Abstract
Purpose
Neuroblastoma, a common pediatric tumor of the sympathetic nervous system, is characterized by clinical heterogeneity, and the Trk family neurotrophin receptors play an important role in this behavior. Expression of TrkA is associated with favorable clinical features and outcome, whereas TrkB expression is associated with an unfavorable prognosis. We wanted to determine if the Trk-selective inhibitor Lestaurtinib had therapeutic efficacy in a preclinical neuroblastoma model.
Experimental Design
We performed intervention trials of Lestaurtinib alone or in combination with other agents in TrkB-overexpressing neuroblastoma xenograft models.
Results
Lestaurtinib alone significantly inhibited tumor growth compared to vehicle-treated animals (p=0.0004 for tumor size, p=0.011 for EFS). Lestaurtinib also enhanced the anti-tumor efficacy of the combinations of topotecan plus cyclophosphamide (p<0.0001 for size, p<0.0001 for EFS), or irinotecan plus temozolomide (p=0.011 for size; p=0.012 for EFS). There was no additive benefit of combining either 13-cis-retinoic acid or fenretinide with Lestaurtinib compared to Lestaurtinib alone. There was dramatic growth inhibition combining Lestaurtinib with Bevacizumab (p<0.0001), but this combination had substantial systemic toxicity.
Conclusions
We show that Lestaurtinib can inhibit growth of neuroblastoma both in vitro and in vivo, and it can substantially enhance the efficacy of conventional chemotherapy, presumably by inhibition of the Trk/BDNF autocrine survival pathway. It may also enhance the efficacy of selected biological agents, but further testing is required to rule out unanticipated toxicities. Our data support the incorporation of Trk inhibitors like Lestaurtinib in clinical trials of neuroblastoma or other tumors relying on Trk signaling pathways for survival.
Keywords: TrkA, TrkB, Lestaurtinib, CEP-701, neuroblastoma, signaling, differentiation
Statement of Translational Relevance
Trk family receptors play an important role in the behavior of neuroblastomas, as well as other pediatric and adult cancers. Activation of these receptors by mutation, translocation, autocrine ligand expression or other mechanisms can enhance survival and favor malignant behavior. Therefore, biologically targeted agents that inhibit Trk activation could be a very effective and nontoxic therapy either alone or in combination with other therapies. Our earlier studies demonstrated the importance of Trk signaling for neuroblastomas, and our results here have demonstrated the therapeutic efficacy of Trk inhibition in a mouse xenograft model of neuroblastoma. Indeed, our findings served as the basis for a Phase I clinical trial of Lestaurtinib for relapsed or refractory neuroblastoma. Given the prevalence of Trk receptor expression in many human cancers, our data support the incorporation of Trk inhibitors like Lestaurtinib in clinical trials of neuroblastoma or other cancers relying on Trk signaling pathways for survival.
BACKGROUND
Neurotrophin signaling through the Trk family of receptor tyrosine kinases (RTKs) plays a critical role in the development, maintenance and function of the nervous system. Activation of these receptors regulates cell survival, proliferation, migration, differentiation, and apoptosis during development. They exert this influence by modulating the responses of neurons to the neurotrophin family of growth factors in a temporally and spatially regulated manner. The neurotrophins nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT3) are the cognate ligands for TrkA (NTRK1), TrkB (NTRK2), and TrkC (NTRK3), respectively (1-3).
Neuroblastoma, a common pediatric tumor of the postganglionic sympathetic nervous system, provides an ideal model for the study of Trk signaling and inhibition in cancer (4). Neuroblastomas are characterized by clinical heterogeneity, from spontaneous regression in infants to relentless progression in older children. The prognosis for these latter patients remains poor, with three-year event-free survival (EFS) probabilities of 30-40% (5-7). Indeed, neuroblastomas can be classified into distinct subsets based on genetic alterations and biologic features (8), and the expression of Trk receptors likely contributes to these distinct behaviors (4, 9-15).
Expression of TrkA in neuroblastoma cell lines has been shown to mediate neuronal differentiation, growth arrest and inhibition of angiogenesis in response to NGF (16, 17). In contrast, unfavorable neuroblastomas frequently express TrkB and its ligand BDNF, which together comprise an autocrine or paracrine survival pathway (11, 18, 19). These tumors typically have gross segmental chromosomal aberrations including amplification of the MYCN proto-oncogene. The TrkB/BNDF pathway promotes cell survival, protects cells from injury, and blocks chemotherapy-mediated cell death in vitro (20-22). Although a number of genes are likely involved in the development and clinical behavior of favorable and unfavorable neuroblastomas, the pattern of Trk gene expression (TrkA versus TrkB) likely plays a role.
Lestaurtinib (CEP-701, Cephalon Inc.) is a small molecule inhibitor of several receptor tyrosine kinases, and it competitively inhibits ATP binding to the Trk kinase domain at nanomolar concentrations. Here we tested the efficacy of Lestaurtinib in a xenograft model of neuroblastoma to determine if it could enhance the antitumor efficacy of conventional chemotherapy, as well as selected, biologically-targeted agents. We first determined the anti-tumor efficacy of Lestaurtinib alone, and then in combination with cyclophosphamide, as well as two pairs of conventional agents (topotecan plus cyclophosphamide, irinotecan plus temozolomide) that are currently used to treat high-risk neuroblastoma patients. We also tested Lestaurtinib in combination with biologically-targeted anticancer agents (13-cis-retinoic acid, fenretinide, bevacizumab) that are currently in use or being developed to treat recurrent or refractory disease.
MATERIAL AND METHODS
Compounds
Lestaurtinib (CEP-701, Cephalon Inc., West Chester, PA) is an orally active, small molecule kinase inhibitor with nanomolar potency against TrkA, TrkB, and TrkC, as well as FLT3 and JAK2 (23-26). Lestaurtinib competitively inhibits the ATP binding site for these kinases, with less potent inhibition of other RTKs. Lestaurtinib was dissolved in a vehicle consisting of 40% polyethylene glycol 100 (Spectrum, Los Angeles, CA) 10% providone C30 (ISP, Bound Brook, NJ), and 2% benzyl alcohol (Spectrum) in distilled water and given subcutaneously at 20 mg/kg twice daily (Monday to Friday) and once daily on Saturday and Sunday. The vehicle alone was used as the control.
Cyclophosphamide (Cyclo) was given at dose of 113 mg/kg intraperitoneally (IP) once a day on days 4 and 6 of Lestaurtinib treatment. When given in combination with Topotecan (Topo), the Cyclo dose was reduced to 75 mg/kg/day; the Topo dose was 0.25 mg/kg/d, and both agents were given together IP on days 5 and 7 of the Lestaurtinib treatment. Irinotecan (Irino) was given at a dose of 0.63 mg/kg daily by oral gavage Monday to Friday of each week. Temozolomide (Temo) was given at a dose of 7.5 mg/kg daily by oral gavage Monday through Friday of each week. The same doses were used when combined with Lestaurtinib. Both Irino and Temo were resuspended in saline for the oral gavage. 13-cis Retinoic acid (13-cRA) was given at a dose of 10 mg/kg/day IP and given daily Monday to Friday. Fenretinide (4-HPR) was given at a dose of 120 mg/kg/day IP and given daily 7 days a week. Bevacizumab was given at a dose of 5 mg/kg IP twice weekly. All chemotherapy and biological agents other than Lestaurtinib were obtained through the pharmacy at the Children’s Hospital of Philadelphia (CHOP). The doses used in these studies were based on published studies with these drugs, and in some cases modified based on our own experience with these drugs in our xenograft model system (Table 1) (27-35). Some doses were reduced from those recommended in the literature, mainly so the chemotherapy alone would not cure all the animals, and so an impact of combining Lestaurtinib with other agents could be assessed.
Table 1.
Drugs and Doses used for Xenograft Studies
| Drug | Route | Solvent | Dose/day this study (literature dose/range) | References |
|---|---|---|---|---|
| Lestaurtinib | SQ | Vehicle | 40 mg/kg (40 mg/kg) | Evans AE, 1999 (27) |
| Cyclophosphamide | IP | Saline | 113 mg/kg; 75 mg/kg (150 mg/kg) | Shusterman S, 2001 (33); Houghton PJ, 2007 (28) |
| Topotecan | IP | Saline | 0.25 mg/kg (0.16-2.0 mg/kg) | Zamboni WC, 1998 (35); Thompson J, 1999 (34) |
| Irinotecan | PO | Saline | 0.63 mg/kg (0.16-2.5 mg/kg) | Houghton PJ, 2000 (29) |
| Temozolomide | PO | Saline | 7.5 mg/kg (14-66 mg/kg) | Houghton PJ, 2000 (29) |
| 13-cis-Retinoic Acid | IP | DMSO | 10 mg/kg (10 mg/kg) | Ponthan F, 2001 (31) |
| Fenretinide (4-HPR) | IP | Saline | 120 mg/kg (120 mg/kg) | Maurer BJ, 2007 (30) |
| Bevacizumab | IP | Saline | 5 mg/kg (5 mg/kg) | Segerstrom L, 2006 (32) |
Cell lines
For most of the xenograft tumor studies, we used SY5Y-TrkB (BR6), a subclone of SY5Y transfected with TrkB that expresses this receptor at high levels (20). This line does not express detectable levels of the TrkAIII isoform at the mRNA or protein levels (36). Some studies were confirmed with other neuroblastoma lines expressing endogenous or exogenous TrkB (11, 20). Cells were grown in RPMI-1640 medium containing 10% fetal bovine serum and 0.3 mg/ml G418, and maintained in 150 cm3 Costar culture flasks in a humidified atmosphere of 95% air and 5% CO2. Cells were harvested using 0.2% tetrasodium EDTA in phosphate buffered saline (PBS).
Animals
Four-week-old athymic nu/nu mice were obtained from Charles River Laboratories. Mice were maintained at five per cage under humidity- and temperature-controlled conditions in a light/dark cycle that was set at 12-hour intervals. The Institutional Animal Care Committee of the Joseph Stokes, Jr. Research Institute at CHOP approved the animal studies described herein.
In Vitro Experiments
To determine the effect of Lestaurtinib on TrkB expressing cells, SY5Y-TrkB (BR6) were grown in 10-cm3 dishes to 70-80% confluency in standard culture medium and harvested for protein extraction. We analyzed TrkB expression by Western Blot using an anti-phospho-Trk antibody (Phospho-TrkA, Tyr-490 Antibody, Cell Signaling Technologies, Danvers, MA) or an anti-pan-Trk antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). We exposed cells to BDNF for 10 minutes in the absence or presence of increasing concentrations of Lestaurtinib to determine the concentration that achieved 50% inhibition of receptor phosphorylation (IC50).
In Vivo Experiments
For the xenograft studies, animals were injected in the flank with 1 × 107 SY5Y-TrkB cells in 0.3 ml of Matrigel (BD Bioscience, Palo Alto, CA). Tumors were measured 2 times a week in 3 dimensions, and the volume calculated as follows: [(d1×d2×d3) × π/6]. Body weights were obtained twice a week, and the dose of compound was adjusted accordingly. Treatment with Lestaurtinib was started about 10 days after tumor inoculation when the average SY5Y-TrkB tumor size was 0.2 cm3.
Statistical Analysis
Comparisons of tumor size results were analyzed by two-sample Student t-test. Comparison of tumor size change over time was analyzed by linear mixed models. For EFS life-table analysis, an event was defined as tumor size that exceeded 3 cm3, or any evidence of animal discomfort resulting from the tumor or the treatment. Kaplan-Meier curves were estimated and compared between groups by log-rank test. All analyses were conducted using SAS-9 or Stata-8.
RESULTS
Effect of Lestaurtinib on TrkB activation in vitro
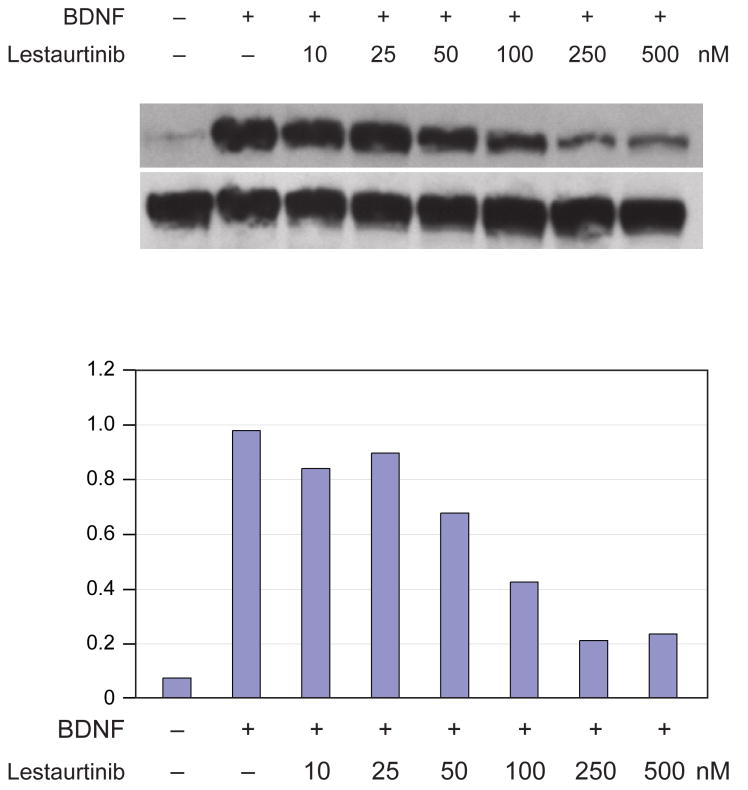
We first tested the ability of Lestaurtinib to inhibit TrkB phosphorylation induced by exogenous BDNF in the SY5Y-TrkB neuroblastoma line. Virtually no TrkB phosphorylation was seen at steady state in the absence of exogenous BDNF (Fig. 1), even though BDNF was expressed in these cells. However, there was intense phosphorylation of TrkB by 10 minutes after the addition of exogenous BDNF in the absence of Lestaurtinib. Increasing concentrations of Lestaurtinib progressively inhibited this ligand-induced TrkB phosphorylation. We saw substantial inhibition of phosphorylation at 10-25 nM Lestaurtinib, with maximal inhibition between 100-250 nM. We did not see complete inhibition of TrkB phosphorylation, presumably because of very high level of TrkB expression in these cells.
Figure 1. Effect of Lestaurtinib on TrkB autophosphorylation induced by BDNF.
A. The SY5Y-TrkB neuroblastoma line was exposed to BDNF in the absence and presence of increasing concentrations of Lestaurtinib. Maximal inhibition of ligand-induced autophosphorylation was seen by 100-200 nM. B. Densitometry of phospho-Trk relative to total Trk for each of the conditions in Fig. 1A.
Effect of single-agent Lestaurtinib on SY5Y-TrkB neuroblastoma xenografts
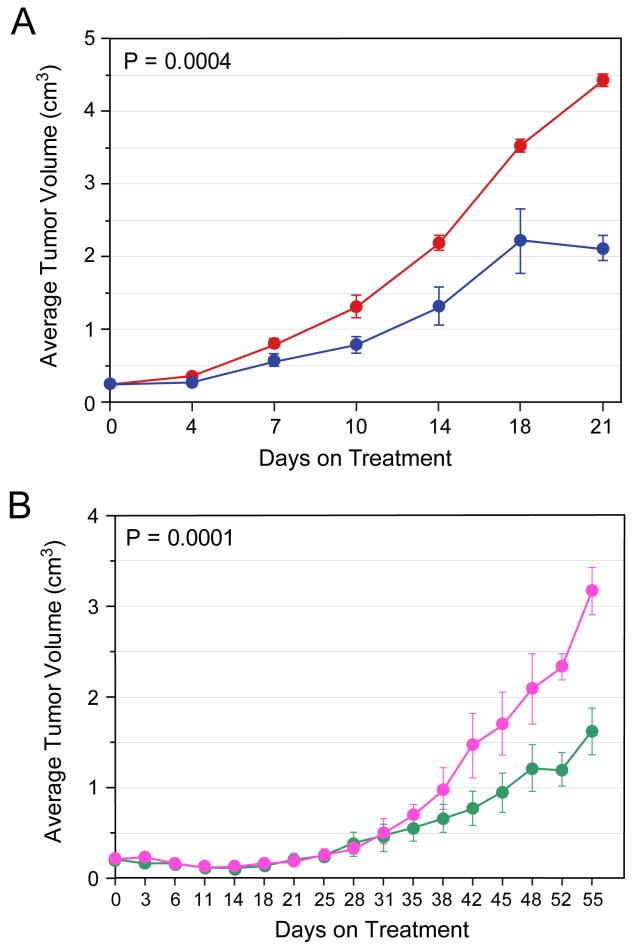
We tested the ability of Lestaurtinib to inhibit the SY5Y-TrkB cells growing as xenografts in athymic nu/nu mice. We injected 1×107 cells subcutaneously in the flank of nude mice. We initiated treatment when the tumor size measured 0.2 cm3, usually 10-14 days from inoculation. We treated mice (10 per group) with either Lestaurtinib or vehicle twice a day (Monday to Friday) and once a day on Saturday and Sunday, and tumors were measured twice a week. Significant tumor growth inhibition was observed with Lestaurtinib treatment (p=0.0004) (Fig. 2A). EFS was also significantly prolonged by administration of Lestaurtinib (P=0.011) (data not shown), with all tumors in control animals reaching or exceeding 3 cm3 by day 18, compared to only 2 of the Lestaurtinib treated animals. Analysis of tumors from control and Lestaurtinib-treated animals showed a modest but consistent decrease in phosphorylation of TrkB and the signaling intermediates MAPK and the PI3K p110a subunit (data not shown).
Figure 2. Effect of Lestaurtinib (± cyclo) on inhibiting growth of SY5Y-TrkB xenografts.
Lestaurtinib significantly slowed the growth of SY5Y-TrkB cells growing as xenografts in athymic nu/nu mice compared to vehicle alone (p= 0.0004, Fig. 2A). Lestaurtinib plus cyclo significantly slowed the growth of SY5Y-TrkB cells growing as xenografts in nude mice compared to Cyclo alone (p= 0.0001, Fig. 2B).
Effect of Lestaurtinib in combination with conventional chemotherapy on SY5Y-TrkB xenografts
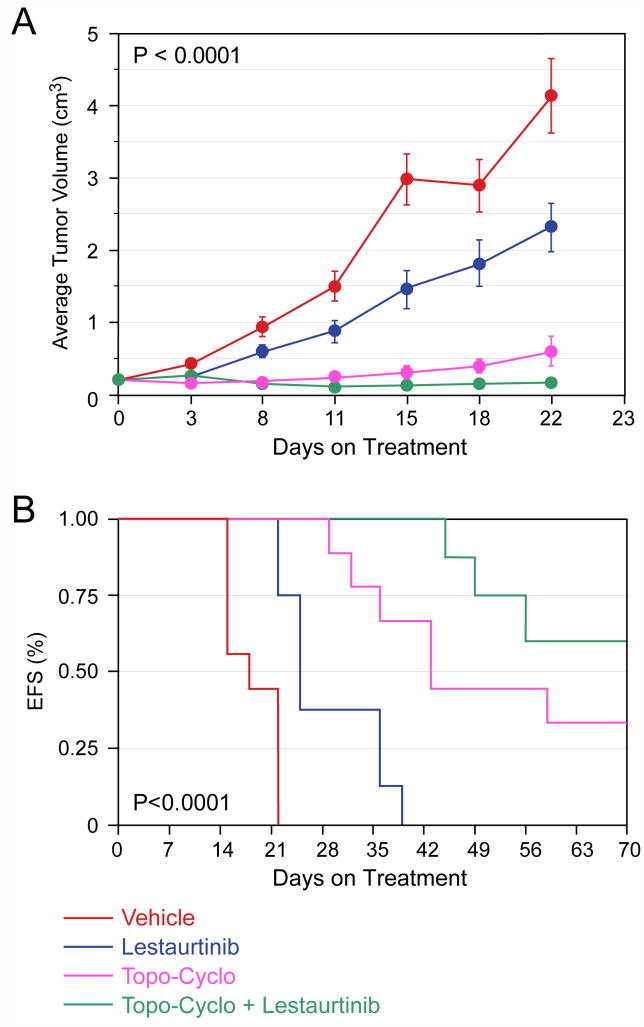
To determine if Lestaurtinib co-treatment enhanced the effect of standard chemotherapy, we treated groups of mice with either vehicle, Lestaurtinib, Cyclo or both. Control tumors grew rapidly, and the growth of Lestaurtinib treated animals was significantly delayed. However, both groups grew more rapidly than tumors in the other two groups, so they were not included to allow us to compare the effect of Lestaurtinib on Cyclo treated tumors. Tumors treated with Cyclo plus Lestaurtinib grew significantly more slowly than tumors treated with Cyclo alone (p=0.0001; Fig. 2B), and there was also a statistically significant difference in EFS (p=0.0014; data not shown). Next we tested the combination of Topo-Cyclo, a combination currently used for neuroblastoma induction therapy, with or without Lestaurtinib. The difference in the slopes of tumor growth was significantly different among the four groups (p<0.0001; Fig. 3A), and the effect on EFS was also highly significant (p<0.0001; Fig. 3B).
Figure 3. Effect of Lestaurtinib in combination with Topo-Cyclo on SY5Y-TrkB xenografts.
Lestaurtinib significantly slowed the growth of SY5Y-TrkB cells growing as xenografts in nude mice compared to vehicle alone, and it significantly enhanced the efficacy of Topo-Cyclo (p< 0.0001, Fig. 3A). Lestaurtinib also significantly improved the EFS of animals treated with either vehicle or Topo-Cyclo alone (p<0.0001, Fig. 3B).
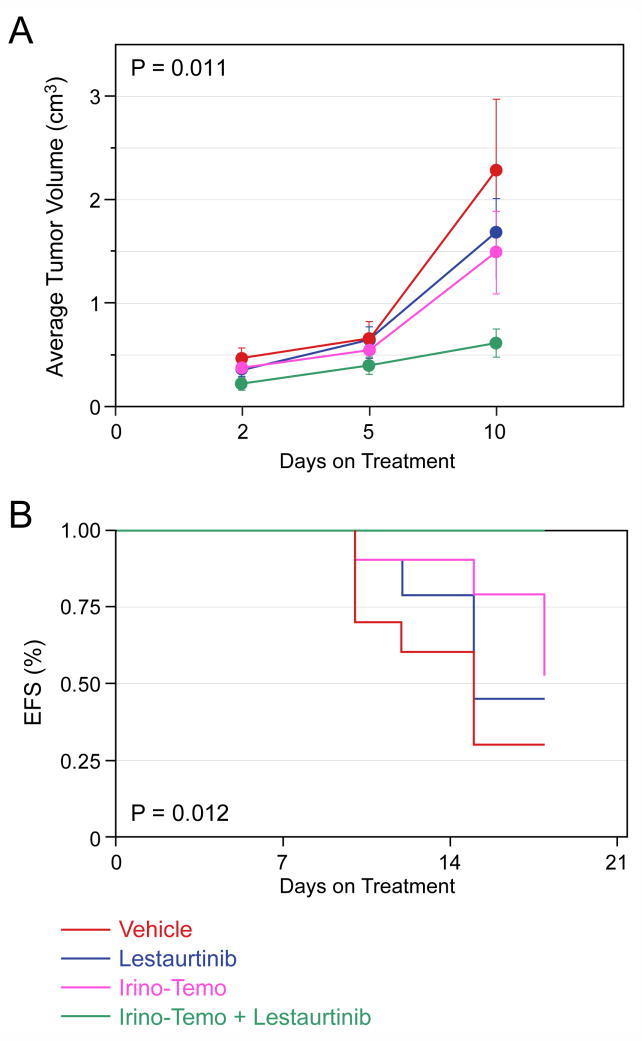
We also tested the combination of Irino-Temo with or without Lestaurtinib. This combination was recently developed to treat for recurrent neuroblastomas. Tumors treated with the combination of Irino-Temo plus Lestaurtinib grew significantly more slowly than with either chemotherapy or Lestaurtinib alone (p=0.011; Fig. 4A). This resulted in a 100% EFS by day 21 in the combination group, which was significantly better than all other groups (p=0.012; Fig. 4B). Thus, co-treatment with Lestaurtinib significantly enhanced the effect of both single agent and paired agent chemotherapy. Furthermore, this was achieved without additional toxicity.
Figure 4. Effect of Lestaurtinib in combination with Irino-Temo on SY5Y-TrkB xenografts.
Lestaurtinib significantly slowed the growth of SY5Y-TrkB cells growing as xenografts in nude mice compared to vehicle alone, and it significantly enhanced the efficacy of Irino-Temo (p=0.011, Fig. 4A). Lestaurtinib also significantly improved the EFS of animals treated with either vehicle or Irino-Temo alone (p=0.012, Fig. 4B).
Effect of Lestaurtinib in combination with retinoids on SY5Y-TrkB xenografts
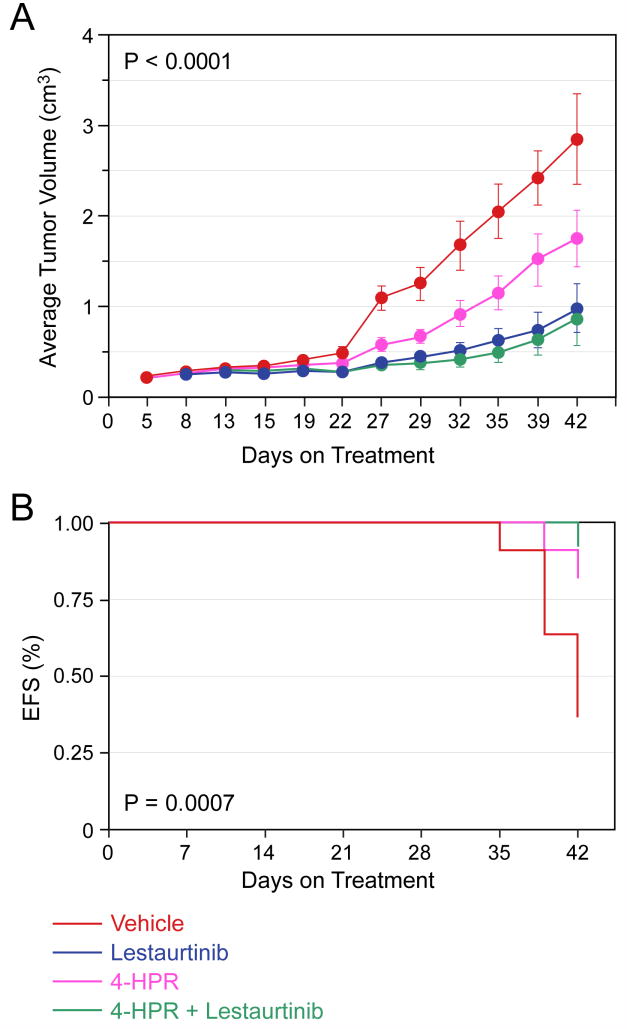
Next we tested the effect of Lestaurtinib in combination with two different retinoids (13-cRA and 4-HPR) that are currently in clinical use to treat neuroblastoma patients. We saw no effect of 13-cRA compared to vehicle on the SY5Y-TrkB cells, and no significant difference when 13-cRA was combined with Lestaurtinib compared to Lestaurtinib alone (data not shown). 4-HPR did have a therapeutic effect when compared to vehicle. However, the effect of Lestaurtinib alone on inhibiting tumor growth was significantly better than vehicle or 4-HPR alone (p<0.0001 for tumor size, p=0.0007 for EFS), and the combination of Lestaurtinib plus 4-HPR was not better than Lestaurtinib alone (Fig. 5A, 5B). Thus, Lestaurtinib was more effective as a single agent than either retinoid alone, and the addition of either retinoid to Lestaurtinib did not provide additional efficacy compared to Lestaurtinib treatment alone.
Figure 5. Effect of Lestaurtinib in combination with 4-HPR on SY5Y-TrkB xenografts.
Lestaurtinib significantly slowed the growth of SY5Y-TrkB cells growing as xenografts in nude mice compared to either vehicle or 4HPR alone, and the addition of 4-HPR did not significantly improve this efficacy (p< 0.0001, Fig. 5A). Lestaurtinib also significantly improved the EFS of animals treated with either vehicle or 4HPR alone (p=0.0007, Fig. 5B).
Effect of Lestaurtinib in combination with Bevacizumab on SY5Y-TrkB xenografts
We also tested the efficacy of combined VEGF and Trk signaling inhibition in our neuroblastoma xenograft model. We performed a four-arm interventional trial (N=10 mice/arm) using the VEGF inhibitor Bevacizumab and the Trk inhibitor Lestaurtinib as single agents or in combination, compared to vehicle control. Bevacizumab or Lestaurtinib alone each caused subtle growth delay of xenografts compared to vehicle, but the combination of the two showed complete and sustained regression of xenografts (p<0.0001 for tumor size). However, there was substantial systemic toxicity experienced in the mice treated with the combination of drugs that was not seen with either as a single agent, and half the animals had died of toxicity (anasarca and death within 24 hr following a Bevacizumab dose) by two weeks of treatment. Indeed, there was no significant difference in EFS between either Bevacizumab or Lestaurtinib and the combination arm due to deaths from toxicity (data not shown), raising questions about this particular combination in clinical trials without further preclinical testing.
DISCUSSION
Neuroblastomas demonstrate clinical heterogeneity, from spontaneous regression to relentless progression. Data from our laboratory and others suggests that Trk receptors play an important role in these disparate clinical behaviors (4, 9, 11-15, 17, 37). TrkA is expressed in favorable tumors, and these tumors are likely to differentiate or regress, depending on the presence or absence of NGF in their microenvironment (9, 12, 14, 37). Conversely, aggressive tumors, especially those with MYCN amplification, express TrkB and its ligand, BDNF (11). The coexpression of TrkB and BDNF comprise an autocrine survival pathway that promotes invasion, metastasis, angiogenesis and drug resistance (17, 19-21). Therefore, targeted therapy aimed at inhibiting the Trk receptor pathways could be a useful adjunct to conventional therapy in these tumors.
Despite improvements in the overall cure rate of neuroblastomas, the progress in curing patients with high-risk disease (stage 4, over 18 months of age; or with MYCN amplification) has been modest. Therapy for these patients includes surgery, local radiation therapy, intensive chemotherapy and one or two autologous stem cell transplantations. These therapies have reached the maximum tolerated intensity, so improvements in the cure rates of these patients will likely require more biologically targeted therapy, and/or therapy that enhances the effectiveness of current therapy without substantially increasing side effects.
Given the important role that Trk receptors play in the behavior of favorable and unfavorable neuroblastomas, we wanted to develop an approach that targeted these receptors. CEP-751 (KT-6587) is a Trk-selective inhibitor provided by Cephalon, Inc. (38). This compound inhibits Trk family kinases at nanomolar concentrations, whereas most other tyrosine kinases are only inhibited at micromolar concentrations. Lestaurtinib (CEP-701, KT-5555) is an active metabolite of CEP-751 that can be administered orally, making it more suitable for clinical trials (23, 25). Previously, we have tested the efficacy of CEP-751 to inhibit Trk-expressing neuroblastomas in vitro and in vivo (27, 39). In this report we examined the efficacy of Lestaurtinib alone and in combination with conventional and biologically targeted therapies in a mouse xenograft model.
We showed that Lestaurtinib dramatically inhibited the autophosphorylation of TrkB (after BDNF exposure) in SY5Y-TrkB cells. Maximal inhibition was seen at concentrations of 100-200 nM (Fig. 1), which is well within the range of what is achievable clinically. These results are also comparable to those we obtained previously with CEP-751 (27, 39). Then, we tested the ability of Lestaurtinib to inhibit the growth of SY5Y-TrkB cells growing in vivo as xenografts in athymic nu/nu mice. The biological effect on phosphorylation of TrkB and signaling intermediates in the xenografts was modest, probably due to the low level of steady-state activation by the autocrine survival pathway. However, significant inhibition of tumor growth was seen with Lestaurtinib treatment compared to a vehicle control (Fig. 2, Fig. 3), and this was achieved without apparent toxicity.
Next we tested the ability of Lestaurtinib to enhance the efficacy of conventional chemotherapy. We tested the ability of Cyclo and/or Lestaurtinib to inhibit the growth of SY5Y-TrkB xenografts. We demonstrated significantly greater inhibition of tumor growth in tumors treated with Lestaurtinib plus Cyclo compared to either alone (Fig. 2). We also tested the antitumor efficacy of Cyclo-Topo with or without Lestaurtinib. Topo-Cyclo has proven to be an effective combination for recurrent or refractory neuroblastomas (Fig. 3), and it is currently being used in front-line therapy for high-risk neuroblastoma patients. The combination of Topo-Cyclo plus Lestaurtinib inhibited tumor growth more effectively than either Topo-Cyclo or Lestaurtinib alone. Furthermore, we tested the combination of Irino-Temo with or without Lestaurtinib. Irino-Temo is an effective combination that is currently in use for high-risk neuroblastoma patients with recurrent or refractory disease. Again, we saw significant inhibition of tumor growth with the combination compared to chemotherapy alone (Fig. 4). Together these results demonstrate that Lestaurtinib enhanced the efficacy of chemotherapy agents in current clinical use, either alone or in pairwise combinations.
Finally, we tested the combination of Lestaurtinib with biologically targeted agents that are in use to treat neuroblastomas. 13-cRA did not have a significant effect on neuroblastoma xenografts in our model system, but 4-HPR did cause a significant inhibition in tumor growth. Nevertheless, Lestaurtinib was more effective at inhibiting tumor growth as a single agent than either 13-cRA or 4-HPR in this system, and the combination was no more effective than Lestaurtinib alone (Fig. 5). The results with Bevacizumab plus Lestaurtinib were more striking. Although both were effective at inhibiting tumor growth as single agents, the combination resulted in very significant growth inhibition. However, this combination was also associated with substantial systemic toxicity in the combination group, which raises questions about using this particular therapeutic combination in clinical trials without further testing.
Lestaurtinib can inhibit neuroblastoma xenograft growth, and it substantially enhances the efficacy of single and pair-wise combinations of chemotherapy agents, without additional toxicities. Lestaurtinib also enhanced the efficacy of Bevacizumab, but no enhancement was seen for the retinoids 13-cRA or 4-HPR. Assuming that the mechanism of Lestaurtinib inhibition is by blocking an important autocrine survival pathway, this agent may have a more profound effect on the growth of neuroblastomas treated with conventional chemotherapy because the pathways affected by 13-cRA or 4-HPR are different. The reason for substantial systemic toxicity with the combination of Lestaurtinib plus Bevacizumab compared to either agent alone is unclear but presumably is a result of a synergistic or off-target effect with the combination.
Brown and colleagues (40) reported that Lestaurtinib combined synergistically with other agents in the treatment of childhood acute lymphoblastic leukemias containing MLL gene rearrangements and FLT3 kinase overexpression. Furthermore, they reported that this synergism was sequence dependent, with the greatest effect seen when chemotherapy was given first, followed by Lestaurtinib. We saw significant inhibition when given simultaneously with chemotherapy (e.g., Cyclo), but there was no added benefit when CEP-751, an analog of Lestaurtinib, was given first, followed by Cyclo (data not shown). We did not test the schedule of chemotherapy followed by Lestaurtinib, but our protocol of continuous administration did continue the Lestaurtinib for weeks after the chemotherapy was given, essentially mimicking the most effective schedule identified by Brown (40).
Lestaurtinib is currently in Phase 3 clinical trials to treat patients with FLT3–positive acute myelogenous leukemia, in combination with conventional induction therapy, and it is in Phase 2 clinical trials to treat patients with myeloproliferative diseases and myelofibrosis (26, 41-43). Furthermore, Lestaurtinib has been tested in a Phase I clinical trials in neuroblastoma patients. Our preclinical studies would suggest that Lestaurtinib will be particularly effective in combination with conventional chemotherapy agents, and may be effective when combined with selective biological agents. However, caution should be used in testing Lestaurtinib plus Bevacizumab in clinical trials without further preclinical studies of this combination.
Acknowledgments
This work was supported in part by grants from the NIH (R01-CA094194, P01-CA097323: GMB), The Richard and Nancy Wolfson Young Investigator Fund (JEM, RH), and the Audrey Evans Endowed Chair (GMB). We are grateful to and Cephalon, Inc (West Chester, PA) for providing us with Lestaurtinib (CEP-701, K5555) for these studies.
References
- 1.Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991;350:158–60. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- 2.Squinto SP, Stitt TN, Aldrich TH, et al. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991;65:885–93. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- 3.Lamballe F, Klein R, Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991;66:967–79. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- 4.Brodeur GM, Minturn JE, Ho R, et al. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 2009;15:3244–50. doi: 10.1158/1078-0432.CCR-08-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George RE, Li S, Medeiros-Nancarrow C, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–6. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 6.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 7.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. New England Journal of Medicine. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 8.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 9.Kogner P, Barbany G, Dominici C, Castello MA, Raschella G, Persson H. Coexpression of messenger RNA for TRK protooncogene and low affinity nerve growth factor receptor in neuroblastoma with favorable prognosis. Cancer Research. 1993;53:2044–50. [PubMed] [Google Scholar]
- 10.Nakagawara A, Arima M, Azar CG, Scavarda NJ, Brodeur GM. Inverse relationship between trk expression and N-myc amplification in human neuroblastomas. Cancer Res. 1992;52:1364–8. [PubMed] [Google Scholar]
- 11.Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol. 1994;14:759–67. doi: 10.1128/mcb.14.1.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 1993;328:847–54. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- 13.Ryden M, Sehgal R, Dominici C, Schilling FH, Ibanez CF, Kogner P. Expression of mRNA for the neurotrophin receptor trkC in neuroblastomas with favourable tumour stage and good prognosis. Br J Cancer. 1996;74:773–9. doi: 10.1038/bjc.1996.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki T, Bogenmann E, Shimada H, Stram D, Seeger RC. Lack of high-affinity nerve growth factor receptors in aggressive neuroblastomas. J Natl Cancer Inst. 1993;85:377–84. doi: 10.1093/jnci/85.5.377. [DOI] [PubMed] [Google Scholar]
- 15.Yamashiro DJ, Nakagawara A, Ikegaki N, Liu XG, Brodeur GM. Expression of TrkC in favorable human neuroblastomas. Oncogene. 1996;12:37–41. [PubMed] [Google Scholar]
- 16.Lucarelli E, Kaplan D, Thiele CJ. Activation of trk-A but not trk-B signal transduction pathway inhibits growth of neuroblastoma cells. Eur J Cancer. 1997;33:2068–70. doi: 10.1016/s0959-8049(97)00266-9. [DOI] [PubMed] [Google Scholar]
- 17.Eggert A, Grotzer MA, Ikegaki N, Liu XG, Evans AE, Brodeur GM. Expression of the neurotrophin receptor TrkA down-regulates expression and function of angiogenic stimulators in SH-SY5Y neuroblastoma cells. Cancer Res. 2002;62:1802–8. [PubMed] [Google Scholar]
- 18.Acheson A, Conover JC, Fandl JP, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–3. doi: 10.1038/374450a0. see comments. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto K, Wada RK, Yamashiro JM, Kaplan DR, Thiele CJ. Expression of brain-derived neurotrophic factor and p145TrkB affects survival, differentiation, and invasiveness of human neuroblastoma cells. Cancer Research. 1995;55:1798–806. [PubMed] [Google Scholar]
- 20.Ho R, Eggert A, Hishiki T, et al. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462–6. [PubMed] [Google Scholar]
- 21.Jaboin J, Kim CJ, Kaplan DR, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3’-kinase pathway. Cancer Res. 2002;62:6756–63. [PubMed] [Google Scholar]
- 22.Middlemas DS, Kihl BK, Moody NM. Brain derived neurotrophic factor protects human neuroblastoma cells from DNA damaging agents. J Neurooncol. 1999;45:27–36. doi: 10.1023/a:1006342423175. [DOI] [PubMed] [Google Scholar]
- 23.George DJ, Dionne CA, Jani J, et al. Sustained in vivo regression of Dunning H rat prostate cancers treated with combinations of androgen ablation and Trk tyrosine kinase inhibitors, CEP-751 (KT-6587) or CEP-701 (KT-5555) Cancer Research. 1999;59:2395–401. [PubMed] [Google Scholar]
- 24.Miknyoczki SJ, Chang H, Klein-Szanto A, Dionne CA, Ruggeri BA. The Trk tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits significant antitumor efficacy in preclinical xenograft models of human pancreatic ductal adenocarcinoma. Clin Cancer Res. 1999;5:2205–12. [PubMed] [Google Scholar]
- 25.Miknyoczki SJ, Dionne CA, Klein-Szanto AJ, Ruggeri BA. The novel Trk receptor tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits antitumor efficacy against human pancreatic carcinoma (Panc1) xenograft growth and in vivo invasiveness. Ann N Y Acad Sci. 1999;880:252–62. doi: 10.1111/j.1749-6632.1999.tb09530.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 27.Evans AE, Kisselbach KD, Yamashiro DJ, et al. Antitumor activity of CEP-751 (KT-6587) on human neuroblastoma and medulloblastoma xenografts. Clinical Cancer Research. 1999;5:3594–602. [PubMed] [Google Scholar]
- 28.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–40. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 29.Houghton PJ, Stewart CF, Cheshire PJ, et al. Antitumor activity of temozolomide combined with irinotecan is partly independent of O6-methylguanine-DNA methyltransferase and mismatch repair phenotypes in xenograft models. Clin Cancer Res. 2000;6:4110–8. [PubMed] [Google Scholar]
- 30.Maurer BJ, Kalous O, Yesair DW, et al. Improved oral delivery of N-(4-hydroxyphenyl)retinamide with a novel LYM-X-SORB organized lipid complex. Clin Cancer Res. 2007;13:3079–86. doi: 10.1158/1078-0432.CCR-06-1889. [DOI] [PubMed] [Google Scholar]
- 31.Ponthan F, Borgstrom P, Hassan M, Wassberg E, Redfern CP, Kogner P. The vitamin A analogues: 13-cis retinoic acid, 9-cis retinoic acid, and Ro 13-6307 inhibit neuroblastoma tumour growth in vivo. Med Pediatr Oncol. 2001;36:127–31. doi: 10.1002/1096-911X(20010101)36:1<127::AID-MPO1030>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 32.Segerstrom L, Fuchs D, Backman U, Holmquist K, Christofferson R, Azarbayjani F. The anti-VEGF antibody Bevacizumab potently reduces the growth rate of high-risk neuroblastoma xenografts. Pediatr Res. 2006;60:576–81. doi: 10.1203/01.pdr.0000242494.94000.52. [DOI] [PubMed] [Google Scholar]
- 33.Shusterman S, Grupp SA, Barr R, Carpentieri D, Zhao H, Maris JM. The angiogenesis inhibitor tnp-470 effectively inhibits human neuroblastoma xenograft growth, especially in the setting of subclinical disease. Clin Cancer Res. 2001;7:977–84. [PubMed] [Google Scholar]
- 34.Thompson J, George EO, Poquette CA, et al. Synergy of topotecan in combination with vincristine for treatment of pediatric solid tumor xenografts. Clin Cancer Res. 1999;5:3617–31. [PubMed] [Google Scholar]
- 35.Zamboni WC, Stewart CF, Thompson J, et al. Relationship between topotecan systemic exposure and tumor response in human neuroblastoma xenografts. J Natl Cancer Inst. 1998;90:505–11. doi: 10.1093/jnci/90.7.505. [DOI] [PubMed] [Google Scholar]
- 36.Tacconelli A, Farina AR, Cappabianca L, et al. TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell. 2004;6:347–60. doi: 10.1016/j.ccr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawara A, Brodeur GM. Role of neurotrophins and their receptors in human neuroblastomas: a primary culture study. Eur J Cancer. 1997;33:2050–3. doi: 10.1016/s0959-8049(97)00280-3. [DOI] [PubMed] [Google Scholar]
- 38.Camoratto AM, Jani JP, Angeles TS, et al. CEP-751 inhibits TRK receptor tyrosine kinase activity in vitro exhibits anti-tumor activity. Int J Cancer. 1997;72:673–9. doi: 10.1002/(sici)1097-0215(19970807)72:4<673::aid-ijc20>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.Evans AE, Kisselbach KD, Liu X, et al. Effect of CEP-751 (KT-6587) on neuroblastoma xenografts expressing TrkB. Medical & Pediatric Oncology. 2001;36:181–4. doi: 10.1002/1096-911X(20010101)36:1<181::AID-MPO1043>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 40.Brown P, Levis M, McIntyre E, Griesemer M, Small D. Combinations of the FLT3 inhibitor CEP-701 and chemotherapy synergistically kill infant and childhood MLL-rearranged ALL cells in a sequence-dependent manner. Leukemia. 2006;20:1368–76. doi: 10.1038/sj.leu.2404277. [DOI] [PubMed] [Google Scholar]
- 41.Brown P, Levis M, Shurtleff S, Campana D, Downing J, Small D. FLT3 inhibition selectively kills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood. 2005;105:812–20. doi: 10.1182/blood-2004-06-2498. [DOI] [PubMed] [Google Scholar]
- 42.Brown P, Meshinchi S, Levis M, et al. Pediatric AML primary samples with FLT3/ITD mutations are preferentially killed by FLT3 inhibition. Blood. 2004;104:1841–9. doi: 10.1182/blood-2004-03-1034. [DOI] [PubMed] [Google Scholar]
- 43.Mead AJ, Gale RE, Kottaridis PD, Matsuda S, Khwaja A, Linch DC. Acute myeloid leukaemia blast cells with a tyrosine kinase domain mutation of FLT3 are less sensitive to lestaurtinib than those with a FLT3 internal tandem duplication. Br J Haematol. 2008;141:454–60. doi: 10.1111/j.1365-2141.2008.07025.x. [DOI] [PubMed] [Google Scholar]