Abstract
Loss of hSNF5 function is usually observed in malignant rhabdoid tumor (MRT), a highly aggressive pediatric neoplasm. Previous studies have shown that reexpression of hSNF5 in MRT cell lines causes G1 cell cycle arrest with p16INK4A, p21CIP1/WAF1 and cyclin D1 playing key roles in MRT cell growth control. However, we have shown that reexpression of hSNF5 induced cell cycle arrest in the absence of p16INK4A expression. These results indicate that the mechanism of hSNF5-induced cell cycle arrest is context dependent. Here, we investigated the relationship between p21CIP1/WAF1 and hSNF5 in the regulation of growth using several MRT cell lines. We found that G1 cell cycle arrest occurred concomitant with an increase in p21CIP1/WAF1 mRNA and protein levels and preceeded p16INK4A mRNA and protein up-regulation. Chromatin immunoprecipitation data confirmed that hSNF5 appeared at both p21CIP1/WAF1 and p16INK4A promoters after reexpression. We further showed that p21CIP1/WAF1 induction showed both p53 dependent and independent mechanisms. We also demonstrated that reduction of p21CIP1/WAF1 expression by RNAi significantly inhibited hSNF5-induced G1 arrest. Our results demonstrate that both p21CIP1/WAF1 and p16INK4A are targets for hSNF5, and that p21CIP1/WAF1 up-regulation during hSNF5-induced G1 arrest precedes p16INK4A up-regulation. These findings indicate that SNF5 mediates a temporally controlled program of CDK inhibition to restrict aberrant proliferation in MRT cells.
Keywords: SNF5/INI1, p21WAF1/CIP1, p16INK4A, chromatin remodeling
Introduction
Malignant rhabdoid tumor (MRT) is a rare and extremely aggressive childhood cancer. MRT was initially described as an unfavorable histologic type of pediatric renal tumor, a variant of Wilms’ tumor (1). While the most common locations occur in the kidney and central nervous system, MRT also arise in almost any site (2, 3). Despite significant advances in the treatment and outcome of other pediatric tumors, for MRTs diagnosed before the age of 6 months, patient survival at 4 years drops to approximately 8.8% (4). Therefore, improved patient outcome requires a better understanding of malignant rhabdoid tumorigenesis and the development of novel therapeutic strategies.
In the past several years, the discovery of deletions and mutations at 22q11.2 involving hSNF5/INI1 has contributed to the clarification of pathogenesis of MRT (5). The finding that genetic alterations in MRTs are usually limited to hSNF5 mutations and deletions implicates the loss of hSNF5 function as the primary cause of these tumors. Now, hSNF5 function is recognized as being lost in almost 100% of MRTs (6, 7). Therefore, the elucidation of hSNF5 function should lead to the identification of the key molecular steps necessary for MRT tumorgenesis.
hSNF5 is one of the core subunits of the SWI/SNF chromatin remodeling complex that also includes an ATPase subunit (either BRG1 or BRM), BAF155, and BAF170. SWI/SNF complexes are ATP-dependent chromatin remodeling complexes that regulate gene transcription by causing conformational changes in chromatin structure, as well as by cooperation with histone acetylation complexes (8). In human cells, studies have shown a role for transcriptional regulation by SWI/SNF complexes in the control of cell growth, tissue differentiation, and embryo development in multiple tissues (9). Furthermore, loss of BRG1 function has been observed in malignant tumors including lung, pancreatic, breast, and prostate cancer (10-13). Several new SWI/SNF members, such as BAF180, have been found to form different subsets of SWI/SNF complexes with distinct functions (14-16). To understand how the SWI/SNF complex regulates gene expression in a complex and precise manner has become increasingly important.
Recently, several reports have shown that hSNF5 plays key roles in cell cycle control, differentiation, and oncogenic transformation. Reexpression of hSNF5 induces G1 cell cycle arrest in MRT cell lines, accompanied by up-regulation of p16INK4A and down-regulation of cyclin D1, cyclin A, and phosphorylated retinoblastoma protein (pRb), suggesting a key role for these genes in MRT cell cycle control (17-20). Kia et al. reported reexpression of hSNF5 mediates eviction of polycomb complex proteins such as BMI-1 from epigenetically silenced promoters of the INK4b-ARF-INK4a locus followed by their activation (21). Furthermore, some reports demonstrated that hSNF5 controls the differentiation of MRT cells (22, 23) and hSNF5 loss changes gene transcription epigenetically and contributes to oncogenesis without genomic instability (24).
Our previous study showed that reexpression of hSNF5 induced cell cycle arrest even in the absence of p16 INK4A expression (25). This finding suggested that other genes besides p16INK4A play a critical role at early time points of G1 cell cycle arrest induced by hSNF5. Therefore, in this study, we determined the mechanism of G1 cell cycle arrest induced by hSNF5 in MRT cells within 24 hours after reexpression using adenoviral vectors. We show that induction of p21WAF1/CIP1 appears at the onset of hSNF5-induced growth arrest and precedes p16INK4A expression. Furthermore, we demonstrate that p21WAF1/CIP1 knock-down inhibits hSNF5-induced G1 cell cycle arrest. We also show differences in the histone methylation changes at these 2 promoters after hSNF5 reexpression. Finally, we demonstrate that p21WAF1/CIP1 shows both p53 dependent and independent mechanisms of induction after hSNF5 reexpression. Our results suggest that p21WAF1/CIP1 plays a key role in hSNF5 control of cell growth, and hSNF5 loss may alter p21WAF1/CIP1 transcription by a different mechanism than that reported for the p16 INK4A promoter in MRT cells.
Materials and Methods
Cell culture and adenovirus infection
A204.1 (ATCC), G401.6 (ATCC), TTC642 (Dr. Timothy Triche- Childrens Hospital of Los Angeles), and NIH3T3 (Dr. Stuart Aaronson-National Cancer Institute) cells were cultured in RPMI 1640 medium and UNC N3T cells in bronchial epithelial growth medium (BEGM) (26). 293FT cells were cultured in DMEM medium containing 10% fetal bovine serum. The Ad/pAdEasyGFPINI-SV+ adenoviral vectors expressing hSNF5 and co-expressing the green fluorescent protein (GFP) (designated Ad-hSNF5) and the Ad/pAdEasyGFP expressing GFP (designated Ad-GFP) were previously published (20). In order to achieve infection of over 90% cells, we infected at a multiplicity of infection (M.O.I.) of 20 for the A204.1 cell line and 200 for the TTC642 cell line.
Protein extracts and Western blotting
Western blotting was carried out as described previously (25). Western analyses of proteins were carried out by using anti-p21CIP1/WAF1 (AB1; Calbiochem), anti-p16INK4a (G175-1239; BD Pharmingen), anti-pRb (G3-245; BD Pharmingen), anti-actin (A2066; Sigma), anti-p53 (DO-1; Santa Cruz), anti–cyclin A (H-432; Santa Cruz Biotechnology), anti-hSNF5 (BD Transduction Laboratories), BMI-1 (upstate cloneF6; Millipore), and horseradish peroxidase–conjugated anti-rabbit or anti-mouse IgG (GE Healthcare).
RNA extraction and Quantitative real-time reverse transcription-PCR analysis
RNA was extracted using the RNeasy mini kit (Qiagen), and 1 μg was used for cDNA synthesis primed with Random Primers (Invitrogen). cDNA was analyzed using TaqMan (Applied Biosystems) quantitative real-time reverse transcription-PCR (QT-PCR) analysis with β-actin as the reference gene in each reaction. Reactions were performed on an ABI 7900 HT sequence detection system (Applied Biosystems) and relative quantification was determined using the 2−ΔΔCt method (27). The primers used for p16INK4a QT-PCR were 5′-CTGCCCAACGCACCGAATA-3′ and 5′-GCGCTGCCCATCATCATGA-3′. The probe used for p16INK4a QT-PCR was 5′-CTGGATCGGCCTCCGACCGTA-3′. The TaqMan gene expression assay primer/probe set (Hs00355782_m1; Applied Biosystems) was used for p21CIP1/WAF1, the primer/probe set (Hs01034249_m1; Applied Biosystems) was used for p53, and the primer/probe set (Hs99999903_m1; Applied Biosystems) was used for β-actin.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIPs) was carried out as described by Donner et al (28). Immunoprecipitation was performed with an antibody specific to hSNF (Dr. Tony Imbalzano), histone H3 trimethylation of lysine 4 (H3K4 me3) (ab8580; Abcam), BRG-1 (J1; Dr. Weidong Wang), BMI-1 (upstate cloneF6; Millipore), normal Rabbit IgG (sc-2027; Santa Cruz Biotechnology), normal mouse IgG (sc-2025; Santa Cruz Biotechnology) or p53 (DO-1; Calbiochem). DNA present in each IP was quantified by QT-PCR using gene-specific primers on an ABI 7000 sequence detection system. All expression values were normalized against input DNA. Antibody specificity was also determined for each cell line (Supplementary Figure 1). The primer sequences are shown in Supplementary Table 1.
Lentiviral procedures and shRNA
Lentivirus was generated using 293FT cells following the protocol of Kafri and co workers (29). Either pLKO.1, a non-target shRNA control vector (SHC002; Sigma), an equal mixture of 5 types of NM_00039 p21 MISSION shRNA Lentiviral Transduction Particles (TRCN0000040123, TRCN0000040124, TRCN0000040125, TRCN0000040126 and TRCN0000040127) or NM_000546 p53 MISSION shRNA Lentiviral Transduction Particles (TRCN0000003756), obtained from Sigma, were co-transfected with the packing construct ΔNRF (from Dr. Tal Kafri, The University of North Carolina) (29), and the VSV-G envelope expression plasmid (pMDK64; from Dr. Matthias Kaeser, The Salk Institute) into 293FT cells with FuGene® (Roche). pLKO.1 is a negative control containing an insert sequence that does not target any human or mouse gene, but will activate the RNAi pathway. For infection, cells were incubated with lentiviral particles and polybrene and then selected with puromycin. At least 3 puromycin-resistant colonies of the A204.1 and TTC642 cells were isolated and expanded for further characterization.
Cell cycle analysis
Cell cycle analyses were performed according to the procedure of Huang S et al. (30). Percentages of cells within each of the cell cycle compartments were determined by flow cytometry (CyAn; Dako) and analyzed with ModFit software (Verity).
Results
The effects of reexpression of hSNF5 on the growth of MRT cell lines
We have previously shown that both hSNF5 and p16INK4A can induce G1 cell cycle arrest in MRT cell lines at 72 hours after transfection (19). However, hSNF5 also induced G1 cell cycle arrest without p16INK4A induction in the same timeframe (25). Because of the extended period between hSNF5 transfection and the characterization of cell cycle arrest in these previous studies, we characterized the effects of hSNF5 expression on the growth of 2 MRT cell lines within 24 of infection with Ad-hSNF5 and Ad-GFP (negative control) adenoviruses.
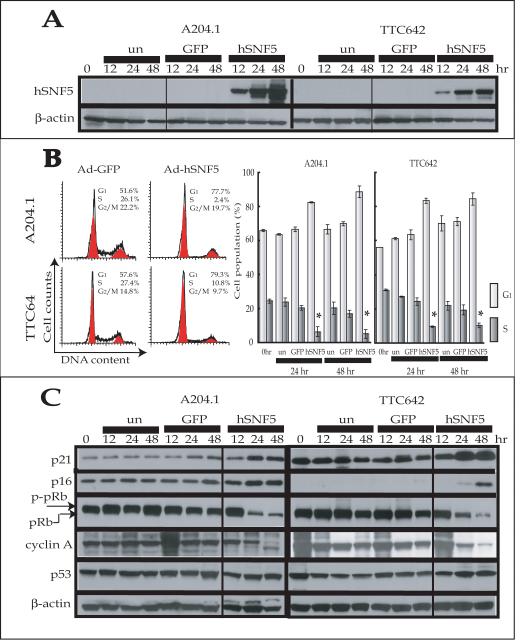
The induction of hSNF5 protein expression in the A204.1 and TTC642 cells by adenoviral infection is shown in Figure 1A. No hSNF5 expression was detected in either MRT cell line in the absence of infection or after Ad-GFP infection. However, infection with Ad-hSNF5 led to hSNF5 expression as early as 12 hours post-infection, followed by a time-dependent increase in hSNF5 expression levels in both MRT cells lines.
Figure 1. G1 cell cycle arrest induced by reexpression of hSNF5.
(A) Cells were harvested at the indicated times after infection with Ad-hSNF5 and Ad-GFP. Total cell protein (30 μg) were separated on a 4-20% SDS-polyacrylamide gel and probed with either anti-SNF5 or anti-ß-actin. un; uninfected control. (B) Twenty-four hours after infection with Ad-hSNF5 or Ad-GFP, cells were harvested and analyzed by flow cytometry. Left, representative profiles; Right, Values are the mean of three independent experiments; bars, ± SD. *, P < 0.05 relative to the number of S-phase of un-infection control. (C) Cells were harvested at the indicated times after infection with Ad-hSNF5 and Ad-GFP. Total cell protein (30 μg) were separated on a 4-20% SDS-polyacrylamide gel and probed with appropriate antibodies. un; uninfected control.
We next tested the effects of hSNF5 reexpression on cell cycle regulation by flow cytometry. Both cell lines infected with Ad-hSNF5 showed cell cycle arrest 24 hours after infection, characterized by the presence of nearly 80% of cells in the G1 phase of the cell cycle and the presence of <10% of cells in the S phase (Fig. 1B). The percentage of Ad-hSNF5- and Ad-GFP-infected cells in S phase was significantly different at 24 hours after infection. Similar results were found at 48 hours post-infection. These results demonstrated that the G1-S cell cycle progression was inhibited by 24 hours after hSNF5 reexpression in these MRT cell lines.
hSNF5-induced p16INK4A and p21CIP1/WAF1 protein expression in MRT cell lines
We previously demonstrated that hSNF5 reexpression induced the down-regulation of Cyclin A, the dephosphorylation of pRb, and up-regulation of p16INK4A and p21CIP1/WAF1 expression at 3 days following transfection (25). To determine whether these changes also occurred simultaneously with hSNF5-induced cell cycle arrest at 24 hours post-infection, we examined the expression of cyclin dependent kinase (CDK) inhibitors, especially p16INK4A and p21CIP1/WAF1, as well as their downstream targets by western blotting (Fig. 1C). We observed increased p21CIP1/WAF1 and decreased phosphorylated-pRb and cyclin A levels at 24 hours after Ad-hSNF5 infection compared to Ad-GFP control and un-infected control in both A204.1 and TTC642 cells. In the A204.1, p16INK4A protein levels were increased slightly at 12 hours after Ad-hSNF5 infection, and showed a further increase at 24 hours after infection compared to control cells. In contrast, p16INK4A protein expression was absent at baseline in the TTC642, with a slight increase at 24 hours, followed by a marked increase at 48 hours after infection. On the other hand, p53 was not significantly changed in both MRT cell lines (Fig. 1C).
Reexpression of hSNF5 induces p21CIP1/WAF1 transcription with or without p53 recruitment in MRT cell lines
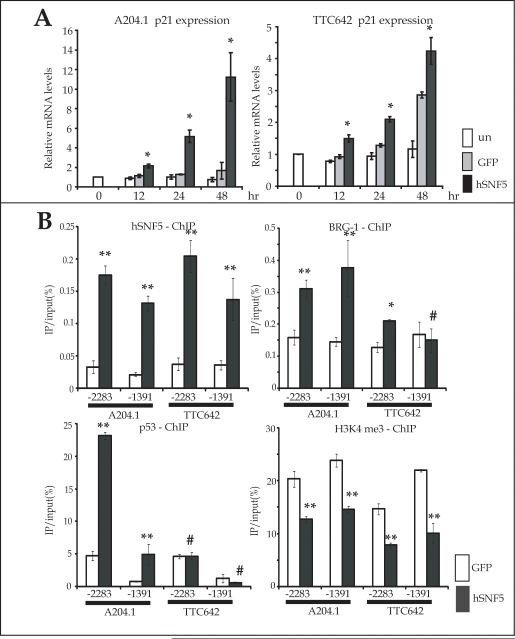
We next examined whether the increase in p21CIP1/WAF1 protein levels resulted from an increase in its mRNA levels by QT-PCR. We found the level of p21CIP1/WAF1 mRNA increased within 12 hours after Ad-hSNF5 infection in comparison with Ad-GFP infection in both MRT cell lines. In the TTC642, p21CIP1/WAF1 also increased more with Ad-GFP infection than un-infected control, especially at 48 hours (Fig. 2A).
Figure 2. hSNF5-induced p21CIP1/WAF1 expression.
(A) RNA was extracted at the indicated times after infection with Ad-hSNF5 and Ad-GFP. The mRNA levels were measured for each gene by QT-PCR and normalized for β-actin expression. Values are the mean of three independent experiments; bars, ± SD. *, P < 0.05 relative to the Ad-GFP and un-infected control. un; uninfected control. (B) At 24 hours after infection with Ad-hSNF5 and Ad-GFP, protein was extracted for ChIPs assays. ChIPs assays were performed using antibodies directed against hSNF5, BRG-1, p53 and H3K4me3 on −2283 kb and −1391 kb of p21CIP1/WAF1 promoter. Values are the mean of triplicates; bars, ± SD. *, P < 0.05 relative to the Ad-GFP control. **, P < 0.01 relative to the Ad-GFP control. #, P > 0.05 relative to the Ad-GFP control.
In our previous study, we showed hSNF5 regulated p21CIP1/WAF1 and p16INK4A transcription in A204.1 at 4 days after hSNF5 plasmid transfection by ChIPs (25). Therefore, we analyzed the chromatin status at p21CIP1/WAF1 promoter, −2283 kb (p53 high-affinity binding site) and −1391 kb (p53 low-affinity binding site) in both A204.1 and TTC642 cells (28), at 24 hours after Ad-hSNF5 infection to clarify the mechanism of p21CIP1/WAF1 activation by hSNF5. ChIPs data confirmed that hSNF5 bound to both −2283 kb site and −1391 kb site in either cell line. Furthermore BRG1 is also recruited by hSNF5 induction to both sites in A204.1, but only at the −2283 site in TTC642 (Fig. 2B).
Moreover, our previous reports also suggested hSNF5 recruits p53 to the p21 promoter (25). Therefore, we determined whether hSNF5 recruitment to the p21 promoter affected p53 binding. In A204.1, p53 binding is increased after hSNF5 reexpression, with a higher amount detected at the high affinity −2283 kb site (Fig. 2B). In contrast, we did not observe a difference in p53 recruitment between Ad-hSNF5 infection and Ad-GFP infection in the TTC642 although the difference in binding between the 2 affinity sites remained (Fig. 2B). We next determined the effect of hSNF5 reexpression on the H3K4me3, a chromatin mark associated with gene activation (31). H3K4me3 decreased after hSNF5 reexpression at both the −2283 kb and −1391 kb sites in both the A204.1 and TTC642 cells (Fig. 2B).
Reexpression of hSNF5 induces p21CIP1/WAF1 transcription through both p53-dependent and p53-independent mechanisms in MRT cell lines
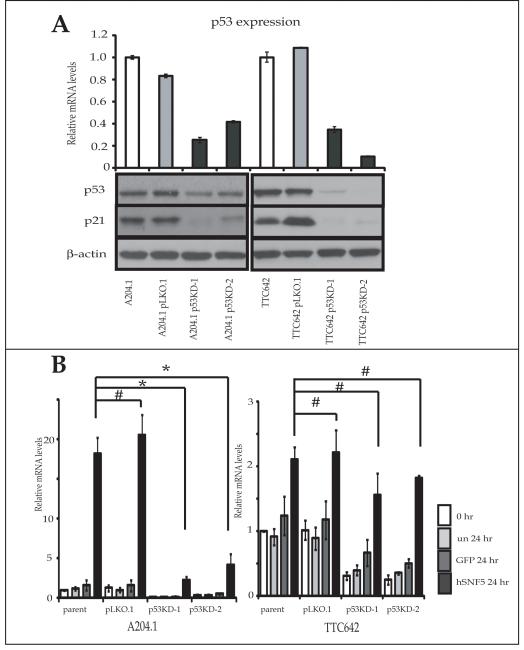
Because our results indicated that hSNF5 reexpression activated p21CIP1/WAF1 transcription with p53 recruitment in A204.1 cells but without p53 recruitment in TTC642 cells, we next assessed the role of p53 in p21CIP1/WAF1 transcription in the MRT cell lines. We established 2 independently derived p53 stable knock-down MRT cell lines from both A204.1 and TTC642 cells using lentiviral vectors encoding a small hairpin RNA (shRNA) targeting p53 mRNAs. We also developed a negative control cell line using a lentiviral vector encoding a small hairpin RNA targeting a non-mammalian sequence (pLKO.1). By QT-PCR and Western blotting, all p53 knock-down cells (A204.1 p21KD and TTC642 p21 KD) showed significant decreases in the p53 mRNA levels along with the protein levels of p53 and p21CIP1/WAF1 compared with the parental cells or the control cells (A204.1 pLKO.1 and TTC642 pLKO.1) (Fig. 3A).
Figure 3. p21CIP1/WAF1 expression after reexpression of hSNF5 in p53 stable knock down MRT cells.
(A) p53 knock-down cells (A204 p53KD and TTC642 p53 KD) and the control cells (A204 pLKO.1 and TTC642 pLKO.1) were harvested and RNA and protein was extracted. The mRNA levels were measured by QT-PCR for each gene and normalized for β-actin expression. Values are the mean of three independent experiments; bars, ± SD. Total protein (30 μg) was separated on a 4-20% SDS-polyacrylamide gel and probed with appropriate antibodies. (B) Every 24 hours after infection with Ad-hSNF5 and Ad-GFP, cells were harvested and RNA was extracted. The mRNA levels were measured for each gene by QT-PCR and normalized for β-actin expression. Values are the mean of three independent experiments; bars, ± SD. *, P < 0.05 relative to the Ad-hSNF5 infected parent cells. #, P > 0.05 the Ad-hSNF5 infected parent cells.
We next determined whether the reduction in p53 expression affected p21CIP1/WAF1 transcription induced by hSNF5. Infection of the pLKO.1 and p53KD cells with Ad-hSNF5 or Ad-GFP resulted in increased levels of p21CIP1/WAF1 mRNA at 24 hours after Ad-hSNF5 infection in pLKO.1 cells as in the parental cell lines (Fig.3B). However, while the increase of p21CIP1/WAF1 mRNA by hSNF5 reexpression was significantly inhibited in all A204.1 p53KD cells, the increase of p21CIP1/WAF1 mRNA by hSNF5 reexpression was not significantly different among TTC642, TTC642 pLKO.1 and all TTC642 p53KD cells (Fig. 3B). These results suggested that the up-regulation p21CIP1/WAF1 transcription by hSNF5 reexpression was operated through p53-dependent mechanism in A204.1 cells and through a p53-independent mechanism in TTC642 cells.
Reexpression of hSNF5 induces p16INK4A transcription through BMI-1 eviction in the TTC642 cell line
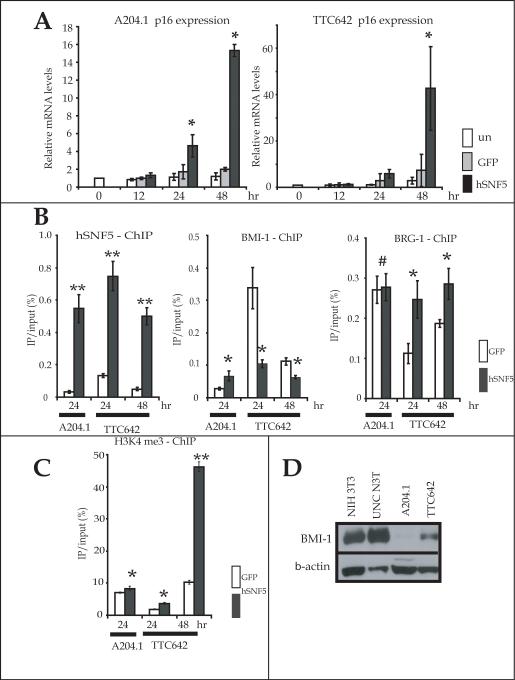
Although the A204.1 expresses p16INK4A mRNA and protein, the TTC642 does not show detectable expression of p16INK4A protein. However, reexpression of hSNF5 caused up-regulation of p16INK4A protein in both MRT cell lines (Fig. 1C). We, therefore, examined whether the increase of p16INK4A protein resulted from an increase in its mRNA levels by QT-PCR. We found that p16INK4A mRNA levels increased within 24 hours and 48 hours after Ad-hSNF5 infection in the A204.1 and in TTC642 cells, respectively (Fig. 4A). These results demonstrated the increase in p21CIP1/WAF1 mRNA occurs earlier than the increase in p16INK4A mRNA.
Figure 4. hSNF5-induced p16INK4A expression.
(A) Cells were infected with Ad-hSNF5 and Ad-GFP. RNA was extracted at the indicated times after infection. The mRNA levels were measured by QT-PCR analysis for each gene and normalized for β-actin expression. Values are the mean of three independent experiments; bars, ± SD. *, P < 0.05 relative to the Ad-GFP and un-infected control. un; uninfected control. (B, C) At 24 and 48 hours after infection with Ad-hSNF5 and Ad-GFP, cells were harvested and protein was extracted for ChIPs assays. ChIPs assays were performed using antibodies directed against hSNF5(B), BMI-1(B), BRG-1(B) and H3K4me3(C) on −450 kb site of p16INK4A promoter. Values are the mean of triplicates; bars, ± SD. *, P < 0.05 relative to the Ad-GFP control. **, P < 0.01 relative to the Ad-GFP control. #, P > 0.05 relative to the Ad-GFP control. (D) Total protein (30 μg) were separated on a 4-20% SDS-polyacrylamide gel and probed with BMI-1 antibody.
Because BMI-1 represses transcription at p16INK4A locus (32) and an earlier report indicated that p16INK4A transcription is activated by induction of hSNF5 via BMI-1 eviction (21), we first confirmed that hSNF5 binding at the p16INK4A promoter increased at 24 hours after Ad-hSNF5 infection in both cell lines (Fig. 4B). We next determined the binding of BMI-1 to the p16INK4A promoter as an indication of polycomb complex silencing. In TTC642, we observed BMI-1 binding was also significantly less after infection of Ad-hSNF 24 and 48 hours after infection compared with control infected cells (Fig. 4B). We also found a modest increase in BRG-1 binding on the p16INK4A promoter after hSNF5 reexpression at 24 hours followed by a dramatic increase in H3K4 me3 binding at 48 hours in TTC642 cells (Fig. 3B, 3C). These results appear consistent with hSNF5 reexpression increasing the binding of the SWI/SNF complex to the p16INK4A promoter accompanied by polycomb eviction at 24 hours followed by H3K4 methylation, and activation of p16INK4A transcription at 48 hours in TTC642 cells.
In contrast, we detected little BMI-1 binding on the p16INK4A promoter in A204.1 cells, even in the absence of hSNF5 expression (Fig. 4B). We also observed that hSNF5 reexpression had little effect on the binding of BRG-1 and the level of H3K4 methyation at this promoter (Fig. 4B, C). These results suggested an absence of polycomb complex silencing at the p16INK4A promoter in the A204.1 cells. Therefore, we examined the expression of BMI-1 in our MRT cell lines by Western blotting. The results in Figure 4F demonstrate that the A204.1 cell line fails to express detectable BMI-1 protein compared to the TTC642 cell line (Fig. 4D). The absence of BMI-1 may explain the basal level of p16INK4A mRNA observed in A204.1 cells.
Reduced p21CIP1/WAF1 expression inhibits the G1 arrest induced by re-expression of hSNF5 in MRT cell lines
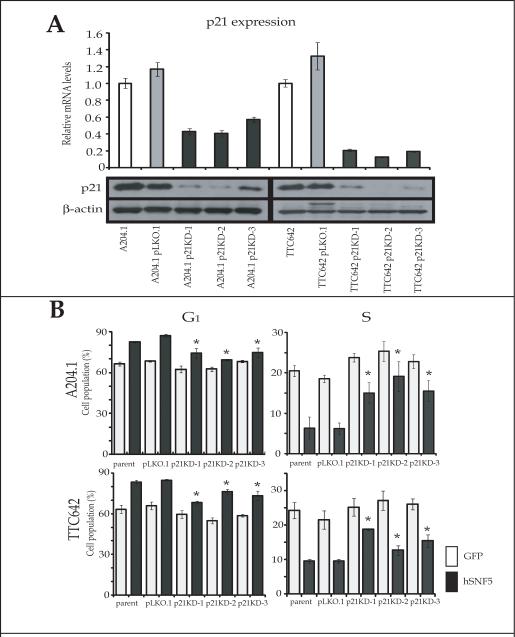
Because our results indicated that hSNF5 reexpression activated p21CIP1/WAF1 transcription earlier than p16INK4A transcription, we next assessed the role of p21CIP1/WAF1 in hSNF5-induced cell cycle arrest in the MRT cell lines. We established 3 independently derived p21CIP1/WAF1 stable knock-down MRT cell lines from both A204.1 and TTC642 cells using lentiviral vectors encoding a small hairpin RNA (shRNA) targeting p21CIP1/WAF1 mRNAs and a negative control cell line (pLKO.1). By QT-PCR and Western blotting, all p21CIP1/WAF1 knock-down cells (A204.1 p21KD and TTC642 p21 KD) showed significant decreases in the mRNA levels along with the protein levels of p21CIP1/WAF1 compared with the parental cells or the control cells (A204.1 pLKO.1 and TTC642 pLKO.1) (Fig. 5A).
Figure 5. Inhibition of G1 cell cycle arrest by reexpression of hSNF5 in p21CIP1/WAF1 stable knock down MRT cells.
(A) p21CIP1/WAF1 knock-down cells (A204 p21KD and TTC642 p21 KD) and the control cells (A204 pLKO.1 and TTC642 pLKO.1) were harvested and RNA and protein was extracted. The mRNA levels were measured by QT-PCR for each gene and normalized for β-actin expression. Values are the mean of three independent experiments; bars, ± SD. Total protein (30 μg) was separated on a 4-20% SDS-polyacrylamide gel and probed with p21CIP1/WAF1 antibodies. (B) Every 24 hours after infection with Ad-hSNF5 and Ad-GFP, cells were harvested and analyzed by flow cytometry. Values are the mean of three independent experiments; bars, ± SD. *, P < 0.05 relative to each Ad-GFP control.
We next determined whether the reduction in p21CIP1/WAF1 expression affected hSNF5 induced cell cycle arrest. Therefore, we infected the pLKO.1 and p21KD cells with Ad-hSNF5 or Ad-GFP, and assayed the effects on cell cycle by flow cytometry. We observed a similar G1 cell cycle arrest induced by Ad-hSNF5 infection in pLKO.1 cells after 24 hours as in the parental cell lines (Fig. 5B). However, the inhibition of the G1-S cell cycle progression by hSNF5 reexpression was significantly inhibited in all A204.1 p21KD cells and TTC642 p21KD cells (Fig. 5B). These results indicated that p21CIP1/WAF1 up-regulation contributes to the inhibition of the G1-S cell cycle progression by hSNF5 at 24 hours after Ad-hSNF5 infection.
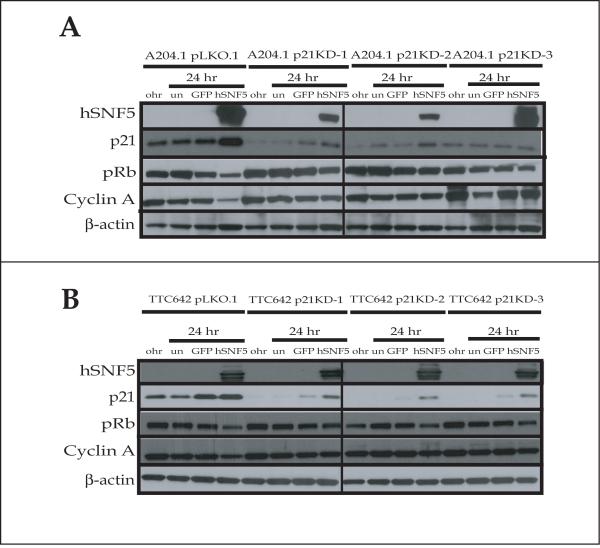
Reduced p21CIP1/WAF1 expression inhibits the dephosphorylation of pRb after hSNF5 reexpression in MRT cell lines
Because p21CIP1/WAF1 knock-down caused inhibition of G1 cell cycle arrest induced by hSNF5 reexpression, we examined whether the effect occurred at the level of pRb phosphorylation. Although p21CIP1/WAF1 expression increased after hSNF5 reexpression in A204.1 pLKO.1 and TTC642 pLKO.1 cell lines, we observed limited increase of p21CIP1/WAF1 and limited reduction of phosphorylated-pRb and cyclin A at 24 hours after Ad-hSNF5 infection in A204 p21KD cells and TTC642 p21KD cells (Fig. 6A & B). The expression of p16INK4A was not significantly increased at 24 hours after Ad-hSNF5 infection in A204.1 p21KD cells. In TTC642 p21KD cells, the p16INK4A protein increased slightly at 24 hours after Ad-hSNF5 infection (data not shown), then increased significantly at 48 hours, similar to the TTC642 parent cell line (data not shown). The change of p21CIP1/WAF1 mRNA was similar to the results seen with p21CIP1/WAF1 protein levels (data not shown). Taken together, these results indicated that the G1 arrest induced by hSNF5 reexpression strongly correlated with dephosphorylation of pRb through p21 CIP1/WAF1 activation.
Figure 6. Inhibition of pRb dephosphorylation after hSNF5 reexpression in p21CIP1/WAF1 knock-down MRT cell lines.
(A, B) p21CIP1/WAF1 knock-down cells (A204 p21KD and TTC642 p21 KD) and the control cells (A204 pLKO.1 and TTC642 pLKO.1) were harvested and protein was extracted. Total cell protein (30 μg) were separated on a 4-20% SDS-polyacrylamide gel and probed with appropriate antibodies.
Discussion
By studying the mechanism of G1 cell cycle arrest induced by hSNF5 at a time point concomitant with the induction of growth arrest, our study shows three important observations. First, hSNF5 directly regulates the p21CIP1/WAF1 and p16INK4A loci through different mechanisms among MRT cell lines. Second, hSNF5 can regulates the p21CIP1/WAF1 through either a p53-dependent or a p53-independent mechanism. Finally, p21CIP1/WAF1 has a key role in hSNF5-induced cell growth arrest in MRT cell lines.
Reexpression of hSNF5 increased p21CIP1/WAF1 transcriptional activity immediately through the recruitment of BRG1 to the p21CIP1/WAF1 promoter. Similarly, some reports have suggested that BRG1 associates with the p21CIP1/WAF1 promoter and activates it along with hSNF5 (33, 34). Does this recruitment of the SWI/SNF complex lead to an interaction with another transcription factor? Lee et al. (35) suggested that the BRG-1 interacts with p53 and activates the p21CIP1/WAF1 promoter in a p53-dependent manner. In contrast, Liu et al. (36) and Hendricks et al. (33) suggested that BRG-1 activates the p21CIP1/WAF1 promoter in p53-independent manner. Indeed, previous reports have shown that multiple mechanisms can activate the p21CIP1/WAF1 promoter including p53-dependent, Sp1- or Sp3-dependent, or CDK8-dependent (37). Our results in the A204.1 cell line appear consistent with the former model. We observed increased p53 levels at the p21CIP1/WAF1 promoter after hSNF5 reexpression and reduction in p53 expression inhibited up-regulation of p21CIP1/WAF1 induced by hSNF5. This finding, at a minimum, supports the notion that reexpression of hSNF5 in these cells facilitates the recruitment of p53 to the p21CIP1/WAF1 promoter. However, our studies did not determine whether this occurs through a direct interaction between p53 and hSNF5. We also need to identify the upstream signal that initiates p53 binding to the p21CIP1/WAF1 promoter.
In contrast, p53 levels on p21CIP1/WAF1 did not change in the TTC642 cells after hSNF5 reexpression nor did decreased p53 levels affect the ability of hSNF5 to increase p21CIP1/WAF1 transcription. While we cannot exclude the possibility that a low level of p53 protein remains at the p21CIP1/WAF1 promoter, sufficient for transcriptional activation, it appears that hSNF5 reexpression may operate through a different mechanism in these cells. While activation of p21CIP1/WAF1 transcription by hSNF5 in both cell lines appears associated with recruitment of BRG-1, the transcription factors recruited to the p21CIP1/WAF1 promoter may differ. Additional ChIPs analyses of the p21CIP1/WAF1 promoter in the TTC642 cell line will clarify this matter. In addition, our result showed a modest increase in p21CIP1/WAF1 transcription after Ad-GFP infection compared to the uninfected control. We believe that the high M.O.I. of adenovirus required for infection of this cell line caused up-regulation of p21CIP1/WAF1 through apoptotic induction (38).
We also showed that reexpression of hSNF5 increases p16INK4A transcriptional activity after p21CIP1/WAF1 up-regulation. In TTC642 cell line, hSNF5 expression caused decrease of BMI-1 and increase of BRG-1 at the p16INK4A promoter. The level of BMI-1 at 48 hours was less than that at 24 hours, and the decrease of BMI-1 was correlated with an increase of H3K4me3. These results concur with the Kia et. al. report showing hSNF5 induced a decrease of BMI-1 and an increase of H3K4me3 on the p16INK4A promoter, and that BRG1 was necessary for activation of the p16INK4A promoter by hSNF5 (21). On the other hand, the A204.1 cell line expresses low basal levels of p16INK4A consistent with a lack of expression of BMI-1. Our results appear in accord with the report that p16INK4A expression is directly regulated by polycomb proteins such as BMI-1 and EZH2 (39). Moreover, while hSNF5 appeared at p16INK4A promoter after reexpression followed by an increase in p16INK4A mRNA in the A204.1 cell line, BRG-1 and H3K4me3 levels at the promoter did not change significantly. Therefore, in the absence of polycomb silencing, p16INK4A mRNA could increase more rapidly in the A204.1 cell line than in the TTC642 cell line. Regardless, the data still show that the hSNF5-induced increase in p16INK4A levels in the A204.1 cell line follows the p21CIP1/WAF1 up-regulation regardless of BMI-1 expression. The mechanism for the increase in p16INK4A mRNA in the A204.1 cell line requires further investigation.
The difference in H3K4me3 patterns between the p21CIP1/WAF1 and p16INK4A promoters was unexpected. The significant increase in H3K4me3 at the p16INK4A promoter (−450 kb) in the TTC642 cell line after hSNF5 reexpression appears consistent with the activation of transcription from this promoter. Although the levels of this modification did not change in the A204.1 cell line, this may reflect the active p16INK4A transcription present in these cells before hSNF5 reexpression. However, in both cell lines, H3K4me3 decreased at the p53 binding sites in the p21CIP1/WAF1 promoter (−2283 kb and −1391 kb) after hSNF5 reexpression, even though p21CIP1/WAF1 transcription increased. One possible explanation for this observation might come from hSNF5 reexpression activating SWI/SNF complex activity resulting in nucleosome repositioning by chromatin remodeling. Therefore, the subsequent change in H3K4me3 positioning on the p21CIP1/WAF1 promoter would be reflected by a decreased signal in our ChIPs assay.
In our study, p21CIP1/WAF1 knock-down experiments showed that inhibition of p21CIP1/WAF1 expression partially inhibited the efficiency of hSNF5-induced G1 cell cycle arrest in MRT cell lines. The failure to completely abrogate the growth arrest may result from the residual activation of p21CIP1/WAF1 protein in the RNAi-expressing MRT cell lines. In addition, the increasing levels of p16INK4A protein may also begin to affect the cells because we observed a complete cell cycle arrest at 48 hours in the p21 knock-down cells (unpublished observations). We also cannot exclude the possibility that altered expression of other cell cycle regulatory genes may contribute the G1 arrest induced by hSNF5 (40, 41). This result indicated that p21CIP1/WAF1 has a key role in hSNF5-induced cell growth arrest in MRT cell lines. Importantly, Smith et al. recently showed cell cycle arrest and cytotoxicity induced by flavopiridol in MRT cell lines correlated with the down-regulation of cyclin D1 and the up-regulation of p21CIP1/WAF1 (42). Taken together, these results support p21CIP1/WAF1 as a relevant target for therapy of MRT.
Many MRTs arise under in infants under the age of 6 months and in neonates (43). Therefore, it seems plausible that a significant number of MRTs arise from the loss of hSNF5 in stem cells or progenitor cells during development. The expression of p16INK4A in stem cells is restricted by the expression of BMI-1 (44). Indeed, mouse embryo cells do not display expression of p16INK4A (45) and stem cells in young mice (8-12 week old) do not express detectable p16INK4A mRNA (46). Thus, a strong possibility exists that p16INK4A was already silenced in the cell that gave rise to the TTC642 cell line before hSNF5 loss occurred.
Our results may indicate that a decrease in p21CIP1/WAF1 expression following hSNF5 loss may signify one key event during MRT development. For example, the absence of p21CIP1/WAF1 expression can increase hematopoietic stem cell proliferation (47) or maintain neural stem cells by playing a significant role in regulating their proliferation (48). Our recent studies showing cooperation between SNF5 loss and pRb family inactivation in the acceleration of formation of spinal cord MRTs in mice support this notion (49). These results suggest that p21CIP1/WAF1 and its down-stream targets may regulate the boundary between quiescence and proliferation in stem cells and progenitor cells.
In conclusion, our results demonstrated that while hSNF5 reexpression in MRT cells increases both p21CIP1/WAF1 and p16INK4A expression during the induction of G1 cell cycle arrest, p21CIP1/WAF1 up-regulation precedes p16INK4A. While our studies firmly substantiate p21CIP1/WAF1 as a key target for hSNF in cell cycle regulation, the role of hSNF5 within the activities of SWI/SNF complex and gene regulation appear complex. Studies from other laboratories also implicate a role for hSNF5 the regulation cellular differentiation, cell migration and DNA repair (40, 41, 50). However, the establishment that SNF5 loss alters p21CIP1/WAF1 expression during MRT tumorigenesis provides an important new target for therapy in a tumor with limited options for treatment.
Supplementary Material
Acknowledgements
We thank Dr. Matthias Kaeser for his excellent technical input and thoughtful discussions, Dr. Cindy Wright, Medical University of South Carolina, for the Ad-SNF5 and Ad-GFP reagents, Dr. Scott Randell, University of North Carolina, for the UNC N3T cell line and Dr. Tony Imbalzano, University of Massachusetts School of Medicine, for the SNF5 antiserum.
This work was supported by Public Health Service grants CA91048 (BEW) and R01-CA104213 (ESK) from the National Cancer Institute and the American Brain Tumor Association Chad Dunbar Postdoctoral Fellowship (AC).
References
- 1.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Wilms’ Tumor Study. Cancer. 1978;41:1937–48. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Hoot AC, Russo P, Judkins AR, Perlman EJ, Biegel JA. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol. 2004;28:1485–91. doi: 10.1097/01.pas.0000141390.14548.34. [DOI] [PubMed] [Google Scholar]
- 3.Biegel JA, Tan L, Zhang F, Wainwright L, Russo P, Rorke LB. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res. 2002;8:3461–7. [PubMed] [Google Scholar]
- 4.Tomlinson GE, Breslow NE, Dome J, et al. Rhabdoid tumor of the kidney in the National Wilms’ Tumor Study: age at diagnosis as a prognostic factor. J Clin Oncol. 2005;23:7641–5. doi: 10.1200/JCO.2004.00.8110. [DOI] [PubMed] [Google Scholar]
- 5.Versteege I, Sevenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–6. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 6.Biegel JA, Kalpana G, Knudsen ES, et al. The role of INI1 and the SWI/SNF complex in the development of rhabdoid tumors: meeting summary from the workshop on childhood atypical teratoid/rhabdoid tumors. Cancer Res. 2002;62:323–8. [PubMed] [Google Scholar]
- 7.Rousseau-Merck MF, Fiette L, Klochendler-Yeivin A, Delattre O, Aurias A. Chromosome mechanisms and INI1 inactivation in human and mouse rhabdoid tumors. Cancer Genet Cytogenet. 2005;157:127–33. doi: 10.1016/j.cancergencyto.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Roberts CW, Orkin SH. The SWI/SNF complex--chromatin and cancer. Nat Rev Cancer. 2004;4:133–42. doi: 10.1038/nrc1273. [DOI] [PubMed] [Google Scholar]
- 9.de la Serna IL, Ohkawa Y, Imbalzano AN. Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat Rev Genet. 2006;7:461–73. doi: 10.1038/nrg1882. [DOI] [PubMed] [Google Scholar]
- 10.Reisman DN, Sciarrotta J, Wang W, Funkhouser WK, Weissman BE. Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: correlation with poor prognosis. Cancer Res. 2003;63:560–6. [PubMed] [Google Scholar]
- 11.Rosson GB, Bartlett C, Reed W, Weissman BE. BRG1 loss in MiaPaCa2 cells induces an altered cellular morphology and disruption in the organization of the actin cytoskeleton. J Cell Physiol. 2005;205:286–94. doi: 10.1002/jcp.20397. [DOI] [PubMed] [Google Scholar]
- 12.Decristofaro MF, Betz BL, Rorie CJ, Reisman DN, Wang W, Weissman BE. Characterization of SWI/SNF protein expression in human breast cancer cell lines and other malignancies. J Cell Physiol. 2001;186:136–45. doi: 10.1002/1097-4652(200101)186:1<136::AID-JCP1010>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Wong AK, Shanahan F, Chen Y, et al. BRG1, a component of the SWI-SNF complex, is mutated in multiple human tumor cell lines. Cancer Res. 2000;60:6171–7. [PubMed] [Google Scholar]
- 14.Xia W, Nagase S, Montia AG, et al. BAF180 is a critical regulator of p21 induction and a tumor suppressor mutated in breast cancer. Cancer Res. 2008;68:1667–74. doi: 10.1158/0008-5472.CAN-07-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue Y, Canman JC, Lee CS, et al. The human SWI/SNF-B chromatin-remodeling complex is related to yeast rsc and localizes at kinetochores of mitotic chromosomes. Proc Natl Acad Sci U S A. 2000;97:13015–20. doi: 10.1073/pnas.240208597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nie Z, Xue Y, Yang D, et al. A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol Cell Biol. 2000;20:8879–88. doi: 10.1128/mcb.20.23.8879-8888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang ZK, Davies KP, Allen J, et al. Cell cycle arrest and repression of cyclin D1 transcription by INI1/hSNF5. Mol Cell Biol. 2002;22:5975–88. doi: 10.1128/MCB.22.16.5975-5988.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Versteege I, Medjkane S, Rouillard D, Delattre O. A key role of the hSNF5/INI1 tumour suppressor in the control of the G1-S transition of the cell cycle. Oncogene. 2002;21:6403–12. doi: 10.1038/sj.onc.1205841. [DOI] [PubMed] [Google Scholar]
- 19.Betz BL, Strobeck MW, Reisman DN, Knudsen ES, Weissman BE. Re-expression of hSNF5/INI1/BAF47 in pediatric tumor cells leads to G1 arrest associated with induction of p16ink4a and activation of RB. Oncogene. 2002;21:5193–203. doi: 10.1038/sj.onc.1205706. [DOI] [PubMed] [Google Scholar]
- 20.Reincke BS, Rosson GB, Oswald BW, Wright CF. INI1 expression induces cell cycle arrest and markers of senescence in malignant rhabdoid tumor cells. J Cell Physiol. 2003;194:303–13. doi: 10.1002/jcp.10201. [DOI] [PubMed] [Google Scholar]
- 21.Kia SK, Gorski MM, Giannakopoulos S, Verrijzer CP. SWI/SNF mediates polycomb eviction and epigenetic reprogramming of the INK4b-ARF-INK4a locus. Mol Cell Biol. 2008;28:3457–64. doi: 10.1128/MCB.02019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albanese P, Belin MF, Delattre O. The tumour suppressor hSNF5/INI1 controls the differentiation potential of malignant rhabdoid cells. Eur J Cancer. 2006;42:2326–34. doi: 10.1016/j.ejca.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Caramel J, Medjkane S, Quignon F, Delattre O. The requirement for SNF5/INI1 in adipocyte differentiation highlights new features of malignant rhabdoid tumors. Oncogene. 2008;27:2035–44. doi: 10.1038/sj.onc.1210847. [DOI] [PubMed] [Google Scholar]
- 24.McKenna ES, Sansam CG, Cho YJ, et al. Loss of the epigenetic tumor suppressor SNF5 leads to cancer without genomic instability. Mol Cell Biol. 2008;28:6223–33. doi: 10.1128/MCB.00658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai J, Charboneau AL, Betz BL, Weissman BE. Loss of the hSNF5 gene concomitantly inactivates p21CIP/WAF1 and p16INK4a activity associated with replicative senescence in A204 rhabdoid tumor cells. Cancer Res. 2005;65:10192–8. doi: 10.1158/0008-5472.CAN-05-1896. [DOI] [PubMed] [Google Scholar]
- 26.Fulcher ML, Gabriel SE, Olsen JC, et al. Novel human bronchial epithelial cell lines for cystic fibrosis research. Am J Physiol Lung Cell Mol Physiol. 2009;296:L82–91. doi: 10.1152/ajplung.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Donner AJ, Szostek S, Hoover JM, Espinosa JM. CDK8 is a stimulus-specific positive coregulator of p53 target genes. Mol Cell. 2007;27:121–33. doi: 10.1016/j.molcel.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu K, Ma H, McCown TJ, Verma IM, Kafri T. Generation of a stable cell line producing high-titer self-inactivating lentiviral vectors. Mol Ther. 2001;3:97–104. doi: 10.1006/mthe.2000.0238. [DOI] [PubMed] [Google Scholar]
- 30.Huang S, Liu LN, Hosoi H, Dilling MB, Shikata T, Houghton PJ. p53/p21(CIP1) cooperate in enforcing rapamycin-induced G(1) arrest and determine the cellular response to rapamycin. Cancer Res. 2001;61:3373–81. [PubMed] [Google Scholar]
- 31.Santos-Rosa H, Schneider R, Bannister AJ, et al. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–11. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–8. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 33.Hendricks KB, Shanahan F, Lees E. Role for BRG1 in cell cycle control and tumor suppression. Mol Cell Biol. 2004;24:362–76. doi: 10.1128/MCB.24.1.362-376.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang H, Cui K, Zhao K. BRG1 controls the activity of the retinoblastoma protein via regulation of p21CIP1/WAF1/SDI. Mol Cell Biol. 2004;24:1188–99. doi: 10.1128/MCB.24.3.1188-1199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee D, Kim JW, Seo T, Hwang SG, Choi EJ, Choe J. SWI/SNF complex interacts with tumor suppressor p53 and is necessary for the activation of p53-mediated transcription. J Biol Chem. 2002;277:22330–7. doi: 10.1074/jbc.M111987200. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Kang H, Liu R, Chen X, Zhao K. Maximal induction of a subset of interferon target genes requires the chromatin-remodeling activity of the BAF complex. Mol Cell Biol. 2002;22:6471–9. doi: 10.1128/MCB.22.18.6471-6479.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gartel AL, Tyner AL. Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp Cell Res. 1999;246:280–9. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- 38.Ae K, Kobayashi N, Sakuma R, et al. Chromatin remodeling factor encoded by ini1 induces G1 arrest and apoptosis in ini1-deficient cells. Oncogene. 2002;21:3112–20. doi: 10.1038/sj.onc.1205414. [DOI] [PubMed] [Google Scholar]
- 39.Bracken AP, Kleine-Kohlbrecher D, Dietrich N, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–30. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts CW, Biegel JA. The role of SMARCB1/INI1 in development of rhabdoid tumor. Cancer Biol Ther. 2009;8:412–6. doi: 10.4161/cbt.8.5.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stojanova A, Penn LZ. The role of INI1/hSNF5 in gene regulation and cancer. Biochem Cell Biol. 2009;87:163–77. doi: 10.1139/O08-113. [DOI] [PubMed] [Google Scholar]
- 42.Smith ME, Cimica V, Chinni S, Challagulla K, Mani S, Kalpana GV. Rhabdoid tumor growth is inhibited by flavopiridol. Clin Cancer Res. 2008;14:523–32. doi: 10.1158/1078-0432.CCR-07-1347. [DOI] [PubMed] [Google Scholar]
- 43.Bourdeaut F, Freneaux P, Thuille B, et al. Extra-renal non-cerebral rhabdoid tumours. Pediatr Blood Cancer. 2008;51:363–8. doi: 10.1002/pbc.21632. [DOI] [PubMed] [Google Scholar]
- 44.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–7. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–11. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 46.Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–6. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 47.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–8. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 48.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–67. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chai J, Lu X, Godfrey V, et al. Tumor-specific cooperation of retinoblastoma protein family and Snf5 inactivation. Cancer Res. 2007;67:3002–9. doi: 10.1158/0008-5472.CAN-06-4207. [DOI] [PubMed] [Google Scholar]
- 50.Ray A, Mir SN, Wani G, et al. hSNF5/INI1, a Component of the Human SWI/SNF Chromatin Remodeling Complex, Promotes Nucleotide Excision Repair by Influencing ATM Recruitment and Downstream H2AX Phosphorylation. Mol Cell Biol. 2009 doi: 10.1128/MCB.00503-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.