Abstract
Exposure to the environmental toxicant arsenic, through both contaminated water and food, contributes to significant health problems worldwide. In particular, arsenic exposure is thought to function as a carcinogen for lung, skin and bladder cancer, via mechanisms that remain largely unknown. More recently, the Hedgehog (HH) signaling pathway has also been implicated in the progression and maintenance of these same cancers. Based on these similarities, we tested the hypothesis that arsenic may act in part through activating HH signaling. Here, we show that arsenic is able to activate HH signaling in a number of primary and established tissue culture cells, as well as in vivo. Arsenic activates HH signaling by decreasing the stability of the repressor form of GLI3, one of the transcription factors that ultimately regulate HH activity. We also show, using tumor samples from a cohort of bladder cancer patients, that high levels of arsenic exposure are associated with high levels of HH activity. Given the important role HH signaling plays in the maintenance and progression of a variety of tumors, including bladder cancer, these results suggest that arsenic exposure may in part promote cancer through the activation of HH signaling. Thus, we provide an important insight into the etiology of arsenic induced human carcinogenesis, which may be relevant to millions of people exposed to high levels of arsenic worldwide.
Keywords: arsenic, Hedgehog, GLI, bladder cancer, toxicant
Introduction
More than 100 million people are currently exposed to drinking water containing inorganic arsenic at the level above 0.13 μM (10 μg/L), the maximum contaminant level set by the World Health Organization (WHO) (1). Contaminant levels of inorganic arsenic as high as 10 μM or more can be found in drinking water throughout the world. Chronic arsenic ingestion causes numerous human health problems (1). In particular, arsenic exposure has a strong association with human cancers (2), including those derived from the lung, skin, bladder, and possibly other sites (1). Consistent with these findings, chronic low-level arsenic treatment has been shown to promote cell proliferation (3) and transform cells in vitro (4). Although arsenic exposure clearly contributes to carcinogenesis, the underlying mechanisms have only recently been described (1).
The secreted protein Hedgehog (HH) was first described as a key factor in metazoan development, determining cell fate, promoting proliferation or differentiation, and acting as a survival factor or a guidance molecule (5). Emerging results now suggest that HH signaling may also play a fundamental role in the maintenance function of adult tissues undergoing continuous proliferation and differentiation (6), perhaps by regulating the small pool of stem/progenitor cells that regulate these processes (7). Consistent with this important role in fetal development and adult tissue maintenance, deregulation of HH signaling leads to a variety of human cancers (8, 9), some of which are commonly associated with arsenic exposure. There are three mammalian HH family members, Sonic Hedgehog (SHH), Indian Hedgehog (IHH) and Desert Hedgehog (DHH), which are thought to function primarily by engaging a common signaling pathway. These proteins, which we collectively refer to as HH, initiate their biological effects through binding to the cell surface receptor Patched (PTCH). Such binding relieves the inhibitory effect of PTCH on a seven transmembrane protein Smoothened (SMO), resulting in modulation of the GLI transcription factors (GLI1, GLI2, and GLI3) (10, 11). GLI1 is a pure transcriptional activator and is itself a HH target gene, whose expression level is thought to be the most reliable marker for HH pathway activity (12). GLI2 and GLI3 function as both positive and negative regulators of the HH pathway, depending on their proteolytic status. Full-length GLI2 and GLI3 are transcriptional activators (GLI-A) (13, 14). However, in the absence of HH, GLI2 and GLI3 are actively converted into partially proteolysed transcriptional repressors (GLI-R), through a processing mechanism regulated by the proteasome (15, 16). The activity of the full-length GLIs is also regulated by controlling their protein stability via their complete proteosomal degradation (17). Ultimately, the overall ratio between GLI-A and GLI-R defines the levels of HH pathway activation (10, 11).
As there are many similarities in outcomes between individuals chronically exposed to arsenic and those harboring mutations that result in deregulated HH signaling (1, 8, 9), we hypothesized that arsenic might act to regulate HH signaling. Here, we provide evidence suggesting that arsenic exposure results in constitutive HH signaling, and that this activation occurs at concentrations relevant to human exposure. Moreover, using an established bladder cancer patient cohort, we show a strong positive association between arsenic exposure and high-level HH signaling, underscoring the physiological relevance of our findings. This is the first report implicating the HH signaling pathway as a physiologically relevant biological target for arsenic, which may begin to explain some of the underlying carcinogenesis found in humans exposed to environmental arsenic.
Material and Methods
Cell culture, reagents, and arsenic treatment
All cells were grown and maintained as previously described (18–20). Cell growth media used in these experiments have undetectable levels of background arsenic (data not shown). SHH conditioned media (SHH CM) were collected from HEK 293 cells stably expressing murine SHH (21). Conditioned media from the parental HEK293 cells were used as the negative control. For the experiments involving chronic arsenic treatment, sodium arsenite (Sigma) was added to NIH3T3 cells to final concentrations of 0, 0.5, 1, or 5 μM for 1 – 8 weeks. For other assays, sodium arsenite was added to cells for 24 hr. Cyclopamine (Toronto Research Chemicals) or vehicle control (dimethyl sulfoxide, DMSO) was added to 8-week chronic arsenic treated NIH3T3 cells in low serum media (0.5% serum) for 24 hr before harvesting cells for RNA isolation. HH reporter assays were performed using a GLIBS-Luc luciferase reporter construct or a miniTK-luciferase control plasmid as described previously (22).
Clonogenic cell survival assay
NIH3T3 cells were seeded into 6-well dishes at 500 cells/well and allowed to grow overnight in normal growth media. Cells were then exposed to 0 – 20 μM sodium arsenite for 24 hr, washed once with phosphate buffered saline (PBS), followed by the addition of fresh growth media. After 5 days post arsenic treatment, cells were stained with 0.4% Giemsa dye (w/v in 70% methanol, Sigma) for 90 min. Clonogenicity was defined as colony numbers formed, as a percent of control untreated cells.
RNA extraction, reverse transcription, and real-time PCR
RNA was extracted using Tri Reagent (Molecular Research Center), followed by a column cleanup step using a RNeasy kit (Qiagen). cDNA was synthesized from 2 μg total RNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystem). Quantitative PCR was performed using a 7500 Fast Real-Time PCR System with inventoried Taqman gene expression assay probes (Applied Biosystem) as described previously (22).
Immunoblot
GLI1 was enriched from one confluent 150 mm dish of cells using Sepharose beads conjugated with a Gli-binding oligonucleotide, or non-specific oligonucleotide, as described previously (16). In all other cases, proteins were directly extracted in 2× Laemmli sample buffer. Immunobloting was performed as described previously (23). Antibodies used were as follows: rabbit polyclonal GLI1 (affinity-purified, raised against a synthetic peptide corresponding to amino acids residue 802–817 of human GLI1, which has no homology with GLI2 or GLI3, and characterized in supplemental Figure S1), rabbit polyclonal GLI3 (24) and mouse monoclonal αTUBULIN (Calbiochem). In certain cases, densitometry analysis was performed on developed X-ray films using ImageQuant (Version 5.2). The quantitation results were expressed as ratios of the intensity score of the anti-GLI3 bands versus the corresponding intensity score of the anti-TUBULIN band. The ratio was used to compare the relative GLI3 levels with or without chronic arsenic exposure. The immunoblot for αTUBULIN was used as a loading control in all cases.
Mice
All mouse studies were performed in accordance with the policies of the Dartmouth IACUC. Seven week-old C57BL/6J male mice (The Jackson Lab) were housed on an AIN-76A diet (ad lib) (Harlan-Teklad Inc) and Carefresh bedding in autoclaved cages. After two weeks, these mice were given drinking water (ad lib, changed weekly) with or without addition of 1.3 μM sodium arsenite for 5 weeks as described previously (25). Tissues were collected from these mice and stored in RNA later (Ambion Inc) before RNA extraction in Tri Reagent using a motor driven homogenizer.
Bladder cancers and GLI1 immunohistochemistry
The study group was comprised of newly diagnosed, histologically confirmed bladder cancer patients on whom we obtained the original diagnostic formalin fixed tumor block. Subjects were interviewed as part of case-control study that involved testing a tap water sample from the participants’ homes for arsenic concentrations using high resolution inductively coupled plasma mass spectrometry (26). Diagnostic slides underwent a standardized histopathology re-review by the study pathologist, who selected the tumor block and outlined regions for cutting 0.6 mm tissue cores for tissue microarrays (TMAs). Immunohistochemistry was performed as described previously (27), using an affinity-purified rabbit polyclonal GLI1 antibody (1:300) characterized in supplemental Figure S1. The GLI1 score from each bladder tumor sample was examined by a research pathologist, who was blinded to the arsenic exposure and tumor invasiveness datasets. The GLI1 scoring criteria were based on an estimate of the percentage of tumor cells with nuclear GLI1 staining: less than 1%; 1 – 5%; 5 – 25%; 25 – 50% (supplemental Figure S2). No tumors with more than 50% nuclear GLI1 staining have been observed. Although we noted that the intensity of nuclear GLI1 staining appeared to positively correlate with the percentage of positively stained cells, this observation was not used as a factor for scoring. A second GLI1 antisera (Abcam) was used to confirm these immunohistochemistry results (supplemental Figure S1). The specificity of this second antisera was previously characterized (22).
Statistics
All experiments were independently performed at least three times. Statistical significance was determined by Student’s T test. For the analysis of TMAs, we compared the proportion of tumors that stained positively for GLI1 among those with high versus low water arsenic concentrations (above and below the median for the study) using a Chi-Squared test. In all analyses, two-tailed P values equal to or smaller than 0.05 were considered statistically significant.
Results
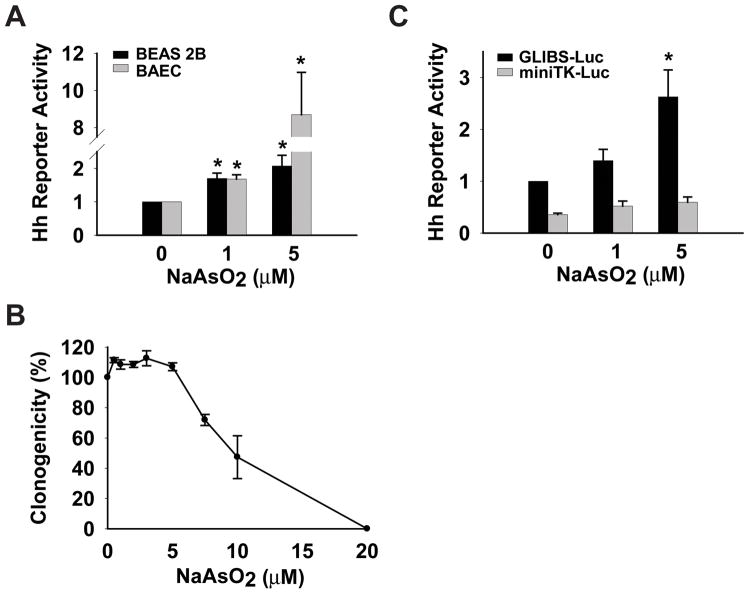
To test the hypothesis that arsenic activates HH signaling, we first evaluated the ability of sodium arsenite to activate a well characterized HH reporter construct in cultured cells originating from several tissues that are known arsenic targets (1, 28). This HH reporter construct was transiently transfected into primary bovine aorta endothelial cells (BAEC) and immortalized bronchial epithelial cells (BEAS 2B), followed by arsenic treatment for 24 hr. 1 μM (77 μg/L) sodium arsenite gave rise to a statistically significant 1.7 fold increase in HH activity in both cell types, whereas treatment with 5 μM arsenic resulted in a 2.1-fold or 8.7-fold increase in HH activity in BEAS 2B or BAEC respectively (Figure 1A). The activity of a constitutively expressed Renilla luciferase construct was not significantly changed in these arsenic exposed cells (data not shown), consistent with these doses of arsenic not inducing a general toxic effect on these cells. Next, we examined the ability of arsenic to activate HH signaling in NIH3T3 fibroblasts, a cell line commonly used to study HH signaling (18). We first determined the tolerable doses of sodium arsenite for NIH3T3 cells using a clonogenic assay, in which the ability of arsenic treated cells to proliferate and form colonies was tested. The clonogenicity of NIH3T3 cells appears unaffected when treated with up to 5 μM sodium arsenite, with an overall IC50 of approximately 9.5 μM (Figure 1B). Similar to the results observed with BAEC and BEAS 2B cells, 5 μM arsenic activated HH signaling 2.6 fold above background in NIH3T3 cells (Figure 1C, black bar). The activation of the HH reporter gene by arsenic appears specific to HH pathway activation, as a similar construct lacking GLI binding sites failed to respond to arsenic exposure (Figure 1C, grey bar). These results suggested that activation of HH signaling was induced by arsenic, and that this activation could occur at arsenic levels similar to those found at contamination levels relevant to human exposure (1).
Figure 1.
Arsenic activates HH signaling in various cells. (A) A HH reporter construct (GLIBS-Luc) was transfected into primary bovine aorta endothelial cells (BAEC) and immortalized human lung epithelial cells (BEAS 2B). Luciferase activity was measured 24 hr after arsenic treatment and normalized to Renilla activity. Normalized luciferase activity in vehicle treated cells was set to 1. (B) Clonogenic assay in NIH3T3 cells exposed to the indicated doses of arsenic for 24 hr. Data are expressed as clonogenicity, which is the number of colonies formed as a percentage of the untreated control cells. (C) GLIBS-Luc or a control construct (miniTK-Luc) was transfected into NIH3T3 cells, followed by measurement of luciferase activity 24hr after arsenic treatment and normalized to Renilla activity. Normalized luciferase activity in vehicle treated GLIBS-Luc transfected cells was set to 1. Error bars indicate s.e.m from three independent experiments. The asterisk indicates statistically significant changes compared to vehicle treated cells.
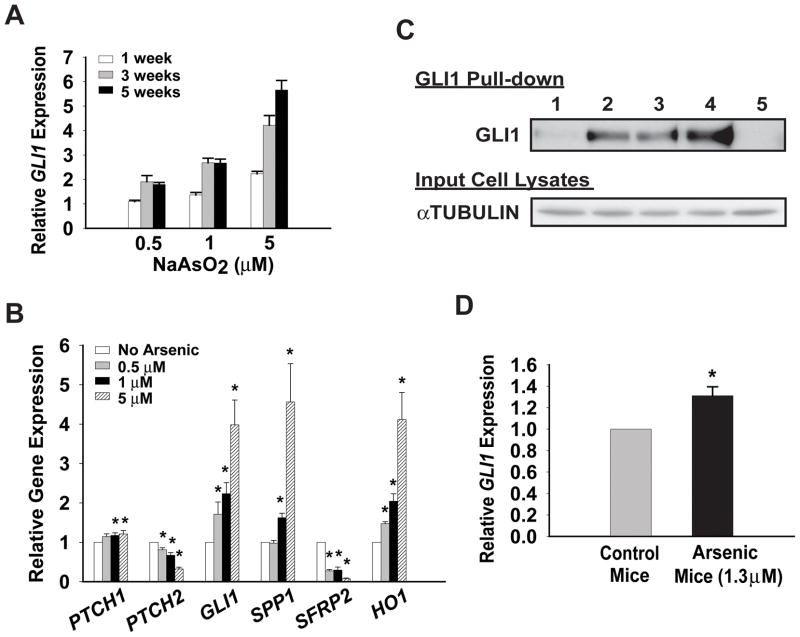
We next examined the ability of arsenic to activate HH signaling when cells were exposed to low levels of arsenic in a chronic manner, which is thought to more closely mimic human exposure in arsenic contaminated areas (1, 2). NIH3T3 cells were cultured either in standard growth media or the same media containing 0.5 μM, 1 μM, or 5 μM sodium arsenite for 1 – 8 weeks. As an endogenous indicator of HH pathway activation, the expression of the HH target gene GLI1 was examined in cells treated with arsenic for one, three, or five weeks, using a quantitative real-time reverse transcription-polymerase chain reaction (real time RT-PCR) assay. After one week of arsenic exposure, 1 μM and 5 μM arsenic treated cells showed a modest increase in the expression of GLI1, 1.4- and 2.2-fold respectively, relative to control cells that were not exposed to arsenic (Figure 2A). This activation became more robust as the time of arsenic treatment increased. Interestingly, although a one week exposure of cells to 0.5 μM arsenic did not cause any increase in GLI1 induction, longer exposure (≥ three weeks) resulted in a 1.7-fold GLI1 induction. This concentration of arsenic is lower than the maximum contaminant level (0.63 μM) allowed in potable water in the USA prior to 2002 (2). Thus, GLI1 expression was activated by chronic arsenic treatment in a dose- and time- dependent manner. To strengthen this analysis, we examined the expression of a panel of HH target genes in NIH3T3 cells exposed to sodium arsenite for 8 weeks. This panel consists of those genes normally up-regulated by HH (GLI1, PTCH1, PTCH2, and Secreted Phosphoprotein 1 (SPP1)) (5, 29, 30), as well as a gene that is repressed by HH in cultured fibroblasts (Secreted Frizzled-Related Protein 2 (SFRP2)) (31). Four out of five of these genes (GLI1, PTCH1, SPP1, and SFRP2) showed dose-dependent changes consistent with activation of HH signaling (Figure 2B). Arsenic effects on NIH3T3 cells were verified by a dose-dependent induction of Heme Oxygenase 1 (HO1) expression, which is a well-established arsenic target (32). As these analyzed genes are all well-characterized HH target genes, their expression serve as physiologically relevant markers of HH pathway activation. We also independently verified the ability of chronic low-dose arsenic to activate HH target genes in a pluripotent mesenchymal mouse embryonic cell line C3H/10T1/2 (data not shown).
Figure 2.
Chronic low-dose arsenic exposure activates HH signaling in vitro and in vivo. (A) NIH3T3 cells were cultured in media with or without 0.5 μM, 1 μM, or 5 μM sodium arsenite. Real-time PCR was performed to determine the expression of the HH target gene, GLI1, relative to expression of 18S rRNA after 1, 3, or 5 weeks of arsenic treatment. A representative experiment of GLI1 expression in arsenic treated cells relative to untreated control cells is shown. (B) RNA was extracted from NIH3T3 cells treated with or without 0.5 μM, 1 μM, or 5 μM sodium arsenite for 8 weeks. The expression levels of indicated genes were examined by real-time PCR relative to the expression of 18S rRNA. The level of expression for each indicated gene in untreated control cells was set to 1. Error bars indicate s.e.m from five independent experiments. The asterisk indicates significant changes compared to control cells. (C) Immunoblot for GLI1 was performed after enrichment of the protein using Sepharose beads conjugated with a Gli-binding oligonucleotide (lane 1 – 4) or non-specific oligonucleotide (lane 5). Lane 1: control cells; lane 2: 0.5 μM arsenic exposed cells; lane 3: 1 μM arsenic exposed cells; lane 4 and 5: 5 μM arsenic exposed cells. The immunoblot for αTUBULIN in lysates prior to GLI pull-down served as an indicator of equal protein input. (D) Nine week-old C57BL/6J male mice were given drinking water with or without 1.3 μM sodium arsenite for 5 weeks, followed by harvesting of RNA from the kidneys. Real-time PCR was performed to determine GLI1 expression relative to expression of 18S rRNA. Expression of GLI1 was set to 1 in control mice that were not exposed to arsenic via drinking water. Six control mice and six arsenic exposed mice were used in each experiment. Error bars indicate s.e.m from three independent experiments. The asterisk indicates statistically significant changes.
To further validate the observed arsenic induced changes in GLI1 expression, we next examined the change in GLI1 protein in cells chronically exposed to arsenic. To detect endogenous GLI1 in lysates of these NIH3T3 cells we first enriched for GLI1 using Sepharose beads conjugated with oligonucleotides encoding a defined GLI-binding site (16), followed by immunoblotting with GLI1 antisera. Using this approach, we observed a dramatic increase in GLI1 protein in response to increasing amount of arsenic exposure, with the strongest induction occurring in 5 μM arsenic exposed cells (Figure 2C, lane 4). Sepharose beads conjugated with non-specific DNA oligonucleotides were used to control the specificity of these pull-downs (Figure 2C, lane 5).
Our findings were also confirmed in a mouse model of chronic arsenic exposure (25). Nine-week old C57BL/6J mice were exposed to 1.3 μM sodium arsenite for five weeks in their drinking water. These animals were sacrificed and various tissues were harvested. Total RNA was extracted from these tissues and the expression of GLI1 was analyzed by real time RT-PCR. A statistically significant increase in the relative expression of GLI1 was observed from the kidneys of arsenic exposed mice, but not from other tissues examined (Figure 2D, and data not shown). This arsenic induced increase in HH signaling is likely an under-estimate of the extent of activation, as only a limited group of adult cells in any tissue may be capable of elaborating a HH response. We did not observe any signs of arsenic induced toxicity in these animals over these five-week studies (data not shown). Thus, these results suggest that chronic arsenic exposure is also able to activate HH signaling in vivo, at least in a subset of tissues.
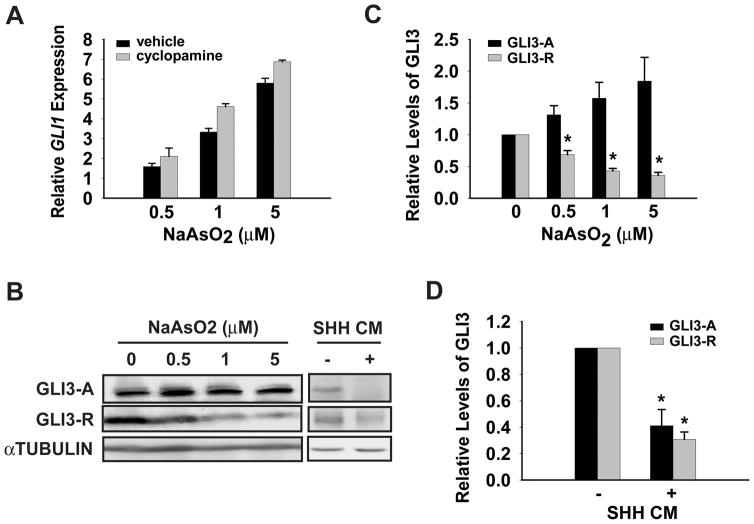
To begin to understand the mechanism of HH pathway activation upon arsenic exposure we determined if the arsenic activation of HH signaling required SMO function, which is a key regulator in the HH pathway (5). Consistent with SMO’s pivotal role in HH signaling, a number of small molecule SMO modulators have been described (33). Moreover, SMO appears to be the most common small-molecule target identified in numerous high-throughput screens, making it a likely arsenic target. Somewhat surprisingly, the SMO antagonist cyclopamine was unable to attenuate arsenic induced HH signaling in NIH3T3 cells (Figure 3A). Cyclopamine decreased overall GLI1 expression in both chronic arsenic exposed cells and control cells to a similar extent (data not shown). Thus, the fold induction of GLI1 expression by arsenic was not inhibited. As a control for the effectiveness of this drug, SHH induced GLI1 expression was completely abolished by cyclopamine (supplemental Figure S3). This result argued for a dispensable role of SMO in arsenic induced HH signaling, thus ruling out an indirect contribution of HH in arsenic mediated pathway activation. HH signaling ultimately results in changes in the levels and activation status of GLI1 – 3 (11). Thus, we hypothesized that arsenic might be activating HH signaling by modulating the processing of GLI2 or GLI3. Interestingly, arsenic attenuated the level of GLI3-R in a dose-dependent manner, but had little effect on the level of GLI3-A (Figure 3B and 3C). Since arsenic treatment also modestly induced the expression of GLI3 (supplemental Figure S4), we speculate that GLI3-R might be selectively degraded upon arsenic exposure. Consistent with this speculation, arsenic is known to regulate the stability of other proteins in both a proteosomal and lysosomal dependent manner (20, 34). Loss of GLI3-R in the context of similar GLI3-A levels would result in a net increase in HH activity, similar to what we have observed. However, the effect of arsenic on GLI3 appears different from the way HH regulates GLI3 in these cells. HH inhibited GLI3 protein processing into GLI3-R and also decreases GLI3 mRNA at the same time (24, 35), resulting in a loss of both GLI3-A and GLI3-R (Figure 3B and 3D). We were unable to detect endogenous GLI2 from these cells. However, the decreases in GLI2 transcript observed in response to arsenic are consistent with GLI3-A driving arsenic-mediated activation of HH signaling (supplemental Figure S4).
Figure 3.
Chronic arsenic exposure reduces the level of GLI3 repressor. (A) Chronic arsenic exposed NIH3T3 cells were treated with 5 μM cyclopamine or vehicle for 24 hr. Real-time PCR was performed to determine the expression of the HH target gene GLI1 relative to expression of 18S rRNA. A representative experiment of GLI1 expression in arsenic treated cells, relative to untreated control cells, is shown here. (B) Immunoblot for full-length GLI3 activator (GLI3-A) and GLI3 repressor (GLI3-R) was performed in total cell lysates from NIH3T3 cells exposed to the indicated doses of arsenic for 8-weeks or SHH conditioned media (SHH CM) or control media for 24 hr. (C, D) Levels of GLI3-A and GLI3-R were quantified by densitometry analysis. Levels of GLI3-A and GLI3-R were set to 1 in control cells that were not exposed to arsenic. Error bars indicate s.e.m from three independent experiments. The asterisk indicates statistically significant changes compared to control cells.
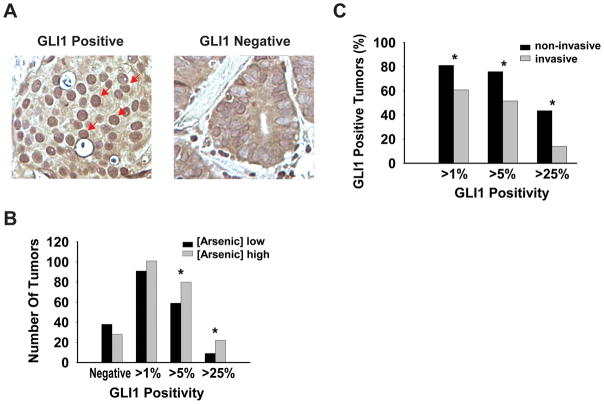
A series of epidemiologic studies have shown an association between arsenic exposure and bladder cancer (1, 36, 37). Moreover, HH signaling has also been implicated in bladder cancer progression (38, 39). Thus, to determine a putative linkage between chronic arsenic exposure and HH signaling in carcinogenesis we utilized tumors from a large population-based bladder cancer patient cohort (26). We examined GLI1 protein level as a readout for HH pathway activity in 265 bladder cancers cases, using a quantitative immunohistochemistry protocol that estimated the percentage of tumor cells with nuclear GLI1 staining (Figure 4A). We defined bladder cancers as negative for GLI1 staining if less than 1% of cancer cells contained nuclear GLI1, while more than 1%, more than 5%, and more than 25% nuclear GLI1 delineated different relative levels of GLI1 positivity. The number of cases with each GLI1 score is shown in supplemental Table S1. Household tap water arsenic concentrations from 97% of the study subjects were measured, ranged from 0.004 μg/L to 160.5 μg/L, with the median concentration being 0.326 μg/L. We classified patients into two categories of “high” or “low” water arsenic exposure using the median arsenic concentration as the cutpoint. In general, the numbers of GLI1 positive tumors were higher in patients with high arsenic exposure, while GLI1 negative tumors resided more in the low arsenic category (Figure 4B). For example, when using the highest level of GLI1 quantitation observed (more than 25%) as the definition of GLI1 positivity, 22 bladder tumors were positive among patients with high water arsenic levels versus 9 among those with low levels (P = 0.0087) (Figure 4B). We also examined the distribution of GLI1 positive cancers in two major clinical categories, non-invasive and invasive bladder cancers, as these two tumor types have different clinical management and prognostic criteria (40). Seventy-one percent of the cancer cohort we used consisted of non-invasive tumors, with the remaining samples being invasive tumors. We observed that a higher percentage of GLI1 positive cancers were non-invasive, regardless of their classification of GLI1 positivity (Figure 4C). In particular, there were 3-fold more GLI1 positive tumors (44% versus 14%) in the non-invasive category when only tumors with highest GLI1 positivity were taken into account. These results suggest that activation of HH signaling by arsenic may play a preferential role in the formation of non-invasive bladder cancers. Indeed, the association between GLI1 positivity and arsenic exposure was also more pronounced in non-invasive tumors, compared to invasive tumors (data not shown). Thus, these results suggest a highly significant positive association between the levels of arsenic exposure and HH pathway activity in bladder cancer, demonstrating the relevance of this work to human health.
Figure 4.
High-level HH signaling positively correlates with arsenic exposure in human bladder cancer. (A) Immunohistochemical staining and scoring were performed on a tumor array consisting of 265 bladder cancers, using an antibody against GLI1. A representative immunohistochemical staining for GLI1 from a positive case (left) and a negative case (right) of bladder cancer are shown. A subset of GLI1 positive cancer cells are indicated by the arrows. (B) Tumors were grouped into “high” or “low” arsenic categories based on the subject’s drinking water arsenic level (high: arsenic concentration ≥ median; low: arsenic concentration < median). Different levels of GLI1 positivity were plotted against the number of tumors in each category. Asterisk indicates statistically significant changes when comparing the distribution of different levels of GLI1 positive tumors to GLI1 negative tumors in each studying category. (C) All tumors were divided into categories of non-invasive tumors and invasive tumors. Different GLI1 classifications were plotted against the percent of GLI1 positive tumors in each category. Asterisk indicates statistically significant changes when comparing the percentage of GLI1 positive tumors in each category.
Discussion
We show here that the environmental toxicant arsenic activates the HH signaling pathway. The activation of this important signaling pathway requires levels of arsenic relevant to human exposure, and occurs in various cell types in vitro, as well as in vivo. Activation of HH signaling occurs in response to acute and chronic arsenic exposure, in a manner that is time and dose dependant, and via a mechanism that does not appear to affect cell viability. The chronic effects of arsenic are particularly relevant to human disease, as most arsenic-related health concerns occur in individuals exposed to environmental levels of arsenic for a long period of time (1, 2). We suggest a model whereby arsenic activates HH signaling by targeting GLI3-R for proteolytic degradation, in a manner that appears independent of SMO. Our results also show a strong association between exposure to arsenic and activation of high-level HH signaling in a cohort of bladder cancer patients, in particular those with non-invasive bladder cancers, demonstrating the significance of our findings to human health.
Although arsenic is able to activate HH signaling in many cell types, the level of activation observed is relatively low compared to those initiated by HH (supplemental Figure S3). However, constitutive low-level HH activation over long periods of time might contribute to significant health problems, as illustrated in individuals diagnosed with Gorlin’s syndrome (41). These individuals harbor loss of function mutations in one copy of PTCH1, which results in constitutive low-level activation of HH signaling (42). These individuals exhibit an increased risk for a number of health problems, including certain types of cancer. Chronic exposure to arsenic also results in an increased risk to a variety of tumors, including those derived from skin, lung and bladder (1). Our results support the hypothesis that chronic activation of HH signaling by arsenic might contribute to the development of a subset of these tumors. Activation of HH signaling is thought to act as a survival factor in tumor cells, with the extent of HH pathway activation approximating tumor progression (43). Recent evidence has also suggested that ectopic HH activation is important to create the microenvironment required for efficient tumor growth (44). Consistent with the frequent requirement of HH activity in carcinogenesis, the HH target gene GLI1 is itself an oncogene. Over-expression of GLI1 results in a transformed phenotype in vitro (45) and is sufficient to drive the formation of many cancers when over expressed in animal models, including those associated with arsenic exposure (17, 46, 47). Therefore, we speculate that arsenic’s ability to function as a carcinogen might act in part through its ability to activate HH signaling, helping to create a microenvironment permissive to tumor development.
Urine is the major route of arsenic excretion in humans, making the bladder a susceptible target for the adverse effects of arsenic (1). A number of epidemiology studies have suggested an increased risk of bladder cancer with chronic arsenic exposure (1, 36, 37). However, the mechanism underlying this increased risk remains unknown. Our results suggest that activation of HH signaling by arsenic might contribute to the etiology of arsenic related bladder cancers. It has been previously suggested that HH signaling may contribute to the development of bladder cancers (38, 39). Deletions in chromosome 9q are the most common genetic alteration and earliest marker for bladder cancers (48). Noticeably, PTCH1 resides in the region of 9q often lost in human bladder cancers (38, 48). Furthermore, gene amplification of a region of chromosome 12q13-q15, which encodes GLI1, has also been found in a subset of bladder cancers (49). Interestingly, loss of PTCH1 and amplification of GLI1 would all result in HH pathway activation. Consistent with the HH pathway playing an important role in the progression of bladder cancer, PTCH1 heterozygous mice exhibit a predisposition to carcinogen induced bladder cancer (50). We have shown here that GLI1 levels are elevated in approximately 75% of bladder cancers (supplemental Table S1, 198 out of 265 cases) and that an environmental toxicant known to function as a risk factor for bladder cancer can also activate HH signaling, albeit through a different mechanism. Combined, these results suggest that the HH signaling pathway plays an important role in bladder cancer progression and that its activation can occur via distinct mechanisms: loss of PTCH1, amplification of GLI1, or degradation of GLI3-R in response to chronic exposure to arsenic.
In conclusion, our study provides for the first time evidence that links activation of the HH pathway with arsenic exposure. This pathway activation occurs in both an acute and chronic manner, and occurs at environmentally relevant levels of arsenic. Furthermore, we show that arsenic exposure correlates with high-level HH activation in bladder cancer samples isolated from a large cohort of such patients. Thus, high-level HH signaling may provide a diagnostic marker for those bladder cancer patients exposed to arsenic, and provides a novel mechanism of how arsenic might function in carcinogenesis.
Supplementary Material
Acknowledgments
This work was supported by a National Institutes of Health grant GM64011 (DJR), an American Lung Association grant LCD-36191-N (DJR), a Dartmouth-Hitchcock Medical Center Prouty grant (DJR), a National Cancer Institute grant ES007373 (MK), and the Hitchcock Foundation (DLF). We thank the members of the Robbins, Karagas, and Hamilton laboratories, X. Liu, M. Zens, B. Ladizinski, A. Andrew, and E. Dmitrovsky for helpful discussions during the course of this work.
References
- 1.International Agency for Research on Cancer (IARC) IARC monographs on the evaluation of carcinogenic risks to humans. France: IARC Press; 2004. Some drinking-water disinfectants and contaminants, including arsenic; pp. 37–270. [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AH, Lopipero PA, Bates MN, Steinmaus CM. Public health. Arsenic epidemiology and drinking water standards. Science. 2002;296:2145–6. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- 3.Barchowsky A, Roussel RR, Klei LR, et al. Low levels of arsenic trioxide stimulate proliferative signals in primary vascular cells without activating stress effector pathways. Toxicol Appl Pharmacol. 1999;159:65–75. doi: 10.1006/taap.1999.8723. [DOI] [PubMed] [Google Scholar]
- 4.Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci U S A. 1997;94:10907–12. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 6.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–75. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 7.Katoh Y, Katoh M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review) Int J Mol Med. 2006;18:1019–23. [PubMed] [Google Scholar]
- 8.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–11. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 9.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8:743–54. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins DJ, Hebrok M. Hedgehogs: la dolce vita. Workshop on Hedgehog-Gli Signaling in Cancer and Stem Cells. EMBO Rep. 2007;8:451–5. doi: 10.1038/sj.embor.7400959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–47. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura H, Stephen D, Joyner A, Curran T. Gli1 is important for medulloblastoma formation in Ptc1+/− mice. Oncogene. 2005;24:4026–36. doi: 10.1038/sj.onc.1208567. [DOI] [PubMed] [Google Scholar]
- 13.Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–15. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 14.Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–72. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- 15.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–77. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, Li Y. Evidence for the direct involvement of {beta}TrCP in Gli3 protein processing. Proc Natl Acad Sci U S A. 2006;103:33–8. doi: 10.1073/pnas.0509927103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huntzicker EG, Estay IS, Zhen H, Lokteva LA, Jackson PK, Oro AE. Dual degradation signals control Gli protein stability and tumor formation. Genes Dev. 2006;20:276–81. doi: 10.1101/gad.1380906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–9. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 19.Martin KA, Rzucidlo EM, Merenick BL, et al. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–17. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kitareewan S, Roebuck BD, Demidenko E, Sloboda RD, Dmitrovsky E. Lysosomes and trivalent arsenic treatment in acute promyelocytic leukemia. J Natl Cancer Inst. 2007;99:41–52. doi: 10.1093/jnci/djk004. [DOI] [PubMed] [Google Scholar]
- 21.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–7. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Z, Goetz JA, Singh S, et al. Frequent requirement of hedgehog signaling in non-small cell lung carcinoma. Oncogene. 2007;26:1046–55. doi: 10.1038/sj.onc.1209860. [DOI] [PubMed] [Google Scholar]
- 23.Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–34. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–34. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 25.Kozul CD, Ely KH, Enelow RI, Hamilton JW. Low Dose Arsenic Compromises the Immune Response to Influenza A Infection in vivo. Environ Health Perspect. 2009 doi: 10.1289/ehp.0900911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karagas MR, Tosteson TD, Blum J, Morris JS, Baron JA, Klaue B. Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a U.S. population. Environ Health Perspect. 1998;106 (Suppl 4):1047–50. doi: 10.1289/ehp.98106s41047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Cherukuri P, Li N, et al. Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res. 2007;67:501–10. doi: 10.1158/0008-5472.CAN-05-4571. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–22. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- 29.Zaphiropoulos PG, Unden AB, Rahnama F, Hollingsworth RE, Toftgard R. PTCH2, a novel human patched gene, undergoing alternative splicing and up-regulated in basal cell carcinomas. Cancer Res. 1999;59:787–92. [PubMed] [Google Scholar]
- 30.Yoon JW, Kita Y, Frank DJ, et al. Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem. 2002;277:5548–55. doi: 10.1074/jbc.M105708200. [DOI] [PubMed] [Google Scholar]
- 31.Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by Sonic Hedgehog in pluripotent mesenchymal cells. Oncogene. 2002;21:8196–205. doi: 10.1038/sj.onc.1205975. [DOI] [PubMed] [Google Scholar]
- 32.Menzel DB, Rasmussen RE, Lee E, et al. Human lymphocyte heme oxygenase 1 as a response biomarker to inorganic arsenic. Biochem Biophys Res Commun. 1998;250:653–6. doi: 10.1006/bbrc.1998.9363. [DOI] [PubMed] [Google Scholar]
- 33.Robbins DJ, Goetz JA, Yuan ZQ, Stegman MA. Inhibitors of the Hedgehog Signal Transduction Pathway. Current Cancer Therapy Reviews. 2005;1:277–88. [Google Scholar]
- 34.Lallemand-Breitenbach V, Zhu J, Puvion F, et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J Exp Med. 2001;193:1361–71. doi: 10.1084/jem.193.12.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marigo V, Johnson RL, Vortkamp A, Tabin CJ. Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev Biol. 1996;180:273–83. doi: 10.1006/dbio.1996.0300. [DOI] [PubMed] [Google Scholar]
- 36.Karagas MR, Tosteson TD, Morris JS, et al. Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire. Cancer Causes Control. 2004;15:465–72. doi: 10.1023/B:CACO.0000036452.55199.a3. [DOI] [PubMed] [Google Scholar]
- 37.Guo HR, Chiang HS, Hu H, Lipsitz SR, Monson RR. Arsenic in drinking water and incidence of urinary cancers. Epidemiology. 1997;8:545–50. doi: 10.1097/00001648-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Aboulkassim TO, LaRue H, Lemieux P, Rousseau F, Fradet Y. Alteration of the PATCHED locus in superficial bladder cancer. Oncogene. 2003;22:2967–71. doi: 10.1038/sj.onc.1206513. [DOI] [PubMed] [Google Scholar]
- 39.McGarvey TW, Maruta Y, Tomaszewski JE, Linnenbach AJ, Malkowicz SB. PTCH gene mutations in invasive transitional cell carcinoma of the bladder. Oncogene. 1998;17:1167–72. doi: 10.1038/sj.onc.1202045. [DOI] [PubMed] [Google Scholar]
- 40.van der Meijden AP. Bladder cancer. Bmj. 1998;317:1366–9. doi: 10.1136/bmj.317.7169.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorlin RJ, Sedano HO. The multiple nevoid basal cell carcinoma syndrome revisited. Birth Defects Orig Artic Ser. 1971;7:140–8. [PubMed] [Google Scholar]
- 42.Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 43.Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 44.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 45.Ruppert JM, Vogelstein B, Kinzler KW. The zinc finger protein GLI transforms primary cells in cooperation with adenovirus E1A. Mol Cell Biol. 1991;11:1724–8. doi: 10.1128/mcb.11.3.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–81. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson M, Unden AB, Krause D, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci U S A. 2000;97:3438–43. doi: 10.1073/pnas.050467397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirao S, Hirao T, Marsit CJ, et al. Loss of heterozygosity on chromosome 9q and p53 alterations in human bladder cancer. Cancer. 2005;104:1918–23. doi: 10.1002/cncr.21423. [DOI] [PubMed] [Google Scholar]
- 49.Simon R, Struckmann K, Schraml P, et al. Amplification pattern of 12q13-q15 genes (MDM2, CDK4, GLI) in urinary bladder cancer. Oncogene. 2002;21:2476–83. doi: 10.1038/sj.onc.1205304. [DOI] [PubMed] [Google Scholar]
- 50.Hamed S, LaRue H, Hovington H, et al. Accelerated induction of bladder cancer in patched heterozygous mutant mice. Cancer Res. 2004;64:1938–42. doi: 10.1158/0008-5472.can-03-2031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.