Abstract
The HER-axis consists of a dynamic, interconnected family of receptors that make critical contributions to a number of malignancies. Therapeutics targeting EGFR, are unable to effectively inhibit tumor growth in a majority of cases. These tumors are assumed to possess primary resistance to anti-EGFR therapies but the consequence of inhibiting EGFR in these tumors is unclear. We established isogenic cell lines by prolonged gefitinib treatment at concentrations that are in excess of that which is required for complete EGFR kinase inhibition but only minimally effected growth. Subsequently, we monitored the ligand-dependent HER profiles based on receptor expression, phosphorylation and dimerization in conjunction with measurements of cellular susceptibility to gefitinib. Chronic EGFR kinase inhibition rapidly switched the HER network from dependence on EGFR to HER2. However, both receptors activated the critical signaling proteins, AKT and MAPK and in both cases HER3 was the common association partner. Remarkably, the switch in receptor dimers caused diminished susceptibility to EGFR-targeted inhibitors, gefitinib and cetuximab, but acquired susceptibility to the HER2-targeted inhibitor, pertuzumab. Overall, our study indicates that the EGFR pathway is responsive to EGFR inhibiting therapies that are not dependent on EGFR for their growth and survival thus challenging the current definition of primary therapeutic resistance. Further, EGFR kinase inhibition induces HER-kinase receptors to engage in alternative dimerization that can ultimately influence therapeutic selection and responsiveness.
Keywords: EGFR, HER2, HER3, homodimerization, heterodimerization, phospho-AKT
Introduction
The human epidermal receptor (HER) axis is a dynamic, interconnected family of receptors that can form receptor complexes that activate both common and distinct downstream signaling events critical for cell growth and proliferation. It includes four, structurally related receptors, HER1 (epidermal growth factor receptor [EGFR]), HER2, HER3 and HER4. These receptors are activated in response to ligand-dependent dimerization that initiates signaling pathways key to cellular proliferation including the RAS/MAPK and PI3K/AKT pathways. However, receptor hyper-activation due to overexpression (1), amplification (2, 3), or activating mutations (4) can cause aberrant cellular proliferation and tumorigenesis.
Constitutively active receptors, such as EGFR or HER2 when over-expressed and/or deregulated, can trigger potent signaling events that confer a selective growth advantage and can lead to tumorigenesis. However, sustained activation also causes cell-dependence on individual signaling pathways. This phenomenon, referred to as “oncogene addiction” (5) predicts targets for directed therapeutics as inhibition of the target oncogene effectively inhibits cellular proliferation. This is exemplified by tumors over-expressing HER2 that can be inhibited by the HER2-directed antibody, trastuzumab (6) as well as EGFR expressing tumors that are inhibited by EGFR tyrosine kinase inhibitors (TKIs) (i.e., erlotinib and gefitinib) or EGFR-directed monoclonal antibodies (cetuximab and panitumumab). However, efficacy for both of these targeted inhibitors is limited to a subset of patients. Although there is some controversy concerning the measurement of HER2 amplification in breast cancers by both standard IHC or FISH techniques (7), it is conventionally accepted that trastuzumab is only effective in a subset of breast cancers (8), those with elevated HER2 expression as assessed by these diagnostic assays. Similarly EGFR-TKIs are most effective in NSCLC tumors in which EGFR is hyperactivated due to gene amplification, kinase domain activating mutations (9-12), EGFR ligand overexpression (13), or activation by non-HER family members (14, 15).
Upon chronic treatment ‘oncogene-addicted’ tumors become resistant to HER-targeted therapeutics. However, tumors are heterogeneous tissues and it is plausible that there is diversity for therapeutic susceptibility within a tumor. As such, it is expected that both oncogene-addicted and non-addicted cells will be exposed to targeted therapies. The oncogene-addicted cells may undergo apoptosis via the recently explained mechanism of ‘oncogenic shock’ (16) while the non-addicted cells may remain viable. Eventually oncogene-addicted cells acquire resistance against the targeted therapeutic through readjustment of signaling networks (17-19), HER reprogramming (20, 21) and acquisition of secondary mutations (22, 23). In contrast, the effect of HER-targeted therapies in the non-addicted cells that express HER family targets at functional but not hyperactive levels has not been thoroughly evaluated. These non-addicted cells may harbor de novo or primary resistance however, it is unclear if the HER network in these cells undergoes modulation in response to HER-targeted therapies. Recent studies have demonstrated that while trastuzumab is ineffective at altering the growth properties of cells that express normal levels of HER2, it causes a shift in HER family expression (24). We queried whether a similar phenomenon occurs in normal EGFR expressing cells in response to EGFR tyrosine kinase inhibitors.
We have developed isogenic cell lines that are not dependent on EGFR for cell survival to evaluate HER-family interactions important for EGFR-TKI sensitivity. We have demonstrated that while cellular proliferation is only moderately effected by prolonged exposure to supraphysiological gefitinib concentrations otherwise known to compromise viability of gefitinib-sensitive, EGFR expressing cells (25), in the androgen-independent prostate cancer cells (22Rv1), the HER family receptor profile was profoundly altered. These gefitinib concentrations were shown previously to rapidly select for gefitinib-resistant cells (25). In response to gefitinib there is a shift in the HER axis, and as EGFR expression is down-regulated, HER2 expression is up-regulated. In parallel, ligand-dependent receptor association patterns are altered and switch from primarily EGFR containing dimers to HER2-HER3 heterodimers. While MAPK activation is not altered by gefitinib exposure, AKT is less efficiently activated by epidermal growth factor (EGF)-dependent EGFR homodimers but is activated more readily by heregulin beta 1 (HRGβ1)-dependent HER2-HER3 heterodimers. Ultimately, cells harboring these receptor profile alterations become susceptible to the HER2 heterodimer-inhibiting antibody, pertuzumab (2C4). Our results demonstrate that EGFR-TKI treatment targets EGFR even in cells that are de novo resistant to EGFR-TKIs and causes a shift in HER axis signaling, potentially “priming” cells for susceptibility to alternative HER targeting strategies.
Materials and Methods
All experiments were performed at least three times. Representative data are shown.
Cell lines and tissue culture
Cells were maintained in RPMI-1640 (ATCC, Manassas, VA) supplemented with 10% FBS (Omega Scientific, Tarzana, CA), 1% Penicillin-Streptomycin (Invitrogen, Carlsbad, CA), and 1% Glutamine (Invitrogen).
Gefitinib selection of 20GS
22Rv1 cells were serially passaged in 20μM gefitinib (a gift from AstraZeneca, Manchester, UK), for ∼4 months until single-cell clones were obtained.
Stable transfection of 20GS
20GS cells were transiently transfected with pEGFP-NI-EGFR (a gift from Alexander Sorkin) (26), using Effectene (Qiagen, Valencia, CA). Single cell clones were selected in presence of G418 and maintained in 200μg/mL G418. Clonal populations were analyzed for EGFR expression, and FACS sorted for GFP expression. GFP-positive population was labeled as 20GS-EGFR.
Ligand and drug treatments
Cells were serum-starved overnight in phenol red-free RPMI-1640 containing 0.1% charcoal-stripped FBS and supplements, incubated with drugs (gefitinib, cetuximab [obtained from CSMC pharmacy] or 2C4 [pertuzumab, gift from Genentech, South San Francisco, CA]) at the indicated concentrations at 37°C for 2 hours before ligand addition. Ligands were 30nM HRG (R&D Systems, Minneapolis, MN) at 37°C, 10 minutes or 4nM EGF (Invitrogen) at 37°C, 5 minutes.
Cell viability assays
Cells were treated with drugs and/or ligands at the indicated concentrations for 5 days at 37°C. Cell viability was assessed using the ATP-lite 1-step Luminescence Assay (Perkin Elmer, Waltham, MA).
Western blotting
Immunoblots were performed as described previously (27). Antibodies used were: phospho-MAPK p44/42 (#9101), phospho-HER3 (Y1289 (#4791), phospho-EGFR (Y1068) (#2236), phospho-AKT (#9271), EGFR (#2232), AKT (#9272) (Cell Signaling, Danvers, MA), HER3 (Neomarkers MS-201), ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA), and β-actin (Sigma, St. Louis, MO).
Real-time quantitative RT-PCR
RNA extraction and real-time quantitative RT-PCR methods were performed as described previously (27) using gene-specific primers (900nM each) and probe (250nM) from Applied Biosystems. Samples were analyzed using the ΔΔCt method and normalized to GAPDH.
Animal studies
All experiments were conducted under CSMC Institutional Animal Care and Use Committee approval. Single-cell tumor suspensions were filtered, incubated for 5 minutes in red blood cell-lysis buffer (Sigma, St. Louis, MO), and washed in 1X PBS. Cell pellets resuspended in 50% RPMI-1640/50% Matrigel (BD Biosciences, San Jose, CA) were injected subcutaneously into female nude mice (Taconic Labs, Hudson, NY) right flank at ∼2-10×106 cells per animal.
Gefitinib tablets were ground, resuspended in 0.5% methylcellulose/0.5% Tween-80 and administered orally 5×/week (100mg/kg animal body weight). 2C4 was administered intraperitoneally 2×/week at 20mg/kg body weight. Control group received vehicle alone. Treatments typically started 3-6 days after tumor implantation. Tumor volumes were measured twice a week with a digital vernier caliper and calculated as: π/6 × larger diameter × (smaller diameter)2. The data represent three to four experiments of 8-10 mice per group, with mice distributed randomly between control and treated groups.
Statistical analysis
We used a Box-Cox transformation (28) of the tumor volume to account for non-normality of the distributions and statistical significance of the treatment effect was tested by a two-sided t test. P values < 0.05 were considered significant. The analyses were performed using the R software package (http://www.r-project.org).
VeraTag lysate HER assays
VeraTag lysate assays were performed using the VeraTag technology (29). These proximity-based assays were used to measure HER protein complexes utilizing tagged receptor-specific antibodies combined with photo-activated cleavage. The detailed protocol is described in the supplemental information. Monoclonal antibodies were purchased from various sources: EGFR-Ab11, EGFR-Ab5, HER2-Ab5, HER2-Ab4, and HER3-Ab7 (Lab Vision), HER3-1B4C3 (Santa Cruz Biotechnology) and PT100 (Cell Signaling Technology). Optimized antibody concentrations were as follows- EGFR multiplex assay: all antibodies at 0.3μg/mL; HER2 multiplex assay: HER2 Ab5-biotin at 1μg/ml, EGFR Ab11-Pro10 and HER3 Ab7-Pro99 at 0.2μg/ml, HER2 Ab4-Pro14 and PT100-Pro2 at 0.1μg/ml; HER3 multiplex assay: HER3 1B4C3-biotin at 2μg/ml, EGFR Ab11-Pro10 and HER2 Ab4-Pro14 at 0.2μg/ml and HER3 Ab7-Pro99, and PT100-Pro2 at 0.1μg/ml. Results are represented as slopes ± SE expressed as relative peak area (RPA) per mg of input protein. The statistical significance of different slopes was generated by linear regression analysis using GraphPad prism software.
Results
Gefitinib blocks EGFR activation and signaling but cell proliferation is only moderately inhibited
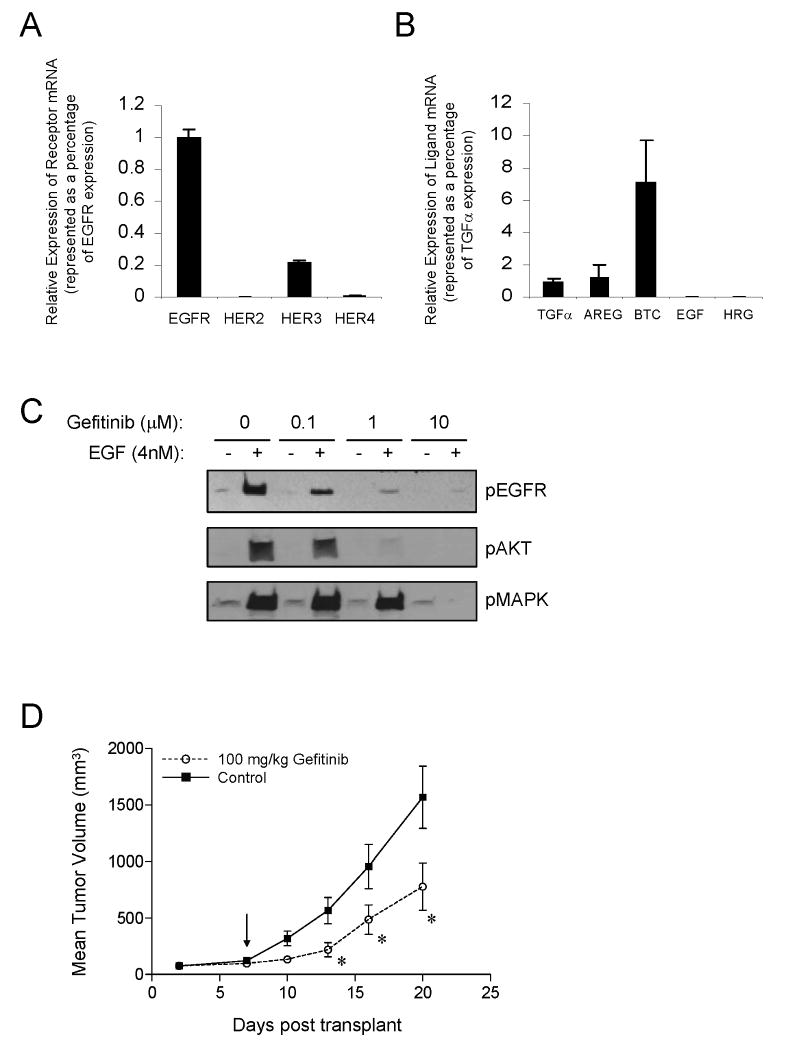
The 22Rv1 cell line expresses a functional EGFR pathway. EGFR is the most highly expressed receptor at the mRNA level while HER3 is expressed approximately 5-fold less and HER2 and HER4 are barely detectable (Fig. 1A). These cells express modest levels of the EGFR-specific ligands, transforming growth factor-alpha (TGFα), amphiregulin (AREG) and betacellulin (BTC). In contrast, EGF and HRGβ1 were not detected (Fig. 1B). The EGFR pathway in 22Rv1 cells is efficiently activated by exogenous EGF, as demonstrated by increased EGFR phosphorylation and activation of both MAPK and AKT, the downstream molecular correlates of cell proliferation and survival (Fig.1C). Moreover, EGF-stimulated EGFR phosphorylation was inhibited with low doses (0.1μM) of the reversible EGFR-TKI, gefitinib. Phosphorylated AKT and MAPK were also inhibited with 1μM and 10μM of gefitinib, respectively. Also, supporting the presence of a functional EGFR signaling pathway, 22Rv1 xenograft tumors subcutaneously grown in immunodeficient nude mice demonstrate ∼50% tumor growth inhibition in the presence of 100mg/kg gefitinib (Fig. 1D). However, it should be noted that the gefitinib-mediated inhibition in tumor growth shown in this system is significantly lower compared to systems that either have gene-amplified EGFR or EGFR mutations, such as in NSCLC. These analyses demonstrate that the 22Rv1 cell line harbors a functional EGFR pathway that is efficiently inhibited with gefitinib, but the effect on growth and survival is minimal. Therefore, the 22Rv1 cell line can serve as a model to study the apparent disconnect between sensitivity to EGFR-TKIs, and the presence of its target, EGFR.
Figure 1.
22Rv1 has a functional EGFR pathway. A, Relative receptor mRNA expression normalized to GAPDH and presented as a percentage of EGFR expression. B, Relative HER ligand mRNA expression normalized to GAPDH and presented as a percentage of TGFα expression. C, Phospho-EGFR, phospho-AKT and phospho-MAPK immunoblots after EGF and gefitinib treatments. D, Gefitinib treated (100mg/kg, 5×/week) 22Rv1 xenograft tumors. Arrow indicates initiation of gefitinib dosing. Data are plotted as mean tumor volumes +/- SEM versus time measured in days. P-values are indicated (* <0.05).
Chronic exposure to gefitinib downregulates EGFR and upregulates HER2
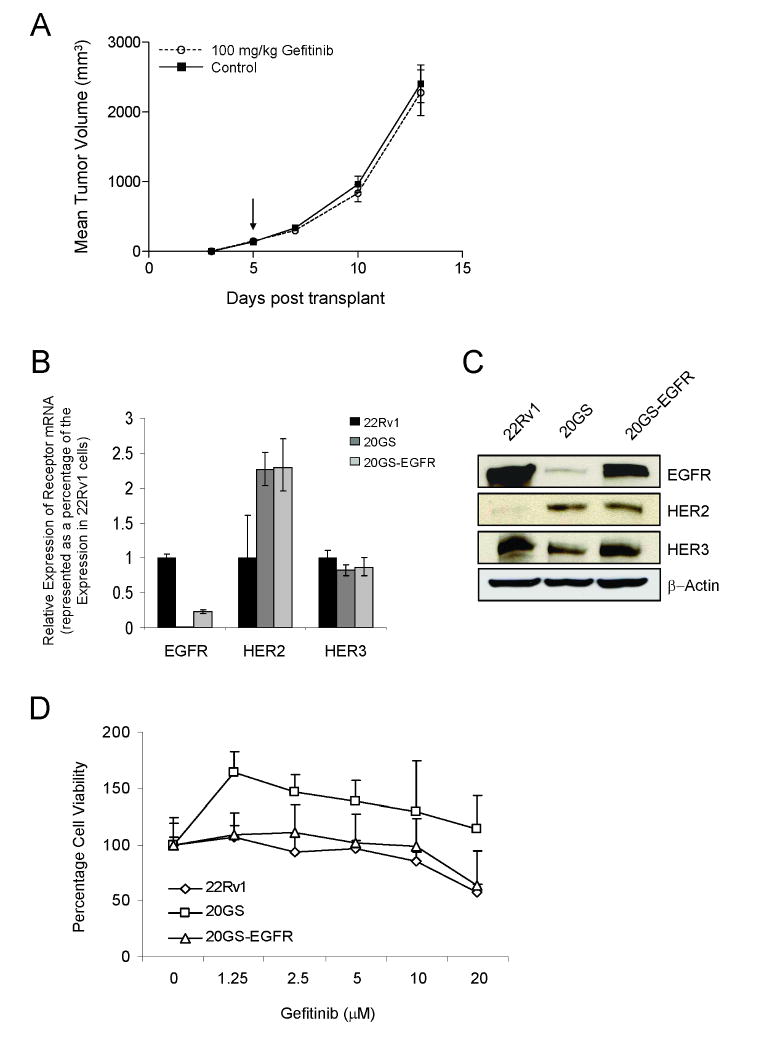
To evaluate the role of EGFR and whether the other HER-kinase axis receptors contribute to the failure of gefitinib to inhibit growth of 22Rv1 cells, cells were treated with gefitinib concentrations reported to kill cells expressing either wild-type or mutant EGFR, with the exception of those that grow as gefitinib-resistant colonies (25). Chronic exposure to 20μM gefitinib rapidly selected for 22Rv1 cell clones (20GS) that no longer exhibited the limited responsiveness to gefitinib, as previously measured in vivo as xenograft tumors (Fig. 2A). The expression pattern of the HER-kinases was altered in the 20GS cell line both at the level of mRNA (Fig. 2B) and protein (Fig. 2C). EGFR mRNA was reduced ∼100-fold, HER2 mRNA was increased ∼2-fold and the HER3 mRNA remained constant. EGFR-specific ligands, EGF and TGFα mRNA levels were increased 10-fold, possibly compensating for reduced EGFR expression (data not shown).
Figure 2.
Characterization of 22Rv1, 20GS and 20GS-EGFR cell lines. A, Gefitinib treated (100mg/kg, 5×/week) 20GS xenograft tumors. Arrow indicates initiation of dosing. B, EGFR, HER2 and HER3 mRNA expression normalized to GAPDH and presented as a percentage of receptor expression in 22Rv1 cells. C, Immunoblot analysis of receptor protein expression in 22Rv1, 20GS and 20GS-EGFR cells with β-actin as the normalization control. D, Cell viability assays in response to increasing doses of gefitinib. Results plotted as percentage of control.
As chronic exposure of cells to gefitinib resulted in reduced EGFR expression (as seen in the 20GS line), recombinant EGFR was stably reintroduced into the 20GS cells generating the 20GS-EGFR cells. The 20GS-EGFR cell line expressed EGFR at ∼25% the level (based both on mRNA and protein expression) of the parental 22Rv1 cells (Fig. 2B and 2C). Reintroduction of EGFR in the 20GS cell line did not alter HER2 and HER3 receptor levels. Protein expression of the HERs in 22Rv1, 20GS and 20GS-EGFR cells was verified using the VeraTag™ assay (Fig. S1). The relative receptor protein expression in the three 22Rv1 cell lines was in concordance with the mRNA measurements (Fig. 2B).
However, modified HER-kinase expression patterns in the cell lines caused only minor modifications in the cellular response to gefitinib (Fig. 2D). As expected, down-regulation of the primary molecular target for gefitinib, EGFR, led to decreased sensitivity to high doses of gefitinib in the 20GS cell line and interestingly conferred a growth advantage at gefitinib concentrations sufficient for inhibition of EGFR signaling. Reintroduction of EGFR in the 20GS cells (20GS-EGFR) was sufficient to restore gefitinib sensitivity that paralleled the parental 22Rv1 cell line (Fig. 2D). However, growth inhibition by gefitinib was minimal for all of the cell lines with only some decrease in growth seen at very high gefitinib doses.
Chronic EGFR kinase inhibition modulates HER ligand-dependent signaling
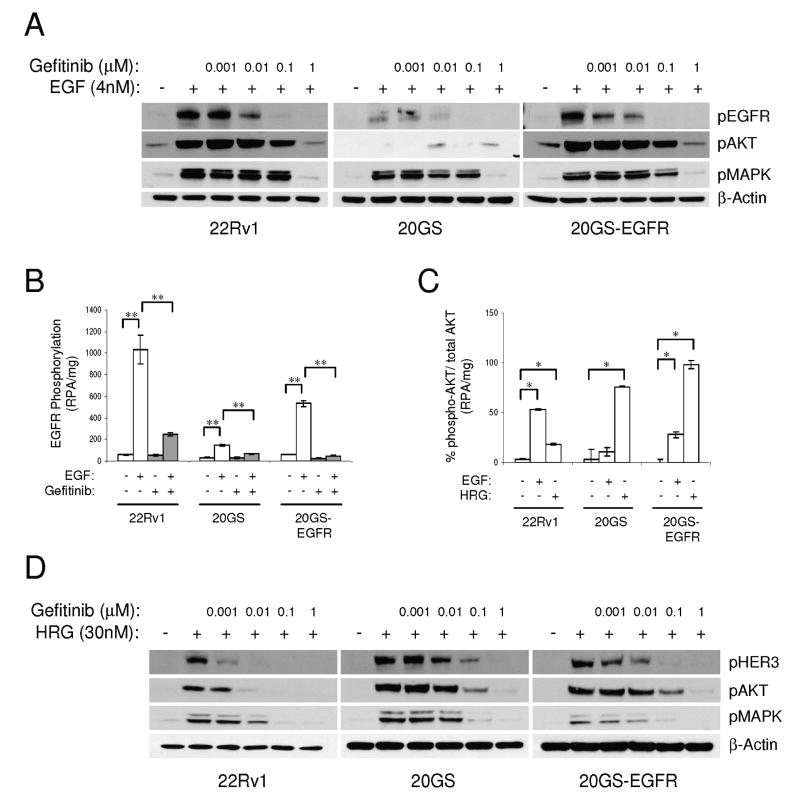
Chronic exposure to gefitinib altered EGFR and HER2 gene expression. As low doses of gefitinib effectively inhibited EGF-dependent signaling in the parental 22Rv1 cells (Fig. 1C), we investigated whether changes in the HER-kinase axis also altered EGFR- and HER2- driven signaling pathways. All cell lines (22Rv1, 20GS and 20GS-EGFR) were growth factor starved and stimulated with either EGF or HRGβ1 in the presence or absence of gefitinib to assess downstream signaling.
The level of EGF-stimulated EGFR phosphorylation, measured by either western blot using phospho site-specific antibodies or by proximity-based pan-phospho antibodies using VeraTag assays, correlated directly with EGFR expression in all cell lines (Fig. 3A and 3B). Furthermore, EGFR phosphorylation was inhibited in a dose-dependent manner that was detected between 10 to 100nM of gefitinib as measured by western blotting (Fig. 3A) or at 100nM using VeraTag assays (Fig. 3B). EGF-dependent AKT activation correlated with both EGFR expression and phosphorylation (Fig. 3A). AKT was activated by EGF in 22Rv1 cells, and was undetectable in 20GS cells, but AKT activation was restored by the reintroduction of recombinant EGFR in the 20GS-EGFR cells. The VeraTag assay corroborated these observations (Fig. 3C). EGF-dependent phospho-AKT signals in both 22Rv1 and 20GS-EGFR cells were inhibited with comparable doses of gefitinib (Fig. 3A). These observations indicate a direct role for EGFR expression in modulating EGF-dependent phospho-AKT signaling that correlates with response to gefitinib.
Figure 3.
Phosphorylation responses to ligand stimulation and EGFR-TKI treatment in the 22Rv1 and derivative cell lines. A, Immunoblot analysis of phospho-EGFR, phospho-AKT and phospho-MAPK in response to gefitinib doses prior to stimulation with 4nM EGF. B, EGFR phosphorylation (RPA/mg) in response to 4nM EGF in the absence or presence of 100nM gefitinib using the VeraTag assays. C, VeraTag (RPA/mg) AKT phosphorylation relative to total AKT measurement after stimulation with ligands, EGF or HRGβ1. Significant differences are indicated (* p<0.05, ** p<0.0001). D, Immunoblot analysis of phospho-HER3, phospho-AKT and phospho-MAPK in response to gefitinib doses prior to HRGβ1 stimulation.
In contrast, HRGβ1-stimulated phospho-AKT was detectable in all cell lines consistent with similar HER3 expression (Fig. 3C and 3D). However, HRGβ1-stimulated phospho-AKT was 4-5 times greater in 20GS and 20GS-EGFR relative to the 22Rv1 (Fig. 3C and 3D). This directly paralleled HRGβ1-mediated HER3 phosphorylation (Fig. 3D). Furthermore, a log-fold greater gefitinib concentration of 100nM was necessary to partially inhibit HRGβ1-stimulated HER3 phosphorylation and phospho-AKT in the 20GS and 20GS-EGFR cells compared to the 22Rv1 cell line. These differences could be due to higher HER2 expression in the 20GS and 20GS-EGFR cells, gefitinib cross-reactivity with HER2 (30, 31) or possibly increased HER2 sensitivity to gefitinib in HER2 over-expressing cells (32). An analysis of the EGFR, HER2 and HER3 phosphorylation patterns in the presence of HRGβ1 stimulation combined with gefitinib inhibition revealed that HER2 phosphorylation was indeed inhibited with 100nM gefitinib (Fig. S2). Furthermore gene-specific knockdown of HER2, but not EGFR, in 20GS cells downregulated HRGβ1-mediated HER3, AKT and MAPK phosphorylation signals demonstrating a role for HER2 in gefitinib-selected cells (Fig. S3).
EGFR and HER2 expression levels influence both HER-kinase receptor phosphorylation and dimerization
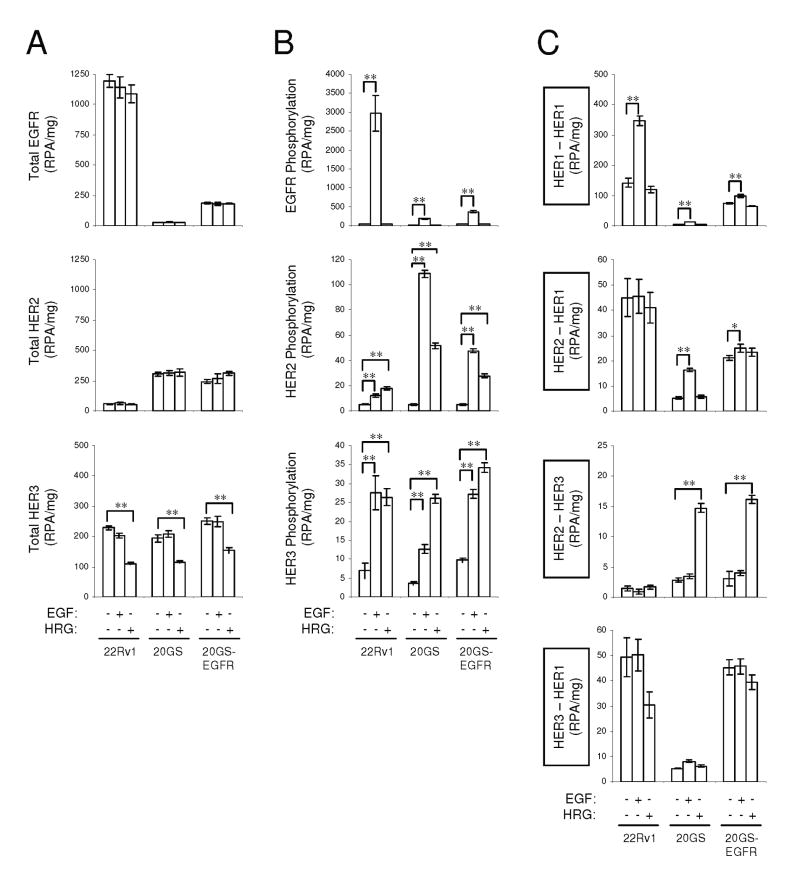
As distinctive receptor profiles were identified in response to chronic gefitinib exposure, we evaluated which receptors were responsible for the altered downstream signaling observed in the 22Rv1, 20GS and 20GS-EGFR cell lines. We compared the relative phosphorylation of EGFR, HER2 and HER3 in response to specific ligand stimulations in all cells using VeraTag assays. EGF stimulation of 22Rv1, 20GS and 20GS-EGFR cells did not alter total protein levels of EGFR, HER2, or HER3. However, in response to HRG, EGFR and HER2 levels were unaltered but there was a small, but reproducible decrease in HER3 protein (Fig. 4A).
Figure 4.
VeraTag assay measurements of total receptor expression (A), receptor phosphorylation (B) and receptor dimerization (C) in response to EGF or HRGβ1 stimulation. Statistically significant differences are indicated (* p<0.05, ** p<0.0001).
EGFR was phosphorylated in all cell lines at levels that paralleled receptor expression (Fig. 4B). Phosphorylation of EGFR occurred exclusively in response to EGF. In contrast, while HER2 phosphorylation tracked with receptor expression, both EGF and HRGβ1 stimulated HER2 phosphorylation. HER3 phosphorylation appeared to follow unique regulation as HRGβ1-dependent HER3 phosphorylation paralleled HER2 expression while EGF-dependent HER3 phosphorylation paralleled EGFR expression. VeraTag assays were used to assess which ligand-stimulated receptor dimers correlated with the observed receptor phosphorylation. In parental 22Rv1 cells, which primarily express EGFR, EGFR-EGFR homodimers were observed exclusively in response to EGF stimulation (Fig. 4C) in agreement with the observation that EGFR phosphorylation was detected only in response to EGF. Although low levels of EGF-stimulated phospho-HER2 and phospho-HER3 were observed, we did not detect ligand-stimulated EGFR-HER2 or EGFR-HER3 heterodimers, but rather there appeared to be baseline ligand-independent heterodimerization in the high EGFR expressing lines. Heregulin stimulation did not induce any detectable receptor dimer partners in the 22Rv1 cells.
Similar analysis on the 20GS cells, showed ∼2-fold increase in EGFR homodimers in response to EGF stimulation. The fold-change value was comparable to homodimer measurements in 22Rv1 cells. However, overall there were fewer homodimers in the 20GS cells relative to the 22Rv1 cells, corresponding to EGFR expression and phosphorylation. Unlike the direct correlation of the EGFR homodimers with EGFR expression, the approximately 3-fold increase in EGF-dependent HER2-EGFR heterodimerization does not correlate with EGFR expression but rather appears to correlate with both EGFR and HER2 phosphorylation. There was very little EGF-stimulated HER3 phosphorylation in 20GS cells and consequently only minor levels of EGF-stimulated phospho-AKT detected. However, HRGβ1 stimulated high levels of HER2-HER3 heterodimers (Fig. 4C) correlating with the observed HRGβ1-dependent HER2 and HER3 phosphorylation.
Lastly, the 20GS-EGFR cells expressed both EGF-stimulated and HRGβ1-stimulated HER dimers in concordance with the observed phosphorylation patterns. These cells displayed EGF-mediated EGFR homodimerization, increased basal ligand-independent EGFR dimerization and, HRGβ1-mediated HER2-HER3 heterodimerization comparable to 20GS cells. Overall, EGFR homodimers are the major HER dimer species of the HER-kinase signaling network in the parental 22Rv1 cells while HER2 heterodimers predominate upon EGFR down regulation in 20GS cells. 20GS-EGFR cells that express both EGFR and HER2, display both EGFR homodimers and HER2 heterodimers upon ligand stimulation.
Receptor dimers determine the dominant HER signaling pathway and subsequent response to HER-targeted therapeutics
In response to chronic gefitinib treatment, EGFR expression is reduced while HER2 expression is increased. This altered receptor profile is less responsive to EGFR-targeted therapies. Therefore, we evaluated whether these cells would be susceptible to HER2-targeting therapies. 22Rv1, 20GS and 20GS-EGFR cells were treated with either EGFR-directed therapeutics including gefitinib or cetuximab or a HER2-targeted therapeutic, pertuzumab (2C4).
22Rv1 cells were serum-starved and stimulated with either EGF or HRGβ1. EGF stimulated EGFR phosphorylation that, in turn, activated AKT and MAPK. Cetuximab and gefitinib, but not pertuzumab, inhibited EGF-stimulated EGFR and AKT phosphorylation (cetuximab was more effective than gefitinib). EGF-stimulated MAPK was not inhibited by any of the HER-targeted therapeutics (Fig. 5A). Alternatively, HRGβ1 stimulated HER3 phosphorylation but caused only a slight increase in the basal EGFR phosphorylation. The HRGβ1-dependent activation of EGFR, HER3, AKT and MAPK, was inhibited by the EGFR inhibitors but not by the HER2 dimerization inhibitor, pertuzumab indicating dependence on EGFR.
Figure 5.
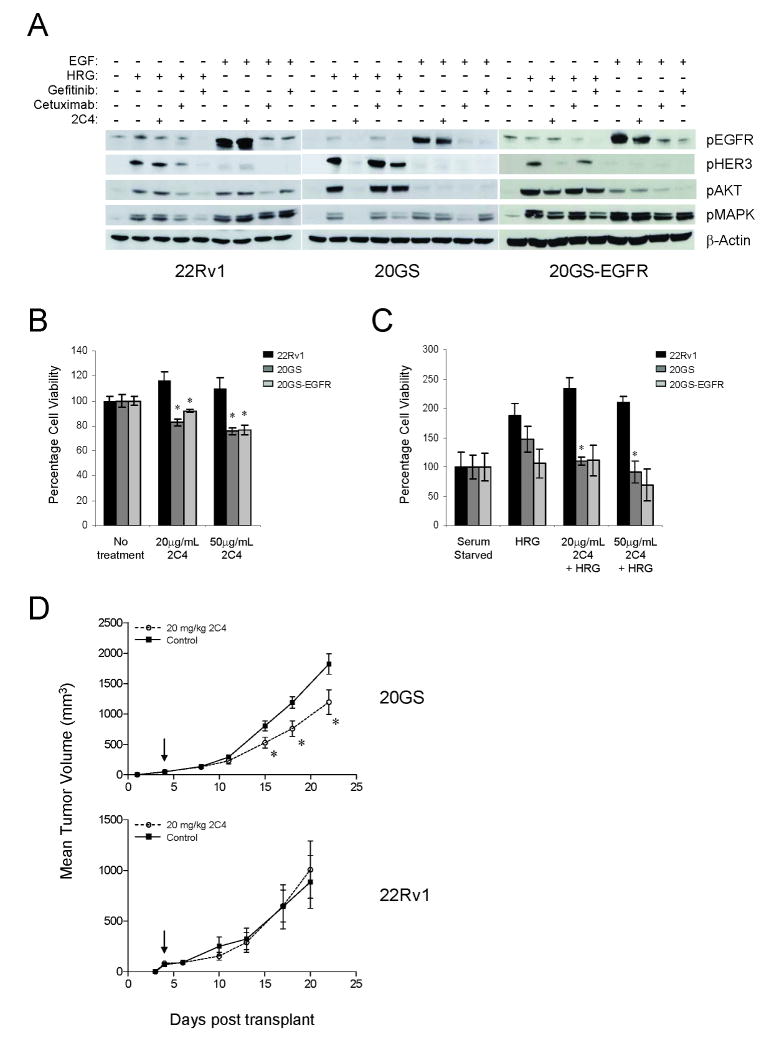
22Rv1, 20GS, and 20GS-EGFR have distinct responses to alternate HER-kinase targeted therapies. A, Immunoblots for phospho-EGFR, phospho-HER3, phospho-AKT, and phospho-MAPK. Serum starved cells were treated as indicated (100nM gefitinib, 2.5μg/mL cetuximab, or 15μg/mL pertuzumab (2C4)) prior to 4nM EGF or 30nM HRGβ1 stimulation. Cell viability analysis in the presence (B) or absence (C) of 10nM HRGβ1 and pertuzumab (2C4) for 5 days. Data are presented as a percentage of cellular viability under serum starved conditions. Significant differences (* p<0.05) are indicated. D, Subcutaneous 20GS (top panel) or 22Rv1 (bottom panel) xenograft tumors treated 2×/week with 20mg/kg of pertuzumab (2C4) antibody. Mean tumor volumes +/- SEM are plotted versus days post transplant. Arrow indicates initiation of dosing. Significant differences with drug treatment are indicated (* p<0.05).
In contrast, 20GS cells in which there is a preponderance of HRGβ1-stimulated HER2 heterodimers, only pertuzumab effectively blocked dimerization as evaluated by inhibition of HRGβ1-induced phospho-HER3, phospho-AKT and phospho-MAPK measurements (Fig. 5A). However, in these cells even though EGF-stimulated EGFR phosphorylation was sensitive to gefitinib and cetuximab, neither HER3 nor AKT were activated in response to EGF. Reintroduction of EGFR in 20GS-EGFR blocked pertuzumab sensitivity as assessed by HRGβ1-stimulated phospho-AKT and phospho-MAPK correlates, but restored gefitinib sensitivity.
Based on the altered inhibition of receptor phosphorylation and downstream molecular correlates by HER targeting agents, cell viability assays were performed to evaluate cell growth sensitivity to pertuzumab. In cycling cells, the effects of 2C4 treatment although significant, were minimal in 20GS and 20GS-EGFR cells (Fig. 5B). Consequently, cell viability assays were performed in the presence of HRGβ1. Although HRGβ1 was able to induce cell growth in both 22Rv1 and 20GS lines, pertuzumab inhibited HRGβ1-induced cell proliferation in a dose dependent manner only in the 20GS cells (25% decrease at 20μg/ml 2C4 and 37% decrease at 50μg/ml 2C4) indicating an enhanced dependence on HER2 heterodimers (Fig. 5C). 20GS-EGFR cell viability was only reduced by 35% at high concentrations of 2C4, 50μg/ml. Furthermore, supporting the dependence on HER2 heterodimers, 20GS tumor growth was inhibited by pertuzumab when grown subcutaneously in immunodeficient mice (Fig. 5D, top panel) while parental 22Rv1 tumors growth was not (Fig. 5D, lower panel).
Discussion
Although there are a number of HER-targeted therapies in clinical development or FDA approved for cancer treatment; some tumors, despite the presence of the HER target are either, not inhibited or, demonstrate a short-term response (23, 33, 34). The current definition of drug responsiveness is based on inhibition of cellular proliferation although HER-targeting inhibitors can cause alterations in the HER axis (24) even in the absence of this critical phenotype. Our data demonstrate that cells that are defined as ‘resistant’ to EGFR-targeted therapeutics undergo similar shifts in receptor profiles as the ‘responsive’ cells. Potentially, these altered receptor profiles, can be used to guide therapy and may be inhibited by alternative HER-targeting therapies.
A functional receptor pathway such as EGFR is often not sufficient to predict sensitivity (35) to tumor growth inhibition by EGFR-targeted therapeutics. However, despite the absence of measurable growth inhibition which typifies ‘primary therapeutic resistance’; the dynamic network of the HER family receptors and ligands (reviewed in (36)) still undergoes transient and long-term adaptation in response to targeted inhibition. This re-programming of the HER axis may not only activate alternate receptor/signaling pathways that contribute to resistance, but may also reveal escape routes from targeted inhibition. Our results demonstrate that in 22Rv1 cells which contain functional EGFR activity, cell proliferation was not dramatically effected by prolonged gefitinib exposure, rather the HER axis was reorganized and EGFR was down-regulated while HER2 was up-regulated. This possible gefitinib escape route through HER2 could allow for the formation of HER2-HER3 heterodimers that could be targeted by pertuzumab. Interestingly, a subtle but reproducible growth advantage was observed in the 20GS cells relative to both the parental 22RV1 or the add-back 20GS-EGFR cells in the presence of gefitinib. The parallel growth pattern of both the EGFR–expressing cell lines as compared to the EGFR-negative, 20GS cell line, supports the dominant role of EGFR for growth. Although growth was only minimally affected in 22Rv1 cells, gefitinib effectively blocked the EGFR pathway based on decreased EGFR phosphorylation, and inhibition of downstream signals including EGF-dependent AKT phosphorylation. However, inhibition of both EGFR and AKT phosphorylation by gefitinib did not accurately reflect gefitinib sensitivity based on cellular proliferation but perhaps may have been an indicator for potential HER axis switching.
Despite the altered HER axis profiles, the ability to activate AKT was sustained in all cell lines consistent with its central role in both tumorigenesis (reviewed in (37)) and resistance (18, 19, 38). However, the mechanism of activation was influenced by altered HER family expression as was susceptibility to alternative targeted inhibitors. HRGβ1-dependent AKT activation was poorly inhibited by gefitinib in cells with higher levels of HER2 (20GS and 20GS EGFR) whereas AKT was potently inhibited in cells with low HER2 expression (22Rv1). At high HER2 expression, the predominant mechanism of HRGβ1-dependent AKT activation is likely to occur in response to HER2-HER3 heterodimerization that is not targeted by gefitinib, whereas at low HER2 expression the HRGβ1-dependent AKT activation could be a consequence of HRG-activated HER3 that comes into contact with over-expressed EGFR, the target of gefitinib. It appears that AKT activation and inhibition may be a downstream marker of receptor switching and consequential susceptibility to alternate HER-targeted therapeutics.
The roles of both EGFR and HER3 in EGFR-TKI susceptibility have been well studied. Co-expression of EGFR and HER3 has been shown previously to correlate with erlotinib sensitivity in both pancreatic and colorectal cell lines (39). Furthermore, inhibition of EGF-stimulated, AKT phosphorylation in EGFR-TKI sensitive systems suggests that signaling occurs between EGFR and HER3 (40). However, the mechanism by which EGF stimulates HER3 phosphorylation and consequently AKT activation is unclear. In our study, EGF-stimulated AKT activation and subsequent inhibition by EGFR-TKI was only observed in the gefitinib-sensitive cell lines (22Rv1 and 20GS-EGFR). EGFR and HER3 were measured in close proximity by VeraTag assays in these cells. However there was no evidence for ligand-dependent EGFR-HER3 heterodimerization. It is possible that a fraction of the detected EGFR-HER3 heterodimers is ligand-activated, as suggested by the complementary receptor phosphorylation measurements, but that these heterodimers are transient. Alternatively, it is possible that the receptors flux between open and closed conformations and in the absence of ligand, particularly at higher receptor expression, both ligand-dependent as well as ligand-independent heterodimers are detected but are indistinguishable in the assay. In cells with high EGFR expression basal EGFR and HER3 phosphorylation was detected that correlated with EGFR-HER3 close proximity dimers. However, in these cell lines, there was no detectable growth-advantage in the presence of gefitinib.
In summary, our study provides insight into EGFR-TKI mediated HER-kinase axis modulations in systems that are supposedly ‘resistant’ to EGFR-kinase inhibition. Our data indicate that receptor-switching mechanisms are likely to occur in tumors both as a resistance mechanism for EGFR-TKI ‘responsive’ cells and as a priming mechanism in receptor-expressing but not growth-inhibited cells (EGFR-TKI ‘resistant’ cells). The heterogeneous nature of tumors coupled with the plasticity of the HER axis make receptor-use switching and altered response to targeted therapies very probable. Multi-node strategies targeting multiple HER family members or the signaling pathways common for the HER axis may lead to more effective treatment.
Supplementary Material
Acknowledgments
We thank James Mirocha, Jeff Sperinde and Agnes Paquet for statistical analysis. John Curran, Jack Altura and Rob Fannon are acknowledged for technical assistance. Gifts of gefitinib and pertuzumab are gratefully acknowledged from AstraZeneca and Genentech, respectively.
Grant support: Jerry and Joyce Monkarsh Young Investigator Award (Prostate Cancer Foundation; A. J.), the National Institutes of Health 1-R21 NS059381-01 (A. J.) and Sumner Redstone Prostate Cancer Research Program (D. B. A. and A. J.).
References
- 1.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37 4:S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 2.Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–7. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 3.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:6899–903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulloy R, Ferrand A, Kim Y, et al. Epidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinib. Cancer research. 2007;67:2325–30. doi: 10.1158/0008-5472.CAN-06-4293. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science (New York, NY. 2002;297:63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. The New England journal of medicine. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. The New England journal of medicine. 2008;358:1409–11. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 8.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 9.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 10.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (New York, NY. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 11.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science (New York, NY. 2004;305:1163–7. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 13.Yonesaka K, Zejnullahu K, Lindeman N, et al. Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin Cancer Res. 2008;14:6963–73. doi: 10.1158/1078-0432.CCR-08-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamoune A, Kassis J, Kharait S, et al. DU145 human prostate carcinoma invasiveness is modulated by urokinase receptor (uPAR) downstream of epidermal growth factor receptor (EGFR) signaling. Experimental cell research. 2004;299:91–100. doi: 10.1016/j.yexcr.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Velling T, Stefansson A, Johansson S. EGFR and beta1 integrins utilize different signaling pathways to activate Akt. Experimental cell research. 2008;314:309–16. doi: 10.1016/j.yexcr.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Sharma SV, Settleman J. Oncogenic shock: turning an activated kinase against the tumor cell. Cell cycle (Georgetown, Tex. 2006;5:2878–80. doi: 10.4161/cc.5.24.3598. [DOI] [PubMed] [Google Scholar]
- 17.Arteaga CL. HER3 and mutant EGFR meet MET. Nature medicine. 2007;13:675–7. doi: 10.1038/nm0607-675. [DOI] [PubMed] [Google Scholar]
- 18.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (New York, NY. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 19.Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. The Journal of clinical investigation. 2008;118:2609–19. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sergina NV, Rausch M, Wang D, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–41. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou BB, Peyton M, He B, et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gow CH, Shih JY, Chang YL, Yu CJ. Acquired gefitinib-resistant mutation of EGFR in a chemonaive lung adenocarcinoma harboring gefitinib-sensitive mutation L858R. PLoS medicine. 2005;2:e269. doi: 10.1371/journal.pmed.0020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 24.Narayan M, Wilken JA, Harris LN, Baron AT, Kimbler KD, Maihle NJ. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer research. 2009;69:2191–4. doi: 10.1158/0008-5472.CAN-08-1056. [DOI] [PubMed] [Google Scholar]
- 25.Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7665–70. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter RE, Sorkin A. Endocytosis of functional epidermal growth factor receptor-green fluorescent protein chimera. The Journal of biological chemistry. 1998;273:35000–7. doi: 10.1074/jbc.273.52.35000. [DOI] [PubMed] [Google Scholar]
- 27.Mumenthaler SM, Ng PY, Hodge A, et al. Pharmacologic inhibition of Pim kinases alters prostate cancer cell growth and resensitizes chemoresistant cells to taxanes. Molecular cancer therapeutics. 2009;8:2882–93. doi: 10.1158/1535-7163.MCT-09-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Box G, Cox D. An analysis of transformations. J Roy Stat Soc B Met. 1964;26:211–46. [Google Scholar]
- 29.Shi Y, Huang W, Tan Y, et al. A novel proximity assay for the detection of proteins and protein complexes: quantitation of HER1 and HER2 total protein expression and homodimerization in formalin-fixed, paraffin-embedded cell lines and breast cancer tissue. Diagn Mol Pathol. 2009;18:11–21. doi: 10.1097/PDM.0b013e31818cbdb2. [DOI] [PubMed] [Google Scholar]
- 30.Moasser MM, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer research. 2001;61:7184–8. [PubMed] [Google Scholar]
- 31.Schaefer G, Shao L, Totpal K, Akita RW. Erlotinib directly inhibits HER2 kinase activation and downstream signaling events in intact cells lacking epidermal growth factor receptor expression. Cancer research. 2007;67:1228–38. doi: 10.1158/0008-5472.CAN-06-3493. [DOI] [PubMed] [Google Scholar]
- 32.Hirata A, Hosoi F, Miyagawa M, et al. HER2 overexpression increases sensitivity to gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, through inhibition of HER2/HER3 heterodimer formation in lung cancer cells. Cancer research. 2005;65:4253–60. doi: 10.1158/0008-5472.CAN-04-2748. [DOI] [PubMed] [Google Scholar]
- 33.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS medicine. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS medicine. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop PC, Myers T, Robey R, et al. Differential sensitivity of cancer cells to inhibitors of the epidermal growth factor receptor family. Oncogene. 2002;21:119–27. doi: 10.1038/sj.onc.1205028. [DOI] [PubMed] [Google Scholar]
- 36.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nature reviews. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 37.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Advances in cancer research. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 38.Wheeler DL, Huang S, Kruser TJ, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–56. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buck E, Eyzaguirre A, Haley JD, Gibson NW, Cagnoni P, Iwata KK. Inactivation of Akt by the epidermal growth factor receptor inhibitor erlotinib is mediated by HER-3 in pancreatic and colorectal tumor cell lines and contributes to erlotinib sensitivity. Molecular cancer therapeutics. 2006;5:2051–9. doi: 10.1158/1535-7163.MCT-06-0007. [DOI] [PubMed] [Google Scholar]
- 40.Engelman JA, Janne PA, Mermel C, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3788–93. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.