Abstract
Red-toothed shrews (Soricidae, subfamily Soricinae) are an intriguing model system to examine the free radical theory of aging in wild mammals, given their short (<18 month) lifespan and high mass-specific metabolic rates. As muscle performance underlies both foraging ability and predator avoidance, any age-related decline should be detrimental to fitness and survival. Muscle samples of water shrews (Sorex palustris) and sympatrically distributed short-tailed shrews (Blarina brevicauda) were therefore assessed for oxidative stress markers, protective antioxidant enzymes and apoptosis. Activity levels of catalase and glutathione peroxidase increased with age in both species. Similarly, Cu,Zn-superoxide dismutase isoform content was elevated significantly in older animals of both species (increases of 60% in the water shrew, 25% in the short-tailed shrew). Only one oxidative stress marker (lipid peroxidation) was age-elevated; the others were stable or declined (4-hydroxynonenal adducts and dihydroethidium oxidation). Glutathione peroxidase activity was significantly higher in the short-tailed shrew, while catalase activity was 2× higher in water shrews. Oxidative stress indicators were on average higher in short-tailed shrews. Apoptosis occurred in <1% of myocytes examined, and did not increase with age. Within the constraints of the sample size we found evidence of protection against elevated oxidative stress in wild-caught shrews.
Keywords: senescence, antioxidant, breath-hold diving, redox, apoptosis, Sorex palustris, Blarina brevicauda
1. Introduction
The `free radical theory of aging' (Harman, 1956; 1994) posits that maximum longevity and aging are limited by free radicals, including reactive oxygen species (ROS). Even under normal physiological conditions free-radical-driven oxidative stress and damage impact cellular apoptotic processes that may be involved in aging (Sohal and Weindruch, 1996; Higami and Shimokawa, 2000). However, much of the empirical data are from studies using domesticated or laboratory animals restricted in activity and lacking natural environmental and biological cues. Little empirical data concerning age-related changes in redox status exist for wild populations. This type of information is essential to examine the classic tenet of aging theory which posits that mortality will be observed prior to senescence in free-ranging populations (e.g., Kirkwood and Austad, 2000; Parsons, 2002). Conversely, it may be more appropriate to apply an aging theory to wild populations which predicts maximal longevity for those groups exposed to low-stress environments (Austad and Fischer, 1991; Parsons, 2002). Given that oxidants participate in mitochondrial dysfunction, fibrosis, and apoptotic signaling pathways, and that dysregulation of mitochondria, fibrosis or apoptosis may be involved in aging, this would imply that low stress cellular environments are those with minimal oxidant exposure. For example, the regulation of cellular oxidant exposure in birds (Herrero and Barja, 1997; 1998) as well as bats (Brunet-Rossini and Austad, 2004) confers maximum longevities to these species that far exceed those of mammals with comparable mass.
Red-toothed shrews are an intriguing model system in this regard given their short (<18 month) lifespan and high mass-specific rates of metabolism (Hindle et al., 2003; Gusztak et al., 2005; McNab, 2008). Concordant with their diminutive size, shrew muscle is distinguished by its extremely high mitochondrial densities and contraction frequency (e.g., Lindstedt et al., 1985; Weibel, 1985; Jürgens, 2002), both of which should translate to elevated production of ROS while active (Reid et al., 1992). Measurements of fiber-type patterns, myosin composition, and enzyme activities moreover suggest the exclusive presence of fast type II fibers in shrew muscle (Savolainen and Vornanen 1995; Peters et al., 1999), cementing this group as a natural aging model (Hindle et al., 2009). Indeed, type II fibers are predominantly targeted by ROS-exposure linked apoptosis and atrophy processes during aging in rodent and human studies (Holloszy et al., 1991). Skeletal muscle was the focus of this study, due to its critical importance to the success of life in the wild. Muscle performance in wild animals underlies both foraging ability and predator avoidance; therefore any age-related performance decline would be detrimental. The loss of muscle quantity and quality (`sarcopenia') with advancing age is well-documented amongst humans and laboratory mammals (Marzetti and Leeuwenburgh, 2006), and senescent changes in muscle morphology in red-toothed shrews have been observed (Hindle et al., 2009). Muscle samples of old (second summer) and young (first summer) water shrews (WS; Sorex palustris) and sympatrically distributed short-tailed shrews (STS; Blarina brevicauda) were therefore assessed for antioxidant capacity, oxidative stress markers, and apoptosis. The maximum lifespan for both species in the wild is about 18 months (George et al., 1986; Beneski and Stinson, 1987), and each can breed in the season or year in which they were born (George et al., 1986; Beneski and Stinson, 1987). These species have similar mass and occupy similar habitats, except that water shrews heavily exploit aquatic food resources, which may also lead to periods of apneustic excercise. As shrews can employ the hindlimb and forelimb in different manners (locomotion on land and in water, digging, prey handling), these muscle groups were also compared. These are the only two shrews of comparable size prevalent in the study region, thus precluding detailed niche-specific data analysis with a larger species sample size. Instead this study focused on documenting the age-related changes in redox profiles of two sympatric wild-caught species which are short-lived (George et al., 1986; Beneski and Stinson, 1987) and display high metabolic rates (Hindle et al., 2003; Gusztak et al., 2005; Gusztak, 2008).
2. Materials and Methods
2.1. Capture, Animal Care and Sampling
Nineteen water shrews (Sorex palustris; nine adults: ~13–15 months of age; ten young-of-the-year: ~1-3 months old) and 15 short-tailed shrews (Blarina brevicauda ;eight adults and seven juveniles) were captured in Whiteshell and Nopoming Provincial Parks (49°49' N, 95°16' W; 50°67'N, 95°28' W) and at the Fort Whyte Centre, Winnipeg, Manitoba (49°50' N, 97°10' W), during July and August of 2005 and 2006 (see Hindle et al., 2003 and Gusztak and Campbell, 2004 for details). All study animals were captured and cared for under Manitoba Conservation permits WPB 20407 and WPB 21706, and in accordance with the principles and guidelines of the Canadian Council on Animal Care (University of Manitoba Animal Use Protocol # F05-014).
Animals were transported immediately upon capture to the Animal Holding Facility, University of Manitoba, and placed in a controlled-environment room (20±1°C; 12L:12D photoperiod). Shrews were maintained in captivity for ~one week as part of another study, during which time STS were housed individually in 76-L plastic terraria, while WS were housed in modified 264-L glass aquaria with terrestrial and aquatic compartments, as previously described (Hindle et al., 2003; Gusztak and Campbell, 2004; Gusztak et al., 2005). Shrews were provided with natural substrate for burrowing and nest construction, aquatic access (for WS), and diet supplementation with live invertebrates, to foster normal exercise opportunities within the enclosure (Hindle et al., 2003; Gusztak and Campbell, 2004).
Shrews were euthanized with an overdose of isoflurane inhalant anesthetic. Given their small size, it was not possible to obtain an adequate homogenate volume from individual muscles to assay the required enzymes. Thus, following Emmett and Hochachka (1981) we excised and analyzed the entire hindlimb and forearm musculature. Musculature from the forelimb and hindlimb (up to the medial end of the humerus and femur, respectively) were dissected quickly, frozen in liquid nitrogen, and stored at −80 °C (for a maximum of three months) prior to biochemical analyses. Gracilis muscle was dissected free from one hindlimb and processed separately for histology. Age determinations (first vs. second year) are described elsewhere (Hindle et al., 2009).
2.2. Tissue Homogenization
Hindlimb and forelimb muscle were homogenized separately on ice in lysis buffer (10 mM HEPES, 350 mM NaCl, 20 % glycerol, 1% Igepal-CA630, 1 mM MgCl2, 0.1 mM DTT, pH 7.5) containing protease inhibitor (Roche Applied Science, Indianapolis, IN, USA; #11836170001), using a glass-on-glass tissue grinder. Muscle homogenate was centrifuged at 12,000 g to remove cellular debris. The supernatant was withdrawn and stored at −80°C until analyses. Total protein content was measured using the Bradford technique (kit #23236, Pierce, Rockford, IL, USA). All enzyme activity assays were performed in triplicate, and were conducted at room temperature. Additionally, we processed all samples for each assay concurrently to reduce reagent variability.
2.3. Citrate Synthase Activity
The activity of citrate synthase (CS; EC 2.3.3.1) was measured as an indicator of oxidative potential. Reaction cocktail (0.1 mM DTNB, 0.07% Triton X-100, 0.1 mM acetyl CoA in 100 mM potassium phosphate buffer with 10 mM EDTA, pH 7.4) was incubated with homogenate for 5 min (modified from Srere, 1969). The substrate oxaloacetate (0.1 mM in buffer) was added and the reaction was followed from 1 to 4 min at 412 nm.
2.4. Antioxidant Enzymes
Muscle homogenate (1:20 dilution in lysis buffer) was incubated 10:1 v/v with ethanol for 30 min on ice, then with 1% Triton-X for 15 min at room temperature. The reaction was started by combining this mixture with 10 mM hydrogen peroxide solution (Aebi, 1984). The activity of catalase (EC 1.11.1.6) was then measured directly by following H2O2 extinction at 280 nm. Glutathione peroxidase (GPx; EC 1.11.1.9) activity was assayed according to the method of Flohé and Günzler (1984). Muscle homogenate was combined with reaction cocktail (0.3 U·mL−1 glutathione reductase (EC 1.8.1.7), 1.25 mM GSH, 0.1875 mM NADPH in 100 mM potassium phosphate buffer with 10 mM EDTA), and incubated for 3 min at room temperature. The reaction was initiated by adding 12 mM t-butyl hydroperoxide, and was monitored at 340 nm over 4 min. Blanks were run with 0.5% bovine serum albumin (in lieu of homogenate) in buffer.
Protein expression of the antioxidant enzymes Cu,Zn-SOD and Mn-SOD (EC 1.15.1.11) were determined through Western immunoblotting. Samples were separated using SDS-PAGE on gradient gels (NextGel (15%): pH 8.3, Amresco, Solon, OH, USA). Thirty μg of protein were mixed with sample buffer (Tris, pH 6.8, 2 % SDS, 60 mM DTT, 25 % glycerol) and separated at 145 V, then transferred onto nitrocellulose membranes and run for 90 min at 45 V (BioRad, Hercules, CA, USA). Upon verifying the presence of consistent protein transfer to the nitrocellulose with a Ponceau S stain, blots were rinsed in PBS and blocked with 5% non-fat milk (in PBS with 0.1% Tween-20) at room temperature for 4 h. Blocked membranes were washed (three × 5 min) in PBS and 0.4% Tween-20, and incubated overnight with specific primary antibodies diluted in PBS. Dilutions for primary antibodies were as follows: Cu,Zn-SOD, 1:500 (rabbit polyclonal, Santa Cruz Biotechnology, Santa Cruz, CA, USA); and Mn-SOD, 1:5000 (rabbit polyclonal, Stressgen, Ann Arbor, MI, USA). Membranes were then washed (three × 5 min) in PBS and 0.4% Tween-20, and incubated at room temperature for 90 min with HRP-conjugated anti-rabbit secondary antibody (Rockland Immunochemical, Gilberstville, PA, USA). Following a final set of washes (see above), protein bands were visualized using enhanced chemiluminescence detection (Supersignal West Pico Chemiluminescent Substrate, Pierce).
Images of the blots were captured with a CCD digital camera in a darkroom with the following exposure times: Cu,Zn-SOD, 30 sec; and Mn-SOD, 10 sec. Images were converted to grayscale and densitometry [area*(density – background for each lane)] was calculated using ImageJ software (version 1.37s, National Institutes of Health, USA).
2.5. Oxidative Stress Indicators
The probe dihydroethidium (DHE) was used as a substrate for the fluorometric detection of reactive oxygen species, principally superoxide and peroxynitrate (ONOO−), but not H2O2 (based on Supinski et al., 1999). The reaction was prepared in 100 mM potassium phosphate buffer, pH 7.4. Muscle homogenate (1:50 dilution in buffer; 160 μL), 240 μL of 1mM purine in buffer, 400 μL of 44 μM DHE, and 400 μL of methanol were thoroughly mixed and incubated for 10 min at 37°C, then read (excitation/emission wavelengths 465/585 nm) using a Farrand Optical fluorometer. Sample fluorescence was quantified in xanthine oxidase (XO) units based on a standard curve. Lipid peroxidation was quantified via xylenol orange oxidation (Hermes-Lima et al., 1995). The reaction constituents were prepared in 100 mM potassium phosphate buffer (pH 7.4), combined in a cuvette and incubated in the dark for 12 h in the following quantities: 225 μL 1mM FeSO4; 90 μL 0.25M H2SO4; 90 μL 1 mM xylenol orange; 468 μL 0.055M H2SO4; 180 μL 1:50 homogenate diluted in buffer. Total hydroperoxides were calculated based on a concurrently run t-butyl hydroperoxide standard curve at 580 nm.
We quantified oxidative protein damage by measuring the presence of protein adducts formed with 4-hydroxynonenal (4-HNE) using Western immunoblotting, as described above. The primary antibody dilution was 1:5000 (rabbit polyclonal, Calbiochem, San Diego, CA, USA). An exposure time of 30 s was used for this assay, with image analyses as described above.
2.6. Markers of Apoptosis
Apoptotic cell death in the cytoplasmic fraction of the muscle homogenate was measured by detection of characteristic mono- and oligonucleosomes with a cell death ELISA kit (Roche #11544675001). This assay was performed according to the directions of the manufacturer, and results are expressed in arbitrary absorbance units per mg protein.
Shrew muscle sections were triply labeled with DAPI (to identify all nuclei), TUNEL (Terminal deoxynucleotidyl transferase-mediated dUTP Nick-End Labeling; to identify apoptotic nuclei) and laminin (to delineate the basal lamina/extracellular matrix). Nuclei were characterized as having a myonuclear origin if they occurred interior to the laminin label (per Siu et al. 2005). Gracilis muscle cross-sections were used for this histochemical analysis. Thin (7–9 μm) sections were cut on a cryostat at −20 °C, and transverse orientation was verified using a standard light microscope. TUNEL fluorescein labeling was performed following the directions of an in situ cell death detection kit (#11684795910, Roche). Positive control slides were treated for 10 min with DNase solution (#4536282001, Roche). Slides were kept in the dark following TUNEL treatment to prevent florescence quenching.
Primary anti-laminin antibody was applied to TUNEL-treated sections for 40 min at room temperature (1:25 dilution in 1 % BSA-TBS of rabbit polyclonal, Sigma). Sections were washed (0.5 % Tween-20 in TBS), incubated with Cy3-labeled Fab' secondary antibody (Sigma), then diluted to 1:200 in 1 % BSA-TBS. After a final wash slides were coverslipped with an aqueous mounting medium containing DAPI (Vector Labs, Burlingame, CA, USA).
Monochrome images for each label were collected from a Spot Pursuit Slider CCD camera and a Nikon E400 microscope, using Nikon filters (UV-2E/C for DAPI, G-2A for laminin, B-2E/C for fluorescein). The images from the three channels were overlaid using SPOT software and the locations of apoptotic cells were counted directly from the images. All myocytes were analyzed, with the exception of those directly adjacent to the edge of the section.
2.7. Statistical Analyses
Mixed homogenate samples containing all the dissected muscles were prepared for the hindlimb and forelimb of each animal; however, available homogenate volume from each animal limited the number of assays that could be conducted. Samples were partitioned amongst assays such that a relatively even distribution from species, age class and sampling site were applied to each. Individual n values for assays are noted in the figure captions or in the text.
SPSS statistical software (V 11.5.1, Chicago, IL, USA) was used for all analyses. Data were tested for normality using the Shapiro-Wilk statistic and homogeneity of variance was confirmed using a modified Levene test. Data were natural log-transformed if necessary to meet the assumptions of parametric tests. Results were initially compared using 3-way ANOVA to separate the effects of species, age and limb. Where no limb effect was noted, pooled data from hindlimb and forelimb were analysed with 2-way ANOVA to compare only species and age class. Posthoc comparisons (LSD) were performed when global F tests showed significance (α=0.05). For TUNEL histology data, percent of cells identified as apoptosis-positive were analyzed using non-parametric 2-way ANOVA to compare age and species effects. Means are presented ± SE.
3. Results
3.1. Study Animals
For the 19 water shrews and 15 short-tailed shrews examined there was no mass difference between old (n=9 WS; n=8 STS) and young (n=10 WS; n=7 STS) individuals from either species (P=0.308 for WS; P=0.210 for STS; Hindle et al., 2009). Pooled mass (both age groups) for wild-caught water and short-tailed shrews were 14.1±0.3 g and 22.4±0.5 g, respectively.
Muscle mass also did not change with age in either species (P=0.72 for WS; P=0.30 for STS). Age-differences were detected in muscle histology, and those results are presented elsewhere (Hindle et al., 2009).
3.2. Muscle Oxidative Capacity
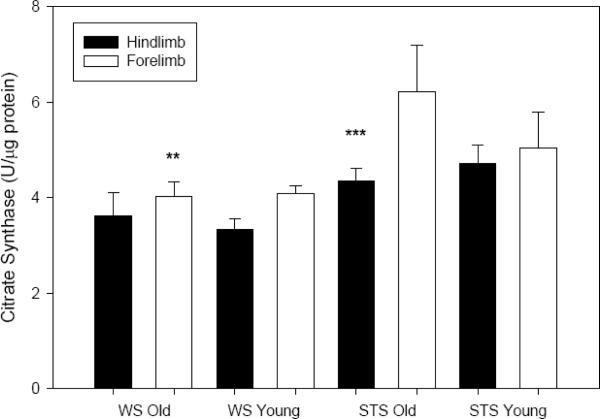
Protein concentration in the soluble fraction of muscle homogenate of all collected samples was statistically similar between the two shrew species and sampling location (hindlimb and forelimb data were therefore pooled for this variable). Protein concentrations appeared consistently lower in older individuals (~10 % decline for both species); this difference was not significant (P=0.146). Activity of the oxidative enzyme citrate synthase activities in shrew forelimb tended to be higher than those in hindlimb (F1,53=4.786, P=0.033; Fig. 1), although this difference displayed post-hoc significance only for adult STS (P=0.012). No effect of age on CS activity was noted for either species (P=0.517). STS (adult forelimb only; Fig. 1) was significantly higher than comparable data from WS (species effect: F1,53=11.878, P=0.001). We noted no significant interaction effects throughout the dataset, therefore between-factor interaction statistics are not presented.
Fig. 1.
Mean (± SE) activity of citrate synthase (U·μg protein−1) in skeletal muscle homogenate from two shrew species. Closed bars represent samples from hindlimb (old: n=8 WS, n=7 STS; young: n=9 WS, n=7 STS), open bars from forelimb (old: n=8 WS, n=8 STS; young: n=6 WS, n=7 STS). LSD post-hoc tests reveal a species effect in adult forelimb (P=0.003, denoted `**') and in limb site (for adult STS only P=0.012, denoted `***').
3.3. Antioxidant Capacity
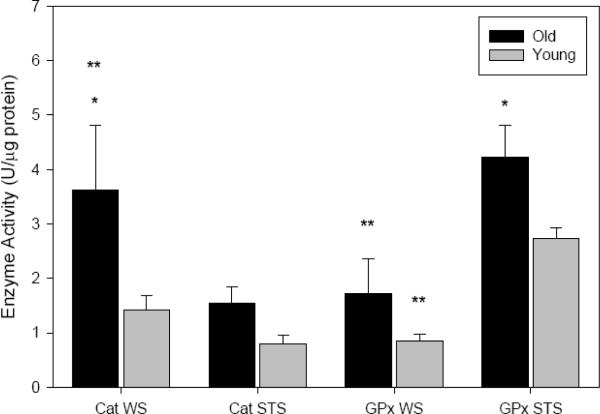
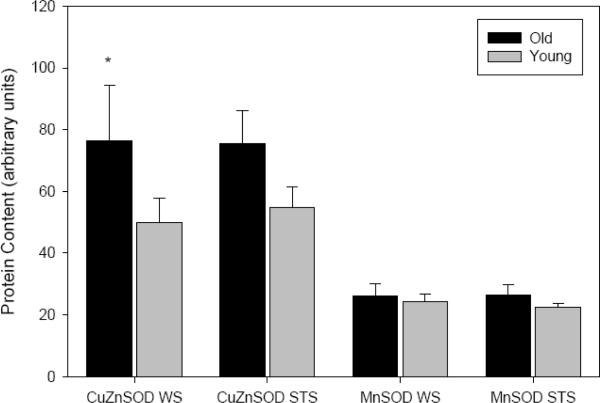
The activities of catalase (F1,28=4.785, P=0.039) and glutathione peroxidase (F1,29=5.715, P=0.024) increased with age in shrew skeletal muscle homogenate. No limb effect was detected for the activity of either antioxidant, and analysis of the pooled limb data revealed that the age-difference in catalase was significant only for WS, while that for GPx was significant only for STS (Fig. 2). In species comparisons, muscle catalase activity was 2× higher in WS (F1,28=4.701, P=0.039; Fig. 2), although this occurred only in adults (P=0.026 vs. P=0.443 for juveniles). Conversely, GPx was nearly 2.2× higher in both cohorts of STS, compared to WS (F1,29=20.542, P<0.001; Fig. 2). The protein contents of Cu,Zn- and Mn-SOD isoforms were assessed via Western immunoblotting, and in the absence of sampling site disparities, the data for both isoforms were pooled with respect to limb. There was no age-effect in Mn-SOD content (P=0.428), while Cu,Zn-SOD was significantly elevated in older animals (F1,24=7.616, P=0.011). Although we noted a 60% age-elevation for WS and a 25% elevation for STS, post-hoc tests revealed that this increase was significant only in WS (P=0.030; Fig. 3). No significant species effect was apparent for either SOD isoform (Cu,Zn-SOD P=0.586; Mn-SOD P=0.843; Fig. 3).
Fig. 2.
Mean (± SE) activities of catalase (CAT) and glutathione peroxidase (GPx) in skeletal muscle homogenate (U·μg protein−1) from two species of shrew. Pooled data from hindlimb and forelimb are presented, with old animals (CAT: n=8 WS, n=8 STS; GPx: n=8 WS, n=8 STS) noted by black bars, and young animals (CAT: n=9 WS, n=7 STS; GPx: n=9 WS, n=7 STS) by gray bars. LSD post-hoc tests revealed a significant age effects (denoted `*') for CAT in WS (P=0.025) and GPx in STS (P=0.046), as well as species effect (denoted `**') for WS CAT (P=0.026) and for GPx in both cohorts (P=0.001 adults; P=0.014 juveniles).
Fig. 3.
Protein contents of Mn-SOD and CuZn-SOD isoforms, expressed in arbitrary units, in skeletal muscle hindlimb homogenate from two species of shrew (means ± SE for pooled forelimb/hindlimb data). Black bars represent data from adult shrews (CuZn: n=5 WS, n=8 STS; Mn: n=6 WS, n=7 STS), and gray bars represent juvenile data (CuZn: n=9 WS, n=6 STS; Mn: n=4 WS, n=5 STS). LSD post-hoc tests revealed a significant age effect for CuZn SOD in WS (P=0.030), denoted `*'.
3.4. Indicators of Oxidative Stress
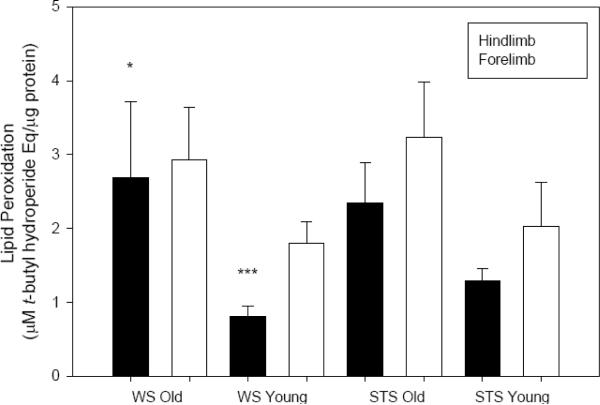
Lipid peroxidation, measured using a ferrous oxide-xylenol orange assay, varied with shrew age and sampling site. Second-year shrews displayed more lipid peroxidation in muscle than did first-year animals (F1,60=12.417, P=0.001; ln-transformed), although post-hoc significance was only detected for WS hindlimb (P=0.003; Fig. 4). Lipid peroxidation was also generally elevated in forelimb compared to hindlimb samples (F1,60=7.740, P=0.007; ln-transformed; Fig. 4). No significant species differences were noted (P=0.117; ln-transformed).
Fig. 4.
Lipid peroxidation (μM t-butyl hydroperoxide Eq·μg protein−1) in skeletal muscle homogenate from two species of shrew (means ± SE). Closed bars represent samples from hindlimb (old: n=9 WS, n=8 STS; young: n=10 WS, n=7 STS), open bars from forelimb (old: n=9 WS, n=8 STS; young: n=10 WS, n=7 STS). LSD post-hoc tests revealed an age effect in WS hindlimb (P=0.003, denoted `*') and in limb site (for juvenile WS only P=0.006, denoted `***').
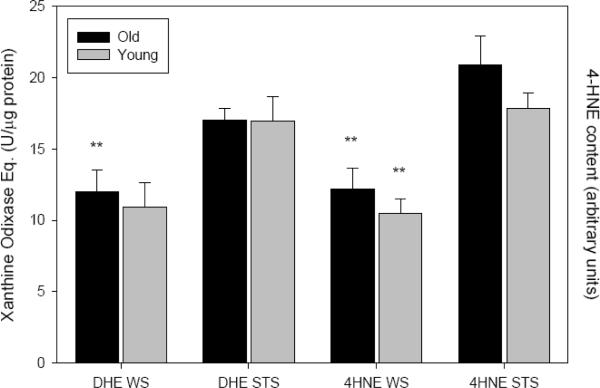
Despite the age-increased lipid peroxidation noted in our dataset, the detection of 4-hydroxynonenol adducts was not elevated in older animals (P=0.104; Fig. 5). In fact, adults of both species displayed an ~15% reduction of 4-HNE content. Further, no differences were detected between forelimb and hindlimb samples. 4-HNE adducts were 70% higher in the STS compared to WS, and this species difference persisted in both cohorts (F1,28=34.250 P<0.001; Fig. 5).
Fig. 5.
Dihydroethidium oxidation (DHE, xanthine oxidase Eq. U·μg protein−1) and 4-hyroxynonenal adducts (4HNE, protein content arbitrary units) in skeletal muscle homogenate from two shrew species (pooled means ± SE from forelimb/hindlimb data). Black bars represent data from adult shrews (DHE: n=7 WS, n=9 STS; 4HNE: n=8 WS, n=8 STS), and gray bars represent juvenile data (DHE: n=10 WS, n=6 STS; 4HNE: n=9 WS, n=7 STS). LSD post-hoc tests revealed a significant species effects for DHE in adults (P=0.016) and 4HNE (P<0.001 for both cohorts), which are indicated with `**'.
Dihydroethidium conversion to its oxidized form was also assayed in muscle homogenate. Similar to the trend found for 4-HNE, this oxidation showed no elevation with age (P=0.734; Fig. 5) nor a difference related to limb in either species. DHE oxidation was higher in STS compared to the WS (F1,27=9.977, P=0.004) although this species difference was statistically significant only in the adult cohort (55% elevation; P=0.016 vs. P=0.063 for juveniles; Fig. 5).
3.5. Apoptosis
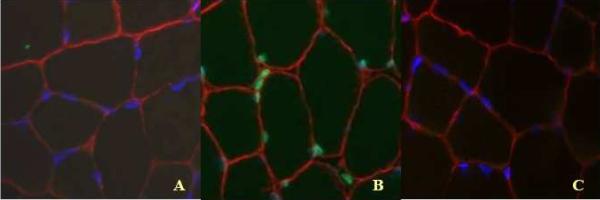
ELISA detection of mono- and oligonucleosomes provided an apoptotic index for shrew muscle (n=7 old, n=8 young WS, n=8 old, n=6 young STS). Although there was a trend for this apoptotic index to be higher in muscle homogenate of STS (20% elevation), no significant differences in this index were noted between species (P=0.099), age class (P=0.999), or limb (P=0.247). When examined in gracilis myonuclei directly via a TUNEL protocol, apoptotic nuclei were apparent in less than 1% of myocytes (Fig. 6). Apoptotic (TUNEL-positive) myonuclei also occurred at similar levels between the two age classes (P=0.337) and the two shrew species (P=0.168).
Fig. 6.
(COLOR FIGURE IN PRINT) Triply labeled gracilis muscle of a representative water shrew. TUNEL-positive areas are labeled with green florescence, laminin with red, and all nuclei (DAPI) with blue florescence. A: representative section of gracilis muscle with triple label; B: positive TUNEL control; C: negative TUNEL control.
4. Discussion
Our results demonstrate that age-related changes in redox status can be detected in two species of short-lived, sympatric wild-caught mammals, although measured species-differences were occasionally larger than within-species age-discrepancies. A consistent skeletal muscle age-elevation of oxidative stress markers was not noted for either species (Figs. 4, 5) concomitant with exceptional increases in antioxidant capacity (Figs. 2, 3). These data suggest that the antioxidant system in shrews retains its functionality with advancing age, and this effectively minimizes an increase in, and pronounced accumulation of, muscle tissue oxidative stress and damage. This antioxidant up-regulation with age further appears protective to pro-apoptotic pathways, resulting in levels of apoptosis in both shrew species that were considerably lower than those observed in captive animals (e.g., Dirks and Leeuwenburgh, 2002). These parallel findings in two species of shrew with different lifestyles support the notion that changes to the redox system can occur within the lifespan of even small, short-lived mammals.
4.1. Biochemical Properties of Shrew Muscle
Citrate synthase activity indicates oxidative metabolic capacity, and scales negatively with body mass (Emmett and Hochachka, 1981; Hochachka et al., 1988). Activity measured in vastus lateralis of young rats was considerably lower per g wet muscle mass (8.2 U·gww−1; Ji et al., 1990), than average shrew hindlimb values measured with comparable methodology in our study (STS: 46.8 U·gww−1, WS: 33.0 U·gww−1, note change in units compared to results). Our values were also somewhat less than those reported for n=2 hindlimb samples of the smaller (3–8 g) vagrant shrew (Sorex vagrans, 61 and 69 U·gww−1; Emmett and Hochachka, 1981), a difference we attribute to both to the lower body mass of the vagrant shrew, and the higher assay temperature (37°C) employed by Emmett and Hochachka (1981).
Mean catalase activity assayed in rat muscle at room temperature ranges from 3.5 U·gww−1 (soleus; Lawler et al., 2003) to 11.4 U·gww−1 (vastus; Ji et al., 1990). Mean activities were again higher in shrews, ranging from 10.4 U·gww−1 (STS) to 17.8 U·gww−1 (WS). GPx activity was 16.6 U·gww−1 in the muscle of young rats (Ji et al 1990), compared to 29.2 U·gww−1 (STS) and 8.2 U·gww−1 (WS). Shrews also displayed higher total hydroperoxide levels compared to published data from rats (STS: 18 300 nmol·gww−1; WS: 13 900 nmol·gww−1; rats: 1770 nmol·gww−1, Lawler et al., 2003). Apoptosis, as measured both quantitatively (cell death ELISA) and qualitatively (TUNEL) was essentially absent in shrews at all ages (Fig. 6). This finding is in stark contrast to data from laboratory species, in which apoptosis can increase by 50% in old rats (Dirks and Leeuwenburgh, 2002). Clearly this indicates that apoptosis is not a primary mechanism driving observed age-associated skeletal muscle remodeling (Hindle et al., 2009) in these short-lived, wild mammals. We suggest this apparent lack of apoptosis may be a function of sustained lifelong activity within the shrews' natural habitat (as opposed to more sedentary laboratory conditions; Song et al., 2006; Kim et al., 2008; Marzetti et al., 2008).
4.2. Biochemical Properties of Aging Shrew Muscle
Shrews do not conform closely to the general redox profile expected from the mammalian aging paradigm, nor do these wild-caught species appear to support the free radical theory of aging. Indeed, shrew muscle displayed large increases in antioxidant capacity (Figs. 2, 3), but inconsistent elevations in oxidative stress markers with age (Figs. 4, 5). Species-differences were generally greater than intraspecies age-discrepancies.
Consistent with human data (Essén-Gustavsson and Borges, 1986), CS activity did not decline significantly with age. Contrary to the expected mass-scaling relationship (Emmett and Hochachka, 1981), however, there was a clear elevation of CS activity (expressed as U·gww−1 or U·μg protein−1) in the larger STS compared to WS. CS activity was moreover higher in forelimb than hindlimb muscle (significant in adult STS), and displayed a greater overall increase with age in the STS (28% vs. 17% increase in WS).
Rat muscle generally increases in antioxidant capacity with advancing age (SOD, CAT, GPx; Ji et al., 1990), despite some fiber-type specificity of the response (e.g., Lawler et al., 1993; Hollander et al., 2000). SOD activities tend to follow this pattern, although the effect generally occurs post-translationally, with SOD mRNA and protein contents unaffected. As with shrews (this study), no elevation of Mn-SOD protein content has been noted in aging rats (Oh-Ishi et al., 1995; Hollander et al., 2000). We did, however, note an age-elevation in shrew Cu,Zn-SOD protein contents (Fig. 3). A similar finding is occasionally noted in laboratory species (e.g., rats; Oh-Ishi et al., 1995; Hollander et al., 2000), although the ~60% overall age-increase observed in WS (Fig. 3) far exceeds any previously documented result.
Consistent with rodent literature (Lawler et al., 1993; Bejma and Ji, 1999), an age-elevation of muscle lipid peroxidation was observed for both shrew species (Fig. 4). We expected other measured indicators of tissue oxidative stress indicators to increase with age (see Goto et al., 1999). By contrast, only the above measure of total hydroperoxides was age-elevated in this study. The detection of 4-HNE protein adducts, a marker of protein oxidative damage, showed no significant change, and in fact was slightly age-decreased (Fig. 5). The capacity of muscle homogenate to oxidize dihydroxyethidine (DHE) was also unchanged in both species (Fig. 5). This implies a comparable cytosolic superoxide presence among the two age classes, a finding at odds with the free radical theory of aging (Huang et al., 2000; Van Remmen et al., 2003; Muller et al., 2007). Taken together, these findings suggest that the expected age-elevation of muscle oxidative damage (based on captive rodent data, see Meydani and Evans, 1993; Bejma and Ji, 1999) is reduced or modulated in wild-caught shrews. It is conceivable that the demonstrated increase in lipid peroxidation represents the predicted age-elevation of oxidative stress, and that the lack of concurrent elevation in other measures of oxidative stress represent some superior scavenging in shrews. Antioxidants such as glutathione-s-transferase catalyze the majority of 4-HNE metabolism via conjugation of the aldehyde with GSH (Yang et al., 2003). Consequently, the lower-than-expected DHE oxidation (which represents superoxide presence) with age in shrews could result from very effective cytosolic scavenging mechanisms (including high Cu,Zn-SOD levels).
4.3. Redox Systems in Wild Species
We conducted this study with two shrew species to ensure that our results were not driven by any species-specific factor, with the corollary that any noted similarities were likely to be “shrew-specific traits”. While our two-species approach does not allow inferences of species-specific adaptation (Garland and Adolph, 1994), it does permit some speculation on the roles of lifestyle and habitat in redox status and aging.
In our analysis, WS displayed generally reduced markers of oxidative stress relative to STS, without an obvious relative antioxidant increase. We speculate that this can be explained in part by the markedly elevated muscle myoglobin contents of WS compared to STS (Gusztak, 2008; Stewart et al., 2005) which are associated with a diving lifestyle requiring episodic apneustic exercise. The direct antioxidant (e.g., Garry et al., 2003; Flögel et al., 2004) and reactive nitrogen species scavenging properties of myoglobin (Ascenzi and Brunori, 2001) could bolster antioxidant capacity of WS muscles. Such protection would be lifelong, assuming no developmental differences (Gusztak, 2008) or a senescent decline of myoglobin content (Dolar et al., 1999; MacArthur et al., 2001).
Further, a paradox of oxidative stress is that ROS can be generated in situations where oxygen is in surplus, or in deficit (Hess and Manson, 1984; Clanton et al., 1999), and both species examined here may routinely operate under hypoxic conditions. Air-breathing divers such as WS operate with low tissue oxygen tensions while submerged, followed by rapid reoxygenation upon arrival at the water surface (e.g., Qvist et al., 1986; Stockard et al., 2005). Using this line of reasoning, the hypothesis that diving mammals are a natural model system for coping with oxidative stress has been advanced (Zenteno-Savín et al., 2002; Johnson et al., 2004). Apnea in large, deep diving animals can last minutes to hours, however, low oxygen tensions should equally occur in small apneustic hunters that breath-hold for seconds to minutes at significantly higher mass-specific metabolic rates. Weighing only 8–18 g, the North American water shrew is the smallest documented homeothermic breath-hold hunter (Beneski and Stinson, 1987). The semi-fossorial nature of the STS could also confer hypoxic exposure underground, albeit of a more consistent, less episodically accentuated nature. The anatomy of the STS lung indicates extended time spent underground in dusty burrows (George et al., 1986). Further, if lung oxygen extraction declines with age, as it does in deer mice (Chappell et al., 2003), the severity of the resulting tissue hypoxia would increase with age in this terrestrial shrew.
Other hypoxia-tolerant animals, such as turtles and hibernating mammals suppress the production of ROS in many organs and ultimately minimize or prevent oxidative damage through elevated anti-oxidant systems (see Hermes-Lima et al., 2001 for review). In turtles at least, a link has also been suggested between longevity and anoxia protective mechanisms in the brain (Lutz et al., 2003). On the other hand, naked mole rats occupy fossorial natural habitats, and are likely faced with similar oxygen-limited conditions as shrews in this study. Naked mole rats demonstrate extreme longevity but display disparate redox patterns compared to other hypoxia-adapted animals (Buffenstein, 2008). No age-affect on antioxidant capacity or oxidative stress is detectable for these rodents (Andziak et al., 2005; 2006), but yet they maintain a surprising resistance to pro-apoptotic stimuli (Labinskyy et al., 2006).
Similar to other hypoxia-tolerant species, measured antioxidants in both cohorts were elevated in shrew muscles (compared to laboratory rodents). Despite this elevated antioxidant profile, lipid peroxidation for shrews in this study was also considerably higher than for laboratory rodents, regardless of age class. Markers of oxidative stress (total hydroperoxides, 4-HNE protein adducts, and DHE oxidation) were somewhat increased in STS relative to WS; possibly related to higher CS activities.
While certain substantial antioxidant age-elevations were noted in our dataset, these did not occur uniformly, but rather appeared to be species-specific (e.g., catalase in WS, GPx in STS). We also failed to note a consistent age-elevation of oxidative stress markers in either species (beyond the relatively high levels vs. rats apparent in both cohorts). Further, skeletal muscle apoptosis was virtually absent in either shrew, regardless of age. These data reflect a non-uniform antioxidant/oxidative stress response in shrews, yet clear apoptotic protection in muscle with respect to aging. On balance, we suggest that shrews may be counted among mammalian species, such as naked mole rats, which are at odds with the free radical theory of aging.
Acknowledgement
The field and laboratory assistance of R. Gusztak and R. Senkiw is gratefully acknowledged. This work was supported in part by a Natural Sciences and Engineering Research Council (Canada) post-graduate scholarship to AGH, a NSERC Discovery Grant to KLC (#238838), and by the Laboratory for Applied Biotelemetry & Biotechnology at Texas A&M University at Galveston.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aebi H. Catalase in vitro. Meth. Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Andziak B, O'Connor TP, Buffenstein R. Antioxidants do not explain the disparate longevity between mice and the longest-living rodent, the naked mole-rat. Mech. Ageing Dev. 2005;126:1206–1212. doi: 10.1016/j.mad.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Andziak B, O'Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Ascenzi P, Brunori M. Myoglobin: a pseudo-enzymatic scavenger of nitric oxide. BAMBED. 2001;29:183–185. [Google Scholar]
- Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J. Gerontol. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J. Appl. Physiol. 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- Beneski JT, Jr., Stinson DW. Mammalian species: Sorex palustris. Am. Soc. Mammal. 1987;296:1–6. [Google Scholar]
- Brunet-Rossinni AK, Austad SN. Ageing studies on bats: a review. Biogerontology. 2004;5:211–222. doi: 10.1023/B:BGEN.0000038022.65024.d8. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B) 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- Chappell MA, Rezende EL, Hammond KA. Age and aerobic performance in deer mice. J. Exp. Biol. 2003;206:1221–1231. doi: 10.1242/jeb.00255. [DOI] [PubMed] [Google Scholar]
- Clanton TL, Zuo L, Klawitter P. Oxidants and skeletal muscle function physiologic and pathophysiologic implications. Proc. Soc. Exp. Biol. Med. 1999;222:253–262. doi: 10.1046/j.1525-1373.1999.d01-142.x. [DOI] [PubMed] [Google Scholar]
- Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am. J. Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- Dolar MLL, Suarez P, Ponganis PJ, Kooyman GL. Myoglobin in pelagic small cetaceans. J. Exp. Biol. 1999;202:227–236. doi: 10.1242/jeb.202.3.227. [DOI] [PubMed] [Google Scholar]
- Emmett B, Hochachka PW. Scaling of oxidative and glycolytic enzymes in mammals. Respir. Physiol. 1981;45:261–72. doi: 10.1016/0034-5687(81)90010-4. [DOI] [PubMed] [Google Scholar]
- Essén-Gustavsson B, Borges O. Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta. Physiol. Scand. 1986;126:107–114. doi: 10.1111/j.1748-1716.1986.tb07793.x. [DOI] [PubMed] [Google Scholar]
- Flögel U, Gödecke A, Klotz L, Schrader J. Role of myoglobin in the antioxidant defense of the heart. FASEB J. 2004;18:1156–1158. doi: 10.1096/fj.03-1382fje. [DOI] [PubMed] [Google Scholar]
- Flohé L, Günzler WA. Assays of glutathione peroxidase. Meth. Enzymol. 1894;105:114–119. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr., Adolph SC. Why not to do two species comparative studies: limitations on inferring adaptation. Physiol. Zool. 1994;67:797–828. [Google Scholar]
- Garry DJ, Kanatous SB, Mammen PPA. Emerging roles for myoglobin in the heart. Trends Cardiovasc. Med. 2003;13:111–116. doi: 10.1016/s1050-1738(02)00256-6. [DOI] [PubMed] [Google Scholar]
- George SB, Choate JR, Genoways HH. Mammalian species: Blarina brevicauda. Am. Soc. Mammal. 1986;261:1–9. [Google Scholar]
- Goto S, Nakamura A, Radak Z, Nakamoto H, Takahashi R, Yasuda K, Sakurai Y, Ishii N. Carbonylated proteins in aging and exercise: immunoblot approaches. Mech. Ageing. Dev. 1999;107:245–253. doi: 10.1016/s0047-6374(98)00133-x. [DOI] [PubMed] [Google Scholar]
- Gusztak RW, Campbell KL. Growth, development and maintenance of American water shrews (Sorex palustris) in captivity. Mammal Study. 2004;29:65–72. [Google Scholar]
- Gusztak RW, MacArthur RA, Campbell KL. Bioenergetics and thermal physiology of American water shrews (Sorex palustris) J. Comp. Physiol. B. 2005;115:87–95. doi: 10.1007/s00360-004-0465-x. [DOI] [PubMed] [Google Scholar]
- Gusztak RW. MSc thesis. University of Manitoba; 2008. Diving physiology and aquatic thermoregulation of the American water shrew (Sorex palustris) [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: prospects for further increases in functional life span. Age. 1994;17:199–146. [Google Scholar]
- Hermes-Lima M, Wilmore WG, Storey KB. Quantification of lipid peroxidation in tissue extracts based on Fe(III) xylenol-orange complex formation. Free Rad. Biol. Med. 1995;19:271–280. doi: 10.1016/0891-5849(95)00020-x. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M, Storey JM, Storey KB. Antioxidant defenses and animal adaptation to oxygen availability during environmental stress. In: Storey KB, Storey JM, editors. Cell and Molecular Responses to Stress. vol. 2. Elsevier; Amsterdam: 2001. pp. 263–287. [Google Scholar]
- Herrero A, Barja G. Sites and mechanisms responsible for the low rate of free radical production of heart mitochondria in the long-lived pigeon. Mech. Ageing Dev. 1997;98:95–11. doi: 10.1016/s0047-6374(97)00076-6. [DOI] [PubMed] [Google Scholar]
- Herrero A, Barja G. H2O2 production of heart mitochondria and aging rate are slower in canaries and parakeets than in mice: sites of free radical generation and mechanisms involved. Mech. Ageing Dev. 1998;103:133–146. doi: 10.1016/s0047-6374(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Hess ML, Manson NH. Molecular oxygen: friend or foe. The role of the oxygen free radical system in the calcium paradox, the oxygen paradox and ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 1984;16:969–985. doi: 10.1016/s0022-2828(84)80011-5. [DOI] [PubMed] [Google Scholar]
- Higami Y, Shimokawa I. Apoptosis in the aging process. Cell Tissue Res. 2000;301:125–132. doi: 10.1007/s004419900156. [DOI] [PubMed] [Google Scholar]
- Hindle AG, McIntyre IW, Campbell KL, MacArthur RA. The heat increment of feeding and its thermoregulatory implications in the short-tailed shrew (Blarina brevicauda) Can. J. Zool. 2003;81:1445–1453. [Google Scholar]
- Hindle AG, Lawler JM, Campbell KL, Horning M. Muscle senescence in short-lived wild mammals, the soricine shrews Blarina brevicauda and Sorex palustris. J. Exp. Zool. A. 2009;311:358–367. doi: 10.1002/jez.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Emmett B, Suarez RK. Limits and constraints in the scaling of oxidative and glycolytic enzymes in homeotherms. Can. J. Zool. 1988;66:1128–1138. [Google Scholar]
- Hollander J, Bejma J, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression in skeletal muscle: fiber-specific effect of age. Mech. Ageing Dev. 2000;116:33–45. doi: 10.1016/s0047-6374(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Mech. Ageing Dev. 1991;60:199–213. doi: 10.1016/0047-6374(91)90131-i. [DOI] [PubMed] [Google Scholar]
- Huang TT, Carlson EJ, Gillespie AM, Shi Y, Epstein CJ. Ubiquitous overexpression of CuZn superoxide dismutase does not extend life span in mice. J. Gerontol. A. 2000;55:B5–B9. doi: 10.1093/gerona/55.1.b5. [DOI] [PubMed] [Google Scholar]
- Ji LL, Dillon D, Wu E. Alteration of antioxidant enzyme with aging in rat skeletal muscle and liver. Am. J. Physiol. 1990;258:R918–R923. doi: 10.1152/ajpregu.1990.258.4.R918. [DOI] [PubMed] [Google Scholar]
- Johnson P, Elsner R, Zenteno-Savín T. Hypoxia-inducible factor in ringed seal (Phoca hispida) tissues. Free Radic. Res. 2004;38:847–854. doi: 10.1080/10715760410001725526. [DOI] [PubMed] [Google Scholar]
- Jürgens KD. Etruscan shrew muscle: the consequences of being small. J. Exp. Biol. 2002;205:2161–2166. doi: 10.1242/jeb.205.15.2161. [DOI] [PubMed] [Google Scholar]
- Kim J-H, Kwak H-B, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp. Gerontol. 2008;43:317–329. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;409:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2−* and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am. J. Physiol. 2006;291:H2698–2704. doi: 10.1152/ajpheart.00534.2006. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Powers SK, Visser T, Van Dijk H, Kordus MJ, Ji LL. Acute exercise and skeletal muscle antioxidant and metabolic enzymes: effects of fiber type and age. Am. J. Physiol. 1993;265:R1344–R1350. doi: 10.1152/ajpregu.1993.265.6.R1344. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radical Biol. Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Lindstedt SL, Hoppeler H, Bard KM, Thronson HA., Jr. Estimate of muscle-shortening rate during locomotion. Am. J. Physiol. 1985;249:R699–703. doi: 10.1152/ajpregu.1985.249.6.R699. [DOI] [PubMed] [Google Scholar]
- Lutz PL, Prentice HM, Milton SL. Is turtle longevity linked to enhanced mechanisms for surviving brain anoxia and reoxygenation? Exp. Gerontol. 2003;38:797–800. doi: 10.1016/s0531-5565(03)00111-6. [DOI] [PubMed] [Google Scholar]
- MacArthur RA, Humphries MM, Fines GA, Campbell KL. Body oxygen stores, aerobic dive limits, and the diving abilities of juvenile and adult muskrats (Ondatra zibethicus) Physiol. Biochem. Zool. 2001;74:178–190. doi: 10.1086/319662. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp. Gerontol. 2006;41:1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Groban L, Wohlgemuth SE, Lees HA, Lin M, Jobe H, Giovannini S, Leeuwenburgh C, Carter CS. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am. J. Physiol. 2008;274:R558–578. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- McNab BK. An analysis of the factors that influence the level and scaling of mammalian BMR. Comp. Biochem. Physiol. B. 2008;115:5–28. doi: 10.1016/j.cbpa.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Meydani M, Evans WJ. Free radicals, exercise, and aging. In: Yu BP, editor. Free Radicals in Aging. CRC Press; Boca Raton, FL: 1993. pp. 183–204. [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Rad. Biol. Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Oh-Ishi S, Kizaki T, Yamashita H, Nagatab N, Suzuki K, Taniguchi N, Ohno H. Alterations of superoxide dismutase iso-enzyme activity, content, and mRNA expression with aging in rat skeletal muscle. Mech. Ageing Dev. 1995;84:65–76. doi: 10.1016/0047-6374(95)01637-f. [DOI] [PubMed] [Google Scholar]
- Parsons PA. Life span: does the limit to survival depend upon metabolic efficiency under stress? Biogerontology. 2002;3:233–241. doi: 10.1023/a:1016271005967. [DOI] [PubMed] [Google Scholar]
- Peters T, Kubis HP, Wetzel P, Sender S, Asmussen G, Fons R, Jurgens KD. Contraction parameters, myosin composition and metabolic enzymes of the skeletal muscles of the Etruscan shrew Suncus etruscus and of the common European white-toothed shrew Crocidura russula (Insectivora: Soricidae) J. Exp. Biol. 1999;202:2461–2473. doi: 10.1242/jeb.202.18.2461. [DOI] [PubMed] [Google Scholar]
- Qvist J, Hill RD, Schneider RC, Falke KJ, Liggins GC, Guppy M, Elliot RL, Hochachka PW, Zapol WM. Hemoglobin concentrations and blood gas tensions of free-diving Weddell seals. J. Appl. Physiol. 1986;61:1560–1569. doi: 10.1152/jappl.1986.61.4.1560. [DOI] [PubMed] [Google Scholar]
- Reid MB, Soji T, Moody MR, Entman ML. Reactive oxygen species in skeletal muscle II: extracellular release of free radicals. J. Appl. Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- Savolainen J, Vornanen M. Myosin heavy chains in skeletal muscle of the common shrew (Sorex araneus): absence of slow isoform and transitions of fast isoform with ageing. Acta Physiol. Scand. 1995a;155:233–239. doi: 10.1111/j.1748-1716.1995.tb09968.x. [DOI] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am. J. Physiol. 2005;288:C338–C349. doi: 10.1152/ajpcell.00239.2004. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Kwak H-B, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antiox. Redox Signal. 2006;8:517–528. doi: 10.1089/ars.2006.8.517. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Meth. Enzymol. 1969;13:3–11. [Google Scholar]
- Stewart JM, Woods AK, Blakely JA. Maximal enzyme activities, and myoglobin and glutathione concentrations in heart, liver and skeletal muscle of the Northern Short-tailed shrew (Blarina brevicauda; Insectivora: Soricidae) Comp. Biochem. Physiol. B. 2005;141:267–273. doi: 10.1016/j.cbpc.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Stockard TK, Heil J, Meir JU, Sato K, Ponganis KV, Ponganis PJ. Air sac PO2 and oxygen depletion during dives of emperor penguins. J. Exp. Biol. 2005;208:2973–2980. doi: 10.1242/jeb.01687. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Design and performance of muscular systems: an overview. J. Exp. Biol. 1985;115:405–412. doi: 10.1242/jeb.115.1.405. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim. Pol. 2003;50:319–336. [PubMed] [Google Scholar]
- Zenteno-Savín T, Clayton-Hernández E, Elsner R. Diving seals: are they a model for coping with oxidative stress? Comp. Biochem. Physiol. C. 2002;133:527–536. doi: 10.1016/s1532-0456(02)00075-3. [DOI] [PubMed] [Google Scholar]