Abstract
The National Institute on Drug Abuse Clinical Trials Network launched the Prescription Opioid Addiction Treatment Study (POATS) in response to rising rates of prescription opioid dependence and gaps in understanding the optimal course of treatment for this population. POATS employed a multi-site, two-phase adaptive, sequential treatment design to approximate clinical practice. The study took place at 10 community treatment programs around the United States. Participants included men and women age ≥18 who met Diagnostic and Statistical Manual, 4th Edition criteria for dependence upon prescription opioids, with physiologic features; those with a prominent history of heroin use (according to pre-specified criteria) were excluded. All participants received buprenorphine/naloxone (bup/nx). Phase 1 consisted of 4 weeks of bup/nx treatment, including a 14-day dose taper, with 8 weeks of follow-up. Phase 1 participants were monitored for treatment response during these 12 weeks. Those who relapsed to opioid use, as defined by pre-specified criteria, were invited to enter Phase 2; Phase 2 consisted of 12 weeks of bup/nx stabilization treatment, followed by a 4-week taper and 8 weeks of post-treatment follow-up. Participants were randomized at the beginning of Phase 1 to receive bup/nx, paired with either Standard Medical Management (SMM) or Enhanced Medical Management (EMM; defined as SMM plus individual drug counseling). Eligible participants entering Phase 2 were re-randomized to either EMM or SMM. POATS was developed to determine what benefit, if any, EMM offers over SMM in short-term and longer-term treatment paradigm. This paper describes the rationale and design of the study.
Keywords: Adaptive Treatment Research Design, Buprenorphine, Opioid, Pain, Drug abuse, Counseling
1. Introduction and Background
While opioids have been used for decades to treat chronic pain, serious concerns about prescription opioid abuse have increased in recent years [1, 2]. In 2006, there were nearly 250,000 emergency department visits related to prescription opioid abuse, a 43% increase since 2004 [3]. An estimated 2.1 million people aged 12 and older used prescription opioids non-medically for the first time in 2007. Among users of all illicit substances, this group represented the largest number of first-time users in that year [4]. According to 2007 data from the Treatment Episode Data Set, 20% of patients entering medication-assisted opioid dependence treatment were primarily using prescription opioids [5].
Although the prevalence rate of prescription opioid dependence is increasing, most treatment studies of opioid-dependent populations have heretofore focused either exclusively or predominantly on heroin users [6–9]. Patients with prescription opioid dependence may differ in some important ways from heroin addicts. In fact, some evidence suggests that traditional treatments for opioid dependence may result in differential outcomes for persons dependent upon heroin versus prescription opioids [10, 11]. For example, Brands et al. [10] compared prescription opioid addicts with those dependent on heroin and those with mixed addictions, and found that those who used prescription opioids exclusively were less likely to have injected drugs, and were less likely to have other co-occurring substance use disorders. Both of these would be good prognostic characteristics, although the greater frequency of pain problems in prescription opioid users [12–14] could counterbalance this, since co-occurring pain has generally been a poor prognostic indicator in patients with substance use disorders [15]. With these differences between prescription opioid users and heroin users, one cannot assume that the same treatment strategy that would be recommended for heroin addicts should be advocated for those dependent upon prescription opioids. Understanding the response to different treatments among patients with prescription opioid dependence is thus important for determining whether specific treatment strategies should be tailored for this population.
Clinical research over the last ten years has established sublingual buprenorphine/naloxone (bup/nx) as a safe and effective pharmacotherapy for opioid dependence. Under the provisions of the Drug Addiction Treatment Act of 2000, qualifying physicians may prescribe and/or dispense bup/nx for the treatment of opioid dependence in an office-based setting [16]. Office-based treatment has created opportunities to intervene earlier in the course of treatment for opioid dependence [17], yet, little is known about the use of bup/nx in patients with prescription opioid dependence. For example, there are no data on the optimal length of bup/nx treatment [18] or the role of additional counseling in the treatment of this population.
In response to this gap in knowledge and the epidemiologic trends described above, the Prescription Opioid Addiction Treatment Study (POATS) was launched by the National Institute on Drug Abuse Clinical Trials Network in 2006 to examine the efficacy of different lengths of bup/nx treatment, paired with different intensities of medical and psychosocial counseling, for patients dependent upon prescription opioids. This paper describes the rationale and design of this study.
2. Research Design and Study Organization
2.1. Research Design
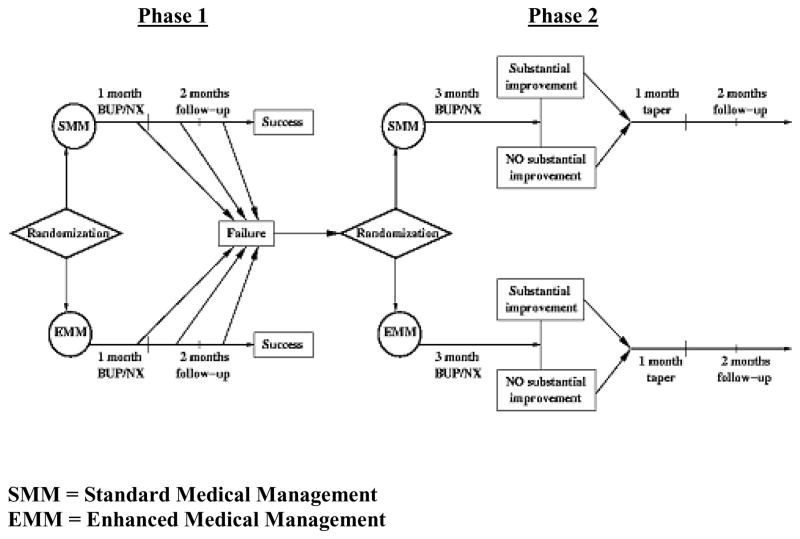
To study different lengths of bup/nx treatment, we created a two-phase adaptive, sequential treatment design (see Figure 1) to approximate what a physician might do in clinical practice – start with a relatively non-intensive treatment approach, and, if unsuccessful, institute more intensive treatment. This type of research design is an example of a practical clinical trial [19], which has been applied elsewhere in psychiatry [20] and general medicine [21]. Phase 1 in our trial consisted of 4 weeks of bup/nx treatment, including a dose taper over 14 days, with 8 weeks of follow-up. Bup/nx induction was designed for rapid dose stabilization (i.e., most participants received a total dose of 12 mg on day 1 and 16 mg on day 2, with flexible dosing adjustments as clinically indicated during the first 14 study days), consistent with bup/nx outpatient clinical practice guidelines [22]. Phase 1 participants were monitored for treatment response during these 12 weeks. Those who relapsed to opioid use and thus met Phase 1 “failure” criteria (See Section 6.1) were then invited to enter Phase 2 of the trial, consisting of a 12-week bup/nx stabilization treatment, followed by a 4-week taper and 8 weeks of post-treatment follow-up.
Figure 1.
Study Design
To examine the role of drug counseling in addition to bup/nx for prescription opioid dependence, participants in each phase were randomized to either a) Standard Medical Management (SMM), meant to approximate typical office-based opioid treatment by a physician, or b) Enhanced Medical Management (EMM), meant to approximate a more intensive opioid dependence treatment, consisting of SMM plus individual drug counseling.
2.2. Research Questions and Hypotheses
The primary research questions for the study are: What benefit, if any, does EMM offer over SMM a) in a short-term treatment paradigm (a 4-week bup/nx treatment, including a 2-week dose taper) and b) in a longer-term treatment paradigm (12 weeks of a stabilization dose of bup/nx) for participants who have not responded successfully to the initial short-term bup/nx treatment?
The primary hypothesis for Phase 1 is that there will be a higher rate of success (defined below) in Phase 1 among participants receiving EMM than among participants receiving bup/nx and SMM alone. For Phase 2, the primary hypothesis is that among participants who have been unsuccessful in Phase 1, the rate of “substantial improvement” (defined below) in Phase 2 will be higher in the group that receives EMM than in the group that receives SMM alone.
Secondary objectives will determine 1) subject characteristics that predict likelihood of successful outcomes (e.g., presence of chronic pain at baseline, reason for initial use of opioids, sociodemographic characteristics, history of lifetime heroin use); 2) the percentage of the study population that responds successfully in Phase 1, either with or without concomitant drug counseling; and 3) whether EMM participants will be more likely than SMM participants to have substantial improvement at the end of 24 weeks of Phase 2, following the taper off bup/nx during weeks 13–16 and 8 weeks of follow-up.
2.3. Study Organization and Sites
POATS is being conducted under the auspices of the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN); the CTN, a partnership between academic research centers and drug abuse community treatment programs (CTPs), conducts multi-site clinical trials with drug-dependent patients in CTPs. The CTN is organized into 16 “nodes,” each of which consists of a research center and several CTPs. POATS has taken place at 10 CTPs around the country: Chestnut Ridge Hospital (Morgantown, WV), San Francisco General Hospital (San Francisco, CA), St. Luke’s Roosevelt Hospital (New York, NY) Long Island Jewish Medical Center-Addiction Recovery Services (Glen Oaks, NY), Bellevue Hospital Center (New York, NY), McLean Hospital (Belmont, MA), East Indiana Treatment Center (Lawrenceburg, IN), ADAPT, Inc. (Roseburg, OR), UCLA Integrated Substance Abuse Programs (Los Angeles, CA), Behavioral Health Service of Pickens County (Pickens, SC), and Providence Behavioral Health Services (Everett, WA). The nodes, based at academic research centers, provide the CTPs with support, technical assistance, and study oversight. For each CTN trial, a node designated as the “lead node” is responsible for providing participating study sites with direction and guidance to ensure consistency of protocol implementation. The Northern New England Node, with its research headquarters located at McLean Hospital, Belmont, MA, has served as the lead node for this trial. CTN trials are also supported by a Clinical Coordinating Center (CCC), the EMMES Corporation (Rockville, MD), and a Data and Statistics Center (DSC), the Duke Clinical Research Institute (Durham, NC). The CCC is an organization selected by NIDA to provide centralized support for regulatory functions, safety and protocol monitoring, training of staff, pharmaceutical supply services, drug testing and analytical laboratory services, and protocol development. The DSC is selected by NIDA to provide centralized support for collecting, managing, and storing study data; designing and performing statistical analyses; reviewing and monitoring data quality; monitoring trial progress; and participating in the protocol development process.
2.4. Site Selection Process
To select sites, we gathered data from public sources of information [3–5]available from the National Institute on Drug Abuse, the Substance Abuse and Mental Health Services Administration, the Department of Justice, the Drug Enforcement Administration, and state and local agencies to identify areas of the country with geographic concentrations of prescription opioid misuse. Next, the lead node solicited sites within the CTN and asked them to complete an in-depth survey that the lead node used to evaluate staffing resources and experience in clinical research. Each potential site was then asked to prospectively monitor its patient case-mix for the presence of patients seeking treatment for prescription opioid dependence. Using information from the surveys, national prevalence data, and the prospective monitoring process, the lead node chose sites in rural, suburban, and urban areas with high rates of prescription opioid misuse, coupled with the resources and capacity to carry out the trial.
2.5. Study Population
The study population included men and women age ≥18 meeting DSM-IV criteria for opioid dependence with physiologic features, excluding prominent heroin use (see below). Participants with pain who had been taking opioid medication as prescribed and simply wanted detoxification were not eligible. Rather, participants had to be abusing their medication to be eligible. Eligible participants who were being prescribed opioids for the treatment of pain needed to consent to have the study physician at the research site consult with their prescribing physician prior to acceptance into the study, to ensure that all participants were sufficiently medically stable to undergo withdrawal from prescribed opioids.
2.6. Inclusion and Exclusion Criteria
Inclusion and exclusion criteria are presented in Table 1. Criteria were defined with a goal of identifying a distinct but generalizable population of treatment-seeking individuals who were dependent upon prescription opioid drugs, by including 1) participants both with and without chronic pain; 2) those who had been prescribed opioids by a physician and those who had obtained them illicitly; and 3) those who had occasionally used heroin, since some patients who seek treatment for prescription opioid dependence have also used heroin [23].
Table 1.
Prescription Opioid Addiction Treatment Study - General Eligibility Criteria
Inclusion Criteria
|
Exclusion criteria
|
2.7. Randomization
For Phase 1, randomization was stratified by two factors expected to have prognostic implications: presence or absence of 1) lifetime heroin use, and 2) current chronic pain. In Phase 2, participants were re-randomized and stratified by the Phase 1 treatment assignment (EMM or SMM). Randomization to treatment assignment was accomplished by using an interactive voice response system operated by Almac Clinical Technologies in partnership with the DSC.
3. Data Management
A web-based distributed data entry model was developed in accordance with 21CFR § 11.1 [24]. Data were collected at the study sites for entry into a secure, password-protected, web-based electronic data capture system. Research staff members were trained in the proper use and navigation of the data entry system via both centralized in-person and web-based training sessions, with booster sessions offered throughout the study.
4. Data and Patient Safety Monitoring
Data monitoring occurred on four levels, via 1) an independent Data Safety and Monitoring Board (DSMB), 2) external Quality Assurance monitoring site visits, 3) routine data entry audits, and 4) reviews of all serious adverse events (SAEs) by an independent medical monitor, and by the study PI and the rest of the lead node team, with subsequent discussion of each SAE on the weekly study national call.
The NIDA-appointed DSMB was responsible for conducting periodic reviews of safety data and was charged with determining whether there was support for continuation of the trial, or if there was evidence that study procedures should be changed, or if the trial should be halted for reasons relating to safety or trial performance. Interim analysis of efficacy data was deemed unnecessary because of the study design and enrollment projection.
Quality Assurance monitors visited all sites to 1) audit subject records to assure that submitted data was accurate and in agreement with source documentation; 2) ensure that the investigational medications were properly stored and accounted for; 3) verify that consent had been properly obtained; 4) confirm that participants met inclusion/exclusion criteria; and 5) assure that Good Clinical Practice [25] guidelines were appropriately followed.
To ensure data entry quality, a random sample of case report forms (CRFs) was selected by the DSC from each CTP for a CRF-to-database audit. The target data audit goal was an error rate of <0.5%, as calculated by the number of data discrepancies divided by the number of data fields audited.
5. Study Treatments
5.1. Pharmacotherapy
All participants received sublingual bup/nx. The maximum allowable dose was 32 mg per day and the minimum allowable dose was 8 mg per day, consistent with current practice guidelines [22]. Prescribing of ancillary comfort medications (e.g., non-steroidal anti-inflammatory drugs for arthralgias, dicyclomine for abdominal cramps, loperamide for diarrhea) during the bup/nx taper was initiated at the physician’s discretion in accordance with clinical need to assist with the management of withdrawal symptoms. All ancillary medications were entered into the database as concomitant medications.
Physicians dispensed a weekly supply of bup/nx, with written dosing instructions at the end of the SMM visit. Participants were instructed to take bup/nx only as prescribed and to secure bup/nx to prevent access to others, particularly children. Participants were also notified that lost or stolen medication would not be replaced, nor would additional bup/nx be provided should they use more than the prescribed amount of bup/nx. Provisions were made for urgent SMM visits and/or between-visit dose adjustments as clinically indicated and allowed by the protocol. Dosing taper was flexible but confined to a 14-day duration in Phase 1 and a 4-week duration in Phase 2.
5.2. Standard Medical Management
Standard Medical Management (SMM) was delivered to all participants, according to the Manual for SMM of Opioid Dependence with Buprenorphine by Fiellin et al. [26]. SMM, originally designed for use by medical personnel in primary care settings, consists of relatively brief (i.e., 15–20 minutes) medically focused visits that combine standard medication (i.e., buprenorphine) management with brief counseling methods to help participants attain and maintain abstinence from illicit opioid use. SMM was able to be delivered with adequate adherence and competence in a previous study by Fiellin et al. [27]. In consultation with the authors, the manuals were modified slightly for this trial, to fit the study design and address specific needs of this population, including individuals with chronic pain.
Key elements of SMM include monitoring substance use and medication adherence; educating participants about opioid dependence and buprenorphine; encouragement to abstain from illicit opioids and other substances of abuse; encouragement to attend Alcoholics Anonymous (AA), Narcotics Anonymous (NA) or other self-help groups; encouragement to make lifestyle changes to facilitate recovery; identification of other medical problems; referral to specialty services if needed; and asking about pain (specifically for this protocol).
SMM was administered by licensed physicians who had received previous training and certification in the use of buprenorphine as well as training and certification in the delivery of SMM. The initial session in Phase 1 was approximately one hour long, during which the physician reviewed the participant’s medical, psychiatric, and substance use problems, discussed the diagnosis of opioid dependence, developed a treatment plan, advised the participant to abstain from all substances of abuse, referred the participant to Narcotics Anonymous or other self-help groups, and answered questions. The initial session in Phase 2 was approximately 30–60 minutes, depending on medical necessity. Subsequent visits in each phase lasted approximately 15–20 minutes each, and included a post-induction follow-up visit in Week 1 of both phases, then weekly visits during the rest of active bup/nx treatment. At these visits, the physician reviewed the participant’s substance use since the previous visit (including urine toxicology results); reviewed the response to bup/nx and associated adverse events; made bup/nx dosing adjustments as clinically indicated and according to protocol allowance; advised abstinence; addressed non-adherence to treatment if indicated; asked about self-help group participation and lifestyle issues; asked about pain, and made referrals as clinically indicated; asked about previous referrals and non-study treatments (e.g., psychiatric maintenance treatment); and dispensed bup/nx.
5.3. Enhanced Medical Management
In choosing a psychosocial treatment to accompany the use of buprenorphine/naloxone, we selected a treatment with the following properties: it was manualized; it had previously been used in a similar trial; it could be used in either a primary care or a specialized drug abuse treatment setting; it could be easily learned and delivered, with minimal specialized training; adherence to the manual was likely to be good; and, if successful, the treatment could be easily disseminated to either primary care or drug abuse treatment settings. The Manual for EMM of Opioid Dependence, by Pantalon et al. [28], was chosen because it met all of the criteria listed above. The individual counseling sessions that were delivered as part of EMM included encouragement to attend treatment, take medication as prescribed, and abstain from opioids and other drugs of abuse; the counselor supported attendance at mutual-help groups such as Narcotics Anonymous, and focused on behavioral and lifestyle changes conducive to recovery. Sessions included education about the processes of addiction and recovery, through a discussion of such topics as “Understanding Addiction,” “The Stages of Recovery,” “Coping with High Drug-Risk Situations,” and “Establishing a Support System.”
In the Fiellin et al. [27] study referenced above, the investigators found lower medication adherence and lower treatment retention with SMM than with EMM, but little between-group difference in the amount of reduction of illicit opioid use. However, the difference in the amount of treatment received by the two groups in that study was rather small: 20 minutes a week for the SMM cohort and 45 minutes a week for the EMM cohort [27]. A greater contrast between SMM and EMM was chosen for our study; rather than simply delivering longer visits in EMM than in SMM, we designed our study so that EMM consisted of additional counseling visits by a separate person. This design reflects a different model of care in SMM vs. EMM (i.e., office-based medical treatment vs. drug abuse treatment program) and provides a greater contrast in amount of treatment exposure. In addition to the weekly SMM visits in both phases, EMM participants received twice a week 45-minute individual drug counseling sessions while receiving medication in Phase 1 (weeks 1–4). In Phase 2, EMM participants received twice a week counseling sessions for the first 6 weeks, and weekly counseling sessions in weeks 7 – 12.
EMM clinicians (i.e., those delivering drug counseling in addition to SMM visits) were substance abuse or mental health professionals (e.g., counselors, social workers, psychologists, or nurses) employed by the CTPs. We considered using cognitive-behavioral therapy as our counseling model. However, a survey of CTPs in the Northern New England node showed that few of the staff delivering treatment in these CTPs had formal cognitive-behavioral training. Additionally, because an important goal of POATS is the development of a treatment that can be disseminated and delivered in a wide variety of treatment settings following completion of the study, the model of treatment in the Pantalon et al. manual [28] was selected.
5.4. Pain Assessment and Intervention
The protocol design included pain assessments, given that 1) some participants entering the study would have been prescribed opioids for pain relief prior to study entry, and 2) opioid dependence itself is often associated with pain syndromes and/or opioid-related hyperalgesia [29]. Because pain intensity was recognized as a potentially important factor affecting treatment outcomes, regular pain assessment was incorporated into the protocol for participants identified with chronic pain at baseline. In these cases, pain assessments also provide a clinical safety monitoring tool for participants at risk for adverse events related to uncontrolled pain (and potentially requiring administrative termination from the study in order to receive opioid pain treatment). POATS was not designed as a pain treatment study but rather, a study examining treatments for opioid dependence; testing treatments for pain per se was beyond the scope of this project.
Physicians and counselors received brief training by an expert in chronic pain management prior to study participation. All clinicians were notified if a participant was identified at baseline as having current chronic pain. Participants with pain were monitored at each medical management visit with regard to their pain, and all participants with pain received and were encouraged to use a self-guided behavioral pain management manual [30]. Material was also added to the EMM manual that addressed the impact of pain on the recovery process from substance dependence (e.g., as a potential trigger for substance abuse relapse); pain was addressed by the counselors when clinically warranted, with a focus on the relation between pain and substance abuse. Study physicians could refer participants with pain to their own physician or to a pain program, but study physicians did not treat participants for pain (other than briefly for withdrawal discomfort) within the context of the study. Importantly, bup/nx adjustments were never made specifically for the purpose of pain relief.
6. Assessments
6.1. Phase 1 and Phase 2 Primary Outcome Measures
The primary outcome in Phase 1 was “success” or “failure,” as defined below. To achieve “Phase 1 success” status, a participant had to meet all of the following criteria: self-reported use of opioids on ≤ 4 days per month (beginning at day 15); absence of 2 consecutive urine screens positive for opioids (beginning at day 15); completion of the 4-week medication regimen and 8-week follow-up period without participating in other formal substance abuse treatment (other than self-help groups); missing no more than one urine sample after day 15. Those who were Phase 1 successes were considered finished with the trial. Participants who did not meet all of these success criteria were classified as “Phase 1 failures,” and in most instances, were eligible for Phase 2. The primary outcome in Phase 2 was presence or absence of “substantial improvement,” defined as abstaining from opioids during week 12 (i.e., the last week of bup/nx stabilization treatment) and for at least 2 of the previous 3 weeks. Abstinence was determined by urine-verified self-reports of opioid abstinence; missing urines were considered positive for opioids. The primary outcome for the overall study was the presence or absence of Phase 2 substantial improvement.
Urine was collected at baseline prior to bup/nx induction, at each weekly visit thereafter, and at each follow-up visit; see Tables 2 and 3 for the schedule of assessment visits. Urine samples were analyzed for drugs of abuse, including prescription opioids (e.g., oxycodone, hydrocodone) in addition to the standard drugs of abuse. Urine screens for bup/nx were performed at Weeks 10 and 12 in Phase 1 and Weeks 22 and 24 in Phase 2, to monitor whether participants defined as “successes” at the end of Phase 1 and “substantially improved” at the end of Phase 2 (in both instances, when they were supposed to have stopped taking study-supplied bup/nx) were, in fact, taking buprenorphine that they had obtained outside the study.
Table 2.
Visit Schedule for Phase 1 of POATS
| Phase 1 (visit week #) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assessments | Screening/Baseline Measures | BUP/NX Treatment, Initial Follow-Up Taper | Final Visit | |||||||||||
| WEEK NUMBER ≫ | 1a | 1b | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | (Week 12 if treatment success, or at time of treatment failure) | |
| SCREENING ASSESSMENTS | ||||||||||||||
| Informed Consent | X | |||||||||||||
| Inclusion/Exclusion Criteria | X | X | ||||||||||||
| Demographics | X | |||||||||||||
| Randomization | X | |||||||||||||
| CIDI/Substance Use Diagnosis | X | |||||||||||||
| SAFETY ASSESSMENTS | ||||||||||||||
| Clinical Opiate Withdrawal Scale (COWS) | X | X | X | X | X | X | X | X | ||||||
| Medical/Psychiatric Evaluation (including physical exam) | X | Physical Exam Only | ||||||||||||
| Vital Signs | X | X | X | X | X | X | X | X | X | X | ||||
| Lab Tests (LFTs, Chemistry, Hematology, Urinalysis) | X | X | ||||||||||||
| Pregnancy Test | X | X | X | |||||||||||
| Adverse Event (AE) Evaluation | X | X | X | X | X | X | X | X | X | |||||
| Serious Adverse Event (SAE) Evaluation | ≪ Completed when necessary ≫ | |||||||||||||
| Concomitant Treatments (including medications & psychosocial) | X | X | X | X | X | X | X | X | X | X | ||||
| EFFICACY ASSESSMENTS | ||||||||||||||
| Craving Visual Analog Scale (VAS) | X | X | X | X | X | X | X | X | X | X | ||||
| Substance Use Report-Baseline | X | |||||||||||||
| Substance Use Report-Follow-up | X | X | X | X | X | X | X | X | X | |||||
| Urine Drug Screen | X | X | X | X | X | X | X | X | X | X | ||||
| PAIN ASSESSMENTS | ||||||||||||||
| Pain & Opioid Analgesic Use History | X | |||||||||||||
| Brief Pain Inventory | X | X | X | X | ||||||||||
| Brief Pain Inventory abbrev (if pain stratification) | X | X | X | X | ||||||||||
| Beck Depression Inventory II | X | X | X | X | ||||||||||
| SF-36 | X | X | ||||||||||||
| OTHER ASSESSMENTS | ||||||||||||||
| Addiction Severity Index (ASI Lite) | X | |||||||||||||
| Addiction Severity Index (ASI Lite) Follow-Up | X | |||||||||||||
| RBS (Risk Behavior Survey) | X | |||||||||||||
| CIDI/Depression & PTSD Diagnosis | X | |||||||||||||
| Fagerstrom Test for Nicotine Dependence | X | X | ||||||||||||
| TREATMENT PLAN | ||||||||||||||
| BUP/NX Induction | X | |||||||||||||
| BUP/NX Dosing or Taper | X | X | X | X | X | |||||||||
| Medication Accountability | X | X | X | X | X | |||||||||
| Standard Medical Management | X | X | X | X | X | X | X | |||||||
| Enhanced Medical Management | 2X | 2X | 2X | 2X | X | X | ||||||||
| VISIT DURATION (minutes) | 240 | 60–90 | 60–90 | 30 | 120 | |||||||||
Table 3.
Visit Schedule for Phase 2 of POATS
| Phase 2 (visit week #) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assessments | Final Visit Phase 1 | BUP/NX Stabilization | BUP/NX Taper | Follow-Up | ||||||||||||||||||||||
| WEEK NUMBER ≫ | 1a | 1b | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| SCREENING ASSESSMENTS | ||||||||||||||||||||||||||
| Consent Review | X | |||||||||||||||||||||||||
| Eligibility Review | X | |||||||||||||||||||||||||
| Randomization | X | |||||||||||||||||||||||||
| SAFETY ASSESSMENTS | ||||||||||||||||||||||||||
| Vital Signs | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Lab Tests (LFTs, Chemistry, Hematology, Urinalysis) | X | X | ||||||||||||||||||||||||
| Pregnancy Test | X | X | X | X | X | |||||||||||||||||||||
| Adverse Event (AE) Evaluation | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Serious Adverse Event (SAE)Evaluation | ≪Completed when necessary≫ | |||||||||||||||||||||||||
| Physical Exam | X | X | ||||||||||||||||||||||||
| Clinical Opiate Withdrawal Scale (COWS) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Concomitant Treatments (including medications &psychosocial) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| EFFICACY ASSESSMENTS | ||||||||||||||||||||||||||
| Craving Visual Analog Scale (VAS) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Substance Use Report Follow-up | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Urine Drug Screen | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| PAIN ASSESSMENTS | ||||||||||||||||||||||||||
| Brief Pain Inventory | X | X | X | X | X | X | X | |||||||||||||||||||
| Brief Pain Inventory abbrev (if pain stratification) | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Beck Depression Inventory II | X | X | X | X | X | X | X | |||||||||||||||||||
| SF-36 | X | X | X | |||||||||||||||||||||||
| OTHER ASSESSMENTS | ||||||||||||||||||||||||||
| Addiction Severity Index (ASI Lite) Follow-up | X | X | ||||||||||||||||||||||||
| Fagerstrom Test for Nicotine Dependence | X | X | ||||||||||||||||||||||||
| TREATMENT PLAN | ||||||||||||||||||||||||||
| BUP/NX Induction (if applicable) | X | |||||||||||||||||||||||||
| BUP/NX Dosing or Taper | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Medication Accountability | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Standard Medical Management | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Enhanced Medical Management | 2X | 2X | 2X | 2X | 2X | 2X | X | X | X | X | X | X | ||||||||||||||
| VISIT DURATION (minutes) | 120 | 60–90 | 60 | 30 | 60 | |||||||||||||||||||||
Substance use data (opioids, other drugs of abuse, and alcohol) were collected in conjunction with weekly medical management visits. A calendar technique based on the Timeline Follow-back (TLFB) [31], which helps to fill in missing data in case of missed visits, was used to fill in each day since the last visit. Baseline data collection reviewed the past 30 days of substance use.
6.2. Secondary Outcomes
Other outcomes that were assessed included 1) opioid craving, as assessed by Visual Analog Scales (VAS); 2) pain (for patients meeting criteria for chronic pain at baseline), assessed by the Brief Pain Inventory (BPI) [32]; 3) depression, assessed by the Beck Depression Inventory [33]; and 4) quality of life, as assessed by the SF-36 [34].
6.3. Other Assessments
6.3.1. Substance Use Assessment
The Composite International Diagnostic Interview (CIDI) [35] was used for diagnosis. The CIDI is a comprehensive, standardized instrument for assessment of mental disorders according to ICD-10 and DSM-IV criteria. CIDI Section J (Alcohol Use Disorders) and Section L (Substance-related Disorders) were used to generate diagnoses to determine study eligibility. In addition, questions from CIDI Section E (Major Depressive Disorders) and Section K (Post-Traumatic Stress Disorder) were administered at baseline to characterize the study population, consistent with the exploratory research question of whether these disorders moderate the effect of treatment on outcome.
The Addiction Severity Index (ASI) [36] was used to assess severity of substance use and its associated problems. The ASI is a standardized, multi-dimensional, semi-structured, comprehensive clinical interview that provides clinical information to assess problem severity in seven areas of functioning that are frequently affected in patients with substance use disorders: drug use, alcohol use, employment, legal issues, medical condition, social/family functioning, and psychological status.
Urine samples were analyzed for drugs of abuse prior to dispensing medication. Urine was collected at baseline, just prior to bup/nx induction, and at all medical management and follow-up visits. Self-reports of substance use were collected in conjunction with weekly medical management visits, using a calendar technique based on the Timeline Follow-back method [31]. Opioid craving was assessed with visual analog scales (VAS), which were completed at medical management and follow-up visits. Opioid withdrawal was assessed with the 11-item Clinical Opiate Withdrawal Scale (COWS) [37], which was completed at each medical management visit during treatment. Smoking was assessed with the Fagerstrom Test for Nicotine Dependence [38], which was completed by participants at baseline and at the final follow-up visit in both Phase 1 and Phase 2.
6.3.2. Pain Assessment
A supplementary battery of assessments was administered to those participants identified as suffering from chronic pain. Pain was assessed by the Brief Pain Inventory and the Pain and Opioid Analgesics Use History. The Brief Pain Inventory-SF (BPI-SF) [32] is a 9-item assessment of intensity of pain and interference of pain in life functioning. The complete BPI-SF was collected at baseline and monthly during Phase 1 and Phase 2. For patients identified at baseline and stratified as chronic pain patients, 4 BPI-SF items were asked weekly throughout treatment as part of their medical management visits for the purpose of monitoring physical pain.
The Pain and Opioid Analgesics Use History was collected at the Phase 1 baseline visit to determine a variety of pain-associated issues, including body region(s) affected by pain; duration of pain; pain treatment history; initial reason for initiating opioid use (i.e., prescribed for pain relief versus illicit use for pain relief versus illicit non-medical use); and current and past sources of prescription opioids.
6.3.3. Mood Assessment
Mood was assessed using the Beck Depression Inventory II (BDI), a 21-item scale [33] used to assess common features of depression on a 4-point severity scale, with a focus on cognitions. The BDI was completed by participants at baseline and then monthly during Phases 1 and 2.
6.3.4. Quality of Life Assessment
Quality of Life was assessed by the SF-36, version 2, a 36-item, participant-administered instrument examining health-related quality of life changes as a function of treatment [34]. This was completed at the beginning and end of both Phase 1 and Phase 2.
6.3.5. Safety Assessment
Safety assessments consisted of physical examinations, vital signs, laboratory tests, pregnancy tests, adverse event reporting, and reporting of concomitant medication use. The research staff asked participants about adverse events (AEs), including serious adverse events (SAEs), at each visit. An AE was defined in the protocol as any reaction, side effect, or untoward event that occurs during the course of the trial, whether or not the event was considered medication- or study-related or clinically significant. SAEs were defined as any fatal event, any immediately life-threatening event, any permanent or substantially disabling event, any event that required or prolonged hospitalization, any congenital anomaly, or any event that required intervention to prevent one of the above outcomes.
7. Data Analyses
As described earlier, this two-phase study was designed to study the additional benefit of EMM, compared to SMM, in both a short-term and a longer-term treatment paradigm. Thus, the primary analyses will focus on estimating success rates in both Phase 1 and Phase 2, and will test the hypotheses that the EMM arm would have higher success rates than the SMM arm in each phase.
In addition to the primary analyses, this study provides the opportunity to explore a broad range of other important research questions. These secondary analyses include a variety of outcomes, such as 1) the comparative probability (SMM vs. EMM) of having positive urine screens over the first 12 weeks of Phase 2; 2) the comparative likelihood of experiencing substantial improvement at the end of phase 2 (week 24); 3) the effect of treatment on pain; 4) the relation between pain and treatment efficacy; 5) the impact of a history of heroin use, post-traumatic stress disorder, or major depressive disorder on treatment outcome; 6) the potential moderating effect of reasons for prescription opioid use on outcome; 7) the impact of opioid craving during treatment on treatment outcome, and 8) the relation between sociodemographic characteristics (e.g., gender, ethnicity, and age) and outcome.
7.1. Statistical Considerations and Analysis
7.1.1. Statistical Modeling
Given the nature of the study, some outcomes will be correlated; correlation exists among outcomes of participants from the same sites as well as repeated measures taken from the same participant over time. To account for the correlation in the analysis, Generalized Estimating Equations (GEE) models will be employed. The GEE approach allows modeling of correlated data that are not necessarily normal, such as the primary outcomes for both Phase 1 and 2, i.e., treatment success and substantial improvement, respectively. It also allows specification of more than one clustering factor, so that both time and site can be treated as clustering factors. GEE methodology provides robust inference with respect to misspecification of the covariance, and allows analysis of continuous, categorical, and count data that may be missing for some participants either because of a missed appointment or study drop-out.
As an illustration, when site is the only clustering factor, such as in the efficacy analysis in Phase 1, the following model will be used to test the hypothesis that there is a higher rate of treatment success among participants receiving EMM than among patients receiving bup/nx and SMM.
The following model will be used to test this hypothesis:
where Yij is a dichotomous measure indicating whether the jth patient of the ith site meets the Phase 1 criteria for success or not, with Yij=1 for success and Yij=0 for failure; txtij is an indicator variable for treatments, with txtij=1 for the EMM group and txtij=0 for the SMM group. The model parameters are estimated by GEE, with site being a cluster. Testing the hypothesis is equivalent to testing for significance of β̂1. The success rates for EMM and SMM as well as the overall success rate will be estimated with point estimate and 95% confidence intervals. Model-based statistics will be considered for inferences. PROC GENMOD in SAS (2003) will be used to carry out these analyses. Analyses for secondary hypotheses are conducted using similar modeling process.
7.1.2. Adjusting for Covariates
The two randomization stratification factors, 1) a lifetime history of heroin use, and 2) current chronic pain status are included in all models.
7.1.3. Handling Missing Data
A weighted GEE model will be used, in which the weight is the inverse of non-missingness. As an alternative, we can fit a Generalized Linear Mixed model; this is a likelihood approach and is valid under the missing-at-random assumption. PROC GLIMMIX in SAS will be used.
8. Sample Size, Power, and Effect Size
Under the two-phase design framework, the sample size for this study was selected to ensure sufficient power (at least 80%) of a two-sided significance test with level of significance α=0.05 to detect clinically meaningful differences between EMM and SMM in Phase 2 with respect to the primary outcome. Based on a test statistic proposed by Liu and Liang [39], employing GEE to account for correlation among measurements of patients from the same site, we determined that a total of 324 subjects would be needed for phase 2.
In estimating the percentage of Phase 1 patients who would enter Phase 2, we reviewed the literature and consulted experts in the field; we assumed a Phase 1 success rate of about 20% overall (averaging EMM and SMM); we also estimated that approximately 40% of the treatment failures (about 30% of all randomized participants) would either be lost to follow-up or ineligible or unwilling to participate in Phase 2. With this conservative estimate of a 50% rate of transition from Phase 1 to Phase 2, it was projected that about 2×324=648 participants would need to enter Phase 1 to achieve our Phase 2 sample size of 324. The success rates for SMM and EMM were expected to be 10%–20% (PSMM) and 20%~30% (PEMM), respectively, and the within-site correlation small (0 ~ 0.1). The proposed sample size (648) gives sufficient power (> 80%) to detect the difference with a two-sided test at α = 0.05.
9. Current Status of the Study
As of the time of the writing of this paper, the study has completed its enrollment, treatment, and follow-up phases. A total of 653 participants were randomized in Phase 1, and 360 participants entered Phase 2. Study results will be reported in future publications.
10. Discussion
The design of the POATS trial has a number of unique characteristics and strengths that will help to answer important questions regarding the treatment of individuals with prescription opioid dependence. The two-phase adaptive treatment research design provides an innovative approach to examining the efficacy of different lengths of bup/nx treatment, paired with different intensities of accompanying counseling. Because there is evidence that prescription drug abusers may have a better prognosis than those with heroin dependence [10, 11], POATS will help to determine the frequency with which a short-term taper (as in Phase 1) with bup/nx could be beneficial to this population, with or without individual counseling. The POATS design is strengthened by the selection of sites from rural, urban, and suburban sites throughout the country, enhancing the generalizability of the study’s results.
The two-phase design that is intended to approximate real-world practice (i.e., start with a relatively non-intensive approach, and then institute more intensive treatment if the first treatment fails) provides ecological validity to the study. However, designing and instituting a two-phase study also poses specific challenges. First, of course, is the choice of the two study phases. We chose a 4-week detoxification period after consulting multiple physicians and CTN community treatment program directors around the country with experience using bup/nx in opioid dependence treatment. When the study was being designed in 2006, detoxification was the most common initial treatment for this population. This design choice was also supported by findings from a study by Gandhi et al. [40], who reported reduced frequency and intensity of drug use at 1-month and 3-month follow-up in a relatively young population (ages 18–25) of heroin addicts after a very brief (3-day) detoxification with buprenorphine. While some practitioners used a rapid taper schedule (as little as several days) at the time of the study design, a 4-week taper was most frequently utilized, and was therefore chosen for POATS. Moore et al. [11] found that participants in their opioid dependence treatment study who were dependent on prescription opioids were significantly younger and had significantly fewer years of opioid use than heroin-dependent individuals; thus, one might expect better outcomes following detoxification in this population than in traditional heroin-dependent populations. In fact, Moore et al. [11] did report substantially better outcomes for those dependent on prescription opioids than for participants dependent on heroin (63% vs. 31% of participants achieving 6 consecutive weeks of opioid-negative urines in a 24-week bup/nx trial); this finding provided additional support for our hypothesis that a substantial minority of individuals dependent on prescription opioids would succeed in Phase 1 of our study.
When considering which options to study in participants who relapsed to opioids during Phase 1, a longer period (12 weeks) of bup/nx stabilization was chosen for Phase 2 to study the impact of additional counseling (i.e., EMM vs. SMM) during both bup/nx detoxification and longer-term bup/nx treatment.
Many of the participants entering this study have received opioids by prescription from a physician; a key question for physicians treating these individuals is thus whether their drug dependence can be managed in a medical office setting (as exemplified by SMM) or whether they should instead be referred to a specialty drug abuse treatment program, a model of care more consistent with EMM. Although SMM and EMM in this study occur in the same setting, the contrast between these two models of care should help to answer the question of the optimal treatment setting for this population. Moreover, since many such patients may resist referral to a drug abuse treatment program, studying the efficacy of SMM could help determine whether treatment in an office setting, without additional specialized counseling, is a reasonable approach for this population.
Another challenge in designing and implementing an adaptive treatment research design of this type involved masking from participants the eligibility criteria that would trigger entry into Phase 2. If these criteria were revealed, then some participants who wanted additional bup/nx treatment could falsify their self-report data (i.e., by reporting more ongoing opioid use than actually occurred) in order to be classified as Phase 1 failures. We managed this situation by emphasizing during initial training and booster sessions with study staff the importance of not disclosing Phase 1 failure criteria to participants; we also engaged in role playing scenarios with staff illustrating ways to respond when study participants asked about criteria for entry into Phase 2.
Another challenge involved powering the study when we were uncertain about how many participants from the Phase 1 population would be eligible for and interested in Phase 2. We were, in essence, working backwards in conducting our power analysis; we knew that we needed 324 participants in Phase 2, but had to estimate the number of Phase 1 participants needed to produce this Phase 2 sample size. We addressed this by making conservative assumptions that approximately 50% of subjects randomized to Phase 1 would meet treatment failure criteria and agree to be randomized in Phase 2.
POATS presents a unique opportunity to evaluate a treatment strategy, rather than a discrete treatment intervention for prescription opioid dependence. This is the largest study yet conducted with this population, and also represents one of the first adaptive treatment research designs to be instituted in a large-scale addiction treatment study. This type of study design with drug dependent patients presents both advantages and challenges, as described above; a clinical trial of this scope should generate important results regarding the delivery of treatment for this important population.
Acknowledgments
This work was supported by NIDA Clinical Trials Network Grants U10 DA015831 (RDW, Northern New England Node); and U10 DA013045 (WL, Pacific Node); NIDA Contract HHSN2712005220HC (Duke Clinical Research Institute); and NIDA Grants K24 DA022288 (RDW) and K23 DA022297 (JSP). Some NIDA personnel were involved in the study and in the authorship of this paper.
We thank the staff and participants at the community treatment programs and regional research and training centers of the National Institute on Drug Abuse Clinical Trials Network for their involvement in this project, including Chestnut Ridge Hospital (Appalachian Tri-State Node), San Francisco General Hospital (California/Arizona Node), St. Luke’s Roosevelt Hospital and Long Island Jewish Medical Center-Addiction Recovery Services (Long Island Node), Bellevue Hospital Center (New York Node), McLean Hospital (Northern New England Node), East Indiana Treatment Center (Ohio Valley Node), Adapt, Inc. (Oregon/Hawaii Node), Integrated Substance Abuse Programs (Pacific Node), Behavioral Health Service of Pickens County (Southern Consortium Node), and Providence Behavioral Health Services (Washington Node). We also thank the staff of the Clinical Coordinating Center (The EMMES Corporation, Rockville, MD) and the Data and Statistics Center (The Duke Clinical Research Institute, Durham, NC), and the staff of the Center for the Clinical Trials Network at the National Institute on Drug Abuse (Rockville, MD) for their work on this project.
Footnotes
Clinical Trials.gov Identifier: NCT00316277
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volkow ND. Confronting the rise in abuse of prescription drugs. NIDA Notes. 2005;19(5):3. [Google Scholar]
- 2.Janofsky M. Drug-Fighters Turn to Rising Tide of Prescription Abuse. The New York Times; Mar 18, 2004. [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. DAWN Series D-30. Rockville, MD: 2008. Drug Abuse Warning Network 2006: National Estimates of Drug-Related Emergency Department Visits. [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration. NSDUH Series H-34. Rockville, MD: Office of Applied Studies; 2008. Results from the 2007 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration Office of Applied Studies. DASIS Series: S-45. National Admissions to Substance Abuse Treatment Services; Rockville, MD: 2009. Treatment Episode Data Set (TEDS). Highlights - 2007. [Google Scholar]
- 6.Amato L, Minozzi S, Davoli M, Vecchi S, Ferri M, Mayet S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev. 2008;(4):CD004147. doi: 10.1002/14651858.CD004147.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Fiellin DA, Pantalon MV, Pakes JP, O’Connor PG, Chawarski M, Schottenfeld RS. Treatment of heroin dependence with buprenorphine in primary care. Am J Drug Alcohol Abuse. 2002;28(2):231–41. doi: 10.1081/ada-120002972. [DOI] [PubMed] [Google Scholar]
- 8.Ling W, Wesson D. Clinical efficacy of buprenorphine: comparisons to methadone and placebo. Drug Alcohol Depend. 2003;70(2 Suppl):S49–57. doi: 10.1016/s0376-8716(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor PG, Oliveto AH, Shi JM, Triffleman EG, Carroll KM, Kosten TR, et al. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. Am J Med. 1998;105(2):100–5. doi: 10.1016/s0002-9343(98)00194-6. [DOI] [PubMed] [Google Scholar]
- 10.Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug Alcohol Depend. 2004;73(2):199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22(4):527–30. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry DT, Beitel M, Joshi D, Schottenfeld RS. Pain and substance-related pain-reduction behaviors among opioid dependent individuals seeking methadone maintenance treatment. Am J Addict. 2009;18(2):117–21. doi: 10.1080/10550490902772470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havens JR, Walker R, Leukefeld CG. Prescription opioid use in the rural Appalachia: a community-based study. J Opioid Manag. 2008;4(2):63–71. doi: 10.5055/jom.2008.0010. [DOI] [PubMed] [Google Scholar]
- 14.Potter JS, Prather K, Weiss RD. Physical pain and associated clinical characteristics in treatment-seeking patients in four substance use disorder treatment modalities. Am J Addict. 2008;17(2):121–5. doi: 10.1080/10550490701862902. [DOI] [PubMed] [Google Scholar]
- 15.Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction. 2007;102(5):752–60. doi: 10.1111/j.1360-0443.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- 16.Drug Addiction Treatment Act of 2000. Public Law No. 106–310, Title XXXV - Waiver authority for physicians who dispense or prescribe certain narcotic drugs for maintenance treatment or detoxification treatment, 2000. [Accessed 2009 March 23]; Available from: http://buprenorphine.samhsa.gov/fulllaw.html.
- 17.Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment? Drug Alcohol Depend. 2005;79(1):113–6. doi: 10.1016/j.drugalcdep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Mendelson J, Flower K, Pletcher MJ, Galloway GP. Addiction to prescription opioids: characteristics of the emerging epidemic and treatment with buprenorphine. Exp Clin Psychopharmacol. 2008;16(5):435–41. doi: 10.1037/a0013637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–32. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 20.Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25(1):119–42. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 21.Materson BJ, Reda DJ, Preston RA, Cushman WC, Massie BM, Freis ED, et al. Response to a second single antihypertensive agent used as monotherapy for hypertension after failure of the initial drug. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Arch Intern Med. 1995;155(16):1757–62. [PubMed] [Google Scholar]
- 22.Center for Substance Abuse Treatment. Treatment Improvement Protocol (TIP) Series 40 DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. [PubMed] [Google Scholar]
- 23.Subramaniam GA, Stitzer MA. Clinical characteristics of treatment-seeking prescription opioid vs. heroin-using adolescents with opioid use disorder. Drug Alcohol Depend. 2009;101(1–2):13–9. doi: 10.1016/j.drugalcdep.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Archives and Records Administration. [Accessed May 7, 2009];Code of Federal Regulations, 21CFR11.1. Revised as of April 1, 2002. p. 119–22. http://www.access.gpo.gov/nara/cfr/waisidx_01/21cfr11_01.html.
- 25.Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance. Rockville, MD: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics and Evaluation; Apr, 1996. [Google Scholar]
- 26.Fiellin DA, Pantalon MV, Schottenfeld RS, Gordon L, O’Connor PG. Manual for Standard Medical Management of Opioid Dependence with Buprenorphine. Yale University; 1999. Unpublished manuscript. [Google Scholar]
- 27.Fiellin D, Pantalon M, Chawarski M, Moore B, Sullivan LE, O’Connor P, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365–74. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 28.Pantalon MV, Fiellin DA, Schottenfeld RS, Gordon L, O’Connor PG. Manual for Enhanced Medical Management of Opioid Dependence with Buprenorphine. Yale University; 1999. Unpublished manuscript. [Google Scholar]
- 29.Hay JL, White JM, Bochner F, Somogyi AA, Semple TJ, Rounsefell B. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J Pain. 2009;10(3):316–22. doi: 10.1016/j.jpain.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Jamison RN. Learning to Master Your Chronic Pain. Sarasota, FL: Professional Resource Press; 1996. [Google Scholar]
- 31.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 32.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 33.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 35.World Health Organization. Composite International Diagnostic Interview (CIDI): Core Version 2.1. Jan, 1997. [Google Scholar]
- 36.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 37.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35(2):253–9. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 38.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu G, Liang K-Y. Sample size calculations for studies with correlated observations. Biometrics. 1997;53:937–947. [PubMed] [Google Scholar]
- 40.Gandhi DH, Jaffe JH, McNary S, Kavanagh GJ, Hayes M, Currens M. Short-term outcomes after brief ambulatory opioid detoxification with buprenorphine in young heroin users. Addiction. 2003;98(4):453–62. doi: 10.1046/j.1360-0443.2003.00334.x. [DOI] [PubMed] [Google Scholar]