Abstract
Nω-Carboxymethyl-arginine (CMA), Nω-carboxyethyl-arginine (CEA) and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1) have been identified as L-arginine-derived advanced glycation end products (AGEs) formed by non-enzymatic reactions between reducing sugars such as glucose and amino groups in proteins. These AGEs are structurally analogous to endogenous inhibitors of nitric oxide synthases (NOS) including NG-monomethyl-L-arginine (L-NMMA) and asymmetric NG,NG-dimethyl-L-arginine (ADMA). Increased plasma levels of these NOS inhibitors, and thus impaired generation of NO in vivo has been associated with the pathogenesis of vascular complications such as kidney failure and atherosclerosis. For these reasons we examined whether L-arginine-derived AGEs inhibit the activities of three L-arginine metabolizing enzymes including three isoforms of NOS (endothelium, neuronal and inducible NOS), dimethylarginine dimethylaminohydrolase (DDAH) that catalyzes the hydrolytic degradation of L-NMMA and ADMA to L-citrulline, and arginase that modulates intracellular L-arginine bioavailability. We found that AGEs inhibited the in vitro activities of endothelium type NOS weakly (IC50 values of CMA, CEA and MG-H1 were 830, 3870 and 1280 µM, respectively) and were also potential endogenous inhibitors for arginase (IC50 values of CMA and CML were 1470 and 1060 µM), but were poor inhibitors for DDAH. These results suggest that the tested L-arginine- and L-lysine-derived AGEs appear not to impair NO biosynthesis directly.
Keywords: advanced glycation end products, Nω-carboxymethyl-arginine, nitric oxide synthase, dimethylarginine dimethylaminohydrolase, arginase
Introduction
Advanced glycation end products (AGEs) are a group of post-translationally modified proteins which are formed from the reactions between amino residues and reducing sugars such as glucose. Levels of glycated proteins increase with aging under normal physiological conditions and alter the molecular structure and biochemical functions of the effected proteins. Hyperglycemia accelerates the glycation and leads to excessive accumulation of AGEs-modified proteins in vessel walls and has been linked to the pathogenesis of diabetic complications, particularly the development of micro- and macrovascular diseases [1, 2]. Previous studies have shown that AGEs-modified proteins quench nitric oxide (NO), an endothelium-derived vasodilator, in vitro [3, 4] and also decrease the enzymatic activities and protein expression of endothelium NO synthase (eNOS) in vascular endothelium cell cultures [5–7]. These results suggest that AGEs impair NO biosynthesis, playing a critical role in diabetic endothelial dysfunction.
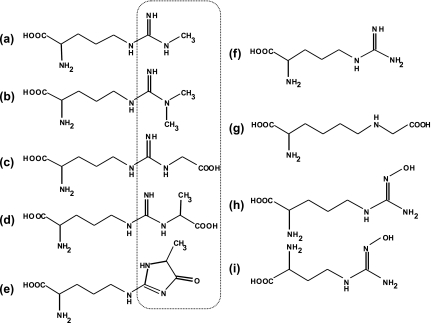
The reactive dicarbonyl intermediates (glyoxal and methylglyoxal) that are generated from auto-oxidation of glucose and the glycolysis pathway [8, 9] rapidly modifies the amino groups of arginine and lysine residues. Some glycated arginine derivatives have been structurally characterized as Nω-carboxymethylarginine (CMA) [10], Nω-carboxyethylarginine (CEA) [11] and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1) [12] (see structures in Fig. 1). Several previous studies have shown that the levels of glycated arginine derivatives are markedly elevated in the plasma of patients with diabetic complications compared with those in healthy individuals [13]. In addition, immunohistochemical studies have demonstrated that CMA accumulates in the atherosclerotic lesions of human aorta, the CMA levels in tissue proteins being positively correlated with the pathogenesis of atherosclerosis [14].
Fig. 1.
Structures of L-arginine-derived AGEs and other compounds used in this study. (a) and (b) endogenous NOS inhibitor, L-NMMA and ADMA, respectively; (c)–(e) L-arginine-derived AGEs, CMA, CEA and MG-H1; (f) L-arginine; (g) L-lysine-derived AGEs, CML; (h) an intermediate metabolite in the L-arginine-NO biosynthesis pathway, NOHA; (i) nor-NOHA, a synthetic analog of NOHA. Dotted circles indicate variable chemical groups of L-arginine derivatives.
NO, identified as an endothelium-derived relaxing factor (EDRF), is a critical signaling molecular in vascular function and homeostasis in mammals [15]. NO is formed during the oxidation of L-arginine to L-citrulline that is catalyzed by NO synthases (NOSs, EC 1.14.13.39). Three NOS isoforms have been characterized and cloned: neuronal NOS (nNOS; type I) and inducible NOS (iNOS; type II), endothelial NOS (eNOS; type III) [16]. Constitutive NOS (eNOS and nNOS) are associated with the regulation of vascular tone in blood vessels and neurotransmission in the central and peripheral nervous tissues. In the immune system, inflammatory cytokines stimulate iNOS expression in macrophages and other cell types to provide defense against pathogens.
NG-Monomethyl-L-arginine (L-NMMA) and NG,NG-dimethyl-L-arginine (asymmetric dimethylarginine; ADMA) are L-arginine analogues and typical endogenous NOSs inhibitors (see structures in Fig. 1). It has been demonstrated that levels of these methylarginines are elevated in plasma and tissues of patients with various pathological conditions, including hypertension [17], chronic renal failure [18] and diabetes mellitus [19], resulting in a deficiency of endogenous biosynthesis of NO due to inhibition of NOSs, especially eNOS.
Methylarginines are generated by the methylation of L-arginine residues in proteins mediated with S-adenosylmethionine: protein arginine methyltransferases (protein methylases). The methyl groups are symmetrically or asymmetrically-adducted to the guanidino group of L-arginine, resulting in formation of L-NMMA and AMDA (see structures in Fig. 1). Endogenous methylarginines are in part excreted by the kidney. However the metabolism of L-NMMA and ADMA, but not NG,NG'-dimethyl-L-arginine (symmetric dimethylarginine, SDMA), mainly occurs via hydrolytic degradation to L-citrulline and either monomethylamine or dimethylamine by the enzyme dimethylarginine dimethylaminohydrolase (DDAH) [20]. Inhibition of DDAH activity increases intracellular concentrations of ADMA and reduces endogenous NO generation [21]. It has been reported that a glucose-induced impairment of DDAH causes the accumulation of ADMA and this may contribute to endothelial vasodilator dysfunction in patients with diabetes [22].
The NO generation depends on the bioavailability of NOSs substrate L-arginine which is shared by arginase, the enzyme metabolizing L-arginine to form urea and L-ornithine via the urea cycle [23]. Arginase is responsible for regulating NO generation by modulating intracellular L-arginine bioavailability. It has been reported that increases in arginase activity diminish plasma levels of L-arginine in human diabetes patients and experimental diabetic animals [24, 25].
The present study has focused on several endogenous AGEs-derived compounds including L-arginine derivatives (CMA, CEA and MG-H1) and an L-lysine derivative (carboxymethyl-lysine, CML). Because these compounds are structurally analogous to endogenous NOSs inhibitors L-NMMA and ADMA, they may be implicated in the pathological mechanism of chronic vasculopathy through competitive inhibition of NOSs activities, especially eNOS. These L-arginine derivatives may impair NO generation by antagonizing L-arginine binding sites of three NOS isoforms. In addition to NOS we have also examined the effects of these compounds on the enzymatic activities of DDAH and arginase, both enzymes indirectly regulating NO bioavailability.
Materials and Methods
Materials
L-Arginine, ADMA, NG-hydroxy-L-arginine (NOHA), nicotinamide adenine dinucleotide phosphate reduced form (NADPH), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), (6R)-5,6,7,8-tetrahydrobiopterin (BH4), recombinant eNOS (from bovine), iNOS (from mouse macrophage), Dowex® AG50W-X8 cation-exchange resin (mesh size, 100–200) were all purchased from Sigma Chemicals (St. Louis, MO). Ultima Gold XR scintillation liquid was from PerkinElmer (Shelton, CA). Recombinant nNOS (from rat) and NG-hydroxy-nor-arginine (nor-NOHA) were obtained from Cayman Chemicals. Arginase (from bovine liver) was from Alexis Biochemicals (Lausen, Switzerland). L-NMMA was from Calbiochem, Darmstadt, Germany. All other chemicals were obtained from Wako Pure Chemical Industries, Osaka, Japan.
Synthesis of L-arginine-derived AGEs
CMA, CEA and MG-H1 were synthesized and purified according to the methods described previously [26]. Their structures were confirmed by nuclear magnetic resonance and electrospray ion mass spectrometry. Purities of these L-arginine derivatives were over 98%, which was verified by high performance liquid chromatography equipped with a fluorescence detector after derivatization with the fluorescent reagent 4-fluoro-7-nitro-2,1,3,-benzoxadiazole [27].
Assay for NOSs activities
The NOS activities were determined by monitoring the conversion of L-[14C(U)]-arginine (Moravek, Brea, CA) to L-[14C(U)]-citrulline as previously described [28]. Recombinant enzymes of three isoforms of NOS (specific activity: eNOS 0.03, nNOS 2.9, iNOS 0.62 units/mg protein) (10 µl) were added to a reaction mixture (final volume of 100 µl) consisting of 50 mM HEPES buffer (pH 7.4), 1 mM NADPH, 4 µM FAD, 4 µM FMN, 10 µM BH4, 1 mg/L calmodulin, 2.5 mM CaCl2, 50 µM L-arginine and 0.5 µCi/ml of L-[14C(U)]-arginine (specific activity: 8.14–11.1 GBq/mmol). After incubation for 30 min at 37°C, the reactions were terminated by addition of 0.5 ml of 20 mM HEPES (pH 5.5) containing 2 mM EDTA and 2 mM EGTA. Samples were passed through a 1 ml column of Dowex AG50W-X8 (Na+-form) to remove unconverted L-[14C(U)]-arginine. L-[14C(U)]-Citrulline was eluted twice with 1 ml of distilled water, and the eluted fractions mixed with 10 ml of cocktail (Ultima Gold XR) were quantified using a liquid scintillation counter. A reaction mixture containing L-[14C(U)]-arginine without the enzyme was incubated as a control to determine background counts. The effects of arginine derivatives on the NOSs activities were examined in the presence and absence of the test compounds at various concentrations.
Assay for arginase activity
Assays for arginase activity were performed by monitoring the conversion of L-[guanidine-14C]-arginine (Moravek, Brea, CA) to [14C]-urea as previously described [28]. Recombinant arginase (0.5 µg of protein, specific activity: 0.094 units/mg protein) (10 µl) was added to a mixture (final volume of 100 µl) containing 10 mM Tris-HCl buffer, pH 9.6, 1 mM MnCl2, and 0.16 µCi/ml of L-[guanidine-14C]-arginine (specific activity: 1.85–2.22 GBq/mmol). After incubation for 1 h at 37°C, the reactions were terminated by addition of 0.4 ml of stop buffer containing 250 mM sodium acetate and 100 mM urea, pH 4.5. Samples were then passed through a 1 ml column of Dowex AG50W-8X (Na+-form) to remove unmetabolized L-[guanidine-14C]-arginine. [14C]-Urea was eluted twice with 1 ml of distilled water, and the eluted fractions mixed with 10 ml of cocktail (Ultima Gold XR) were quantified using a liquid scintillation counter. A reaction mixture containing L-[guanidine-14C]-arginine in the absence of enzyme was incubated as a control to determine background counts. The inhibitory effects of arginase were examined in the presence and absence of the test compounds at various concentrations.
Assay for dimethylarginine dimethylaminohydrolase (DDAH) activity
The DDAH activity was determined by monitoring the conversion of L-[4,5-3H]-NMMA (American Radiolabeled Chemicals, Inc., St. Louis, MO) to L-[3H]-citrulline as previously described [28, 29]. Rat kidney tissues (0.5 g) were homogenized in 1 ml of ice-cold 0.1 M sodium phosphate buffer (SPB, pH 6.5), and then centrifuged at 10,000 rpm for 5 min at 4°C. The supernatants were analyzed for protein content using a BCA protein reagent kit with bovine serum albumin as the standard. The supernatants were diluted with SPB to 1 mg protein in 50 µl, and then were added to a reaction mixture (a final volume of 100 µl) containing 0.1 M SPB (pH 6.5) and 0.1 µCi/ml of L-[3H]-NMMA (specific activity: 1.48–2.96 TBq/mmol). After incubation for 1 h at 37°C, the reactions were terminated by keeping the tubes on ice for 5 min. Samples were then passed through a 1 ml column of Dowex AG50W-8X (Na+-form) to remove unmetabolized L-[3H]-NMMA. L-[3H]-Citrulline was eluted twice with 1 ml of distilled water, and the eluted fractions mixed with 10 ml of cocktail (Ultima Gold XR) were quantified using a liquid scintillation counter. A reaction mixture containing L-[3H]-NMMA in the absence of enzyme was added to the Dowex column to determine background counts. The effects of arginine derivatives on the enzyme activities in the presence and absence of the test compounds at various concentrations were examined.
Statistical analysis
All experiments were repeated 3~8 times. Representative results are shown as means ± standard deviations (SD). For statistical comparison between two groups, an unpaired t test was used.
Results
Effects of L-arginine-derived AGEs on activities of three NOS isoforms
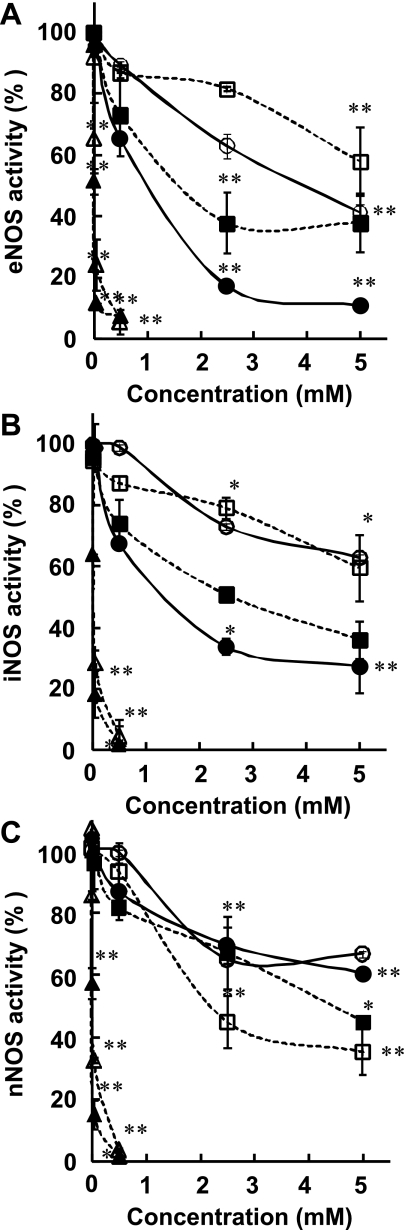
The effects of four AGEs compounds (CMA, CEA, MG-H1 and CML) on the activities of three NOS isoforms were examined at final concentrations of 5, 50, 500, 2500, and 5000 µM. The conversion of L-[14C(U)]-arginine to [14C(U)]-citrulline by NOS isoforms was decreased in a concentration-dependent manner when the enzymatic reaction was carried out in the presence of these AGEs. In particular, the eNOS activity was efficiently inhibited by CMA (IC50 value of 830 ± 36 µM) and MG-H1 (IC50 value of 1280 ± 75 µM), but not by CEA (IC50 value of 3870 ± 680 µM) and CML, a glycated L-lysine derivative (IC50 value >5000 µM) (Fig. 2A, Table 1). On the other hand, L-NMMA and ADMA, well known inhibitors for NOSs, inhibited the activities of eNOS strongly with IC50 values being 5 ± 1 and 16 ± 9 µM, respectively. Similar to eNOS, the iNOS activity was also inhibited by CMA (1160 ± 40 µM), MG-H1 (2970 ± 560 µM), but not by CEA and CML (both IC50 values of >5000 µM) whereas L-NMMA and ADMA inhibited iNOS activities with IC50 values of 10 ± 3 and 24 ± 1 µM, respectively (Fig. 2B, Table 1). The nNOS activity was moderately inhibited by CML (1930 ± 300 µM), MG-H1 (4170 ± 380 µM), and very little by CMA and CEA (with IC50 values of >5000 µM (Fig. 2C, Table 1). L-NMMA and ADMA inhibited the activities of nNOS with IC50 values being 8 ± 2 and 23 ± 0 µM, respectively.
Fig. 2.
Inhibitory effects of various AGEs and other compounds on the enzymatic activities of three isoforms of NOSs, (A) eNOS, (B) iNOS and (C) nNOS. A reaction mixture containing a recombinant NOS, necessary cofactors, 50 µM L-arginine (including L-[14C(U)]-arginine) and a test compound at indicated concentration was incubated for 30 min at 37°C. Results are shown as the means ± S.D normalized to a value of 100% for control (the absence of compounds) (n = 4–8). Significantly different when compared with the control, *p<0.05, **p<0.01. L-NMMA (closed triangle), ADMA (open triangle), CMA (closed circle), CEA (open circle), MG-H1 (closed square) and CML (open square).
Table 1.
The IC50 values of various arginine derivatives for inhibition of three isoforms of NOS
| Compounds | IC50 (µM) |
||
|---|---|---|---|
| eNOS | iNOS | nNOS | |
| L-NMMA | 5 ± 1 | 10 ± 3 | 8 ± 2 |
| ADMA | 16 ± 9 | 24 ± 1 | 23 ± 0 |
| CMA | 830 ± 36 | 1160 ± 40 | >5000 |
| CEA | 3870 ± 680 | >5000 | >5000 |
| MG-H1 | 1280 ± 75 | 2970 ± 560 | 4170 ± 380 |
| CML | >5000 | >5000 | 1930 ± 300 |
The effects of various L-arginine derivatives on the activities of three isoforms of NOSs were measured as described in Materials and Methods. The values correspond to concentrations necessary for 50% inhibition of enzyme activities. Results are means ± SD (n = 4).
Effects of L-arginine-derived AGEs on activities of arginase and DDAH
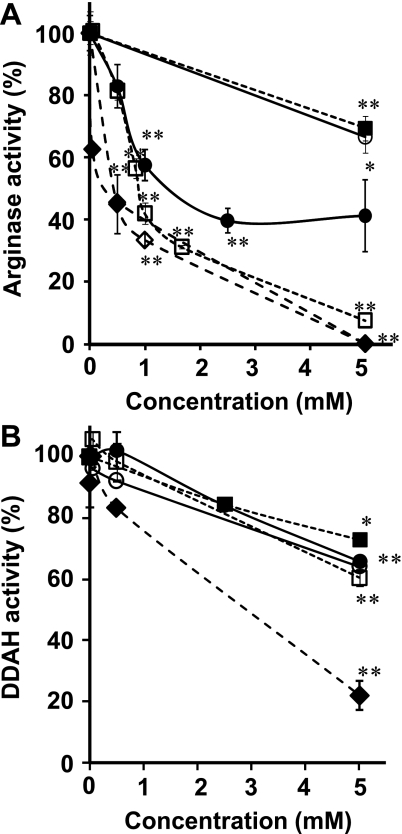
The metabolism of L-[guanidine-14C]-arginine to [14C]-urea mediated by arginase was diminished by the presence of AGEs compounds. In particular, CML suppressed the formation of [14C]-urea in a dose-dependent manner with an IC50 value of 1060 ± 60 µM, whereas CMA inhibited the arginase activity with an IC50 value of 1470 ± 120 µM. CEA and MG-H1 showed very little inhibition at the concentrations tested with IC50s >5000 µM (Fig. 3A, Table 2). NOHA, an intermediate metabolite in the L-arginine-NO biosynthesis pathway, and nor-NOHA, a synthetic analog of NOHA, are known inhibitors for arginase [30] and efficiently inhibited the activity of arginase in a dose-dependent manner, with the IC50 values being 230 ± 26 and 340 ± 12 µM, respectively (Fig. 3A, Table 2).
Fig. 3.
Inhibitory effects of various AGEs and other compounds on the enzymatic activities of arginase and DDAH. (A) The enzymatic activities of arginase were measured by incubation of a reaction mixture containing recombinant arginase, 1 mM MnCl2, substrate of L-[guanidine-14C]-arginine and test compounds at the indicated concentrations. (B) The enzymatic activities of DDAH were measured by incubation of a reaction mixture containing rat kidney extracts, L-[3H]-NMMA and test compounds at the indicated concentrations in SPB (pH 6.5). Results are shown as the means ± S.D normalized to a value of 100% for control (the absence of compounds) (n = 4–8). Significantly different when compared with the control, *p<0.05, **p<0.01. nor-NOHA (closed diamond), NOHA (open diamond), CMA (closed circle), CEA (open circle), MG-H1 (cloaed square) and CML (open square).
Table 2.
The IC50 values of various arginine derivatives for inhibition of arginase and DDAH.
| Compounds | IC50 (µM) |
|
|---|---|---|
| Arginase | DDAH | |
| Nor-NOHA | 230 ± 26 | 1770 ± 250 |
| NOHA | 340 ± 12 | ND |
| CMA | 1470 ± 120 | >5000 |
| CEA | >5000 | >5000 |
| MG-H1 | >5000 | >5000 |
| CML | 1060 ± 60 | >5000 |
The effects of various L-arginine derivatives on the activities of arginase and DDAH were measured as described in Materials and Methods. The values correspond to concentrations necessary for 50% inhibition of enzyme activities. Results are means ± SD (n = 4). ND: not determined.
The enzymatic activity of DDAH was assayed by counting the amounts of L-[3H]-citrulline catalytically formed from L-[3H]-NMMA by a homogenate of rat kidney tissue [28, 29]. AGEs (CMA, CEA, MG-H1 and CML) were found to exhibit weak inhibitory effects on DDAH activity with IC50 values being >5000 µM for each compound (Fig. 3B, Table 2). We have also examined the inhibitory effect of nor-NOHA, a structurally-analogous compound to a potent DDAH inhibitor S-2-amino-4(5-methylguanidino) butamoic acid (4124W) [21]. nor-NOHA inhibited DDAH activity with an IC50 value of 1770 ± 250 µM.
Discussion
We have examined our hypothesis that L-arginine-derived AGEs, CMA, CEA and MG-H1 may inhibit L-arginine metabolism such as the biosyntheses of NO and urea, and contribute to the pathogenesis of vascular complications such as kidney failure and atherosclerosis in diabetes patients. L-Arginine-derived AGEs are structurally analogous to the endogenous NOS inhibitor methylarginine and their levels have been reported to be markedly elevated in the plasma of patients with diabetes compared with those in healthy individuals [13, 31]. For these reasons we have synthesized CMA, CEA and MG-H1 and examined their effects on the enzymatic activities of three isoforms of NOS as well as DDAH and arginase that are enzymes which indirectly regulate NO bioavailability.
We found that glycated L-arginine derivatives (CMA, CEA and MG-H1) inhibited all of three NOS isoforms (eNOS, nNOS, and iNOS) as well as arginase, whereas they did not show inhibitory effects on DDAH activity. The glycated L-lysine derivative (CML) inhibited arginase, but not NOSs and DDAH. However, the IC50 values of these L-arginine derivatives were 3 to 1000-fold higher than those of the synthetic endogenous NOS inhibitors such as ADMA and NOHA.
Reactive α-oxoaldehydes of glyoxal and methylglyoxal (the major AGEs precursors) react with L-arginine and L-lysine residues in protein to form AGEs such as CMA, CEA, MG-H1 and CML. AGEs-modified proteins accumulate in tissues age-dependently and their accumulation is increased under some pathophysiological conditions. Thornalley et al. [31] determined the concentrations of 12 AGEs including MG-H1 and CML by liquid chromatography-mass spectrometry (LC-MS) after enzymatic hydrolysis and found significantly increased levels of AGEs residues in plasma proteins (up to 7-fold) in patients with renal failure compared to those in normal healthy subjects. Similarly the concentrations of CMA residues in serum protein analyzed by LC-MS were ~1.5-fold higher in patients with diabetes without renal failure than in normal subjects [13]. In addition, the levels of free MG-H1 and CML were reported to be 0.11 µM and 0.023 µM in plasma of normal individuals, whereas their levels rose to approximately 5.5 µM and 0.2 µM, respectively, in patients with end-stage renal disease [31]. Under our in vitro assay conditions, these AGEs compounds inhibited NOS activities with IC50 values being >830 µM. It is therefore unlikely that glycated L-arginine and L-lysine derivatives such as CMA, CEA, MG-H1 and CML competitively inhibit the enzymatic activities of the three isoforms of NOS.
Many studies have demonstrated that elevated plasma levels of ADMA might contribute at least in part to the molecular mechanisms of vasculopathy by impairment of NO-dependent vasodilation under various pathophysiological states [17–19]. The plasma concentrations of ADMA found in healthy populations are 0.5 ~ 1.2 µM, whereas they increase up to 10-fold in patients with end-stage renal disease and more moderately (2–3 fold) in many other disease states including chronic heart failure and hypercholesterolemia [32, 33].
Free methylarginines, derived from the degradation of methylated proteins and more than 90% of L-NMMA and ADMA, but not symmetric dimethylarginine (SDMA), are mainly metabolized by the enzyme DDAH, which catalyzes the degradation of L-NMMA and ADMA to form L-citrulline and monomethylamine or dimethylamine, respectively. DDAH expression is found in many tissues (endothelial cells, brain etc.), but the liver and kidney are the primary organs responsible for the metabolism of L-NMMA and ADMA [34]. The concentrations of endogenous NOS inhibitors modulated by DDAH are associated with L-arginine/NO-dependent vasodilation. Furthermore recent studies suggest that ADMA accumulation caused by an impairment of DDAH due to down-regulation of protein expression and its decreased activity contributes to abnormal endothelium-dependent vasodilation in diabetes mellitus [22, 35]. For these reasons, we have investigated the effects of AGEs (CMA, CEA, MG-H1 and CML) on the enzymatic activities of DDAH. The DDAH activity was slightly inhibited by the test compounds (CMA, MG-H1 and CML) with an IC50 value being >5000 µM for each compound (Fig. 3B, Table 2). Even in the presence of the highest concentrations (5000 µM) of these compounds, the DDAH activity was inhibited only by approximately 60%. Because the DDAH inhibitor 4124W [21] could not be obtained commercially, we examined the effect of nor-NOHA, a structural analogue to 4124W, on the DDAH activity. We found that nor-NOHA showed a dose-dependent inhibition of the conversion of L-[3H]-NMMA to L-[3H]-citrulline with an IC50 value of 1770 µM. Compared with nor-NOHA, AGEs exhibited weaker inhibitory effects on the DDAH activity.
The generation of vasodilator (NO) depends also on the bioavailability of the NOS substrate, L-arginine, which is shared by an enzyme arginase forming urea and L-ornithine via the urea cycle [36]. It has been reported that an increased activity of arginase causes diminished levels of plasma L-arginine in human diabetes patients and experimental diabetic animals [24, 25]. For these reasons, we examined the effects of AGEs compounds on the arginase activity. CML and CMA (IC50 values of 1060 and 1470 µM, respectively), but not CEA and MG-H1 (IC50 values >5000 µM), were found to be moderate inhibitors of arginase, although the inhibitory efficiencies were approximately 3-fold weaker than a well known arginase inhibitor, NOHA (IC50 value 340 µM), that is an intermediate of the L-arginine/NO pathway.
In conclusion, the present study demonstrated that glycated L-arginine derivatives (CMA, CEA and MG-H1) and an L-lysine derivative (CML) are potential endogenous inhibitors for three NOS isoforms (eNOS, nNOS, and iNOS) and arginase, but are very poor inhibitors for DDAH. The IC50 values were much greater than the well-known endogenous NOSs and arginase inhibitors such as ADMA and NOHA. Further studies are needed to determine whether AGEs (CMA, CEA, MG-H1 and CML) inhibit other arginine metabolizing enzymes such as argininosuccinate synthase and argininosuccinate lyase and also affect L-arginine transport into cells, which is also known to play a role in the regulation of NO production by cells.
Acknowledgments
This work was supported in part by the Global COE program from the Ministry of Education, Science, Culture, and Sport and by Japanese Society for the Promotion of Science (JSPS) with a grant No of 20.11572. The authors thank Dr. Kohji Ichimori for valuable discussions.
Abbreviations
- AGEs
Advanced glycation end products
- ADMA
asymmetric NG,NG-dimethyl-L-arginine
- CMA
carboxymethyl-arginine
- CEA
carboxyethyl-arginine
- CML
carboxymethyl-lysine
- DDAH
dimethylarginine dimethylaminohydrolase
- EDRF
endothelium-derived relaxing factor
- MG-H1
Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine
- NOHA
NG-hydroxy-L-arginine
- nor-NOHA
NG-hydroxy-nor-arginine
- LC-MS
liquid chromatography-mass spectrometry
- L-NMMA
NG-monomethyl-L-arginine
- NOS
NO synthase
- nNOS
neuronal NOS
- iNOS
inducible NOS
- eNOS
endothelial NOS
- SDMA
symmetric NG,NG'-dimethyl-L-arginine
- SPB
sodium phosphate buffer
- BH4
(6R)-5,6,7,8-tetrahydrobiopterin
References
- 1.Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 2.Kiuchi K., Nejima J., Takano T., Ohta M., Hashimoto H. Increased serum concentrations of advanced glycation end products: a marker of coronary artery disease activity in type 2 diabetic patients. Heart. 2001;85:87–91. doi: 10.1136/heart.85.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucala R., Tracey K.J., Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J. Clin. Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauer T., Rassaf T., Planitz C., Preuss R., Krause R., Henle T., Deussen A. Evidence against nitric oxide-quenching effects of chemically defined Maillard reaction products. Horm. Metab. Res. 2008;40:233–238. doi: 10.1055/s-2008-1058062. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarthy U., Hayes R.G., Stitt A.W., McAuley E., Archer D.B. Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes. 1998;47:945–952. doi: 10.2337/diabetes.47.6.945. [DOI] [PubMed] [Google Scholar]
- 6.Xu B., Ji Y., Yao K., Cao Y.X., Ferro A. Inhibition of human endothelial cell nitric oxide synthesis by advanced glycation end-products but not glucose: relevance to diabetes. Clin. Sci. 2005;109:439–446. doi: 10.1042/CS20050183. [DOI] [PubMed] [Google Scholar]
- 7.Xu B., Chibber R., Ruggiero D., Kohner E., Ritter J., Ferro A. Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. FASEB J. 2003;10:1289–1291. doi: 10.1096/fj.02-0490fje. [DOI] [PubMed] [Google Scholar]
- 8.Thornalley P.J., Langborg A., Minhas H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999;344:109–116. [PMC free article] [PubMed] [Google Scholar]
- 9.Babaei-Jadidi R., Karachalias N., Ahmed N., Battah S., Thornalley P.J. Prevention of Incipient Diabetic Nephropathy by High-Dose Thiamine and Benfotiamine. Diabetes. 2003;52:2110–2120. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- 10.Iijima K., Murata M., Takahara H., Irie S., Fujimoto D. Identification of N(omega)-carboxymethylarginine as a novel acid-labile advanced glycation end product in collagen. Biochem. J. 2000;347:23–27. [PMC free article] [PubMed] [Google Scholar]
- 11.Gruber P., Hofmann T. Chemoselective synthesis of peptides containing major advanced glycation end-products of lysine and arginine. J. Pept. Res. 2005;66:111–124. doi: 10.1111/j.1399-3011.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 12.Westwood M.E., Thornalley P.J. Molecular characteristics of methylglyoxal-modified bovine and human serum albumins. Comparison with glucose-derived advanced glycation endproduct-modified serum albumins. J. Protein Chem. 1995;14:359–372. doi: 10.1007/BF01886793. [DOI] [PubMed] [Google Scholar]
- 13.Odani H., Iijima K., Nakata M., Miyata S., Kusunoki H., Yasuda Y., Hiki Y., Irie S., Maeda K., Fujimoto D. Identification of N(omega)-carboxymethylarginine, a new advanced glycation endproduct in serum proteins of diabetic patients: possibility of a new marker of aging and diabetes. Biochem. Biophys. Res. Commun. 2001;285:1232–1236. doi: 10.1006/bbrc.2001.5322. [DOI] [PubMed] [Google Scholar]
- 14.Mera K., Fujiwara Y., Otagiri M., Sakata N., Nagai R. Immunological detection of N omega-(Carboxymethyl) arginine by a specific antibody. Ann. NY Acad. Sci. 2007;1126:155–157. doi: 10.1196/annals.1433.000. [DOI] [PubMed] [Google Scholar]
- 15.Förstermann U., Closs E.I., Pollock J.S., Nakane M., Schwarz P., Gath I., Kleinert H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 16.Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surdacki A., Nowicki M., Sandmann J., Tsikas D., Boeger R.H., Bode-Boeger S.M., Kruszelnicka-Kwiatkowska O., Kokot F., Dubiel J.S., Froelich J.C. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J. Cardiovasc. Pharmacol. 1999;33:652–658. doi: 10.1097/00005344-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Vallance P., Leone A., Calver A., Collier J., Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 19.Fard A., Tuck C.H., Donis J.A., Sciacca R., Di Tullio M.R., Wu H.D., Bryant T.A., Chen N.T., Torres-Tamayo M., Ramasamy R., Berglund L., Ginsberg H.N., Homma S., Cannon P.J. Acute elevations of plasma asymmetric dimethylarginine and impaired endothelial function in response to a high-fat meal in patients with type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 2000;20:2039–2044. doi: 10.1161/01.atv.20.9.2039. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa T., Kimoto M., Sasaoka K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J. Biol. Chem. 1989;264:10205–10209. [PubMed] [Google Scholar]
- 21.MacAllister R.J., Parry H., Kimoto M., Ogawa T., Russell R.J., Hodson H., Whitley G.S., Vallance P. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br. J. Pharmacol. 1996;119:1533–1540. doi: 10.1111/j.1476-5381.1996.tb16069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin K.Y., Ito A., Asagami T., Tsao P.S., Adimoolam S., Kimoto M., Tsuji H., Reaven G.M., Cooke J.P. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–992. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 23.Wu G., Morris S.M. Jr. Arginine metabolism: nitric oxide and beyond. Biochem. J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pieper G.M., Dondlinger L.A. Plasma and vascular tissue arginine are decreased in diabetes: acute arginine supplementation restores endothelium-dependent relaxation by augmenting cGMP production. J. Pharmacol. Exp. Ther. 1997;283:684–691. [PubMed] [Google Scholar]
- 25.Hagenfeldt L., Dahlquist G., Persson B. Plasm a amino acids in relation to metabolic control in insulin-dependent diabetic children. Acta Paediatr. Scand. 1989;78:278–282. doi: 10.1111/j.1651-2227.1989.tb11070.x. [DOI] [PubMed] [Google Scholar]
- 26.Alt N., Schieberle P. Model studies on the influence of high hydrostatic pressure on the formation of glycated arginine modifications at elevated temperatures. J. Agric. Food Chem. 2005;53:5789–5797. doi: 10.1021/jf050615l. [DOI] [PubMed] [Google Scholar]
- 27.Aoyama C., Santa T., Tsunoda M., Fukushima T., Kitada C., Imai K. A fully automated amino acid analyzer using NBD-F as a fluorescent derivatization reagent. Biomed. Chromatogr. 2004;18:630–636. doi: 10.1002/bmc.365. [DOI] [PubMed] [Google Scholar]
- 28.Imamura M., Waseda Y., Marinova G.V., Ishibashi T., Obayashi S., Sasaki A., Nagai A., Azuma H. Alterations of NOS, arginase, and DDAH protein expression in rabbit cavernous tissue after administration of cigarette smoke extract. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R2081–R2089. doi: 10.1152/ajpregu.00406.2007. [DOI] [PubMed] [Google Scholar]
- 29.Loyaga-Rendon R.Y., Sakamoto S., Beppu M., Aso T., Ishizaka M., Takahashi R., Azuma H. Accumulated endogenous nitric oxide synthase inhibitors, enhanced arginase activity, attenuated dimethylarginine dimethylaminohydrolase activity and intimal hyperplasia in premenopausal human uterine arteries. Atherosclerosis. 2005;178:231–239. doi: 10.1016/j.atherosclerosis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Boucher J.L., Custot J., Vadon S., Delaforge M., Lepoivre M., Tenu J.P., Yapo A., Mansuy D. N omega-hydroxyl-L-arginine, an intermediate in the L-arginine to nitric oxide pathway, is a strong inhibitor of liver and macrophage arginase. Biochem. Biophys. Res. Commun. 1994;203:1614–1621. doi: 10.1006/bbrc.1994.2371. [DOI] [PubMed] [Google Scholar]
- 31.Thornalley P.J., Battah S., Ahmed N., Karachalias N., Agalou S., Babaei-Jadidi R., Dawnay A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003;375:581–592. doi: 10.1042/BJ20030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Böger R.H. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc. Res. 2003;59:824–833. doi: 10.1016/s0008-6363(03)00500-5. [DOI] [PubMed] [Google Scholar]
- 33.Vallance P., Leiper J. Cardiovascular biology of the asymmetric dimethylarginine: dimethylarginine dimethylaminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 34.Leiper J.M., Santa Maria J., Chubb A., MacAllister R.J., Charles I.G., Whitley G.S., Vallance P. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem. J. 1999;343:209–214. [PMC free article] [PubMed] [Google Scholar]
- 35.Sorrenti V., Mazza F., Campisi A., Vanella L., Li Volti., DiGiacomo C. High glucose-mediated imbalance of nitric oxide synthase and dimethylarginine dimethylaminohydrolase expression in endothelial cells. Curr. Neurovasc. Res. 2006;3:49–54. doi: 10.2174/156720206775541778. [DOI] [PubMed] [Google Scholar]
- 36.Boucher J.L., Moali C., Tenu J.P. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cell Mol. Life Sci. 1999;55:1015–1028. doi: 10.1007/s000180050352. [DOI] [PMC free article] [PubMed] [Google Scholar]