-IRS antibodies, respectively. (B, C, E, F) IRS phosphorylation level and the amount of IRS-associated p85 were quantified and expressed as relative values to cells treated with insulin and without PQ (Ins+, PQ 0 mM). Values are means ± SEM of three dishes. #Significantly different from cells without insulin treatment (p<0.05). *Significantly different between the two groups by PQ treatment (p<0.05).
Effects of PQ on insulin-dependent activation of PI 3-kinase
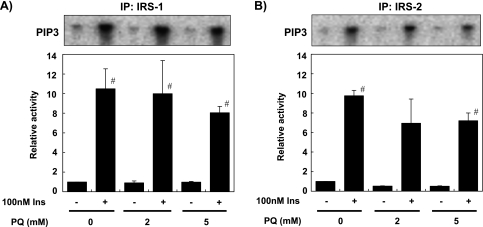
As shown in Fig. 4, treatment of the cells with 100 nM insulin for 6 h increased PI 3-kinase activity bound to IRS-1 or IRS-2. Pretreatment with PQ slightly reduced insulin-dependent PI 3-kinase activity by about 20%; however, this reduction was not statistically significant for PI 3-kinase activity bound to IRS-1 as well as IRS-2 (Fig. 4).
Fig. 4.
PQ treatment did not affect insulin-dependent activation of PI 3-kinase. Primary cultured rat hepatocytes were pre-treated with 0, 2, or 5 mM PQ for 6 h and then treated with/without 100 nM insulin for 1 min. Protein lysate was prepared from the cells, immunoprecipitated with anti-IRS-1 antibody (A) or anti-IRS-2 antibody (B), and PI 3-kinase activity was analyzed by measuring 32P incorporation into phosphatidylinositol (upper panels). 32P incorporated into phophatidylinositol 3,4,5-triphosphate was quantified and expressed as relative values to cells treated without insulin and PQ (Ins-, PQ 0 mM) (lower panels). Values are means ± SEM of three dishes. #Significantly different from cells without insulin treatment (p<0.05).
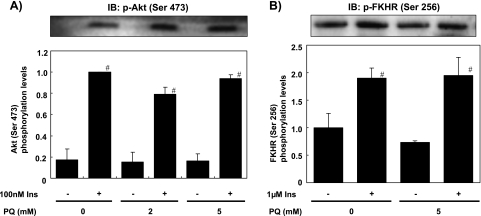
Effects of PQ on insulin-dependent phosphorylation of Akt and FKHR
We then examined changes of the downstream signals along the PI 3-kinase pathway. Treatment of the cells with 100 nM insulin for 1 min increased the phosphorylation of Akt (Ser 473) which reflects Akt activity (Fig. 5A). Pretreatment with 2 mM or 5 mM PQ for 6 h did not affect insulin-dependent Akt phosphorylation in the same manner with PI 3-kinase activity bound to IRS-1 and IRS-2. In addition, treating the cells with 1 µM insulin for 15 min increased Ser 256 phosphorylation of FKHR, which is one of the substrates of Akt, and increased transcription of IGFBP-1 and G6Pase genes with the non-phosphorylated form [27, 29]. Pretreatment with 5 mM PQ for 6 h did not inhibit insulin-dependent FKHR phosphorylation (Fig. 5B).
Fig. 5.
PQ treatment did not affect insulin-dependent phosphorylation of Akt and FKHR. Primary cultured rat hepatocytes were pre-treated with 0, 2, or 5 mM PQ for 6 h and then treated with/without insulin for 1 min for Akt and 15 min for FKHR. Protein lysate was prepared from the cells, and Ser 473 phosphorylation of Akt (A) and Ser 256 phosphorylation of FKHR (B) was analyzed by Western blot analysis with anti-phospho-Akt (Ser 473) or anti-phospho-FKHR (Ser 256) antibody (upper panels). Phosphorylated levels were quantified and expressed as relative values to cells without insulin and PQ treatment (Ins-, PQ 0 mM) (lower panels). Values are means ± SEM of three dishes. #Significantly different from the cells without insulin treatment (p<0.05).
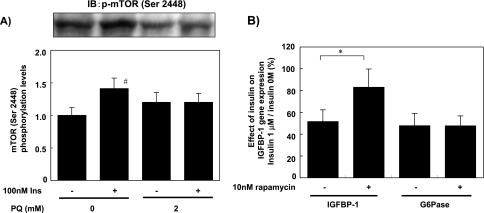
Effect of PQ on insulin-dependent phosphorylation of mTOR
Last, we measured the activity of another downstream Ser/Thr kinase, mTOR, by its phosphorylation. Treatment of the cells with 100 nM insulin for 10 min increased the phosphorylation of mTOR (Ser 2448). Interestingly, pretreatment with 2 mM PQ for 6 h abolished insulin-dependent mTOR phosphorylation (Fig. 6A). In order to investigate the role of mTOR in insulin-dependent regulation of gene expression, we analyzed the effect of rapamycin, an mTOR inhibitor, on insulin-dependent regulation of IGFBP-1 and G6Pase mRNA content. As shown in Fig. 6B, regulation of IGFBP-1 by insulin was rapamycin-sensitive while that of G6Pase was not, indicating that insulin signals through mTOR play an important role in down-regulation of the IGFBP-1 mRNA level.
Fig. 6.
PQ treatment impaired insulin-induced phosphorylation of mTOR. (A) phosphorylation of mTOR was analyzed with primary cultured rat hepatocytes pre-treated with/without 2 mM PQ for 6 h and then treated with/without 100 nM insulin for 10 min. Protein lysate was analyzed by Western blot analysis with anti-phospho-mTOR (Ser 2448) antibody. Phosphorylated levels were quantified and expressed as relative values to cells without insulin and PQ treatment (Ins-, PQ 0 mM). Values are means ± SEM of three dishes. #Significantly different from the cells without insulin treatment (p<0.05). (B) The effect of rapamycin treatment on the IGFBP-1 mRNA level was measured by RT-PCR with primary cultured rat hepatocytes treated with/without 10 nM rapamycin and 1 µM insulin for 6 h simultaneously. Values represent the repressive effect of insulin on the IGFBP-1 mRNA level (1 µM insulin/0 M insulin) and are expressed as means ± SEM of three dishes. *Significantly different between the two groups by rapamycin treatment (p<0.05).
Discussion
PQ produces oxidative stress by redox cycling with a variety of diaphorases and oxygen to produce supreoxide radicals [14–16] and was used as an intracellular radical generator in the present study. PQ treatment for 6 h, the experimental condition in this study, did not decrease cell viability and slightly increased leakage of LDH from the cells (Fig. 1). The effect of PQ-induced oxidative stress was not so severe as to reduce cell viability and was almost equal to that seen in our previous study, in which we demonstrated the repressive effect of oxidative stress on insulin regulation of gene expression [13]. The results of this study showed that PQ treatment inhibited insulin-dependent regulation of IGFBP-1 but not G6Pase, suggesting that the effect of PQ was specific to a signaling pathway that regulates IGFBP-1 gene expression. As the inhibitory effect of PQ on insulin-dependent regulation of IGFBP-1 gene expression was repressed by the antioxidant NAC, the effect was mediated by ROS or changes of intracellular redox status (Fig. 2). Since PQ is an intracellular radical generator, we assumed that PQ-derived ROS attacked insulin signaling molecules before being quenched by cellular antioxidative systems.
Among the intracellular insulin signaling molecules investigated, our results demonstrated that PQ treatment did not affect insulin signal transduction at the early stage, such as phosphorylation of IR and IRS, activation of PI 3-kinase, and phosphorylation of Akt. In adipocytes and muscle cells, oxidative stress has been shown to disrupt subcellular redistribution of IRS-1 and PI 3-kinase induced by insulin treatment [11, 12]. Since the insulin-induced increase and activation of IRS-1 and PI 3-kinase in low-density microsome fractions is important in glucose transporter 4 translocation and glucose uptake in these tissues, impairment of this process may have an important role in inducing insulin resistance. In the case of liver, H2O2 has been shown to act as a stimulator of IR tyrosine kinase by inhibiting protein-tyrosine-phosphatase activity [43]. These results suggest that H2O2-derived ROS influences the early stage of insulin signaling in a tissue-specific manner. As PQ treatment did not up-regulate the early stages of insulin signaling, such as phosphorylation of IR, IRS, PI 3-kinase, and Akt/PKB in this study (Figs. 3, 4, and 5), the severity or duration of ROS generation may be important to induce the up-regulation of insulin signaling.
Based on the fact that PQ treatment did not affect insulin-dependent phosphorylation of FKHR, we conclude that the effects of PQ treatment on inhibition of insulin-dependent repression of IGFBP-1 gene expression are not mediated by the PI 3-kinase-Akt-FKHR pathway. On the other hand, another interesting target of PQ-induced ROS among insulin signaling molecules that we found in this study is mTOR. That is, phosphorylation of mTOR Ser 2448, which reflects its activation, was impaired by PQ treatment (Fig. 6). Since the insulin regulation of IGFBP-1 was sensitive to rapamycin treatment but that of G6Pase was not (Fig. 6B), it is conceivable that the inhibition of insulin-dependent mTOR phosphorylation caused impairment of insulin regulation of only IGFBP-1 gene expression. It has been reported that insulin-regulation of IGFBP-1 gene expression is not involved in FKHR binding [34] but is dependent on mTOR activation [32, 34]. These findings support the present results showing that inhibition of insulin-dependent mTOR phosphorylation causes impairment of insulin-dependent regulation of IGFBP-1 gene expression. This is the first report to indicate that ROS impairs insulin-dependent mTOR phosphorylation directly, although it has also been reported that H2O2 impairs mTOR dependent pathways in hepatocytes [32]. Therefore, we conclude that insulin-dependent mTOR phosphorylation is a target of oxidative stress in liver.
Phosphorylation of mTOR is regulated not only by insulin, but also by growth factors, energy, or the amino acid supply. Signals from insulin and growth factor receptors via the PI 3-kinase/Akt pathway phosphorylate and inactivate tuberous sclerosis complex (TSC) 2 and convert Ras homolog-enriched in brain to its active GTP-bound form, leading to activated mTOR [44]. Energy or amino acid restriction has been reported to impair mTOR phosphorylation through activation of AMP-activated protein kinase which can activate TSC2 [45–48]. By these mechanisms, protein synthesis of cells is regulated in response to nutritional stress using mTOR as an essential regulator. Whether the same mechanism is involved in the impairment of mTOR phosphorylation under PQ-induced oxidative stress is unclear at present. The results of the present study suggest for the first time that ROS-derived stress suppresses mTOR phosphorylation as in the case of energy- or amino acid-deprivation.
The mechanism underlying mTOR regulation of IGFBP-1 gene expression is unknown at present. As a transcription factor regulated by mTOR, hypoxia-inducible factor-1α has been reported to regulate IGFBP-1 gene expression in response to hypoxia in zebra fish [49]. Under hypoxia, IGFBP-1 mRNA expression is increased through the elevation of HIF-1α. However, we found that the HIF-1α level in the cells did not change with PQ treatment (data not shown). Insulin-dependent mTOR activation may regulate IGFBP-1 gene expression by a different mechanism from that of hypoxia, and elucidation of this mechanism is in progress in our laboratory.
The impairment of insulin regulation of IGFBP-1 synthesis helps maintain hepatic IGFBP-1 synthesis and plasma IGFBP-1 at higher level, which inhibits the protein synthesis activity of IGF-I in peripheral tissues. Furthermore, mTOR by itself is a serine/threonine protein kinase which phosphorylates and regulates the activity of eukaryotic translation initiation factor 4E-binding protein 1 and p70 S6 kinase, inducing mRNA translation and resulting in an increase in cellular protein synthesis [50]. Therefore, oxidative stress may suppress cellular protein synthesis through the inhibition of insulin-dependent mTOR phosphorylation in hepatocytes. The present results therefore suggest a novel mechanism of oxidative stress to down-regulate protein synthesis via IGFBP-1 production.
In conclusion, PQ-induced oxidative stress impaired insulin-dependent repression of IGFBP-1 gene expression, not by changes in tyrosine phosphorylation of IR or IRSs, or activation of PI 3-kinase and Akt/PKB, but by the impairment of insulin-dependent mTOR activation. The present study strongly suggests that this novel mechanism of oxidative stress to inhibit mTOR activity leads to a reduction of whole body protein synthesis through inhibition of IGF activity by increases in IGFBP-1 as well as inhibition of mTOR-mediated protein synthesis.
Acknowledgment
We are grateful to Yuka Toyoshima for technical assistance and helpful discussions. This study was supported by a Grant-in-Aid for Scientific Research from Japanese Ministry of Education, Culture, Sports, Science and Technology [(C) 19580150, 2007-2008; A. Takenaka].
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- FKHR
forkhead in rhabdomyosarcoma
- G6Pase
glucose-6-phosphatase
- HBSS
Hanks’ balanced-salt solution
- IGFBP-1
insulin-like growth factor-binding protein-1
- IGFs
insulin-like growth factors
- IR
insulin receptor
- IRS
insulin receptor substrate
- LDH
lactate dehydrogenase
- mTOR
mammalian target of rapamycin
- NAC
N-acetyl-L-cystein
- NBS
newborn bovine serum
- PCR
polymerase chain reaction
- PI 3-kinase
phosphatidylinositol 3-kinase
- PMSF
phenylmethylsulfonyl fluoride
- PNPP
p-nitrophenyl phosphate
- PQ
paraquat, 1,1-dimethyl-4,4-dipyridynium
- ROS
reactive oxygen species
- RT-PCR
reverse transcription-polymerase chain reaction
- TSC2
tuberous sclerosis complex
- WE
Wiiliams’ medium E
References
- 1.Dandona P., Thusu K., Cook S., Snyder B., Makowski J., Armstrong D., Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444–445. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 2.Nourooz Z.J., Rahimi A., Tajaddini S.J., Tritschler H., Rosen P., Halliwell B., Betteridge D.J. Relationship between plasma measures of oxidative stress and metabolic control in NIDDM. Diabetologia. 1997;34:171–175. doi: 10.1007/s001250050729. [DOI] [PubMed] [Google Scholar]
- 3.Baynes J.W., Thorpe S.R. Role of oxidative stress in diabetic complications; a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Paolisso G., D’Amore A., Giugliano D., Ceriello A., Varrricchio M., D’Onofrio F. Pharmacologic doses of vitamin E improve insulin action in healthy subjects and non-insulin-dependent diabetic patients. Am. J. Clin. Nutr. 1993;57:650–656. doi: 10.1093/ajcn/57.5.650. [DOI] [PubMed] [Google Scholar]
- 5.Faure P., Rossini E., Lafond J.L., Richard M.J., Favier A., Halimi S. Vitamin E improves the free radical defence system potential and insulin sensitivity of rats fed high fructose diets. J. Nutr. 1997;127:103–107. doi: 10.1093/jn/127.1.103. [DOI] [PubMed] [Google Scholar]
- 6.Khamaisi M., Kavel O., Rosenstock M., Porat M., Yuli M., Kaiser N., Rudich A. Effect of inhibition of glutathione synthesis on insulin action; in vivo and in vitro studies using buthionine sulfoxyimine. Biochem. J. 2000;349:579–586. doi: 10.1042/0264-6021:3490579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houstis N., Rosen E.D., Lander E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 8.Hansen L.L., Ikeda Y., Olsen S.S., Busch A.K., Mosthaf L. Insulin signaling is inhibited by micromolar concentrations of H2O2. J. Biol. Chem. 1999;274:25078–25084. doi: 10.1074/jbc.274.35.25078. [DOI] [PubMed] [Google Scholar]
- 9.Maddux B.A., See W., Lawrence J.C., Goldfine A.L., Goldfine I.D., Evance J.L. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by micromolar concentrations of α-lipoic acid. Diabetes. 2001;50:404–410. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- 10.Tirosh A., Rudich A., Potashnik R., Bashan N. Oxidative stress impairs insulin but not platelet-derived growth factor signaling in 3T3-L1 adipocytes. Biochem. J. 2001;355:757–763. doi: 10.1042/bj3550757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tirosh A., Potashnik R., Bashan N., Rudich A. Oxidative stress disrupts insulin-induced cellular redistribution of insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. J. Biol. Chem. 1999;274:10595–10602. doi: 10.1074/jbc.274.15.10595. [DOI] [PubMed] [Google Scholar]
- 12.Ogihara T., Asano T., Katagiri H., Sakoda H., Anai M., Shojima N., Ono H., Fujishiro M., Kushiyama A., Fukushima Y., Kikuchi M., Noguchi N., Aburatani H., Gotoh Y., Komuro I., Fujita T. Oxidative stress induces insulin resistance by activating the nuclear factor-kB pathway and disrupting normal subcellular distribution of phosphatidylinositol 3-kinase. Diabetologia. 2004;47:794–805. doi: 10.1007/s00125-004-1391-x. [DOI] [PubMed] [Google Scholar]
- 13.Kimura K., Tawara S., Igarashi K., Takenaka A. Effect of various radical generators on insulin-dependent regulation of hepatic gene expression. Biosci. Biotechnol. Biochem. 2007;71:16–22. doi: 10.1271/bbb.60016. [DOI] [PubMed] [Google Scholar]
- 14.Krall J., Bagley A.C., Mullenbach G.T., Hallewell R.A., Lynch R.E. Superoxide mediates the toxicity or paraquat for cultured mammalian cells. J. Biol. Chem. 1988;263:1910–1914. [PubMed] [Google Scholar]
- 15.Clejan L., Cederbaum A.I. Synergistic interactions between NADPH-cytochrome P-450 reductase, paraquat, and iron in the generation of active oxygen radicals. Biochem. Pharmacol. 1989;38:1779–1786. doi: 10.1016/0006-2952(89)90412-7. [DOI] [PubMed] [Google Scholar]
- 16.Shimada H., Hirai K.I., Shimamura E., Pan J. Mitochondrial NADH-quinone oxidoreductase of the outer membrane is responsible for paraquat cytotoxicity in rat livers. Arch. Biochem. Biophys. 1998;351:75–81. doi: 10.1006/abbi.1997.0557. [DOI] [PubMed] [Google Scholar]
- 17.Bus J.S., Gibson J.E. Paraquat: model for oxidant-initiated toxicity. Environ. Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olesen B.T., Clausen J., Vang O. Characterization of the transctiprional profile in primary astrocytes after oxidative stress induced by paraquat. Neurotoxicology. 2008;29:13–21. doi: 10.1016/j.neuro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Yeh S.T., Guo H.-R., Su Y.-S., Lin H.-J., Hou C.-C., Chen H.-M., Chang M.-C., Wang Y.-J. Protective effects of N-acetylcycteine treatmenet post acute paraquat intoxication in rats and in human lung epithelial cells. Toxicology. 2006;223:181–190. doi: 10.1016/j.tox.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Choi H.S., An J.J., Kim S.Y., Lee S.H., Kim D.W., Yoo K.-Y., Won M.H., Kan T.-C., Kwon H.J., Kang J.H., Cho S.-W., Kwon O.-S., Park J., Eum W.S., Choi S.Y. PEP-1-SOD fusion protein efficiently protects against paraquat-induced dopaminergic neuron damage in a Parkinson disease mouse model. Free Radic. Biol. Med. 2006;41:1058–1068. doi: 10.1016/j.freeradbiomed.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Hausburg M.A., DeKrey G.K., Salmen J.J., Oallic M.R., Gardiner C.S. Effects of paraquat on development of preimplantation embryos in vivo and in vitro. Reprod. Toxicol. 2005;20:239–246. doi: 10.1016/j.reprotox.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien R.M., Granner D.K. Regulation of gene expression by insulin. Physiol. Rev. 1996;76:1109–1161. doi: 10.1152/physrev.1996.76.4.1109. [DOI] [PubMed] [Google Scholar]
- 23.Hwa V., Oh Y., Rosenfeld R.G. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr. Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 24.Rajkumar K., Krsek M., Dheen S.T., Murphy L.J. Impaired glucose homeostasis in insulin-like growth factor binding protein-1 transgenic mice. J. Clin. Invest. 1996;98:1818–1825. doi: 10.1172/JCI118982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinh K.Y., O’Doherty R.M., Anderson P., Lange A.J., Newgard C.B. Pertubationof fuel homeostasis caused by overexpression of the glucose-6-phosphatase catalytic subunit in liver of normal rats. J. Biol. Chem. 1998;273:31615–31620. doi: 10.1074/jbc.273.47.31615. [DOI] [PubMed] [Google Scholar]
- 26.Cichy S.B., Uddin S., Danilkovich A., Guo S., Klippel A., Unterman T.G. Protein kinase B/Akt mediates effects of insulin on hepatic insulin-like growth factor-binding protein-1 gene expression through a conserved insulin response sequence. J. Biol. Chem. 1998;273:6482–6487. doi: 10.1074/jbc.273.11.6482. [DOI] [PubMed] [Google Scholar]
- 27.Durham S.K., Suwanichkul A., Scheimann A.O., Yee D., Jackson J.G., Barr F.G., Powell D.R. FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology. 1999;140:3140–3146. doi: 10.1210/endo.140.7.6856. [DOI] [PubMed] [Google Scholar]
- 28.Kops G.J., de Ruiter N.D., DeVries-Smith A.M., Powell D.R., Bos J.L., Burgering B.M. Direct control of the forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 29.Barthel A., Schmoll D., Kruger K.-D., Bahrenberg G., Walther R., Roth R.A., Joot H.-G. Differential regulation of endogenous glucose-6-phosphatase and phosphoenolpyruvate carboxykinase gene expression by the forkhead transcription factor FKHR in H4IIE-hepatoma cells. Biochem. Biophys. Res. Commun. 2001;285:897–902. doi: 10.1006/bbrc.2001.5261. [DOI] [PubMed] [Google Scholar]
- 30.Streeper R.S., Eaton E.M., Ebert D.H., Chapman S.C., Svitek C.A., O’Brien R.M. Hepatocyte nuclear factor-1 acts as an accessory factor to enhance the inhibitory action of insulin on mouse glucose–6-phosphatase gene transcription. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9208–9213. doi: 10.1073/pnas.95.16.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel S., Lochhead P.A., Rena G., Sutherland C. Antagonistic effects of phorbol esters on insulin regulation of insulin-like growth factor-binding protein-1 (IGFBP-1) but not glucose-6-phosphatase gene expression. Biochem. J. 2001;359:611–619. doi: 10.1042/0264-6021:3590611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel S ., Rena G., Fumagalli S., Pende M., Kozma S.C., Thomas G., Sutherland C. Insulin regulation of insulin-like growth factor-binding protein-1 gene expression is dependent on the mammalian target of rapamycin, but independent of ribosomal S6 kinase activity. J. Biol. Chem. 2002;277:9889–9895. doi: 10.1074/jbc.M109870200. [DOI] [PubMed] [Google Scholar]
- 33.Patel S., Van Der Kaay J., Sutherland C. Insulin regulation of hepatic insulin-like growth factor-binding protein-1 (IGFBP-1) gene expression and mammalian target of rapamycin (mTOR) signaling is impaired by the presence of hydrogen peroxide. Biochem. J. 2002;365:537–545. doi: 10.1042/BJ20020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petal S., Lipina C., Sutherland C. Different mechanisms are used by insulin to repress three genes that contain a homologous thymidine-rich insulin response element. FEBS Letters. 2003;549:72–76. doi: 10.1016/s0014-5793(03)00774-9. [DOI] [PubMed] [Google Scholar]
- 35.Ogihara T., Shin B.C., Ani M., Katagiri H., Inukai K., Funaki M., Fukushima Y., Ishihara H., Takata K., Kikuchi M., Yazaki Y., Oka Y., Asano T. Insulin receptor substrate (IRS)-2 is dephosphorylated more rapidly than IRS-1 via its association with phosphatidylinositol 3-kinase in skeletal muscle cells. J. Biol. Cell. 1997;272:12868–12873. doi: 10.1074/jbc.272.19.12868. [DOI] [PubMed] [Google Scholar]
- 36.Kato H., Takahashi S.-I., Takenaka A., Funabiki R., Noguchi T., Naito H. Degradation of endogenous proteins and internalized asialofetuin in primary cultured hepatocytes of rats. Int. J. Biochem. 1989;21:483–495. doi: 10.1016/0020-711x(89)90128-6. [DOI] [PubMed] [Google Scholar]
- 37.Chopmczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 38.Carpenter C.L., Duckworth B.C., Auger K.R., Cohen B., Schaffhausen B.S., Cantley L.C. Purification and characterization of phosphoinositide 3-kinase from rat liver. J. Biol. Chem. 1990;265:19704–19711. [PubMed] [Google Scholar]
- 39.Unterman T.G., Oehler D.T., Murphy L.J., Lacson R.G. Multihormonal regulation of insulin-like growth factor-binding protein-1 in rat H4IIE hepatoma cells: the dominant role of insulin. Endocrinology. 1991;128:2693–2701. doi: 10.1210/endo-128-6-2693. [DOI] [PubMed] [Google Scholar]
- 40.Powell D.R., Suwanichkulk A., Cubbage M.L., DePaolis L.A., Snuggs M.B., Lee P.D.K. Insulin inhibits transctiprion of the human gene for insulin-like growth factor-binding protein-1. J. Biol. Chem. 1991;266:18868–18876. [PubMed] [Google Scholar]
- 41.Orlowski C.C., Ooi G.T., Brown D.R., Yan Y.W., Tseng L.Y., Rechler M.M. Insulin rapidly inhibits insulin-like growth factor-binding protein-1 gene expression in H4-II-E rat hepatoma cells. Mol. Endocrinol. 1991;5:1180–1187. doi: 10.1210/mend-5-8-1180. [DOI] [PubMed] [Google Scholar]
- 42.Streeper R.S., Svitek C.A., Chapman S., Greenbaum L.E., Taub R., O’Brien R.M. A multicomponent insulin response sequence mediates a strong repression of mouse glucose-6-phosphatase gene transcriptin by insulin. J. Biol. Chem. 1997;282:11398–11701. doi: 10.1074/jbc.272.18.11698. [DOI] [PubMed] [Google Scholar]
- 43.Mahadev K., Zilbering A., Zhu L., Goldstein B.J. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1B in vivo and enhances the early insulin action cascade. J. Biol. Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 44.Sarbassov D.D., Ali S.M., Sabatini D.M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Cheng S.W.Y., Fryer L.G.D., Carling D., Shepherd P.R. The2446 is a novel mammalian target of rapamaycin (mTOR) phosphorylation site regulated by nutrient status. J. Biol. Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- 46.Long X., Ortiz-Vega S., Lin Y., Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J. Biol. Chem. 2005;280:23433–23436. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 47.Smith E.M., Finn S.G., Tee A.R., Browne G.J., Proud C.G. The Tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acid and certain cellular stresses. J. Biol. Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 48.Sofer A., Lei K., Johannessen C.M., Ellisen L.W. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol. Cell Biol. 2005;25:5834–5845. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kajimura S., Aida K., Duan C. Understanding hypoxia-induced gene expression in early development: in vitro and in vivo analysis of hypoxia-inducible factor 1-regulated zebra fish insulin-like growth factor binding protein 1 gene expression. Mol. Cell Biol. 2006;26:1142–1155. doi: 10.1128/MCB.26.3.1142-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Proud C.G. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem. J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]