Abstract
The GD2 ganglioside, displayed by five carbohydrate Neu5Acα2-8Neu5Acα2-3(GalNAcβ1-4)Galβ1-4Glcβ residues attached to a ceramide chain that anchors the ganglioside in the cell membrane, is expressed on neuroectodermally derived tumors. GD2 has been used as a target for passive and active immunotherapy in patients with malignant melanoma and neuroblastoma. We have generated 47-LDA mimotope of GD2 by screening a phage display peptide library with anti-GD2 mAb 14G2a and reported that vaccination with the 47-LDA mimotope elicited GD2 cross-reactive IgG antibody responses as well as MHC class I-restricted CD8+ T cells to syngeneic neuroblastoma tumor cells. The cytotoxic activity of the vaccine-induced CTLs was independent of GD2 expression, suggesting recognition of a novel tumor-associated antigen cross-reacting with 47-LDA. Immunoblotting studies using 14G2a mAb demonstrated that this antibody cross-reacts with a 105 kDa glycoprotein expressed by GD2+ and GD2− neuroblastoma and melanoma cells. Functional studies of tumor cells grown in three-dimensional (3D) collagen cultures with 14G2a mAb showed decreases in matrix metalloproteinase-2 activation, a process regulated by 105 kDa activated leukocyte cell adhesion molecules (ALCAM/CD166). The CD166 glycoprotein was shown to be recognized by 14G2a antibody, and inhibition of CD166 expression by RNA interference ablated the cell sensitivity to lysis by 47-LDA-induced CD8+ T cells in vitro and in vivo. These results suggest that the vaccine-induced CTLs recognize a 47-LDA cross-reactive epitope expressed by CD166 and reveal a novel mechanism of induction of potent tumor-specific cellular responses by mimotopes of tumor-associated carbohydrate antigens.
Keywords: T Cells, Cytotoxic, Adhesion molecules, Tumor immunity, Vaccination
Introduction
Targeting carbohydrate antigens expressed on tumor cells represents a challenge for the immunotherapy of cancer since aberrant glycosylation exhibited by tumor cells is considered a factor of their uncontrolled cell growth, invasiveness, and increased metastatic potential [1]. However, carbohydrates are typically poorly immunogenic, difficult to synthesize and purify in large quantities, and usually induce short-lived IgM-type antibodies in vaccinated hosts. Furthermore, most carbohydrate antigens are T cell independent, reflecting their inability to stimulate an anamnestic immune response. For this reason, strategies have been developed to convert carbohydrates to T cell-dependent antigens, including carbohydrate–protein conjugates, anti-idiotypes, and peptides resembling a carbohydrate structure as surrogate antigens. Mimicking peptides represent a very promising tool to overcome T cell-independence and may have significant advantage as vaccines compared with carbohydrate–protein conjugates or anti-idiotypic antibodies as (1) The chemical composition and purity of synthesized peptides can be precisely defined, and immunogenicity significantly enhanced by polymerization. (2) Peptide synthesis may be more practical than synthesis of carbohydrate–protein conjugates or the production of anti-idiotypes. (3) Peptide mimotopes can also be engineered into plasmids for genetic vaccination alone or in combination with genetic adjuvants to increase the level and persistence of carbohydrate-specific immune responses [2, 3].
In an effort to define strategies to induce cell-mediated immunity to carbohydrate antigens expressed on tumor cells, we have been developing peptide mimics of GD2 ganglioside expressed on neuroectodermal tumor cells including neuroblastoma, melanoma, and glioma [4]. We have shown that a 47-LDA peptide mimic of GD2 ganglioside expressed in plasmid DNA and delivered to mice in combination with IL-15 and IL-21 genes induced GD2 cross-reactive antibody responses that inhibited tumor growth of human MV3 melanoma cells in the SCID mouse xenograft model [4, 5]. Unexpectedly, this vaccine also activated potent CD8+ T cell responses when delivered simultaneously or 1 day after challenge with GD2+ syngeneic NXS2 neuroblastoma tumor cells [5]. Adoptive immunotherapy with CD8+ T cells isolated from 47-LDA-immunized and cured mice exhibited antitumor activity associated with regression of NXS2 tumor growth and tumor-free survival [5]. The isolated CD8+ T cells lyzed syngeneic GD2+ and also GD2− (Neuro2a) neuroblastoma cells in a MHC class I-restricted manner [5], which indicated that the cellular ligand shared by both neuroblastoma tumor cells is distinct from GD2 ganglioside.
To clarify the possibility of therapeutic application of the 47-LDA mimetic vaccine-induced cellular responses for malignant tumors, we analyzed the antitumor activity and antigenic epitope recognized by 47-LDA vaccine-induced CD8+ T cell responses in tumor-free mice. This study was done to exclude the possibility that the generation of the neuroblastoma-specific CTLs in NXS2-challenged and immunized mice could be affected by antibody-mediated targeting of tumor antigens, which in the presence of NK cells, would lead to a greater accumulation of antibody-coated antigenic tumor cell debris for cross-priming by dendritic cells [6–8]. The most intriguing finding in the process of identifying the target molecule for the 47-LDA vaccine-induced CTLs was the discovery that the GD2-specific mAb 14G2a, which was originally used for isolation of the peptide mimic 47-LDA, cross-reacted with an epitope expressed by 105 kDa CD166 cell adhesion molecules. Stable silencing of CD166 expression in GD2− Neuro2a cells by CD166-specific shRNA not only decreased reactivity with 14G2a mAb but also abolished recognition by 47-LDA vaccine-induced CD8+ T cells. The latter effect was also associated with resistance of Neuro2a cells with down-regulated CD166 expression to 47-LDA vaccine-induced antitumor protection in syngeneic mice.
Immunization with the 47-LDA vaccine elicits antitumor CD8+ T cell responses in syngeneic mice
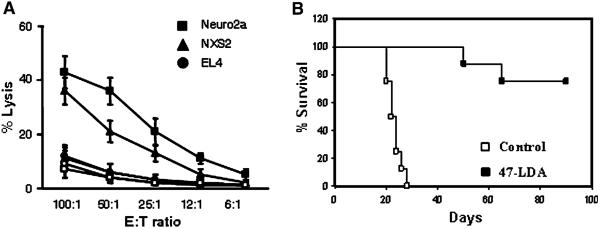
To characterize the mechanisms of the generation of NXS2 neuroblastoma-specific CTLs by the 47-LDA vaccine, we investigated whether the antitumor CTL responses could be induced during prophylactic immunization of syngeneic mice. A/J mice were immunized three times with the 47-LDA mimotope DNA vaccine or sham vector in combination with IL-15 and IL-21 expression vectors in a 2-week period of interval as described [5]. The effector function of CD8+ T cells was analyzed in a standard 51Cr-release assay against syngeneic GD2+ NXS2 and GD2− Neuro2a neuroblastoma cells. Allogeneic GD2+ EL4 lymphoma (H-2b) cells were used as a negative control. Figure 1a shows that both NXS2 and Neuro2a cells were efficiently killed by the vaccine-induced CD8+ T cells, whereas EL4 cells were resistant to the CTL-mediated killing. The cytotoxic activities against NXS2 and Neuro2a tumor cells were approximately threefold higher over a broad range of the effector-to-target (E:T) ratios compared to those measured in control animals, confirming that the antigen recognized by the 47-LDA vaccine-induced CTLs is distinct from GD2 ganglioside.
Fig. 1.
Analyses of tumor-reactive CD8+ T cell responses induced by the 47-LDA vaccine. a CTL activities against NXS2 and Neuro2a neuroblastoma cells as well as EL4 lymphoma (GD2+, H-2b) cells in A/J mice immunized with the 47-LDA (closed symbols) or sham (open symbols) vector in the presence of IL-15 and IL-21 genes were analyzed in a standard 51Cr-release assay. All determinants were made in triplicate samples, and the SD was <10%. Results are presented as the means ± SD of three independent experiments. b CD8+ T cells from A/J mice (n = 8), which had been immunized with the 47-LDA vaccine in combination with the IL-15 and IL-21 genes and rejected s.c. NXS2 tumor challenge, were used for adoptive transfer (■). For the adoptive cell transfer, mice that were challenged s.c. with 106 NXS2 cells were sublethally irradiated (500 rad) and treated by i.v. injection with freshly isolated CD8+ T cells from the cured mice (2 × 107 cells). All recipient mice were vaccinated every 2 weeks by i.v. injection of 47-LDA-transfected DCs (2 × 106 cells) and i.m. injection of IL-15 and IL-21 genes delivered at the time of immunization and 5 days latter, respectively. NXS2-challenged mice that received CD8+ T cells from untreated animals served as controls (□). Survival was defined as the point at which mice were killed due to extensive tumor growth. Kaplan–Meier survival plots were prepared, and significance was determined using logrank Mantel–Cox method
In a separate study, we investigated antitumor activities of CD8+ T cells in vivo. A/J mice (n = 8), which had been immunized with the 47-LDA minigene in combination with IL-15 and IL-21 genes and rejected the s.c. NXS2 tumor challenge, were used as a source of CD8+ splenocytes. The freshly isolated CD8+ T cells were used for adoptive transfer to NXS2-challenged and sublethally irradiated (500 rad) naïve mice that were vaccinated every 2 weeks by i.v. injection of 47-LDA-transfected DCs (2 × 106 cells) and i.m. injection of IL-15 and IL-21 genes. Figure 1b shows that six of eight NXS2-challenged mice that received CD8+ T cells from 47-LDA-vaccinated and cured mice had tumor-free survival for greater than 90 days, reflecting a significant antitumor influence of the transferred cells compared to control animals (P < 0.001).
Reactivity of 14G2a mAb with a 105 kDa antigen expressed by GD2+ and GD2− tumor cells
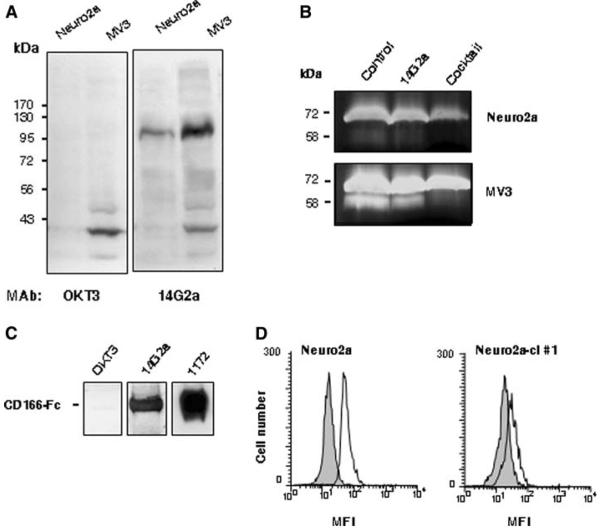
In a search of the putative 47-LDA cross-reactive epitope whose expression by tumor cells would facilitate recognition by 47-LDA vaccine-induced CTLs, we performed immunoblotting analyses using 14G2a mAb and lysates from Neuro2a neuroblastoma cells. Because Neuro2a cells do not express GD2 ganglioside due to lack of GD3 synthase [9], but are efficiently killed by 47-LDA vaccine-induced CTLs, we hypothesized that these cells express a carbohydrate GD2-like motif that is recognized by 14G2a mAb. To address this possibility, cell lysates from Neuro2a were analyzed by Western blotting with 14G2a antibody in addition to the human CD3-specific mAb OKT3, which served as an isotype control. Lysates from the weakly GD2+ human MV3 melanoma cells were also included in the analysis to determine whether the same antigen is expressed by other malignancies of neuroectodermal origin. As shown in Fig. 2a, 14G2a mAb recognized a 105 kDa band in the analyzed tumor cells irrespective of the expression of GD2 ganglioside. The recognition seems to be specific since the 105 kDa band was not detected with OKT3 mAb and other bands, including the one of 39 kDa molecular mass that was prominent in cell lysates of MV3 melanoma, were recognized by both antibodies. The deglycosylation of the cell lysates with enzymes that remove all N-linked, simple O-linked, and complex Core 2 O-linked carbohydrates [N-glycanase, PNGase F, sialidase A, O-glycanase, beta(1–4) galactosidase, beta-N-acetylglucosaminidase] did not affect recognition by 14G2a mAb, except for the ~15 kDa faster migration of the deglycosylated bands compared to their 105 kDa control counterparts (not shown), implying recognition of a non-glycan epitope on CD166. These results indicated that 14G2a mAb recognizes a glycosylated protein with molecular mass of 105 kDa expressed by human and mouse neuroblastoma and melanoma cells.
Fig. 2.
Interaction of 14G2a mAb with recombinant and cellular CD166 glycoprotein. a Immunoblotting of cell lysates from Neuro2a neuroblastoma and MV3 melanoma cells with 14G2a and OKT3 mAbs. The molecular masses of the standard proteins are shown on the left of the panels. b Inhibition of MMP-2 activation by 14G2a mAb. Neuro2a and MV3 cell lines were cultured in collagen gels for 72 h. Conditioned media were analyzed by gelatin zymography to determine the level of MMP-2 activation. Position of 72 kDa pro-MMP-2 and 58 kDa active MMP-2 is indicated. c Western blotting analyses of recombinant CD166-Fc fusion protein (2 μg/lane) with mAbs specific for CD3 (OKT3), GD2 (14G2a), and CD166 (1172). d Intracellular staining of Neuro2a and Neuro2a-cl #1 cells with 14G2a mAb. In the flow cytometry figure, light gray area denotes cell stained with the secondary antibody only
14G2a antibody inhibits 3D collagen gel culture stimulation of pro-MMP-2 processing and reacts with CD166 glycoprotein
Previous studies have shown that cell-to-matrix interactions that lead to clustering of integrin β1 and activation of gelatinase A/matrix metalloproteinase-2 (MMP-2) are influenced by cell-to-cell adhesion and controlled by the 105 kDa glycoprotein known as ALCAM/CD166 [10]. Therefore, we next assessed the effect of 14G2a mAb on pro-MMP-2 processing using conditioned media from the 3D collagen cultures of Neuro2a and MV3 cells. The cells were plated at a high density of 106 cells per gel in the presence of OKT3 (control), 14G2a mAb, or protease inhibitor cocktail, and conditioned media collected after 72 h of incubation were analyzed by gelatin zymography. Figure 2b shows that media from the control cultures treated with OKT3 mAb exhibited reproducible conversion of pro-MMP-2 (72 kDa) to active (58 kDa) MMP-2. The human MV3 melanoma cells showed the strongest expression of pro-MMP-2 with a prominent band corresponding to the active MMP-2 in control cultures, whereas MMP-2 activation was less efficient in Neuro2a cells. As expected, MMP-2 activation was barely detectable in cultures treated with the protease inhibitor cocktail. Addition of 14G2a mAb at a concentration of 30 μg/ml reduced two- to threefold expression of MMP-2 compared to levels detected in the control cultures, with the most prominent effect detected in MV3 cells (Fig. 2b).
Current models state that the assembly of a ternary complex of MT1-MMP, TIMP-2, and pro-MMP-2 involved in the MMP-2 activation cascade requires cell-to-cell adhesion, intact CD166 expression, and integrin clustering [10]. The analyzed Neuro2a and MV3 cells were found to express CD166 based on staining with CD166-specific 1172 mAb followed by flow cytometry analysis (data not shown). These results together with the ability of 14G2a mAb to bind to the 105 kDa glycoprotein in Neuro2a and MV3 cells and interfere with the CD166-mediated network of MMP-2 activation [10] suggested that 14G2a recognizes an antigenic epitope expressed by the CD166 glycoprotein. To address this hypothesis experimentally, we investigated the binding of 14G2a mAb to the recombinant CD166 glycoprotein in the Western blotting analysis. CD166-specific 1172 mAb and OKT3 mAb were included in the assay as positive and negative controls, respectively. The recombinant CD166 glycoprotein used in the study was a glycosylated disulfide-linked homodimeric protein expressed as an extracellular domain of human CD166 (aa. 1–526) fused to the carboxy-terminal 6× histidine-tagged Fc region of human IgG1 via a polypeptide linker (CD166-Fc). Figure 2c shows that both 1172 and 14G2a mAbs recognized a 120 kDa band corresponding to the CD166-Fc fusion protein, whereas immunoblotting with OKT3 mAb was at a background level. The binding of 1172 antibody to CD166-Fc was approximately threefold higher than that of 14G2a reflecting either differences in reactivity of these antibodies under denaturing conditions or a higher specificity of the former antibody for its cognate epitope on CD166.
Effect of down-regulation of CD166 expression on recognition by 47-LDA vaccine-induced CTLs
We next investigated whether down-regulation of CD166 expression in Neuro2a cells by the RNA interference approach would ablate binding of 14G2a mAb. In the CD166 knockout experiments, Neuro2a cells were transduced with a lentivirus plasmid DNA expressing shRNA for stable CD166 gene silencing. Cells transduced with a vector containing a non-targeting shRNA that activates the RNAi pathway but does not target any human and mouse gene (Neuro2a-control) were included as a control to determine whether the reduction in CD166 expression was specific for CD166. The transduced cells were selected in puromycin-containing medium and analyzed for CD166 expression by intra-cellular staining with 1172 mAb. In all CD166 shRNA-transduced Neuro2a clones, the reduction in CD166 expression levels ranged from 40 to 70% and correlated with lower 14G2a antibody staining (data not shown). Among the analyzed clones, Neuro2a-cl #1 cells that exhibited the lowest intracellular expression of CD166 compared to the parental Neuro2a and Neuro2a-control cells, as determined by indirect immunofluorescence staining with 1172 mAb, had also reduced reactivity with 14G2a mAb (Fig. 2d).
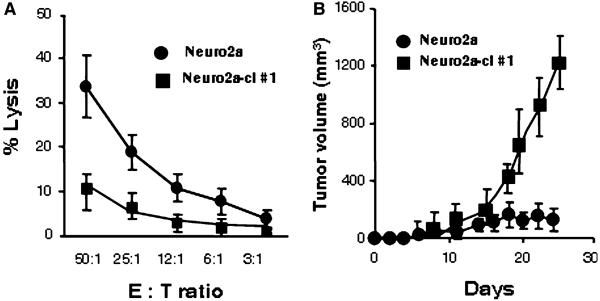
To examine the effect of reduced CD166 expression on recognition by the 47-LDA vaccine-induced CD8+ T cells, we performed a standard 51Cr-release assay against Neuro 2a and Neuro2a-cl #1 cells. For the cytotoxic assay, CD8+ splenocytes were isolated from 47-LDA- or sham plasmid-immunized A/J mice. As shown in Fig. 3a, Neuro2a cells were efficiently killed by the 47-LDA vaccine-induced CD8+ T cells, whereas these responses were low with Neuro2a-cl #1 cells used as targets. These results indicate that the down-regulation of CD166 expression rendered the tumor cells resistant to killing by 47-LDA-induced CTLs. The significance of the evasion of Neuro2a cells with down-regulated CD166 from recognition by the 47-LDA vaccine-induced CTLs was also investigated during challenge studies by analyzing the ability of Neuro2a-cl #1 cells to form tumor in 47-LDA-immunized mice. Groups of A/J mice (n = 5) were injected s.c. with 2 × 106 Neuro2a or Neuro2a-cl #1 cells 2 weeks after the third booster immunization with the 47-LDA vaccine delivered in combination with IL-15 and IL-21 genes. Four out of five mice immunized with the 47-LDA vaccine and challenged with Neuro2a cells remained tumor-free, and only one mouse developed palpable tumor within 2 weeks after the challenge (Fig. 3b). In contrast, all 47-LDA-immunized mice that were challenged with Neuro2a-cl #1 developed progressive tumors by day 15 after tumor inoculation and were killed by day 26 (Fig. 3b). No significant decreases in the mean rate of Neuro2a-cl #1 tumor growth were observed in the 47-LDA-immunized mice compared to control animals that were immunized with the sham plasmid (not shown), indicating that the down-regulation of CD166 expression conferred resistance to the 47-LDA vaccine-induced antitumor protection.
Fig. 3.
Effect of down-regulation of CD166 expression on recognition by 47-LDA-induced CTLs in vitro and in vivo. a CTL activities of 47-LDA vaccine-induced CD8+ splenocytes in A/J mice against Neuro2a and Neuro2a-cl #1 cells were analyzed in a standard 51Cr-release assay. All determinations were made in triplicate samples, and the SD was <10%. Results are presented as the means ± SD of three independent experiments. b The effect of a prophylactic 47-LDA vaccine on Neuro2a and Neuro2a-cl #1 tumor growth in A/J mice. Groups of A/J mice (n = 5) were immunized i.m. with the 47-LDA vaccine delivered in combination with IL-15 and IL-21 genes and injected s.c. with Neuro2a or Neuro2a-cl #1 tumor (2 × 106 cells per injection) 2 weeks after the third booster immunization. Animals were examined daily until the tumor became palpable, after which tumor growth was monitored by measuring s.c. tumors once to thrice a week
Conclusions
For many years, the GD2 ganglioside has been considered to be a marker of neuroectoderm-derived human cancers without specific biological function in the malignant properties of human cancer cells. Co-localization of GD2 ganglioside at the adhesion plaques of melanoma cells and its functional involvement in a cell adhesion process mediated by the interaction between integrins and the extracellular matrix was first reported by Cheresh et al. [11]. More recent studies revealed that GD2, integrin β1, and focal adhesion kinase (FAK) form a molecular complex across the plasma membrane, and antibody binding to GD2 induces conformational changes in integrin molecules leading to dephosphorylation of FAK and apoptosis associated with disruption of cell–matrix interaction, known as anoikis [12]. Although results of this study elucidated, at least partly, the molecular association of GD2 with integrin and FAK molecules, it remains to be determined whether this interaction is direct or mediated by other cell adhesion molecules.
Cell adhesion molecules are involved in cell–cell and cell–matrix interactions, and are among the primary determinants of tissue architecture. ALCAM/CD166 is a member of the immunoglobulin superfamily belonging to a subgroup with five extracellular immunoglobulin-like domains (VVC2C2C2), which includes MCAM/CD146/MUC18 and B-CAM/Lutheran. ALCAM/CD166 mediates cell–cell clustering through homophilic (ALCAMALCAM) and heterophilic (ALCAM-CD6) interactions, and while present in a wide variety of tissues, expression of CD166 is usually restricted to subsets of cells involved in dynamic growth and/or migration (reviewed in ref. [13]). Recent structure–function analyses have shown that homophilic CD166-mediated cell–cell adhesion is regulated through actin cytoskeleton-dependent clustering of CD166 molecules at the cell surface and that this clustering of CD166 is necessary to obtain stable adhesive interactions. In addition, CD166 regulates matrix metalloproteinase activity and acts as a cell sensor for cell density, controlling the transition between local cell proliferation and tissue invasion in melanoma progression. In human melanoma cells, expression of CD166 glycoprotein and CD166-dependent MMT-2 activation correlates strongly with metastatic potential and advanced melanoma tumor progression. The similarity between CD166 and GD2 ganglioside in their expression profile and involvement in cell adhesion process mediated by the interaction between integrins and the extracellular matrix suggests that both molecules could contribute to the metastatic process of tumor cell dissemination. The ability of GD2-specific mAb 14G2a to cross-react with CD166 and to inhibit MMT-2 activation in 3D collagen lattices may unravel complexity of this network [14]. Furthermore, identification of the putative carbohydrate motif of GD2 within CD166, or perhaps other members of the cell adhesion molecules with a high sequence homology to CD166, would be critical in understanding its functional significance in cell–cell and cell–matrix interactions and may lead to discovery of therapeutic agents that disrupt adhesion or normalize signaling. Thus, our results which demonstrated that the vaccine-induced CTLs recognize a 47-LDA cross-reactive epitope expressed by CD166 may represent a novel mechanism of induction of tumor-specific cellular responses by mimotopes of tumor-associated carbohydrate antigens [14].
Acknowledgments
We are grateful to Dr R. A. Reisfeld and Dr G. van Muijen for the reagents. We thank Earl Timm for help with a flow cytometric analysis. This work was supported by the National Institutes of Health grants R21EB008071 and Roswell Park Alliance Foundation.
References
- 1.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 2.Kieber-Emmons T, Monzavi-Karbassi B, Wang B, Luo P, Weiner DB. Cutting edge: DNA immunization with minigenes of carbohydrate mimotopes induce functional anti-carbohydrate antibody response. J Immunol. 2000;165(2):623–7. doi: 10.4049/jimmunol.165.2.623. [DOI] [PubMed] [Google Scholar]
- 3.Monzavi-Karbassi B, Luo P, Jousheghany F, et al. A mimic of tumor rejection antigen-associated carbohydrates mediates an antitumor cellular response. Cancer Res. 2004;64(6):2162–6. doi: 10.1158/0008-5472.can-03-1532. [DOI] [PubMed] [Google Scholar]
- 4.Bolesta E, Kowalczyk A, Wierzbicki A, et al. DNA vaccine expressing the mimotope of GD2 ganglioside induces protective GD2 cross-reactive antibody responses. Cancer Res. 2005;65(8):3410–8. doi: 10.1158/0008-5472.CAN-04-2164. [DOI] [PubMed] [Google Scholar]
- 5.Kowalczyk A, Wierzbicki A, Gil M, et al. Induction of protective immune responses against NXS2 neuroblastoma challenge in mice by immunotherapy with GD2 mimotope vaccine and IL-15 and IL-21 gene delivery. Cancer Immunol Immunother. 2007;56(9):1443–58. doi: 10.1007/s00262-007-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhodapkar KM, Dhodapkar MV. Recruiting dendritic cells to improve antibody therapy of cancer. Proc Natl Acad Sci USA. 2005;102(18):6243–4. doi: 10.1073/pnas.0502547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groh V, Li YQ, Cioca D, et al. Efficient cross-priming of tumor antigen-specific T cells by dendritic cells sensitized with diverse anti-MICA opsonized tumor cells. Proc Natl Acad Sci USA. 2005;102(18):6461–6. doi: 10.1073/pnas.0501953102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202(2):203–7. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kojima N, Kurosawa N, Nishi T, Hanai N, Tsuji S. Induction of cholinergic differentiation with neurite sprouting by de novo biosynthesis and expression of GD3 and b-series gangliosides in Neuro2a cells. J Biol Chem. 1994;269(48):30451–6. [PubMed] [Google Scholar]
- 10.Lunter PC, van Kilsdonk JW, van Beek H, et al. Activated leukocyte cell adhesion molecule (ALCAM/CD166/MEMD), a novel actor in invasive growth, controls matrix metalloproteinase activity. Cancer Res. 2005;65(19):8801–8. doi: 10.1158/0008-5472.CAN-05-0378. [DOI] [PubMed] [Google Scholar]
- 11.Cheresh DA, Harper JR, Schulz G, Reisfeld RA. Localization of the gangliosides GD2 and GD3 in adhesion plaques and on the surface of human melanoma cells. Proc Natl Acad Sci USA. 1984;81(18):5767–71. doi: 10.1073/pnas.81.18.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aixinjueluo W, Furukawa K, Zhang Q, et al. Mechanisms for the apoptosis of small cell lung cancer cells induced by anti-GD2 monoclonal antibodies: roles of anoikis. J Biol Chem. 2005;280(33):29828–36. doi: 10.1074/jbc.M414041200. [DOI] [PubMed] [Google Scholar]
- 13.Swart GW. Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol. 2002;81(6):313–21. doi: 10.1078/0171-9335-00256. [DOI] [PubMed] [Google Scholar]
- 14.Wierzbicki A, Gil M, Ciesielski M, et al. Immunization with a mimotope of GD2 ganglioside induces CD8+ T cells that recognize cell adhesion molecules on tumor cells. J Immunol. 2008;181(9):6644–53. doi: 10.4049/jimmunol.181.9.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]