Abstract
Although the role of astrocyte glutamate transporters in glutamate clearance is well illustrated, the role of glutamine synthetase (GS) that influences this process remains to be elucidated. We examined whether GS affected the uptake of glutamate in astrocytes in vitro. The glutamate uptake was assessed by measuring the concentration of glutamate and glutamine in culture medium in the presence or absence of glutamate. We demonstrated that inhibition of GS in astrocytes by MSO significantly impaired glutamate uptake and glutamine release. Conversely, induction of GS expression in astrocytes by gene transfer significantly enhanced the glutamate uptake and glutamine release. When an inflammatory cytokine tumor necrosis factor-α (TNF-α) was applied to the cultures, it significantly reduced GS expression and inhibited glutamate-induced GS activation resulting in increased excitotoxicity to neurons. These results suggest that GS in astrocytes may represent a novel target for neuroprotection against neuronal dysfunction and death that occur in many neurological disorders.
Keywords: glutamate, glutamate uptake, glutamine, glutamine synthetase, TNF-α, astrocyte, neuroexcitotoxicity
1. Introduction
Astrocytes have been demonstrated to protect against excitotoxicity by clearing excessive excitatory neurotransmitters from the extracellular space (Maragakis et al., 2004). The glutamate transporters in astrocytes have been shown to be the main mediators of glutamate clearance (Maragakis et al., 2004). Additionally, the glutamate-glutamine metabolic cycle between astrocytes and neurons is believed to be vital for preventing neuronal excitotoxicity (Broer and Brookes, 2001). In astrocytes, glutamate is converted rapidly into glutamine by GS (Choi et al., 1987), an astrocyte-specific enzyme (Shaked et al., 2002). The coupling of GS and glutamine traffic from glia to neurons permits glutamate passage in the extracellular compartment in a non-neuroactive form (glutamine) thus avoiding toxicity (Muse et al., 2001).
Glutamate neurotoxicity has been implicated in many neurological disorders (Vardimon, 2000). A decline in GS activity and/or expression has been reported in these disorders (Oliver et al., 1990;Grosche et al., 1995;Robinson, 2000;Lievens et al., 2001). Considering the role of GS in glutamate conversion, changes in GS expression may affect the concentration of glutamate in astrocytes as well as their capacity to capture extracellular glutamate. Although a previous report demonstrated that GS activity significantly influenced glutamate uptake by neural retina (Shaked et al., 2002), the role of GS on glutamate uptake and metabolism in astrocytes remains to be elucidated.
Neurodegenerative diseases often occur along with inflammation bursts, including the release of proinflammatory cytokines which may contribute to the neurotoxicity (Kreutzberg, 1996;McGeer and McGeer, 2002;Nelson et al., 2002;Orr et al., 2002;Block and Hong, 2005). Although astrocytes proliferation and activation are found in these diseases, glutamate is always found at a high level which leads to neuronal death. The cause may be due to the interaction between inflammatory cytokines and astrocytes. Previous studies have found that tumor necrosis factor-α (TNF-α) can suppress glutamate uptake by astrocytes via negative regulation of glutamate transporters (Wang et al., 2003;Sitcheran et al., 2005), thus potentiating glutamate neurotoxicity (Zou and Crews, 2005). TNF-α may also have an effect on GS activity. For example, treatment with TNF-α markedly decreased GS activity in C6 glial cells (Kazazoglou et al., 1996) and suppressed dexamethasone induced GS in primary mouse astrocytes (Huang and O'Banion, 1998). These results suggest that the neurotoxic effects of TNF-α may be due, at least in part, to its interaction with astrocytes.
Although the importance of GS in glutamate-glutamine cycle has been implicated, its effect on glutamate uptake in primary astrocytes has not been clearly demonstrated. In addition, the relationship between TNF-α expression and GS activity in regulating glutamate metabolism in primary astrocytes remains unclear. The present study was designed to address these issues by testing a central hypothesis that TNF-α mediates neuronal glutamate excitotoxicity through its interaction with astrocytes and that astrocytic GS mediates neuroprotection against excitotoxicity.
2. Materials and methods
2.1 Culture of astrocytes and neurons
Purified astrocyte cultures were obtained from postnatal (P) day 2 Sprague Dawley (SD) rat cerebral cortices according to a previously established protocol (Smith et al., 1990). Briefly, cerebral cortices were isolated, freed of meninges, and digested using a fire-polished Pasteur pipette. Cells were dissociated and plated into 75 cm2 tissue culture flasks at a density of 2.0 × 107 cells/flask. After 24 hrs, non-adherent cells were removed by shaking and the remaining cells were incubated with DMEM/10% FBS medium (Gibco, Grand Island, NY). Once cells reached confluence (10-14 days), microglia and oligodendrocytes were removed from the culture after shaking. Astrocytes were purified further by more vigorous shaking. The resulting cultures contained predominantly GFAP-immunoreactive astrocytes (>95%), and a small number of oligodendrocytes and glial progenitors. The cultures were virtually free of neurons and microglia. Upon reaching confluence, cells were trypsinized and replated. After the second passage, cells were seeded into 3.5 cm dishes (Corning, NY). After reaching subconfluence, 10 μg/ml mitomycin C (Sigma Chemical Co., St. Louis) was added to cultures for 2 hrs to avoid cell proliferation (Okada et al., 2006). Cells were maintained with serum free DMEM medium supplemented with TNF-α (Sigma), L-methionine-S-sulfoximine (MSO, Sigma), and glutamate (Sigma) alone or in combination as indicated.
Rat primary neuron cultures were derived from the cerebral cortices of embryonic day (E) 18 SD rats according to a previously described protocol (Jiang et al., 2006) with minor modifications. Briefly, cerebral cortices were dissected and freed of meninges. Cells were dissociated by trypsinization, followed by triturating and passing through a 70 μm nylon mesh. After adhering in 37°C for 30 minutes to eliminate the glial cells and fibroblasts, neurons (5×104) were plated either on poly-L-lysine (Sigma) coated 12 mm glass coverslips (Becton Dickinson Labware, USA) for co-culture experiments or poly-L-lysine-coated 24 well culture plates for experiments with neurons alone. Neurons were maintained in neurobasal media (Invitrogen Corp., Grand Island, NY), 2% B27 supplement (Invitrogen) and 0.5 mM glutamine (Invitrogen) with the absence of serum, mitotic inhibitors, or antibiotics. On the third day, 5 μM arabinosylcytosin (Sigma) was added to the culture to inhibit glia. On the sixth day, the resulting cultures contained predominantly βIII-tubulin (1:800, Sigma) immunoreactive neurons (>95%). For the final 48 hrs, neurobasal medium was supplemented with either glutamine, TNF-α, MSO or glutamate according to the experimental design.
For astrocyte-neuron co-culture, neuron-containing coverslips at 6–7 days in vitro were placed into astrocyte-seeded wells in the presence of neurobasal medium supplemented with glutamine, MSO, TNF-α, and glutamate, alone or in combination, for 48 hrs before neuron viability assay. Neurons and astrocytes were in close apposition but with no direct cell-cell contact. This would allow analysis of neurons and astrocytes separately.
2.2 Construction of recombinant GS plasmid and transfection
Total RNA was extracted from samples of normal rat cortical astrocytes with TRI REAGENT (Invitrogen) and was reversed to cDNA by AMV reverse transcriptase (Promega, Madison, WI). PCR was used to amplify GS total cDNA fragment. The amplified products were recovered and purified using DNA Purification kit (Qiagen, Santa Clarita, CA) according to the manufacturer's instructions. Both the amplified products and plasmid vector pEGFP-N3 (Clontech) were digested with restriction endonuclease HindIII and BamHI (TaKaRa Biotechnology, Dalian, China) and linked with T4 DNA ligase (TaKaRa). The new recombinant plasmid vectors were transferred into the E.Coil (DH5α) strain, analyzed using restriction endonuclease and DNA sequencing methods.
Astrocytes were cultured in DMEM containing 10% FBS. A total of 2×105 cells per well were seeded in 6-well plates. The cells were allowed to grow to 80% confluence and medium removed and transfected with 2.4 μg total recombinant DNA using Lipofectamine™ 2000 (Invitrogen) according to the manufacture's protocol. The mixtures were incubated for 4 hrs before being replaced with a fresh medium to stop the transfection. 48 hrs later, glutamate or TNF-α was added to the wells and the cultures were maintained for an additional 48 hrs. All transfections were performed in triplicate.
2.3 Glutamate uptake assay
To evaluate the glutamate clearance capacity, astrocyte cultures were grown in 6-well dishes transfected as described above. Briefly, medium in each well was replaced with 1.5 ml of serum free HBSS containing 2 mM glutamate. After incubation for 2 hrs at 37°C, the medium was removed and 12.5 μl culture supernatant was transferred to each of the 96-well culture plates and glutamate remaining in the medium was determined using the Glutamate Colorimetric Assay kit (Genmed Scientific Inc., MA). The absorbance of the product was measured at 492 nm using a microplate reader. A standard curve was constructed in each assay using cell-free culture media containing known concentrations of glutamate. The concentration of extracellular glutamate in the samples was estimated from the standard curve. As a control for each experiment, serum-free medium containing 2 mM glutamate were added to empty wells (free of astrocytes) of a 6-well dish and processed together with those containing astrocytes. In all experiments described in Fig. 2C and E, the concentration of glutamate in dishes without astrocytes remained at ≈1.8 mM.
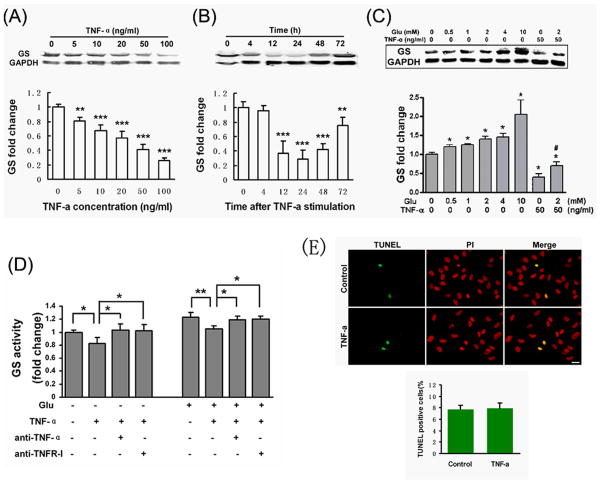
Fig.2. Astrocytic GS was down-regulated by TNF-α.
(A-B) Western-blotting showed that TNF-α inhibited GS expression in a dose- and time-dependent manner. (A) A dose-dependent effect of TNF-α on GS expression in astrocytes which were incubated with indicated concentrations of TNF-α for 48 hrs. (B) A time-dependent effect of TNF-α on GS expression in astrocytes which were incubated with 50 ng/ml of TNF-α for indicated time points. GS expression levels were quantified by densitometry and corrected for GAPDH levels. GS expression levels in untreated controls were set to 1. Values are means ± SD (n=4). **: p < 0.01, ***: p < 0.001, compared to control. (C) The inhibitory effects of TNF-α on GS expression can not be abolished in the presence of glutamate. Astrocytes were maintained with either TNF-α (50 ng/ml) alone or in combination with glutamate (0.5-2 mM) for 48 hrs and were analyzed for GS protein levels by Western blotting. Values are means ± SD (n=4). *: p < 0.01, treatment vs. control, #: p < 0.01 vs 2 mM glutamate (Student-Newman-Keuls test). (D) GS activity was decreased by TNF-α in the absence or presence of glutamate. Astrocytes were incubated in 2 mM glutamate for 2 hrs with or without 50 ng/ml TNF-α, anti-TNF-α-neutralizing antibody or anti-TNF RI-neutralizing antibody. Values are means ± SD (n=4). *: p < 0.05; **: p < 0.01. (E) Astrocytes, immuno-labeled with GFAP, showed a marked cytoplasm retraction and process extension after exposure to 50 ng/ml TNF-α for 48 hrs compared to the control. (F) Exposure astrocytes to TNF-α (50 ng/ml) for 48 hrs did not affect cell death or apoptosis, assessed by TUNEL staining (green), as compared to the control. PI, a nuclear marker. Scale bars in E and F, 20 μm.
For measuring glutamine, the medium was collected and measured by the colorimetric assay (GENMED). The concentration of total cell protein was used as a reference, which was determined using BCA protein estimation kit (Pierce, Rockland, IL). Data were expressed as the mean percentage (± SD) of respective control value.
2.4. GS activity
The specific activity of GS was measured in cell lysates by a colorimetric assay based on the catalysis of γ-glutamylhydroxamate from glutamine and hydroxylamine (Sher and Hu, 1990). GS activity was expressed as micromolar c-glutamylhydroxamate per hr per mg of cell protein. Data were presented as percentage (± SD) of respective control (0 mM) value.
2.5. Apoptosis Assay
Apoptosis of astrocytes treated with TNF-α for 2 days was assessed by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining using R&D TdT In Situ Apoptosis Detection Kit (R&D system Inc., MN, USA). The results were examined using a fluorescence microscope. Apoptotic cells were stained by TUNEL staining. Propidium iodide (PI) labeled cell nucleolus.
2.6. Western blot analysis
The western blotting assay has been described previously (Yan et al., 2003). Briefly, astrocytes at varying times or treatments (n = 4 per time point, or per treatment) were homogenized by sonication in a lysis buffer. Twenty micrograms of protein, measured using BCA method, from the supernatant of each sample and Full Range Rainbow marker were loaded onto 12% polyacrylamide gel, separated by SDS/PAGE, and transferred to PVDF membranes by electrophoresis. The membranes were blocked in 5% milk in TBST for 1 h at room temperature. Mouse anti-GS (1:2,000; BD Biosciences, San Jose, CA) and mouse anti-GAPDH (1:1,000; Chemicon, Temecula, CA) was added to the membrane and incubated at 4°C overnight. The membrane was washed with TBST 3 times at 10 min intervals and incubated with the secondary antibody, goat anti-mouse IgG conjugated with horseradish peroxidase (1:2,000; Amersham Pharmacia Biotech, Amersham, UK) at RT for 2 hr. The membrane was then washed 3 times with TBST at 10 min intervals and the proteins were detected by enhanced chemiluminescence (Pierce). One-way ANOVA was used for statistical comparison of the means with the sham-operated controls as baseline controls (100%; arbitrary unite). Significant results were followed by Tukey's post hoc tests.
2.7. Neuronal degeneration and cell viability assays
Degenerated neurons were identified by their disrupted or reduced neurites and condensed or fragmented nuclear DNA using a method described previously (Shin et al., 2005). Cultured neurons were fixed and stained with an antibody against MAP-2 (1:200, Sigma) for neuronal cells and Hoechst 33342 (Sigma) for nuclear DNA. Fluorescent images were captured and neurons with normal or degenerative morphology were counted. To count cells after immunocytochemistry, random 10-20 pictures of each experimental group were taken with a 20× objective. The imaged areas were chosen randomly from at least three different wells per experimental group. The controls were neurons that had not been treated. The numbers of MAP-2-positive neurons and degenerative neurons were expressed as the percentage of total neurons counted. Data were obtained from 4 independent culture experiments.
2.8. Statistical analysis
Data were expressed as mean ± standard deviation of the mean (SD) values. Two-tailed Student's t-test was used for statistical comparison of paired data and one way ANOVA followed by Dunnett's post hoc test was used for statistically multiple comparisons to determine whether there were significant differences between individual groups. Statistical significance was established when p < 0.05.
3. Results
3.1. GS influences glutamate uptake and glutamine production
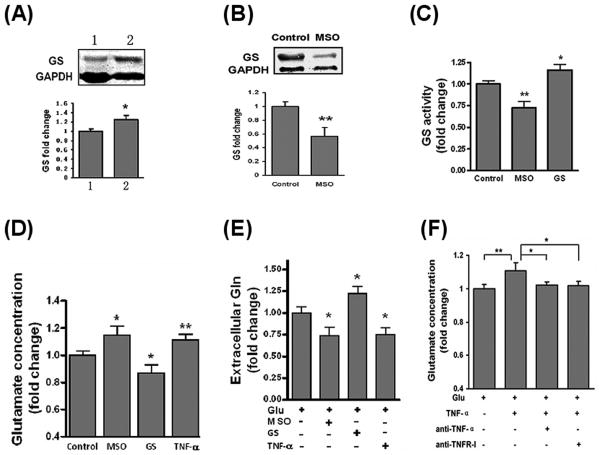
Since GS is localized to astrocytes and astrocytes are the primary sites of glutamate conversion to glutamine, we examined the effects of GS on glutamate uptake and glutamine production in astrocytes. We used a GS inhibitor MSO or GS gene transfer approach to change GS levels in astrocytes (Fig.1A-C). Since inflammatory cytokines were shown to have negative effects on glutamate uptake and GS expression in astrocytes (Huang and O'Banion, 1998;Sitcheran et al., 2005), we used TNF-α to determine whether an inflammatory cytokine could inhibit glutamate uptake and downregulate GS expression in astrocytes. 7 day cultured astrocytes were incubated with 1 mM MSO or 50 ng/ml of TNF-α for 30 min before the addition of 2 mM glutamate. After 2 hrs incubation, the medium was assayed for glutamate concentration. In a pilot study, we tested possible interaction between glutamate and MSO or TNF-α. The results showed that, in the absence of astrocytes, neither MSO nor TNF-α had an effect on glutamate concentration (data not show). As shown in Fig. 1D, inhibited GS activity by 1 mM MSO weakened glutamate clearance by astrocytes. In contrast, induction of GS expression by gene transfer enhanced the clearance of extracellular glutamate. These results demonstrate that GS activity influences the uptake of glutamate by cortical astrocytes and suggest that this enzyme could be an important target for neuroprotection. In physiological conditions, astrocytes surround glutamatergic synapses and express glutamate transporters and glutamine synthetase. Glutamate is transported into glial cells and amidated by GS into non-toxic glutamine, which is then released by the glial cells and taken up by neurons. Thus, changes in GS level may influence glutamine production by astrocytes. To test this possibility, the extracellular glutamine content was determined by a colorimetric assay in astrocyte cultures incubated with 2 mM glutamate for 48 hrs (Fig. 1E). The data showed that decreased GS activity by MSO significantly inhibited glutamine production and release. Conversely, astrocytes with high GS level released more glutamine, indicating that GS level strongly influence astrocytic glutamine production. These data collectively indicate that GS activity can influence glutamate uptake and regulate glutamine production in astrocytes. Interestingly, TNF-α seems to play a role in weakening glutamate uptake and glutamine production (Fig. 1D, E). Using neutralizing antibody to TNF-α and TNF Receptor-I (TNFR-I), such inhibitory effects of TNF-α on glutamate uptake in astrocytes were confirmed (Fig. 1F). Previous studies found that TNF-α decreased glutamate clearance ability of astrocytes by negatively regulating the expression of glutamate transporter, such as glutamate transporter-1 (GLT-1) and excitatory amino acid transporters 1 (GLAST) (Wang et al., 2003;Sitcheran et al., 2005). Here we suggest that down-regulation of GS activity can be another mechanism of TNF-α action on reducing glutamate clearance. TNF-α may inhibit glutamine production through the suppression of GS activity.
Fig.1. GS influences glutamate clearance and glutamine production by astrocytes.
(A) Western-blotting results of GS over-expressed astrocytes. 1, control vector transfected astrocytes; 2, GS expressing vector transfected astrocytes. GS immunoreactive protein bands (45kD) were quantified by densitometry and corrected for GAPDH (36kD) levels. Values are means ± SD (n=3). (B) The inhibitory effects of MSO on GS expression in astrocytes. Astrocytes were stimulated with or without 1 mM MSO for 2 hrs and GS protein level were assayed by western-blot. Values are means ± SD (n=3). (C) Astrocytes were treated with 1 mM MSO for 2 hrs or over-expressed GS (denoted as GS) for 72 hrs. GS activity was determined by colorimetric assay of accumulated cell protein. Values are means ± SD (n=4). (D) GS activity influenced the clearance of extracellular glutamate by astrocytes. Astrocytes were incubated with 2 mM glutamate for 2 hrs in the absence (control) or 30 min pre-incubation of 1 mM MSO, 50 ng/ml TNF-α or over-expressed GS. Glutamate concentration in accumulated supernatant was determined by colorimetric assay. Values are means ± SD (n=4). (E) GS activity affects glutamine (Gln) release by astrocytes. Astrocytes were incubated with 2 mM glutamate for 48 hrs in the presence or absence of MSO, TNF-α or over-expressed GS. Extracellular glutamine content was determined by colorimetric assay respectively. Values are means ± SD (n=3). (F) TNF-α impaired glutamate uptake in astrocytes. Astrocytes were incubated in 2 mM glutamate for 2 hrs with or without 50 ng/ml TNF-α, anti-TNF-α or anti-TNF RI-neutralizing antibody. Values are means ± SD, (n=4). *: p < 0.05; **: p < 0.01.
3.2 TNF-α decreases GS level in astrocytes
In addition to its regulatory effects on glutamate transporters or uptake, TNF-α may influence GS expression and/or activity. To address this issue, we first examined the response of astrocytes to TNF-α stimulation. After 48 hrs of TNF-α exposure, astrocytes showed a marked cytoplasm retraction and process extension, characteristic of astrocyte stellation (data not show). However, TNF-α treatment did not influence the survival or death of astrocytes (Fig. 2E). Then, we used Western-blotting to determine whether TNF-α affects GS expression in cultured cortical astrocytes (Fig. 2A, B). Treatment astrocytes with different concentrations of TNF-α for 48 hrs resulted in a dose-dependent reduction of GS expression (Fig. 2A). Likewise, TNF-α treatment also resulted in a time-dependent decrease of GS expression (Fig. 2B). Further, we examined GS expression in astrocytes treated with or without different concentrations of glutamate. As showed in Figure 2 C, glutamate treatments increased the expression of GS in a dose-dependent manner. When TNF-α was added to the culture, it resulted in a pronounced decline (≈60%) of the basal GS expression. The effect of TNF-α was not abolished in the presence of 2 mM glutamate. Similarly, GS activity could be decreased by TNF-α in the presence or absence of 2 mM glutamate (Fig. 2D). These effects can be further clarified by using TNF-α and TNF RI neutralizing antibody. Collectively, these data indicate that TNF-α inhibits GS expression in cultured cortical astrocytes both in the presence and absence of glutamate.
3.3. TNF-α reduces astrocytic protection against glutamate excitotoxicity
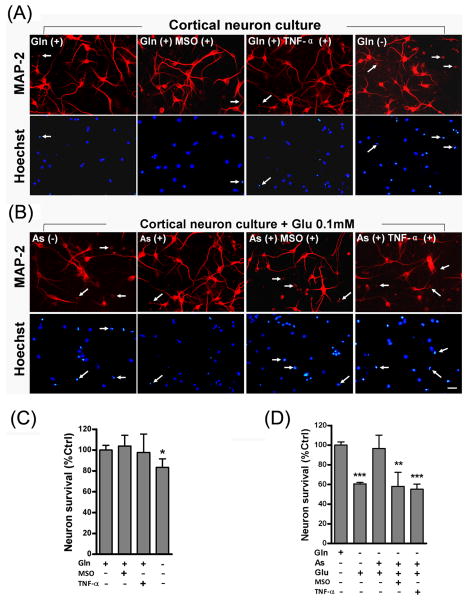
To determine whether glutamine is indispensable to neuronal survival, neurons were treated with or without glutamine for 48 hours at 6 days in vitro (DIV). In the absence of glutamine, neurons generally showed a decrease in MAP2 staining (Fig. 3A), a neuron-specific marker (Shin et al., 2005). Degenerating neurons with shrinking cell bodies and pyknotic nuclei (Fig. 3A, arrows) were clearly seen. In the presence of glutamine, neurotoxicity was not prominent even after the addition of TNF-α or MSO (Fig. 3A, C). Next, we examined neuronal protection by astrocytes and the effect of TNF-α on astrocyte-mediated neuronal protection. In this experiment, neuron-containing coverslips at 6 days were co-cultured with astrocytes, stimulated with glutamate for 48 hours, and stained with MAP2 (Fig. 3B). Since astrocytes display larger nuclei than neuronal nuclei and lack MAP2 immunostaining, they can be easily distinguished from neurons. In the absence of astrocytes, a significant decrease in the number of MAP2-positive neurons was found when stimulated with 0.1 mM glutamate (Fig. 3B, D). On the contrary, significant neuronal loss was not found in neurons co-cultured with astrocytes even under the stimulation with 0.1mM glutamate (Fig. 3B, D), indicating that astrocytes can reduce neuronal glutamate excitotoxicity in the neuron-astrocyte co-cultures. To assess the specific protective effect of astrocytes in the metabolism of glutamate and release of glutamine, MSO was added to inhibit GS activity 30 min prior to the stimulation of glutamate. The addition of MSO significantly diminished the astrocytes protection on neurons. Since TNF-α was found to weaken glutamate uptake (Fig.1 D, F) as well as glutamine release (Fig. 1E) by astrocytes, we examined whether addition of TNF-α would reduce astrocyte-mediated neural protection. Indeed, addition of TNF-α at 30 min prior to the glutamate stimulation significantly increased neuronal excitotoxicity. Thus, the mechanism by which astrocytes mediate neuronal protection may result from their capability of removing extracellular glutamate and releasing glutamine through intracellular glutamate metabolism.
Fig.3. Reduced astrocytic protection against glutamate excitotoxicity by TNF-α.
Neuronal degeneration and viability was assayed by a neuronal marker MAP-2 counterstained with a nuclear marker Hoechst. Degenerating neurons with shrinking cell bodies and pyknotic nuclei (arrows showed in A and B) were clearly seen. (A) Cultured rat cortical neurons were stimulated for 48 hours with TNF-α or MSO in the presence or absence of glutamine (Gln). In the presence of glutamine, neurons survived after TNF-α or MSO stimulation whereas in the absence of glutamine, more neurons degenerated and lost MAP2 staining. (B) Cultured neurons were stimulated with 0.1 mM glutamate (Glu) for 48hrs in the presence or absence of astrocytes. Astrocytes protected neurons from glutamate neural toxicity; however, pre-treatment with MSO or TNF-α, showed a decrease of protection. (C, D) Percentage values of MAP2-positive neurons showed respectively in A and B. The control represents the number of cells without excitotoxin stimulation. Values are means ± SD (n=4). **, p < 0.01; ***, p <0.001, as compared with the control. Scale bars, 20 μm.
4. Discussion
The present finding suggests that GS in astrocytes plays an important role in protection against neuronal dysfunction and excitotoxicity. GS activity or expression can influence glutamate uptake as well as glutamine accumulation and release by astrocytes. Decreased GS activity or expression induced by TNF-α seems to aggravate the neural excitotoxicity.
It has been suggested that glutamate uptake by glutamate transporter is regulated by the extracellular and intracellular glutamate concentrations (Ma et al., 2006). In brain, excitatory amino acid transporters (EAAT1 and EAAT2) are expressed in glial cells, and EAAT3 and EAAT4 are found mainly in neurons. Although neurons also express glutamate transporters, their glutamate sequestration capacity is incomparable to that of astrocytes (data not show). Consequently, neurons are unable to withstand even a relatively low concentration (25 μM) of glutamate insult whereas astrocytes can sustain much higher concentrations of glutamate stimulation. One explanation for their difference in response to glutamate stimulation is that neurons and astrocytes utilize glutamate for different purposes. For example, astrocytes are the main protectors of neurons against excitotoxicity and such protection is conferred by clearance of extracellular glutamate. Astrocytes possess two important mechanisms of maintaining the level of extracellular glutamate. One is an uptake system located on plasma membrane and the other is the intracellular metabolic enzyme that converts glutamate to glutamine by coupling glutamate with ammonium cation (Broer and Brookes, 2001). The neurotransmitter glutamate, which is released by glutamatergic neurons, can be taken up by astrocytes where it is converted to glutamine by GS. Glutamine is then released by astrocytes and reabsorbed by neurons where it is hydrolyzed by glutaminase to form glutamate again. Thus, the two key enzymes, GS and glutaminase contribute to the glutamate–glutamine cycle. In the present study, we found that excessive glutamate level induced GS activity and protein expression. In several neurological disorders such as ischemic (Hoshi et al., 2006) and hypoxic (Sher and Hu, 1990) brain injuries when energy levels were low, increased extracellular glutamate was found to stimulate GS activity. Since intracellular glutamate concentration may be dependent on the activity of glutamate metabolizing pathways, it is likely that these pathways may also influence the uptake of extracellular glutamate.
Due to its important role in glutamate metabolism (Vardimon, 2000;Hertz and Zielke, 2004), GS constitutes an endogenous protective mechanism against glutamate neurotoxicity by catalyzing the conversion of a toxic amino acid glutamate to a non-toxic amino acid glutamine (Linser and Moscona, 1979;Gorovits et al., 1997;Hoshi et al., 2006). In the present study, we demonstrated that GS was neuroprotective and that its activation induced a clearance of excessive glutamate. Previously, it was shown that a decrease in GS activity, caused by MSO, led to a significant decline in glutamate uptake in retina cells (Shaked et al., 2002). When GS expression was induced by increased uptake of glutamate, the total amount of intracellular glutamate was not affected, but rather led to a dramatic increase in the amount of glutamine released. Our results support the previous finding that MSO reduced GS activation which in turn decreased the uptake of extracellular glutamate in cortical astrocytes. In our case, overexpression of GS increased glutamate uptake at a level even higher than that seen in normal astrocytes. This indicates that GS induction in astrocytes can modulate glutamate uptake capacity. In cells expressing high level of GS, the metabolic conversion of glutamate to glutamine may reduce the extracellular glutamate to hold the relative constant intracellular glutamate concentration and thereby provide a driving force for continued uptake of extracellular glutamate. A recent study showed that subjecting cultured cortical astrocytes to increasing glutamate concentrations of 0.5-20 mM resulted in a prolonged increase of GS expression in contrast to a dramatic loss of glutamate transporter protein levels (Lehmann et al., 2009). It seems that the glutamate mediates astrocytic glutamate transporters by a glutamate receptor-independent mechanism. Thus, maintaining a physiological glutamate concentration gradient between the extracellular and intracellular milieu is critical in regulating glutamate transporter and glutamate uptake.
The present study also indicates that the intracellular enzyme GS in astrocytes is a key contributor of neuroprotection. GS exerts its function mainly by influencing the clearance of extracellular glutamate and efflux of glutamine in cortical astrocytes. In the present study, MSO, an analogue of glutamate, inhibited GS activity in astrocytes. In addition to its action as an inhibitor of GS, MSO may have other actions. For example, MSO reduces an increase in the number of swollen astrocytes in rat cortex after hyperammonemia (Tanigami et al., 2005). However, a puzzling finding is that injection of MSO into the striatal tissue induced glial glutamate release (Rothstein and Tabakoff, 1985). The diversity roles of MSO may lie in the usage of different experimental subjects, doses, or approaches. It also indicates that the application of MSO as an inhibitor of GS should be used cautiously. In our study, astrocytes were pre-treated with MSO before the addition of glutamate. In this case, 1 mM MSO can effectively inhibit GS activity and protein expression. We surmise that glutamate uptake and metabolism are two important mechanisms that interact with each other to maintain the glutamate homeostasis.
Defect in glutamate uptake is critical to the development of glutamate-mediated excitotoxicity (Lievens et al., 2001;Zou and Crews, 2005;Mallolas et al., 2006;Tilleux and Hermans, 2007). Such a defect in glutamate uptake is suggested to be mediated by a decrease in EAATs (Werner et al., 2001;Vercellino et al., 2007). Increased proinflammatory cytokines have also been implicated in various neurodegenerative and post-traumatic disorders (Bartholdi and Schwab, 1997;Yan et al., 2003;Tilleux and Hermans, 2007) in which defects in glutamate uptake were found (Cavaliere et al., 2007;Tilleux and Hermans, 2007). In pathological conditions, neurological dysfunction has often been attributed to changes in amino acid neurotransmitter metabolism (Vardimon, 2000). Although proinflammatory cytokines have been implicated in these conditions, limited information is available concerning the effects of these cytokines on neurotransmitter metabolism, especially on GS mediated glutamate metabolism. In the present study, we found that TNF-α, a key cytokine mediating post-traumatic inflammation, reduced astrocytic GS activity and expression which may lead to neuronal excitotoxicity. Previously, it was suggested that TNF-α could enhance excitotoxicity through synergistic stimulation of TNFR and NMDA receptor (Floden et al., 2005;Jara et al., 2007) and inhibition of astroglial glutamate uptake via regulating glutamate transporters (Wang et al., 2003;Sitcheran et al., 2005;Zou and Crews, 2005). Here we report another possible mechanism of TNF-α action on neuronal excitotoxicity, by directly down-regulating GS level and reducing glutamate-induced GS up-regulation. TNF-α may not only reduce glutamate uptake but also inhibit glutamine accumulation and production in astrocytes. Thus, in addition to down-regulating glutamate transporters, as reported previously, TNF-α may play an important role in glutamate metabolism in astrocytes.
To demonstrate the protective effects of GS, we established a neuron-astrocyte co-culture system which allows observation of a close interaction between neurons and astrocytes. We found that in neuronal cultures, about 20% of neurons die of malnutrition in the absence of glutamine. Addition of glutamate into neuronal culture induced further death of neurons. However, when glutamate was added in the presence of astrocytes, the neuronal survival was protected. These data support the previous finding that astrocytes play a key role in glutamate-glutamine cycle. The neuroprotective role of astrocytes not only lies in the clearance of excessive glutamate, but also in the production of glutamine for neurons via its metabolic pathway, especially by GS. The exocytosis of glutamine from astrocytes and its uptake by neurons are integral steps in the glutamate-glutamine cycle, a major pathway for the replenishment of neuronal glutamate. However, once this cycle is disrupted by inhibition of the key enzyme GS, it may lead to neuronal degeneration. Indeed, when GS activity in astrocytes was inhibited by MSO, as shown above, the neuroprotection of astrocytes against glutamate toxicity was diminished. Thus, GS may play a key role in the glutamate-glutamine cycle between astrocytes and neurons. Although how TNF-α exerts neurotoxicity remains controversial, our study suggests that a direct toxic effect of TNF-α on neurons is relatively weak since TNF-α can also activate some neuroprotective factors such as mitogen-activated kinase (MAPKs) and nuclear factor-κB (NF-κB) (Ghezzi and Mennini, 2001;Kamata et al., 2005). In our neuron-astrocyte co-culture system, TNF-α not only impaired neuroprotection of astrocytes but also intensified neuronal death when exposed to glutamate. Thus, TNF-α induced neuronal death may be mediated, at least in part, indirectly through its action on astrocytes. Such an effect may be mediated by two mechanisms. First, TNF-α can weaken astrocytic glutamate uptake activity by down-regulating glutamate transporters. Secondly, TNF-α can down-regulate GS activity regardless of the presence or absence of glutamate. These two mechanisms may act synergistically in mediating TNF-α induced neurotoxicity. Although in the presence of glutamate, decreased levels of GS may be caused by TNF-α inhibition on glutamate transporters or receptors, the possibility that TNF-α down-regulates GS in astrocytes cannot be ruled out. In fact, the effect of TNF-α on GS activity can be an important and direct cause of neuronal excitotoxicity. If so, regulating GS levels may lead to neuroprotection against CNS disorders or injuries.
In physiological conditions GS expression is tightly controlled by several factors which can be altered following CNS injuries or other neurological disorders. For example, glucocorticoids, the endogenous activators of GS gene, can induce GS expression only in quiescent but not proliferating glial cells (Shaked et al., 2002). This indicates that increased proliferation of glial cells does not necessary induce increased GS expression. Such antagonism between GS expression and glial cell proliferation appears to occur at the site of injury where glial cell proliferation (gliosis) might cause a decline in GS expression (Kruchkova et al., 2001). Moreover, GS expression could be affected by inflammatory cytokines, particularly TNF-α, which has been shown to increase following CNS injuries (Xu et al., 1998;Yan et al., 2001) or degenerative disorders (Tilleux and Hermans, 2007). Two factors may contribute to TNF-α mediated decrease in GS expression. First, TNF-α may influence glial cell proliferation, differentiation and maturation (Kazazoglou et al., 1996). Secondly, TNF-α may regulate GS transcriptional activity. Since GS plays an important role in glutamate uptake, as suggested by the present study, a decline in GS expression at the site of injury may significantly reduce the ability of glial cells to remove extracellular glutamate, thereby exacerbating the process of neuronal degeneration. Since astrocytes are the key producer of TNF-α after CNS injury, understanding the relationship between the production of inflammatory cytokines and their effects on glutamate metabolism may shed new light on the underlying mechanism of inflammation-mediated neuronal survival or death.
In conclusion, our data suggest that the neuroprotective effects of astrocytes are not only dependent on glutamate uptake but also on GS activity. For the latter, the capacity for storage and release of glutamine in astrocytes is critical. It is conceivable that interrupting the glutamate-glutamine cycle may represent an important neurotoxic mechanism that occurs in many neuropathological conditions. Our data also suggest that the inflammatory cytokine TNF-α has an inhibitory role on astrocyte GS. Since cytokines such as TNF-α is increasingly expressed in many neurological disorders, the neurotoxicity induced by inflammatory cytokines may be induced indirectly by the regulation of GS levels in astrocytes. In this regard, research ways to increase GS at the site of CNS injuries and other neurological disorders may offer novel strategies for neuroprotection.
Acknowledgments
This work was supported by the grants from Major State Basic Research Development Program of China (973 Project 2003CB515302); NIH grants NS36350, NS52290, NS50243, the Indiana Spinal Cord and Brain Injury Research Fund, and the Mari Hulman George Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9:1422–1438. doi: 10.1111/j.1460-9568.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77:705–719. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Cavaliere C, Cirillo G, Rosaria Bianco M, Rossi F, De Novellis V, Maione S, Papa M. Gliosis alters expression and uptake of spinal glial amino acid transporters in a mouse neuropathic pain model. Neuron Glia Biol. 2007;3:141–153. doi: 10.1017/S1740925X07000695. [DOI] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Li S, Combs CK. Beta-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor alpha and NMDA receptors. J Neurosci. 2005;25:2566–2575. doi: 10.1523/JNEUROSCI.4998-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P, Mennini T. Tumor necrosis factor and motoneuronal degeneration: an open problem. Neuroimmunomodulation. 2001;9:178–182. doi: 10.1159/000049024. [DOI] [PubMed] [Google Scholar]
- Gorovits R, Avidan N, Avisar N, Shaked I, Vardimon L. Glutamine synthetase protects against neuronal degeneration in injured retinal tissue. Proc Natl Acad Sci U S A. 1997;94:7024–7029. doi: 10.1073/pnas.94.13.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosche J, Hartig W, Reichenbach A. Expression of glial fibrillary acidic protein (GFAP), glutamine synthetase (GS), and Bcl-2 protooncogene protein by Muller (glial) cells in retinal light damage of rats. Neurosci Lett. 1995;185:119–122. doi: 10.1016/0304-3940(94)11239-f. [DOI] [PubMed] [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Hoshi A, Nakahara T, Kayama H, Yamamoto T. Ischemic tolerance in chemical preconditioning: possible role of astrocytic glutamine synthetase buffering glutamate-mediated neurotoxicity. J Neurosci Res. 2006;84:130–141. doi: 10.1002/jnr.20869. [DOI] [PubMed] [Google Scholar]
- Huang TL, O'Banion MK. Interleukin-1 beta and tumor necrosis factor-alpha suppress dexamethasone induction of glutamine synthetase in primary mouse astrocytes. J Neurochem. 1998;71:1436–1442. doi: 10.1046/j.1471-4159.1998.71041436.x. [DOI] [PubMed] [Google Scholar]
- Jara JH, Singh BB, Floden AM, Combs CK. Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK-dependent death. J Neurochem. 2007;100:1407–1420. doi: 10.1111/j.1471-4159.2006.04330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XY, Fu SL, Nie BM, Li Y, Lin L, Yin L, Wang YX, Lu PH, Xu XM. Methods for isolating highly-enriched embryonic spinal cord neurons: a comparison between enzymatic and mechanical dissociations. J Neurosci Methods. 2006;158:13–18. doi: 10.1016/j.jneumeth.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kazazoglou T, Fleischer-Lambropoulos E, Geladopoulos T, Kentroti S, Stefanis C, Vernadakis A. Differential responsiveness of late passage C-6 glial cells and advanced passages of astrocytes derived from aged mouse cerebral hemispheres to cytokines and growth factors: glutamine synthetase activity. Neurochem Res. 1996;21:609–614. doi: 10.1007/BF02527760. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kruchkova Y, Ben-Dror I, Herschkovitz A, David M, Yayon A, Vardimon L. Basic fibroblast growth factor: a potential inhibitor of glutamine synthetase expression in injured neural tissue. J Neurochem. 2001;77:1641–1649. doi: 10.1046/j.1471-4159.2001.00390.x. [DOI] [PubMed] [Google Scholar]
- Lehmann C, Bette S, Engele J. High extracellular glutamate modulates expression of glutamate transporters and glutamine synthetase in cultured astrocytes. Brain Res. 2009;1297:1–8. doi: 10.1016/j.brainres.2009.08.070. [DOI] [PubMed] [Google Scholar]
- Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, Bates GP. Impaired glutamate uptake in the R6 Huntington's disease transgenic mice. Neurobiol Dis. 2001;8:807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- Linser P, Moscona AA. Induction of glutamine synthetase in embryonic neural retina: localization in Muller fibers and dependence on cell interactions. Proc Natl Acad Sci U S A. 1979;76:6476–6480. doi: 10.1073/pnas.76.12.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Zheng S, Zuo Z. The transcription factor regulatory factor X1 increases the expression of neuronal glutamate transporter type 3. J Biol Chem. 2006;281:21250–21255. doi: 10.1074/jbc.M600521200. [DOI] [PubMed] [Google Scholar]
- Mallolas J, Hurtado O, Castellanos M, Blanco M, Sobrino T, Serena J, Vivancos J, Castillo J, Lizasoain I, Moro MA, Davalos A. A polymorphism in the EAAT2 promoter is associated with higher glutamate concentrations and higher frequency of progressing stroke. J Exp Med. 2006;203:711–717. doi: 10.1084/jem.20051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Dykes-Hoberg M, Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann Neurol. 2004;55:469–477. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- Muse ED, Jurevics H, Toews AD, Matsushima GK, Morell P. Parameters related to lipid metabolism as markers of myelination in mouse brain. J Neurochem. 2001;76:77–86. doi: 10.1046/j.1471-4159.2001.00015.x. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Soma LA, Lavi E. Microglia in diseases of the central nervous system. Ann Med. 2002;34:491–500. doi: 10.1080/078538902321117698. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990;87:5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Halliday GM. An inflammatory review of Parkinson's disease. Prog Neurobiol. 2002;68:325–340. doi: 10.1016/s0301-0082(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Robinson SR. Neuronal expression of glutamine synthetase in Alzheimer's disease indicates a profound impairment of metabolic interactions with astrocytes. Neurochem Int. 2000;36:471–482. doi: 10.1016/s0197-0186(99)00150-3. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Tabakoff B. Glial and neuronal glutamate transport following glutamine synthetase inhibition. Biochem Pharmacol. 1985;34:73–79. doi: 10.1016/0006-2952(85)90102-9. [DOI] [PubMed] [Google Scholar]
- Shaked I, Ben-Dror I, Vardimon L. Glutamine synthetase enhances the clearance of extracellular glutamate by the neural retina. J Neurochem. 2002;83:574–580. doi: 10.1046/j.1471-4159.2002.01168.x. [DOI] [PubMed] [Google Scholar]
- Sher PK, Hu SX. Increased glutamate uptake and glutamine synthetase activity in neuronal cell cultures surviving chronic hypoxia. Glia. 1990;3:350–357. doi: 10.1002/glia.440030506. [DOI] [PubMed] [Google Scholar]
- Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. Embo J. 2005;24:510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Rutishauser U, Silver J, Miller RH. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990;138:377–390. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- Tanigami H, Rebel A, Martin LJ, Chen TY, Brusilow SW, Traystman RJ, Koehler RC. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience. 2005;131:437–449. doi: 10.1016/j.neuroscience.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Vardimon L. Neuroprotection by glutamine synthetase. Isr Med Assoc J. 2000 2 Suppl:46–51. [PubMed] [Google Scholar]
- Vercellino M, Merola A, Piacentino C, Votta B, Capello E, Mancardi GL, Mutani R, Giordana MT, Cavalla P. Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J Neuropathol Exp Neurol. 2007;66:732–739. doi: 10.1097/nen.0b013e31812571b0. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol. 2001;50:169–180. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
- Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res. 1998;59:135–142. doi: 10.1016/s0169-328x(98)00142-9. [DOI] [PubMed] [Google Scholar]
- Yan P, Li Q, Kim GM, Xu J, Hsu CY, Xu XM. Cellular localization of tumor necrosis factor-alpha following acute spinal cord injury in adult rats. J Neurotrauma. 2001;18:563–568. doi: 10.1089/089771501300227369. [DOI] [PubMed] [Google Scholar]
- Yan P, Liu N, Kim GM, Xu J, Xu J, Li Q, Hsu CY, Xu XM. Expression of the type 1 and type 2 receptors for tumor necrosis factor after traumatic spinal cord injury in adult rats. Exp Neurol. 2003;183:286–297. doi: 10.1016/s0014-4886(03)00135-3. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]