Abstract
Transplantation of neural cells is a potential approach for stroke treatment, but disruption of tissue architecture may limit transplant efficacy. One strategy for enhancing the ability of transplants to restore brain structure and function is to administer cells together with biomaterial scaffolding. We electrocoagulated the distal middle cerebral artery in adult rats and, 3 weeks later, injected one of the following into the infarct cavity: artificial cerebrospinal fluid, Matrigel scaffolding, human embryonic stem cell-derived neuronal precursor cells, scaffolding plus cells, or cells cultured in and administered together with scaffolding. Five weeks after transplantation, the latter two groups showed ∼50% and ∼60% reductions, respectively, in infarct cavity volume. Rats given cells cultured in and administered together with scaffolding also showed (1) survival and neuronal differentiation of transplanted cells shown by immunostaining for neuronal marker proteins and cleaved caspase-3, and by patch-clamp recording, 8 weeks after transplantation and (2) improved outcome on tests of sensorimotor and cognitive functions, 4 to 9 weeks after transplantation. These results indicate that transplantation of human neural cells together with biomaterial scaffolding has the potential to improve the outcome from stroke, even when treatment is delayed for several weeks after the ischemic event.
Keywords: ischemia, neurogenesis, neuronal progenitor cell, stem cells, stroke, transplantation
Introduction
Stroke is a common and often incapacitating disorder without satisfactory treatment, for which cell replacement might provide effective therapy. At least two approaches are possible. Endogenous neurogenesis generates new brain neurons throughout adult life. Their production is increased after cerebral ischemia and is associated with migration to ischemic brain regions (Gu et al, 2000; Jin et al, 2001, 2003; Zhang et al, 2001, 2004; Arvidsson et al, 2002; Parent et al, 2002). Moreover, blocking endogenous neurogenesis impairs recovery from ischemia (Raber et al, 2004), consistent with a role in brain repair (Magavi et al, 2000). A second approach involves cell transplantation from exogenous sources, the advantages of which include the ability to expand and differentiate cells in vitro, engineer cells, and administer cells to the sites of injury (Lindvall et al, 2004). A critical step in evaluating the feasibility of this approach is to understand the interaction between human neural cells and the ischemic brain.
Transplanting neuronal precursor cells (NPCs) can improve the outcome from focal cerebral ischemia in rodents. Neuronally differentiated mouse embryonic stem cells (ESCs) transplanted into the ischemic adult rat brain can proliferate, differentiate, and improve recovery (Wei et al, 2005). Transplanting NPCs from E14 rat hippocampus into the ischemic rat striatum also improves neurologic function after middle cerebral artery occlusion (MCAO) (Zhu et al, 2005). Monkey ESC-derived NPCs transplanted into the ischemic mouse striatum express neuronal markers and project to the thalamus and substantia nigra (Hayashi et al, 2006).
Human fetal NPCs have also been transplanted into the adult rodent brain after stroke. In one study (Kelly et al, 2004), NPCs injected into the perilesional cortex of rats expressed βIII-tubulin and migrated toward the ischemic lesion. In another study (Ishibashi et al, 2004), human NPCs injected into the gerbil caudate after ischemia stained for NeuN (neuronal nuclei), reduced infarct volume and improved behavioral outcome.
As stroke disrupts tissue architecture, biodegradable scaffolds may enhance the ability of transplanted NPCs to restore brain structure and function (Steindler, 2002). Possible mechanisms include imposing histologic structure, promoting donor–recipient cell interactions and graft vascularization, offering a haven from cytotoxic or growth-inhibitory factors, helping to direct cell fate, and providing neuroprotective or neurotrophic molecules (Ourednik et al, 2002; Park et al, 2002; Steindler, 2002; Silva et al, 2004; Ford et al, 2006; Goetz et al, 2006).
Biodegradable scaffolds have been used for reconstruction after trauma to the optic tract (Ellis-Behnke et al, 2006), brain (Hou et al, 2005; Deguchi et al, 2006), or spinal cord (Teng et al, 2002), but less attention has been given to their role in brain ischemia. Park et al (2002) seeded clonal murine NPCs onto polyglycolic acid, which was transplanted into the infarct cavity of neonatal mice. These cells expressed neuronal and glial markers, formed projections, and acquired a vascular supply.
To help elucidate the potential of cell replacement therapy in stroke, we transplanted human NPCs into the infarct cavity after MCAO in rats. We report that transplantation of human ESC-derived NPCs with Matrigel scaffolding is associated with improved histologic and behavioral outcome.
Materials and methods
Cell Culture
Neural stem/progenitor cells (NPCs) derived from the human ESC line BG01 were obtained from Aruna Biomedical (Athens, GA, USA). Cells were seeded on polyornithine- and laminin-coated dishes and cultured in proliferation medium, consisting of Neurobasal medium with B27 supplementation containing 2 mmol/L -glutamine, 50 μg/mL Pen/Strep (Invitrogen, Carlsbad, CA, USA), 10 ng/mL leukemia inhibitory factor, and 20 ng/mL fibroblast growth factor-2 (R&D Systems, Minneapolis, MN, USA) (Shin et al, 2006). Cells were propagated in the proliferation medium and, on reaching 90% to 100% confluence, were triturated to detach them from the dish. After centrifugation at 200 × g for 4 mins, cells were resuspended in a fresh medium and replated. Some NPCs were cultured on uncoated dishes for 1 week with Matrigel (BD Biosciences, San Diego, CA, USA; 1:1 in culture medium) before transplantation. Other cells were transfected with pEGFP-N1 vector under the control of the cytomegalovirus (CMV) promoter using a Nucleofection V kit and Nucleofector II Device (program B-16; Amaxa Biosystems, Gaithersburg, MD, USA).
Focal Cerebral Ischemia
Animal procedures were conducted in accordance with the National Institutes of Health Guidelines and with the approval of the Institutional Animal Care and Use Committee. Male Sprague–Dawley rats weighing 280 to 310 g were anesthetized with 4% isoflurane in 70% N2O/30% O2 using a mask. Permanent distal MCAO was performed as described previously (Nawashiro et al, 1997; Won et al, 2006). A 2-cm incision was made between the right orbit and tragus under a surgical microscope. The temporal muscle was retracted laterally and a 3-mm diameter craniotomy was made just rostral to the foramen ovale. The dura was incised with a 26-gauge needle and the MCA was exposed. The MCA was occluded by electrocoagulation without damaging the brain surface. Interruption of blood flow was confirmed visually under a microscope. The temporal muscle was repositioned and the skin was closed. The rectal temperature was measured and maintained at 37±0.2°C with a heating blanket. In sham-operated controls, the MCA was visualized but not occluded. Functional deficits associated with MCAO were confirmed by motor asymmetry at 3 h, and the infarct size was measured as described below.
Cell Transplantation
Cells were transplanted 3 weeks after the induction of focal ischemia. Rats (4 to 12 per group) were reanesthetized with 4% isoflurane in 70% N2O/30% O2 and placed in stereotaxic frames with a rat head holder. Burr holes were drilled using a dental drill, which was irrigated continuously with 0.9% saline at room temperature to prevent overheating of the underlying cortex. Experimental groups received one of the following: (1) artificial cerebrospinal fluid (aCSF) vehicle, (2) Matrigel scaffold (2 × 2 × 2 mm3), (3) NPCs (1.2 × 105 cells per μL) in aCSF, (4) NPCs plus Matrigel scaffold, or (5) NPCs cultured in and administered together with Matrigel scaffold (NPC/Matrigel cultures). For groups (1 to 4), these were injected using a Hamilton syringe into the center of the cortical infarct cavity (−0.3 mm posterior to the bregma, 3 mm lateral to midline 1.8 mm below the dura) in a volume of 50 μL over a period of 5 mins. In each case, the needle was left in place after injection for 5 mins before being slowly withdrawn. For group (5), a portion of the skull overlying the infarct cavity was lifted using a surgical blade and NPC/Matrigel cultures were inserted into the cavity using a 0.3-mm glass micropipette. After the injections were completed, bone wounds were closed with bone wax, anesthesia was discontinued, and animals were returned to their cages. Rats were perfused with 0.9% saline and 4% paraformaldehyde in phosphate-buffered saline (pH 7.5) 5 to 8 weeks after transplantation.
Measurement of Infarct Cavity Volume
For infarct volume measurement, rats were killed 5 weeks after transplantation. The brains were removed and 40-μm-thick coronal brain sections were stained with hematoxylin and eosin. The areas of the infarct cavity, left hemisphere, and total brain were measured by a blinded observer using the NIH Image program (National Institutes of Health, Bethesda, MD, USA), and areas were multiplied by the distance between sections to obtain the respective volumes. The volume of the infarct cavity was calculated as a percentage of the volume of the contralateral hemisphere as described previously (Swanson et al, 1990).
Neurobehavioral Evaluation
For functional assessment, a comprehensive analysis of long-term effects of NPC transplantation after focal ischemia in rats was carried out 4 to 9 weeks after transplantation. The experimenters conducting behavioral testing and neurobehavioral scoring were blind to the experimental conditions. Each experimental group comprised 10 to 11 rats.
Cylinder test
The cylinder test (Schallert et al, 1986) was used as a measure of forelimb use asymmetry. When placed in a transparent Plexiglas cylinder, the animal rears and explores the cylinder walls using its forepaws, allowing three categories of placements to be recorded, namely independent ipsilateral limb use, independent contralateral limb use, and use of both paws in unison or in quick succession. Animals were tested 4 to 9 weeks after transplantation. Forelimb preference was expressed as the percentage of the total number of placements made.
Adhesive-removal test
The adhesive-removal (sticky-tape) test, a measure of somatosensory dysfunction after cerebral ischemia in rats (Sughrue et al, 2006), was conducted 4 weeks after transplantation. Adhesive-backed paper dots (70.6 mm2) were used as tactile stimuli to the distal–radial region of the wrist, and the mean time to remove each dot was recorded.
Y maze
Y-maze testing was conducted 8 weeks after transplantation. Normal rats tend to visit the arms of a Y maze one after the other. This behavior (known as spontaneous alternation) was used to evaluate the working memory of rats placed in a new environment (Lalonde, 2002). The SDI Y Maze (San Diego Instruments, San Diego, CA, USA) comprised three black plastic arms (16 inches × 12 inches × 12 inches). The rat was placed in arm 1, offering three possibilities, namely staying in arm 1, moving into arm 2, or moving into arm 3. An alternation was scored if the rat visited a new arm rather than returning to a previously visited arm. The ratio of correct alternations to the total number of visits during an 8-min observation period was calculated to yield the frequency of alternation. A frequency of >50% indicates normal spontaneous alternation.
Immunohistochemistry
Rat brain sections were embedded in paraffin and 6-μm-thick coronal and sagittal sections were prepared. Immunohistochemistry was performed as described previously (Jin et al, 2002, 2003). Primary antibodies were rabbit polyclonal anti-Sox2 (Abcam, Cambridge, MA, USA, 1:200), guinea pig polyclonal anti-doublecortin (DCX; Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:500), mouse monoclonal anti-microtubule-associated protein-2 (MAP-2; Sigma-Aldrich, St Louis, MO, USA, 1:100), rabbit polyclonal anti-glial fibrillary acidic protein (GFAP; Sigma-Aldrich, 1:400), rabbit polyclonal anti-calbindin (Upstate, Billerica, MA, USA, 1:400), and mouse monoclonal anti-human nuclei (HuNu; Chemicon, Billerica, MA, USA, 1:250). The secondary antibodies were Alexa Fluor 488-, 594-, or 647-conjugated donkey anti-mouse, anti-rabbit, or anti-goat IgG (Molecular Probes, Carlsbad, CA, USA, 1:200 to 500). Fluorescence signals were detected using an LSM 510 NLO Confocal Scanning System mounted on an Axiovert 200 inverted microscope (Carl Zeiss, Thornwood, NY, USA) equipped with a two-photon Chameleon laser (Coherent, Santa Clara, CA, USA). Images were acquired using LSM 510 Imaging Software (Carl Zeiss). Cells were counted in at least three 400 × fields per rat, and three rats per condition, using an eyepiece grid covering 0.0625 mm3. Controls included omitting or preabsorbing the primary antibody or omitting the secondary antibody.
Preparation of Acute Brain Slices
Rats were anesthetized with chloral hydrate and decapitated 8 weeks after transplantation. The brains were removed and coronal slices (of 300-μm thickness) were prepared with a Vibratome, in ice-cold 75 mol/L sucrose slicing aCSF containing (in mol/L): 85 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose, 0.5 CaCl2, and 4 MgCl2, equilibrated with 95% O2 and 5% CO2. Slices were incubated at 28°C for 30 mins, and the sucrose slicing solution was replaced with standard aCSF containing (in mol/L): 125 NaCl, 2.4 KCl, 1.2 NaH2PO4, 25 NaHCO3, 25 glucose, 1 CaCl2, and 2 MgCl2, supplemented with 10 μmol/L ,-aminophosphonovaleric acid and 100 μmol/L kynurenic acid. One slice at a time was transferred into a recording chamber and submerged in continuously flowing aCSF containing 2 mol/L CaCl2 and 1 mol/L MgCl2.
Whole-Cell Patch-Clamp Recording
Current- and voltage-clamp recordings were obtained using the whole-cell patch-clamp technique. Electrodes were pulled from thick-walled borosilicate glass on a Flaming/Brown P-97 pipette puller (Sutter Instruments, Novato, CA, USA) and pipettes had resistances of 4 to 5 MΩ when filled with an intracellular solution containing 140 mol/L K-gluconate, 0.1 mol/L CaCl2, 2 mol/L MgCl2, 1 mol/L EGTA, 2 mol/L ATP·K2, 0.1 mol/L GTP·Na3, and 10 mol/L HEPES (pH adjusted to 7.25 with KOH). Whole-cell recordings were performed using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA), and current signals were low-pass filtered at 2 kHz and digitized online at 5 to 50 kHz using a Digidata 1322A digitizing board interfaced to a computer (Axon Instruments, Foster City, CA, USA). Data were analyzed using pClamp 9.2 software (Molecular Devices, Sunnyvale, CA, USA). Action potentials were elicited by applying 100-msecs depolarizing current pulses ranging from −0.06 to 0.16 nA. When spontaneous excitatory postsynaptic currents (sEPSCs) were recorded, cells were held at −70 mV. All recordings were performed at room temperature.
Statistical Analysis
Quantitative data were expressed as mean±s.e.m. from at least three experiments. Data were analyzed by one-way ANOVA (analysis of variance) followed by Fisher's protected least squares difference (PLSD) post hoc tests. P-values <0.05 were considered significant.
Results
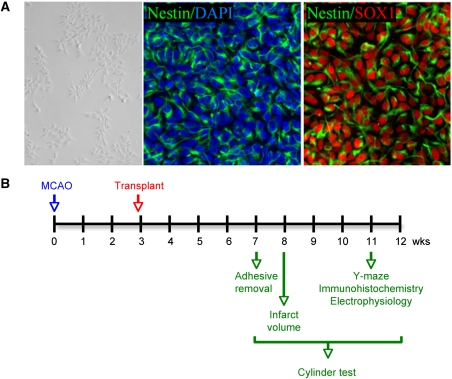
Human ESC line BG01-derived NPCs were maintained in the proliferation medium (Figure 1A, left) in which more than 90% of cells expressed both nestin and Sox2 (Figure 1A, middle and right). Under these conditions, markers of mature neural lineage, including the neuronal marker calbindin, the astroglial marker GFAP, and the oligodendroglial marker OX4, were detected in <1% of cells. Permanent occlusion of the left distal MCA in adult rats produced extensive infarction in the ipsilateral cerebral cortex, leading to evolution over weeks of a large cystic cavity reflecting loss of the brain parenchyma. The contralateral hemisphere remained intact.
Figure 1.
Culture of hESC-derived NPCs and timeline of experiments. (A) Phase-contrast (left), anti-nestin immunostained (green) and DAPI stained (blue) (center), and anti-nestin (green) and anti-Sox2 (red) immunostained (right) images of hESC-derived NPCs in culture. (B) Distal MCAO was performed at time 0, hESC-derived NPCs were transplanted into the infarct cavity 3 weeks later, and outcome measures were assessed at the indicated intervals thereafter.
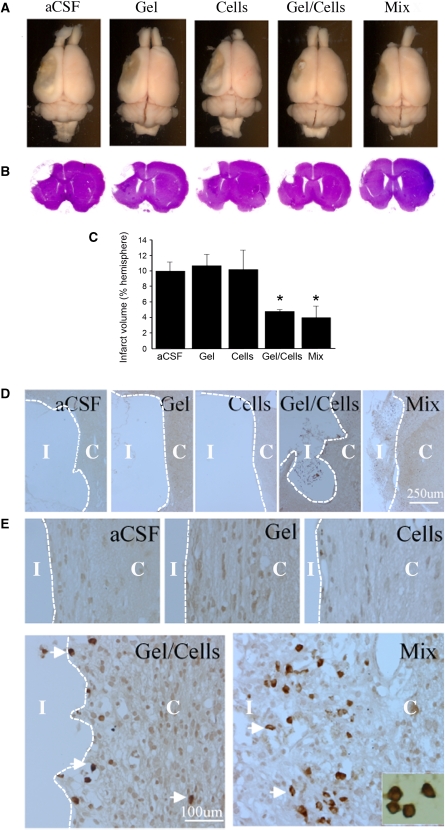
To investigate whether the ischemic infarct cavity could be reconstructed by transplantation, animals were treated 3 weeks after MCAO (Figure 1B) by administration into the infarct cavity of (1) aCSF vehicle, (2) Matrigel scaffold, (3) NPCs, (4) NPCs plus Matrigel scaffold, or (5) NPC/Matrigel cultures. Rats were killed 5 weeks after transplantation and the brains were removed and examined (Figure 2A), serially sectioned, and stained with hematoxylin and eosin (Figure 2B). The size of the infarct cavity was similar in animals treated with aCSF, Matrigel alone, or cells alone, and smaller in animals treated with either NPCs plus Matrigel or NPC/Matrigel cultures (Figure 2C). HuNu-immunopositive cells were absent from the vicinity of the infarct in animals treated with aCSF or Matrigel alone, rare in animals treated with cells alone, more numerous in animals administered both cells and Matrigel, and most abundant (∼300 cells per mm2 at 8 weeks) after transplantation of NPC/Matrigel cultures (Figures 2D and 2E).
Figure 2.
Effects of transplantation conditions on infarct size and survival of transplanted cells after MCAO. (A) Dorsal view and (B) H&E-stained coronal sections of the rat brain 8 weeks after left MCAO and 5 weeks after transplantation, showing differences in infarct size (unstained areas in panel B). (C) Comparison of infarct volumes, 8 weeks after left MCAO and 5 weeks after transplantation, in rats treated with artificial CSF (aCSF), Matrigel alone (Gel), human ESC-derived NPCs alone (Cells), Matrigel and human ESC-derived NPCs added together at the time of transplantation (Gel/Cells), and NPC/Matrigel cultures (Mix). *P<0.05 compared with aCSF. (D) Low- and (E) high-magnification views of the infarct core (I) and adjacent cerebral cortex (C) stained with anti-HuNu (brown, arrows) to label surviving transplanted cells (inset). Treatments were as for panel C above.
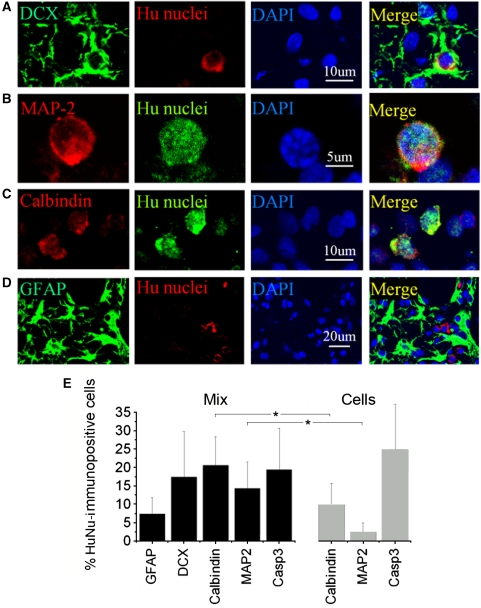
To determine the phenotypic fate of cells from NPC/Matrigel transplants, double-label immunohistochemistry was performed 8 weeks after transplantation using antibodies against various immature and mature neural lineage markers. Subpopulations of HuNu-positive cells expressed the immature neuronal marker DCX (Figure 3A) or the mature neuronal marker MAP-2 (Figure 3B) or calbindin (Figure 3C), whereas only a few HuNu-positive cells expressed the astrocytic marker GFAP (Figure 3D), suggesting a predominantly neuronal phenotype of transplanted cells at the transplant site. Cell counts showed that ∼5% of HuNu-immunopositive cells in the periinfarct cerebral cortex coexpressed GFAP; 15% to 20% coexpressed DCX, calbindin, or MAP-2; and ∼15% showed caspase-3 cleavage, consistent with cell death (Figure 3E). By comparison, after transplantation of NPCs alone, the proportion of HuNu/calbindin- and HuNu/MAP-2-immunopositive cells was ∼50% and ∼80% lower, respectively, and the proportion of HuNu-immunopositive cells with caspase-3 cleavage was ∼25% higher.
Figure 3.
Cell type-specific marker expression in transplanted NPCs in vivo. The brains were removed 8 weeks after transplantation of NPC/Matrigel cultures, sectioned through the frontal cortex adjacent to the lesion cavity, and stained with antibodies against cell-type markers (left panels) and HuNu (panels second from left). Nuclei were counterstained with DAPI (blue; panels second from right). HuNu-immunopositive cells expressed neuronal markers, including (A) DCX (green), (B) MAP-2 (red) and (C) calbindin (red), but not (D) the astroglial marker GFAP (green). (E) Cell counts in the periinfarct cortex after transplantation of human ESC-derived NPCs alone (Cells) or NPC/Matrigel cultures (Mix) were expressed as a percentage of all HuNu-immunopositive cells (mean±s.e.m., n=3 rats per condition). *P<0.05.
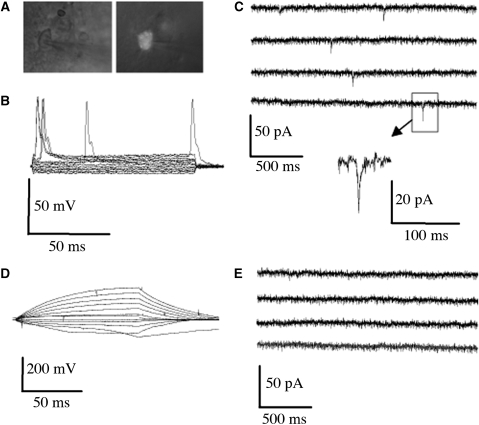
To further determine whether transplanted cells could differentiate sufficiently to exhibit neuronal function, some human ESC-derived NPCs were transfected with a vector containing GFP (green fluorescent protein) under the control of the CMV promoter, cultured in Matrigel, and transplanted into the infarct cavity after MCAO. Brain slices were prepared 8 weeks later for whole-cell patch-clamp recording from GFP-positive cells (Figure 4A). We identified 20 GFP-positive cells, and found that 40% of patched cells displayed spontaneous (although slow, ∼10 msecs) action potentials (Figure 4B) and excitatory postsynaptic currents (Figure 4C), consistent with physiologic neuronal properties. However, other transplanted cells failed to show spontaneous action potentials (Figure 4D) or excitatory postsynaptic currents (Figure 4E), consistent with a less mature neuronal or nonneuronal phenotype.
Figure 4.
Electrophysiological properties of transplanted NPCs in acute brain slices. Human ESC-derived NPCs, transfected with a GFP-expressing vector, were transplanted as NPC/Matrigel cultures 3 weeks after MCAO. Brain slices were prepared 8 weeks later for whole-cell patch-clamp recording from GFP-positive (transplanted) cells (A; phase contrast on left, GFP fluorescence on right). Approximately 40% of patched GFP-positive cells showed (B) spontaneous action potentials elicited by applying 100-msecs depolarizing current pulses ranging from −0.06 to 0.16 nA and (C) excitatory postsynaptic currents measured with voltage held at −70 mV, whereas the remainder did not (D, E).
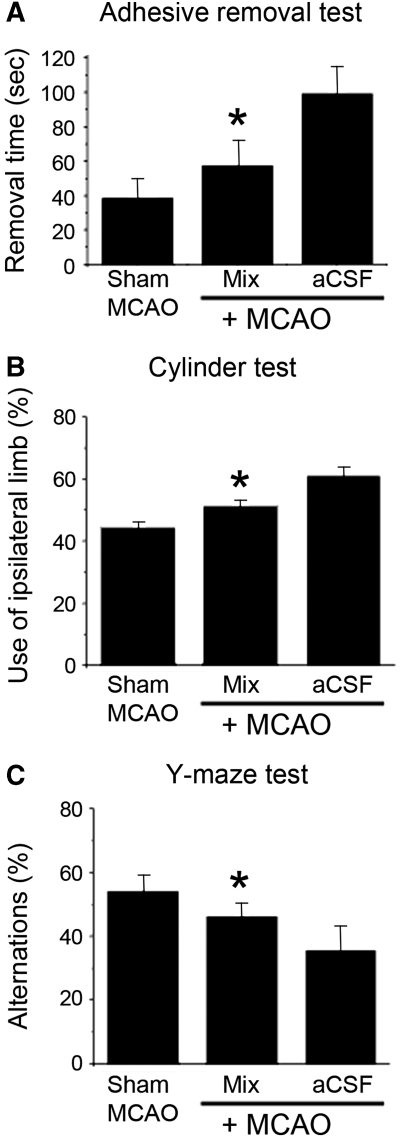
Next, we asked whether administration of NPCs cultured in a Matrigel scaffold could improve functional outcome after MCAO. The adhesive-removal test (Figure 5A), cylinder test (Figure 5B), and Y-maze task (Figure 5C) all showed improvement with NPC/Matrigel treatment, with performance intermediate between that observed in nonischemic control (sham MCAO) and aCSF vehicle-treated ischemic rats.
Figure 5.
Effects of NPC/Matrigel culture transplants on behavioral function after MCAO. Rats underwent MCAO followed 3 weeks later by transplantation of aCSF alone (vehicle) or NPC/Matrigel cultures, and 4 to 9 weeks later by behavioral testing. In some (sham MCAO) rats, the MCA was visualized but not occluded, and these rats served as nonischemic controls in tests of behavioral function. (A) Adhesive-removal test, showing the time required to remove a 70.6-mm2 adhesive-backed paper dot from the distal–radial region of the wrist. (B) Cylinder test, showing the percentage use of the limb ipsilateral to (unaffected by) MCAO when the animal attempted to explore a transparent cylinder. (C) Y-maze test, showing percentage of correct alternations between different arms of the maze (normal rats alternate arms of the maze, i.e., enter a previously unexplored arm, ≥50% of the time). Error bars represent s.e.m. *P<0.05 compared with aCSF+MCAO.
Discussion
In this study, we report that implantation into a cortical infarct cavity of human ESC-derived NPCs, together with the Matrigel matrix in which they are grown, results in (1) reduced cavity size, (2) survival of transplanted cells, (3) primarily neuronal differentiation of these cells, (4) acquisition of electrophysiological neuronal properties by some transplanted cells, and (5) improved behavioral outcome. The most closely related previous study is that by Park et al (2002), who used the C17.2 immortalized murine NPC line and a polyglycolic acid scaffold, given 7 days after ischemia, to study the effects on cerebral ischemic injury in neonatal mice. They found reduction in infarct cavity size, survival of transplanted cells, neuronal and glial cell differentiation, vascularization of transplants, axonal projections into the host brain, and attenuated rotational behavior. Our study differs in that we transplanted cells of human origin, performed transplants 3 weeks after ischemia, studied adult rats, found electrophysiological evidence of functional neuronal differentiation, and conducted a more extensive battery of neurobehavioral tests, but the results of both reports are highly consistent.
Transplantation of NPCs is effective in ameliorating deficits resulting from injury to the central nervous system in various experimental settings, and the efficacy of transplantation can be improved in some cases by the coadministration of biomaterial scaffolds (Potter et al, 2008). Cerebral ischemia may be a particularly suitable target for such combined cell and scaffold treatment, because it affects all cell types in the ischemic zone and severely disrupts tissue architecture. This means that, in contrast to disorders that produce selective degeneration of a single cell type, ischemia may require not only replacement of cells, but also restoration of a panoply of extracellularly derived chemotactic and haptotactic influences.
In this study, we used human NPCs, because understanding how human cells interact with the ischemic brain is likely to be a prerequisite for clinical application of stem-cell therapy in stroke. We used Matrigel—an extract of tumor-cell basement membrane known to promote cell attachment, survival, growth, differentiation and migration, as well as tissue vascularization (Kleinman and Martin, 2005)—as a source of scaffolding. The complex composition and properties of Matrigel make it difficult to establish its mechanism of action in this context with certainty. However, previous reports have suggested that several factors, including physical effects mediated through cell–matrix and cell–cell contact, as well as chemical effects of matrix proteins and secreted trophic factors, may be involved (Kleinman and Martin, 2005).
In initial experiments, we compared transplantation after MCAO under five conditions, involving the delivery of (1) aCSF, (2) Matrigel alone, (3) NPCs alone, (4) NPCs plus Matrigel, or (5) NPCs cultured in and administered together with Matrigel. Neither Matrigel alone nor NPCs alone reduced the size of the infarct cavity, and when NPCs were given alone, few survived. When NPCs and Matrigel were added together at the time of transplantation, there was increased cell survival, and when NPC/Matrigel cultures were used, cell survival was greater. These observations may shed light on the mechanism through which the NPC/Matrigel cultures elicited functional improvement. First, although acutely filling the infarct cavity alone might be expected to afford some benefit, such as by providing structural integrity, interfering with inflammation or the formation of a glial scar, or releasing bioactive molecules (Potter et al, 2008), none of these are sufficient to explain the observed findings, as Matrigel alone exerted no effect on infarct size; the administration of NPCs alone also did not reduce infarct size, perhaps because so few cells survived. It has been previously suggested that the cerebral infarct cavity may represent a hostile environment for cell transplantation, and that the death of transplanted cells may be a major factor limiting posttransplant recovery from ischemia. In support of this notion, overexpression of the antiapoptotic gene Bcl2 in murine ESCs was associated with improved histologic and functional outcome after transplantation in rats with MCAO (Wei et al, 2005). Thus, at least one method by which Matrigel seems to have enhanced recovery in our rats is by enhancing cell survival.
The increased survival of transplanted cells and smaller infarct size observed after transplantation of NPCs cultured in Matrigel, compared with those added together with Matrigel at the time of transplantation, suggests that physical or chemical factors that required some time to develop in vitro may be involved. This could be related to the survival-promoting effects of Matrigel mediated through its influence on cell differentiation, morphology, adhesion, or gene expression (Kleinman and Martin, 2005). In embryonic rat cortical cultures, detachment of NPCs from an extracellular collagen matrix leads to dissociation-induced programmed cell death (anoikis), by inducing the cell-surface expression of Fas (Cheema et al, 2004). A similar phenomenon is observed with murine ESC-derived NPCs: dissociation of NPC-containing neurospheres causes anoikis in vitro, which can be blocked by inhibiting Rho or Rho kinase (ROCK) (Koyanagi et al, 2008); it is noteworthy that Rho and ROCK are also mediators of Fas-induced cell death (Sarrabayrouse et al, 2007; Hebert et al, 2008). Moreover, inhibition of Rho/ROCK signaling greatly reduces the number of cells that die after transplantation of dissociated neurospheres into the mouse striatum (Koyanagi et al, 2008). Therefore, one possibility is that culturing NPCs in Matrigel enhances cell survival on transplantation by preventing dissociation-induced cell death.
Neuronal precursor cell/Matrigel transplants also reduced the size of the infarct cavity, although the number of cells administered was clearly too small, even if all had survived, to replace the volume of the brain that was lost. Moreover, transplantation was not performed until 3 weeks after MCAO, when death of brain cells is no longer occurring, brain atrophy is maximal, and inflammation has resolved (Clark et al, 1993); therefore, inhibiting one of these processes is not a likely explanation. Nevertheless, previous studies have also shown decreased brain atrophy after transplantation of NPCs and scaffolding after ischemia (Park et al, 2002) or trauma (Deguchi et al, 2006). As in those instances, it is likely that the relative preservation of brain volume seen after transplantation reflects the combined contributions of donor and host tissues, including neural cells derived from the transplant, vascular elements from the host, and processes extended from both (Park et al, 2002). One method by which the transplant might promote these events is by the production of trophic and angiogenic factors (Chu et al, 2005; Zhu et al, 2005).
Finally, NPC/Matrigel transplants improved neurobehavioral outcome after MCAO. Park et al (2002) reported that C17.2/polyglycolic acid transplants decreased rotational behavior induced by cerebral ischemia in neonatal mice. We also found that transplantation improved sensorimotor function (cylinder and adhesive-removal tests), as well as spatial memory and learning (Y maze). Although these tests often show improvement with treatment of cerebral ischemia in rodents, it is surprising that they did so in this study, in which treatment was not instituted until 3 weeks after ischemia. Again, the mechanism is unclear, but may involve changes in axonal sprouting, synaptogenesis, or somatotopic reorganization, which are especially active around this poststroke interval (Stroemer et al, 1995; Carmichael et al, 2001). The design of this study does not permit us to answer how much time was required for the behavioral improvement afforded by transplantation to be manifested, as we did not conduct serial behavioral testing. However, this might be a useful approach in the future insofar as it may help to shed light on the mechanism involved.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis. 2001;8:910–922. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- Cheema ZF, Santillano DR, Wade SB, Newman JM, Miranda RC. The extracellular matrix, p53 and estrogen compete to regulate cell-surface Fas/Apo-1 suicide receptor expression in proliferating embryonic cerebral cortical precursors, and reciprocally, Fas-ligand modifies estrogen control of cell-cycle proteins. BMC Neurosci. 2004;5:11. doi: 10.1186/1471-2202-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Park KI, Lee ST, Jung KH, Ko SY, Kang L, Sinn DI, Lee YS, Kim SU, Kim M, Roh JK. Combined treatment of vascular endothelial growth factor and human neural stem cells in experimental focal cerebral ischemia. Neurosci Res. 2005;53:384–390. doi: 10.1016/j.neures.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Clark RK, Lee EV, Fish CJ, White RF, Price WJ, Jonak ZL, Feuerstein GZ, Barone FC. Development of tissue damage, inflammation and resolution following stroke: an immunohistochemical and quantitative planimetric study. Brain Res Bull. 1993;31:565–572. doi: 10.1016/0361-9230(93)90124-t. [DOI] [PubMed] [Google Scholar]
- Deguchi K, Tsuru K, Hayashi T, Takaishi M, Nagahara M, Nagotani S, Sehara Y, Jin G, Zhang H, Hayakawa S, Shoji M, Miyazaki M, Osaka A, Huh NH, Abe K. Implantation of a new porous gelatin-siloxane hybrid into a brain lesion as a potential scaffold for tissue regeneration. J Cereb Blood Flow Metab. 2006;26:1263–1273. doi: 10.1038/sj.jcbfm.9600275. [DOI] [PubMed] [Google Scholar]
- Ellis-Behnke RG, Liang YX, You SW, Tay DK, Zhang S, So KF, Schneider GE. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc Natl Acad Sci USA. 2006;103:5054–5059. doi: 10.1073/pnas.0600559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MC, Bertram JP, Hynes SR, Michaud M, Li Q, Young M, Segal SS, Madri JA, Lavik EB. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proc Natl Acad Sci USA. 2006;103:2512–2517. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz AK, Scheffler B, Chen HX, Wang S, Suslov O, Xiang H, Brustle O, Roper SN, Steindler DA. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:11063–11068. doi: 10.1073/pnas.0510926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J Cereb Blood Flow Metab. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, Takahashi J, Hashimoto N, Nozaki K. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26:906–914. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- Hebert M, Potin S, Sebbagh M, Bertoglio J, Breard J, Hamelin J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J Immunol. 2008;181:5963–5973. doi: 10.4049/jimmunol.181.9.5963. [DOI] [PubMed] [Google Scholar]
- Hou S, Xu Q, Tian W, Cui F, Cai Q, Ma J, Lee IS. The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J Neurosci Methods. 2005;148:60–70. doi: 10.1016/j.jneumeth.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Sakaguchi M, Kuroiwa T, Yamasaki M, Kanemura Y, Shizuko I, Shimazaki T, Onodera M, Okano H, Mizusawa H. Human neural stem/progenitor cells, expanded in long-term neurosphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. J Neurosci Res. 2004;78:215–223. doi: 10.1002/jnr.20246. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Sun Y, Xie L, Greenberg DA. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest. 2002;110:311–319. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Takahashi J, Arakawa Y, Doi D, Fukuda H, Hayashi H, Narumiya S, Hashimoto N. Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res. 2008;86:270–280. doi: 10.1002/jnr.21502. [DOI] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10 (Suppl:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor and amelioration of brain infarction in mice. J Cereb Blood Flow Metab. 1997;17:229–232. doi: 10.1097/00004647-199702000-00013. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20:1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- Potter W, Kalil RE, Kao WJ. Biomimetic material systems for neural progenitor cell-based therapy. Front Biosci. 2008;13:806–821. doi: 10.2741/2721. [DOI] [PubMed] [Google Scholar]
- Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004;55:381–389. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- Sarrabayrouse G, Synaeve C, Leveque K, Favre G, Tilkin-Mariame AF. Statins stimulate in vitro membrane FasL expression and lymphocyte apoptosis through RhoA/ROCK pathway in murine melanoma cells. Neoplasia. 2007;9:1078–1090. doi: 10.1593/neo.07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Hernandez TD, Barth TM. Recovery of function after brain damage: severe and chronic disruption by diazepam. Brain Res. 1986;379:104–111. doi: 10.1016/0006-8993(86)90261-1. [DOI] [PubMed] [Google Scholar]
- Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao R, Stice SL. Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells. 2006;24:125–138. doi: 10.1634/stemcells.2004-0150. [DOI] [PubMed] [Google Scholar]
- Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- Steindler DA. Neural stem cells, scaffolds, and chaperones. Nat Biotechnol. 2002;20:1091–1093. doi: 10.1038/nbt1102-1091. [DOI] [PubMed] [Google Scholar]
- Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- Sughrue ME, Mocco J, Komotar RJ, Mehra A, D'Ambrosio AL, Grobelny BT, Penn DL, Connolly ES., Jr An improved test of neurological dysfunction following transient focal cerebral ischemia in rats. J Neurosci Methods. 2006;151:83–89. doi: 10.1016/j.jneumeth.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, Adams LD, Gottlieb DI, Johnson EM, Jr, Yu SP, Choi DW. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. 2005;19:183–193. doi: 10.1016/j.nbd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Won SJ, Xie L, Kim SH, Tang H, Wang Y, Mao X, Banwait S, Jin K. Influence of age on the response to fibroblast growth factor-2 treatment in a rat model of stroke. Brain Res. 2006;1123:237–244. doi: 10.1016/j.brainres.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhu W, Mao Y, Zhao Y, Zhou LF, Wang Y, Zhu JH, Zhu Y, Yang GY.2005Transplantation of vascular endothelial growth factor-transfected neural stem cells into the rat brain provides neuroprotection after transient focal cerebral ischemia Neurosurgery 57325–333.discussion 325–333 [DOI] [PubMed] [Google Scholar]