Abstract
Photodynamic therapy (PDT) is an FDA-approved modality that rapidly eliminates local tumors, resulting in cure of early disease and palliation of advanced disease. PDT was originally considered to be a local treatment; however, both pre-clinical and clinical studies have shown that local PDT treatment of tumors can enhance systemic anti-tumor immunity. The current state of investigations into the ability of PDT to enhance anti-tumor immunity, the mechanisms behind this enhancement and the future of PDT as an immunotherapy are addressed in this review.
Keywords: PDT, Neutrophil, Vaccine, T cell
Photodynamic therapy
Photodynamic therapy (PDT) is an anti-tumor modality that is approved for clinical use in a number of countries, including the US, for the elimination of early-stage malignancies and the palliation of symptoms in patients with late stage tumors [1, 2]. The treatment involves the systemic or topical application of a photoreactive drug known as a photosensitizer, which is inert until activated by the light of a specific wavelength [3]. Photosensitizers generally localize within cellular organelles including mitochondria and lysosomes and have some selectivity for tumor cells; however, selectivity is generally achieved by directed light delivery. Directed light delivery is obtained by placement of optical fibers at the tumor site. Light activation of the of the photosensitizer leads to the production of reactive oxygen species and direct tumor cell death.
The first photosensitizer approved for clinical use in the US was Photofrin® or Porfimer sodium, which was discovered and developed at Roswell Park Cancer Institute. Photofrin-PDT is approved by the FDA for the treatment of early and late stage non-small-cell lung carcinoma and the treatment of high-grade dysplasia associated with Barrett’s esophagus [2, 4]. Since the discovery and approval of Porfimer sodium, a number of second generation photosensitizers, which have increased tumor selectivity and reduced normal tissue phototoxicity, have been developed and are currently in clinical trials [1, 2, 5].
Tumor destruction by PDT is not only a result of direct tumor destruction via generation of reactive oxygen. PDT also induces secondary events including microvascular disruption and local acute inflammation that are known to contribute significantly to the long-term tumor growth control by PDT [6].
The local acute inflammatory response to PDT is characterized by increased expression of several pro-inflammatory cytokines, including IL-1β, TNF-α and IL-6 [7–10], adhesion molecules E-selectin and ICAM-1 [9] and rapid leukocyte infiltration into the treated tumor site [11–14]. The initial infiltrating leukocyte population was identified as CD11b+Gr1Hi and classified as neutrophils. Depletion of Gr1-expressing cells resulted in diminished long-term tumor growth control by PDT [12, 14–16], leading to the hypothesis that neutrophils are critical to PDT outcome. Recently, however, characterization of cell populations have revealed that multiple leukocyte populations express Gr1, including neutrophils, inflammatory and plasmacytoid dendritic cells (DCs), as well as granulocytic myeloid derived suppressor cells [17–19], thus one of the current focuses of our laboratory is distinguishing which of the Gr1-expressing leukocyte populations is critical to long-term tumor control by PDT.
Although PDT was initially considered a local treatment, several studies have indicated that local PDT treatment can result in systemic neutrophilia [20], induction of acute phase proteins [9, 20], increased circulating levels of complement proteins [21] and systemic release of pro-inflammatory cytokines [9, 22–26], all of which indicate the presence of a systemic inflammatory response. Subsequent studies showed that local PDT treatment of murine tumors could lead to the induction of anti-tumor immunity [27, 28].
PDT enhancement of anti-tumor immunity
Induction of anti-tumor immunity by PDT of tumors was first postulated by Canti et al. who showed that cells isolated from the tumor-draining lymph nodes of PDT-treated mice were able to suppress subsequent tumor challenge when transferred to naïve hosts [29], and that PDT-treated mice that remained tumor free for 100 days were able to resist subsequent tumor challenge. Korbelik and Dougherty [13] later demonstrated the presence of immune memory following PDT. The importance of the immune response in PDT was definitively shown by a series of experiments in immunocompromised scid (severe combined immunodeficient) and nude mice [11, 16]. PDT treatment, at a dose that was curative in immunocompetent BALB/c mice, provided only short-term cures of EMT6 tumors in scid and nude mice. The ability to provide long-term cures was restored when immunodeficient animals were reconstituted with bone marrow cells from BALB/c mice.
The ability of PDT to induce systemic anti-tumor immunity led us to hypothesize that PDT treatment may have an effect on established tumors present outside the local treatment field. Previous studies that examined the effect of local PDT on distant tumors did so in the absence of an intact immune system [30], were unable to detect the control of distant disease [31], performed PDT prior to establishment of distant tumors [32], or did not discerned a mechanism by which PDT inhibits the growth of tumors outside the treatment field [33–35]. We demonstrated that control of the growth of tumors present outside the treatment field was dependent upon an intact immune system, was mediated by CD8+ cells and was accompanied by the induction of anti-tumor immunity [36]. Furthermore, while it is generally accepted that the generation and maintenance of effective immune memory CD8+ cells is dependent upon the presence of CD4+ T cells (reviewed in [37]), there are conflicting reports [38, 39], and we showed that in the absence of CD4+ T cells, local PDT treatment is able to stimulate the generation of effective and persistent CD8+ T cell-mediated immune memory responses. We further showed that PDT induction of CD8+ T cell-dependent control of distant tumor growth requires NK cells. These studies demonstrated that PDT-induced anti-tumor immunity can be independent of CD4+ T cells and suggested that PDT may be beneficial in the control of distant disease.
Establishment of CD8+ T cell memory responses in the absence of CD4+ T cells also appears to be related to the extent and nature of the inflammatory response generated at the time of antigen exposure, which can affect the activation status of antigen-presenting cells [40–42]. The activation status of antigen-presenting cells (APC), particularly that of DCs is critical to their ability to fully stimulate the generation of CD8 memory T cells [43, 44]. We have shown that PDT enhances maturation and activation of DCs and that DCs isolated from PDT-treated mice are able to stimulate T cell effector functions [45].
Although these pre-clinical studies establish that PDT of tumors leads to enhancement of anti-tumor immunity, the mechanisms are unclear. Innate immune cell presence and activation, a hallmark of PDT, is critical to the development of immunity [46], and elimination of Gr1-expressing leukocytes reduces long-term tumor growth control by PDT. The role of Gr1+CD11b+ cells in induction of anti-tumor immunity is not well defined. However, mouse tumors genetically modified to increase Gr1HiCD11b+F4/80− leukocyte infiltration into the tumor bed exhibited enhanced T cell infiltration and subsequent tumor regression [47–49]. Furthermore, depletion of Gr1HiCD11b+F4/80− leukocytes reduced the number of CD8+ T cells infiltrating the tumor and abrogated tumor regression.
We have recently shown that PDT regimens that induce a high level of Gr1HiLy6-GHiCD11b+F4/80− leukocytes, hereafter referred to neutrophils, infiltration into the treated tumor, lead to enhancement of anti-tumor immunity and strong primary and memory CD8+ T cell responses [50]. Mice defective in neutrophil homing to peripheral tissues (CXCR2−/− mice) or mice depleted of Gr1Hi leukocytes were unable to mount strong anti-tumor CD8+ T cell responses following PDT. The mechanism by which Gr1HiLy6GHiCD11b+F4/80− leukocytes regulate anti-tumor immunity following PDT is unclear. These cells have been shown to influence pathogen immunity through (1) secretion of chemotactic signals that recruit monocytes and immature DCs [51], (2) activation of DCs via cell-to-cell contact and secretion of TNF-α [52, 53] and (3) stimulation of monocyte and T cell differentiation through secretion of interferon (IFN)-γ [51, 54]. We have shown that Gr1HiLy6GHiCD11b+F4/80− leukocytes express cell surface TNF-α following PDT [50] and have proposed a working model, depicted in Fig. 1, in which Gr1HiLy6GHiCD11b+F4/80− leukocytes infiltrate the PDT-treated tumor bed within minutes following treatment, peaking between 4 and 8 h following treatment. The migration of these cells into the tumor bed following PDT is regulated, at least in part, by the chemokines KC and MIP-2 and their cognate receptor CXCR2. Following migration into the tumor, the Gr1HiLy6GHiCD11b+F4/80− leukocytes express TNF-α on their cell surface and within 4 h, this population of cells can be detected in the tumor-draining lymph node. We predict that these TNF-α-expressing leukocytes interact with DCs, licensing them and promoting CD8+ T cell activation and increased anti-tumor immunity. The current questions being addressed in the laboratory are (1) how do Gr1HiLy6-GHiCD11b+F4/80− leukocytes gain access to the tumor-draining lymph node; (2) what regulates Gr1HiLy6GHiCD11b+F4/80− leukocytes access to the tumor-draining lymph node and (3) where and by what mechanism do Gr1HiLy6GHiCD11b+F4/80− leukocytes license DCs?
Fig. 1.
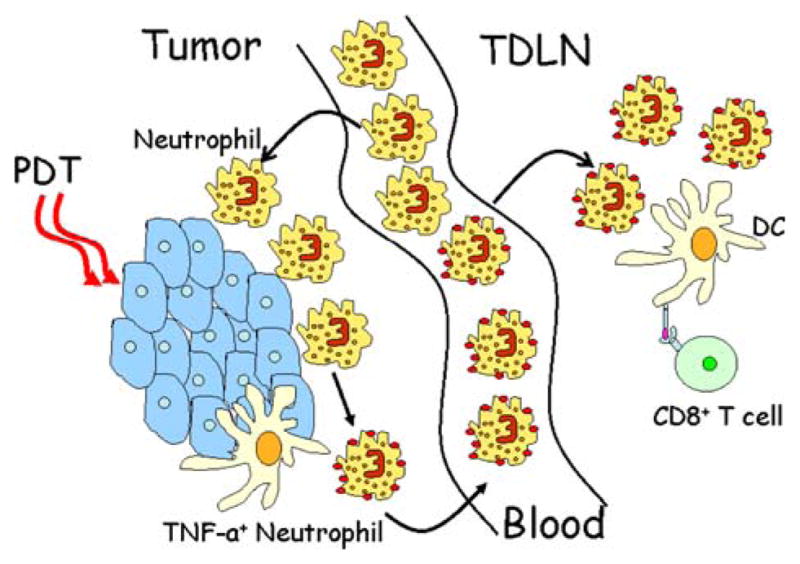
Working model of PDT-induced anti-tumor immunity: PDT treatment of tumors leads to acute local inflammation characterized by infiltration of tumor by Gr1HiLy6GHiCD11b+F4/80− leukocytes. These cells become activated and express TNF-a and migrate to the tumor-draining lymph node, where they interact with dendritic cells. Gr1Hi leukocytes interaction with dendritic cells leads to dendritic cell activation, stimulation of CD8+ T cells and enhanced anti-tumor immunity
PDT-generated anti-tumor vaccines
We have shown that effective anti-tumor vaccines can be generated by in vitro PDT treatment of tumor cells [55]. Our studies demonstrated that PDT-treated tumor cells were more effective as preventative vaccines than tumor cells treated with UV or ionizing irradiation or than cells subjected to freeze–thaw (F/T) cycles. Cell lysates generated by ex vivo PDT of tumor cells were able to stimulate the maturation and activation of DCs as measured by increased expression of major histocompatibility complex (MHC) class II and co-stimulatory (CD80, CD86 and CD40) molecules and the expression of IL-12. Increased DC maturation and activation resulted in greater T cell stimulation, and vaccination with PDT-treated tumor cells resulted in increased activation and function of tumor-specific CD8+ T cells. Since our initial findings, other groups have recapitulated our findings in other models [56] and have shown that PDT-treated tumor cells could also act as therapeutic anti-cancer vaccines [57, 58]. A study by Jalili et al. [59] showed that immature DCs engulf PDT-treated tumor cells. Thus, it appears as though PDT treatment of tumor cells enhances their ability to be recognized by DCs and that this recognition leads to DC maturation, activation and increased ability to stimulate T cells. However, the mechanisms by which PDT-treated tumor cells are able to stimulate functional maturation of DCs are unclear.
DC maturation and activation is critical to initiation of immunity, as mature activated or “licensed” DCs are uniquely able to activate naïve T cells and provide critical signals for determining the nature of the adaptive immune response [60–62]. DC maturation and activation are stimulated by recognition of “danger signals” that indicate the presence of infection or cell stress [41, 63–67]. Membrane bound and cytoplasmic danger signal sensors/receptors mediate danger signal recognition. Activation of immunity by recognition of danger signals forms the core of the “danger” model of immunity [68–70]. Danger signal sensors/receptors are traditionally grouped into three categories: (1) Toll-like receptors (TLRs), which are type I integral membrane glycoproteins; (2) NOD-like receptors (NLRs), which can be divided into two subfamilies: the NOD subfamily and the NALP (NACHT-LRR-PYD-containing protein) subfamily; and (3) the RLH [retinoic-acid-inducible gene (RIG)-like helicase] family [67, 71, 72]. TLRs are localized to either cell surface or organelle (ER and endosome) membranes, while NLRs and RLHs are cytoplasmic sensors.
Danger signal receptors play a critical role in recognition of stressed and dying cells [67, 73]. PDT treatment of tumor cells causes both cell death and cell stress [6, 74, 75], and we and others have hypothesized that the activation of DCs by PDT-treated cells is the result of recognition of danger signals release by PDT from dying cells [27, 28, 45, 76, 77].
Initial attempts to identify the active component in PDT-generated vaccines focused on the role of TLR ligands released during cell stress/death and in particular on heat shock protein 70 (HSP70). HSP70 is a well-characterized danger signal that interacts with TLRs 2 and 4 [78] and is induced by PDT [79]. The level of expression of HSP70 in PDT-treated tumor cells appears to correlate with an ability to stimulate DC maturation [80]. In addition, Korbelik et al. demonstrated that secretion of TNF and expression of complement protein genes by macrophages incubated with PDT-treated cells were blocked in the presence of antibodies to HSP70 [76, 81]. However, our subsequent studies indicated that elimination of HSP70 from PDT-treated cells did not impair DC activation [19]. Additionally, Jalili et al. [59] showed that PDT treatment of tumor cells could stimulate DC maturation in a HSP70-independent manner, and Korbelik et al. showed that blocking of TLRs 2 and 4 only partially inhibited TNF-α secretion by macrophages incubated with PDT-treated cells [76].
It is important to recognize that cell surface receptors other than members of the TLR/NLR/RLH families may also act as danger sensors for dying cells. Members of the scavenger receptor and C-lectin families have been associated with phagocytosis of apoptotic cells[73, 82], and Korbelik et al. [58] have suggested that opsonization of PDT-treated tumor cells by complement proteins is critical to the efficacy of PDT-generated vaccines. Thus, it appears that PDT treatment of tumor cells induces multiple danger signals capable of triggering antigen-presenting cell activation critical for activation of anti-tumor immunity.
The promise of PDT-generated vaccines has encouraged us to begin to determine whether such vaccines have clinical efficacy. Toward that end, we have recently begun initiation of a Phase I clinical trial to ascertain whether PDT-generated anti-tumor vaccines can augment immune responses to melanoma in a clinical setting. Autologous vaccines will be generated by PDT treatment of surgically removed melanomas and used to treat patients with advanced stage II in transit melanoma.
Clinical studies
Although numerous pre-clinical studies have demonstrated that PDT can result in an increase in anti-tumor immunity (reviewed in [28]), until recently few studies have examined the ability of PDT to enhance anti-tumor immunity in a clinical setting. In a study of PDT of vulval intraepithelial neoplasia (VIN), Abdel-Hady et al. [83] were the first to provide evidence that anti-tumor immunity following PDT may be important to clinical outcome. They showed that patients VIN who did not respond to ALA-PDT were more likely to have MHC-I negative tumors than patients who responded to ALA-PDT. Patient response was accompanied by increased CD8+ T cell infiltration into the treated tumors when compared to nonresponders. A study on the use of ALA-PDT to treat actinic keratoses and Bowen’s disease in immunosuppressed and immunocompetent patients showed that while both patient groups had similar initial response rates of greater than 80% and immunosuppressed patients exhibited greater persistence of disease or appearance of new lesions [84]. Finally, a recent case report shows that PDT of multifocal angiosarcoma of the head and neck resulted in increased immune cell infiltration into distant untreated tumors that was accompanied by tumor regression [77].
While these reports supported the pre-clinical studies indicating that PDT of tumors could enhance anti-tumor immunity, the lack of known tumor antigens associated with tumors commonly treated with PDT prevented more controlled mechanistic studies. To address this deficiency, we examined the effect of PDT of basal cell carcinoma (BCC) on immune reactivity to Hip1, a type 1 transmembrane protein, which binds to all members of the hedgehog signaling pathway transcription factor family. Hip1 is a new member of the hedgehog signaling pathway receptor family that is encoded by the HIP (hedgehog-interacting protein) gene [85].
Mutations in the gene for the receptor for sonic hedgehog protein (SHH), patched-1 (PTCH1) are causally involved in the development of basal cell carcinoma (BCC) [86, 87]. Patched-1 negatively regulates the function of smoothen and mutations of PTCH1, which primarily result in truncated nonfunctional proteins, lead to unregulated activation of the hedgehog signaling pathway transcription factor family known as GLI. mRNA levels for PTCH1 and the GLI family members, GLI1, GLI2 and GLI3, have all been shown to be up regulated in BCC [88, 89]. Hip1 binds to all members of the hedgehog family with an affinity similar to that of patched-1 and is thought to have a similar negative regulatory function [85]. HIP is also over expressed in BCC [88, 89], but it appears to have lower expression in normal skin than PTCH1 and does not appear to be mutated in BCC. Thus, it is possible that Hip1 can act as a tumor-associated antigen (TAA), and that its over expression can provide a target for the immune response. Vogt et al. [90] demonstrated that immunization of mice prone to BCC (Ptch1+/− mice) with Hip1 resulted in increased immune reactivity to Hip1 and reduced incidence of BCC, further indicating the potential of this protein as a TAA. Kabingu et al. [91] we showed that treatment of BCC with either Porfimer sodium or ALA-PDT resulted in an enhancement of the ability of immune cells to recognize and respond to the tumor-associated antigen, Hip1. This increase in reactivity was significantly greater than reactivity observed in patients whose lesions were surgically removed.
Previous clinical studies showed increased CD8+ T cell infiltration into the treated tumor following PDT [77, 83]. Kabingu et al. [91] demonstrated that PDT enhanced recognition of MHC-I: antigen complexes by immune cells, suggesting that PDT of BCC enhances activation of tumor-specific CD8+ T cells, which require MHC-I: antigen recognition for activation. The effects of PDT on immune recognition appeared to be greater in patients with superficial lesions when compared to those with nodular lesions. These patients also exhibited better clinical responses 6 months following treatment, suggesting that immune reactivity may contribute to outcome.
PDT enhancement of anti-tumor immunity appears to be inversely correlated with both fluence and fluence rate in preclinical and clinical studies [50, 92]. Treatment with higher light doses and fluence rates leads to vascular shut down and limited immune cell infiltrate into the treated area [14, 50] and subsequent lower anti-tumor immunity [50]. In clinical settings, treatments with lower light dose and fluence rate resulted in remission of neighboring and distant untreated lesions [92] and greater enhancement of immune recognition of tumor-associated antigens (TAA) [91],
Certain PDT regimens have been shown to systemically suppress immune reactivity in pre-clinical models (reviewed in [93, 94]). The switch from immune enhancing to immune-suppressing effects of PDT appears to be linked to the area of skin treated; whole body light irradiation in combination with photosensitizer resulted in immune suppression and reduction in autoimmunity in several model systems [93, 94]. Enhancement of anti-tumor immunity following PDT of BCC was inversely related to the area treated [91], which may indicate that treatment of large-surface areas leads to immune suppression rather than immune stimulation.
The ability of PDT to enhance anti-tumor immunity suggests that this treatment modality may be used in an adjuvant setting with treatments that have either no or a negative effect on the patients’ immune response, such as surgery. Friedberg et al. [95] reports increased survival for patients with non-small-cell lung cancer with pleural spread who receive surgery and PDT when compared to patients receiving surgery alone.
Although each of the studies discussed above support a role for PDT enhancement of anti-tumor immunity in a clinical setting, they are largely based upon relatively small patient samples, and confirmation of the findings in larger trials is needed. This is particularly important in determining whether enhanced immune responses contribute to improved local or systemic tumor control, which will require well-designed clinical trials with large enough patient populations to provide statistical relevance and correlative immunologic endpoints.
Summary
PDT has proven to be an effective therapy for a growing number of malignancies, and its clinical use has increased dramatically, since PDT was first employed. Pre-clinical and clinical studies have suggested that in addition to its direct effects on tumor cell, PDT augments anti-tumor immunity and enhances tumor cell immunogenicity. However, the optimization of PDT regimens that lead to enhanced anti-tumor immunity have been limited due to a lack of mechanistic understanding and the complexity of the effects of PDT on both tumor and host cells. The long-range goal of this laboratory is to define the mechanisms by which PDT is able to enhance tumor cell immunogenicity and anti-tumor immunity and to use this information to develop protocols that optimally control primary tumor growth and augment systemic anti-tumor immunity for control of distant disease. We further hope to develop protocols in which PDT or PDT-generated vaccines are used as adjuvants in a clinical setting to enhance anti-tumor immunity. We predict that understanding of the mechanisms used by PDT to augment anti-tumor immunity will permit optimization and exploitation of this aspect of PDT in a clinical setting.
Acknowledgments
This work was supported by NIH Grants CA55791 and CA98156 and in part by the Roswell Park Cancer Center Support Grant CA16056.
References
- 1.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg. 2002;20:3–7. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphy Phthalocyan. 2001;5:105–29. [Google Scholar]
- 4.McBride G. Studies expand potential uses of photodynamic therapy. J Natl Cancer Inst (Bethesda) 2002;94:1740–2. doi: 10.1093/jnci/94.23.1740. [DOI] [PubMed] [Google Scholar]
- 5.Patrice T, Olivier D, Bourre L. PDT in clinics: indications, results, and markets. J Environ Pathol Toxicol Oncol. 2006;25:467–85. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.300. [DOI] [PubMed] [Google Scholar]
- 6.Henderson BW, Gollnick SO. Mechanistic principles of photodynamic therapy. In: Vo-Dinh T, editor. Biomedical photonics handbook. Boca Raton: CRC Press; 2003. [Google Scholar]
- 7.Evans S, Matthews W, Perry R, Fraker D, Norton J, Pass HI. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J Natl Cancer Inst. 1990;82:34–9. doi: 10.1093/jnci/82.1.34. [DOI] [PubMed] [Google Scholar]
- 8.Kick G, Messer G, Goetz A, Plewig G, Kind P. Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-kB DNA binding. Cancer Res. 1995;55:2373–9. [PubMed] [Google Scholar]
- 9.Gollnick SO, Evans SE, Baumann H, et al. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br J Cancer. 2003;88:1772–9. doi: 10.1038/sj.bjc.6600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gollnick SO, Liu X, Owczarczak B, Musser DA, Henderson BW. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 1997;57:3904–9. [PubMed] [Google Scholar]
- 11.Korbelik M, Krosl G, Krosl J, Dougherty GJ. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996;56:5647–52. [PubMed] [Google Scholar]
- 12.Korbelik M. Induction of tumor immunity by photodynamic therapy. J Clin Laser Med & Surg. 1996;14:329–34. doi: 10.1089/clm.1996.14.329. [DOI] [PubMed] [Google Scholar]
- 13.Korbelik M, Dougherty GJ. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999;59:1941–6. [PubMed] [Google Scholar]
- 14.Henderson BW, Gollnick SO, Snyder JW, et al. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64:2120–6. doi: 10.1158/0008-5472.can-03-3513. [DOI] [PubMed] [Google Scholar]
- 15.de Vree WJ, Essers MC, De Bruijn HS, Star WM, Koster JF, Sluiter W. Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer Res. 1996;56:2908–11. [PubMed] [Google Scholar]
- 16.Korbelik M, Cecic I. Contribution of myeloid and lymphoid host cells to the curative outcome of mouse sarcoma treatment by photodynamic therapy. Cancer Lett. 1999;137:91–8. doi: 10.1016/s0304-3835(98)00349-8. [DOI] [PubMed] [Google Scholar]
- 17.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–408. [PubMed] [Google Scholar]
- 18.Nakano H, Yanagita M, Gunn MD. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–8. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano H, Lin KL, Yanagita M, et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cecic I, Parkins CS, Korbelik M. Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochem Photobiol. 2001;74:712–20. doi: 10.1562/0031-8655(2001)074<0712:iosnri>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Cecic I, Stott B, Korbelik M. Acute phase response-associated systemic neutrophil mobilization in mice bearing tumors treated by photodynamic therapy. Int Immunopharmacol. 2006;6:1259–66. doi: 10.1016/j.intimp.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Cecic I, Korbelik M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett. 2002;183:43–51. doi: 10.1016/s0304-3835(02)00092-7. [DOI] [PubMed] [Google Scholar]
- 23.de Vree WJ, Essers MC, Koster JF, Sluiter W. Role of interleukin 1 and granulocyte colony-stimulating factor in photofrin-based photodynamic therapy of rat rhabdomyosarcoma tumors. Cancer Res. 1997;57:2555–8. [PubMed] [Google Scholar]
- 24.Ziolkowski P, Symonowicz K, Milach J, Szkudlarek T. In vivo tumor necrosis factor-alpha induction following chlorin e6-photodynamic therapy in Buffalo rats. Neoplasma. 1996;44:192–6. [PubMed] [Google Scholar]
- 25.Nseyo UO, Whalen RK, Duncan MR, Berman B, Lundahl SL. Urinary cytokines following photodynamic therapy for bladder cancer. Urology. 1990;36:167–71. doi: 10.1016/0090-4295(90)80220-h. [DOI] [PubMed] [Google Scholar]
- 26.Yom SS, Busch TM, Friedberg JS, et al. Elevated serum cytokine levels in mesothelioma patients who have undergone pleurectomy or extrapleural pneumonectomy and adjuvant intraoperative photodynamic therapy. Photochem Photobiol. 2003;78:75–81. doi: 10.1562/0031-8655(2003)078<0075:esclim>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Canti G, De Simone A, Korbelik M. Photodynamic therapy and the immune system in experimental oncology. Photochem Photobiol Sci. 2002;1:79–80. doi: 10.1039/b109007k. [DOI] [PubMed] [Google Scholar]
- 28.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–45. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canti G, Lattuada D, Nicolin A, Taroni P, Valentini G, Cubeddu R. Immunopharmacology studies on photosensitizers used in photodynamic therapy (PDT) Proc SPIE Photodyn Ther Cancer. 1994;2078:268–75. [Google Scholar]
- 30.Schreiber S, Gross S, Brandis A, et al. Local photodynamic therapy (PDT) of rat C6 glioma xenografts with Pd-bacteriopheophorbide leads to decreased metastases and increase of animal cure compared with surgery. Int J Cancer. 2002;99:279–85. doi: 10.1002/ijc.10299. [DOI] [PubMed] [Google Scholar]
- 31.van Duijnhoven FH, Aalbers RI, Rovers JP, Terpstra OT, Kuppen PJ. Immunological aspects of photodynamic therapy of liver tumors in a rat model for colorectal cancer. Photochem Photobiol. 2003;78:235–40. doi: 10.1562/0031-8655(2003)078<0235:iaopto>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Momma T, Hamblin MR, Wu HC, Hasan T. Photodynamic therapy of orthotopic prostate cancer with benzoporphyrin derivative: local control and distant metastasis. Cancer Res. 1998;58:5425–31. [PubMed] [Google Scholar]
- 33.Gomer CJ, Ferrairo A, Murphree AL. The effect of localized porphyrin photodynamic therapy on the induction of tumour metastasis. Br J Cancer. 1987;56:27–32. doi: 10.1038/bjc.1987.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blank M, Lavie G, Mandel M, Keisari Y. Effects of photodynamic therapy with hypericin in mice bearing highly invasive solid tumors. Oncol Res. 2001;12:409–18. doi: 10.3727/096504001108747864. [DOI] [PubMed] [Google Scholar]
- 35.Castano AP, Gad R, Zahra T, Hamblin MR. Specific anti-tumor immune response with photodynamic therapy mediated by benzoporphyrin derivative and chlorin(e6). In: Jacques SL, Duncan DD, Kirkpatrick SJ, Kriete A, editors. The International Society for Optical Engineering. Proceedings of SPIE; 2003. [Google Scholar]
- 36.Kabingu E, Vaughan L, Owczarczak B, Ramsey KD, Gollnick SO. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br J Cancer. 2007;96:1839–48. doi: 10.1038/sj.bjc.6603792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–40. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 38.Marzo AL, Vezys V, Klonowski KD, et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol. 2004;173:969–75. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Santosuosso M, Ngai P, Zganiacz A, Xing Z. Activation of CD8 T cells by mycobacterial vaccination protects against pulmonary tuberculosis in the absence of CD4 T cells. J Immunol. 2004;173:4590–7. doi: 10.4049/jimmunol.173.7.4590. [DOI] [PubMed] [Google Scholar]
- 40.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 41.Kaisho T, Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr Mol Med. 2003;3:373–85. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- 42.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol. 2005;174:710–7. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 43.Ridge JP, DiRosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 44.van Mierlo GJ, Boonman ZF, Dumortier HM, et al. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. J Immunol. 2004;173:6753–9. doi: 10.4049/jimmunol.173.11.6753. [DOI] [PubMed] [Google Scholar]
- 45.Gollnick SO, Owczarczak B, Maier P. Photodynamic therapy and anti-tumor immunity. Lasers Surg Med. 2006;38:509–15. doi: 10.1002/lsm.20362. [DOI] [PubMed] [Google Scholar]
- 46.Lee HK, Iwasaki A. Innate control of adaptive immunity: dendritic cells and beyond. Semin Immunol. 2007;19:48–55. doi: 10.1016/j.smim.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Cavallo F, Giovarelli M, Gulino A, et al. Role of neutrophils and CD4+ T lymphocytes in the primary and memory response to nonimmunogenic murine mammary adenocarcinoma made immunogenic by IL-2 gene. J Immunol. 1992;149:3627–35. [PubMed] [Google Scholar]
- 48.Graf MR, Prins RM, Merchant RE. IL-6 secretion by a rat T9 glioma clone induces a neutrophil-dependent antitumor response with resultant cellular, antiglioma immunity. J Immunol. 2001;166:121–9. doi: 10.4049/jimmunol.166.1.121. [DOI] [PubMed] [Google Scholar]
- 49.Stoppacciaro A, Melani C, Parenza M, et al. Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-T cell cooperation and T cell-produced interferon gamma. J Exp Med. 1993;178:151–61. doi: 10.1084/jem.178.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic therapy (PDT) enhancement of anti-tumor immunity is regulated by neutrophils. Cancer Res. 2007;67:10501–10. doi: 10.1158/0008-5472.CAN-07-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 52.van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. Close encounters of neutrophils and DCs. Trends Immunol. 2005;26:626–31. doi: 10.1016/j.it.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Bennouna S, Denkers EY. Microbial antigen triggers rapid mobilization of TNF-alpha to the surface of mouse neutrophils transforming them into inducers of high-level dendritic cell TNF-alpha production. J Immunol. 2005;174:4845–51. doi: 10.4049/jimmunol.174.8.4845. [DOI] [PubMed] [Google Scholar]
- 54.Ethuin F, Gerard B, Benna JE, et al. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–71. doi: 10.1038/labinvest.3700148. [DOI] [PubMed] [Google Scholar]
- 55.Gollnick SO, Vaughan LA, Henderson BW. Generation of effective anti-tumor vaccines using photodynamic therapy. Cancer Res. 2002;62:1604–8. [PubMed] [Google Scholar]
- 56.Zhang H, Ma W, Li Y. Generation of effective vaccines against liver cancer by using photodynamic therapy. Lasers Med Sci. 2009;24:549–52. doi: 10.1007/s10103-008-0609-4. [DOI] [PubMed] [Google Scholar]
- 57.Korbelik M, Cecic I. Mechanism of tumor destruction by photodynamic therapy. In: Nalwa HS, editor. Handbook of photochemistry and photobiology. Stevenson Ranch: American Scientific Publishers; 2003. [Google Scholar]
- 58.Korbelik M, Sun J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol Immunother. 2006;55:900–9. doi: 10.1007/s00262-005-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jalili A, Makowski M, Switaj T, et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin Cancer Res. 2004;10:4498–508. doi: 10.1158/1078-0432.CCR-04-0367. [DOI] [PubMed] [Google Scholar]
- 60.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 61.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 62.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 63.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 64.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 65.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–54. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–7. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 67.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 68.Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Fuchs EJ, Matzinger P. Is cancer dangerous to the immune system? Semin Immunol. 1996;8:271–80. doi: 10.1006/smim.1996.0035. [DOI] [PubMed] [Google Scholar]
- 70.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 71.Bowie A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol. 2000;67:508–14. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- 72.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 73.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–35. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 74.Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oleinick NL, Evans HH. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150(5 Suppl):S146–56. [PubMed] [Google Scholar]
- 76.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–26. [PubMed] [Google Scholar]
- 77.Thong PS, Ong KW, Goh NS, et al. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol. 2007;8:950–2. doi: 10.1016/S1470-2045(07)70318-2. [DOI] [PubMed] [Google Scholar]
- 78.Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol. 2002;270:169–84. doi: 10.1007/978-3-642-59430-4_11. [DOI] [PubMed] [Google Scholar]
- 79.Gomer CJ, Ryter SW, Ferrairo A, Ryffel N, Woodard A, Fisher AMR. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56:2355–60. [PubMed] [Google Scholar]
- 80.Gollnick SO, Kabingu E, Kousis PC, Henderson BW. Stimulation of the host immune response by photodynamic therapy (PDT). In: Jacques SL, Duncan DD, Kirkpatrick SJ, Kriete A, editors. The International Society for Optical Engineering. Proceedings of SPIE; 2004. [Google Scholar]
- 81.Stott B, Korbelik M. Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol Immunother. 2006;56:649–58. doi: 10.1007/s00262-006-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 83.Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001;61:192–6. [PubMed] [Google Scholar]
- 84.Dragieva G, Hafner J, Dummer R, et al. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen’s disease in transplant recipients. Transplantation. 2004;77:115–21. doi: 10.1097/01.TP.0000107284.04969.5C. [DOI] [PubMed] [Google Scholar]
- 85.Chuang P-T, McMahon AP. Vertebrate hedgehog signalling modulated by induction of a hedgehog-binding protein. Nature. 1999;397:617–21. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 86.Ingham PW. The patched gene in development and cancer. Curr Opin Genet Dev. 1998;8:88–94. doi: 10.1016/s0959-437x(98)80067-1. [DOI] [PubMed] [Google Scholar]
- 87.Wicking C, Smyth I, Bale A. The hedgehog signalling pathway in tumorigenesis and development. Oncogene. 1999;18:7844–51. doi: 10.1038/sj.onc.1203282. [DOI] [PubMed] [Google Scholar]
- 88.Bonifas JM, Pennypacker S, Chuang P-T, et al. Activation of expression of hedgehog target genes in basal cell carcinomas. J Invest Dermatol. 2001;116:739–42. doi: 10.1046/j.1523-1747.2001.01315.x. [DOI] [PubMed] [Google Scholar]
- 89.Tojo M, Kiyosawa H, Iwatsuki K, Kaneko F. Expression of a sonic hedgehog signal transducer, hedgehog-interacting protein, by human basal cell carcinoma. Br J Derm. 2002;146:69–73. doi: 10.1046/j.1365-2133.2002.04583.x. [DOI] [PubMed] [Google Scholar]
- 90.Vogt A, Chuang PT, Hebert J, et al. Immunoprevention of basal cell carcinomas with recombinant hedgehog-interacting protein. J Exp Med. 2004;199:753–61. doi: 10.1084/jem.20031190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kabingu E, Oseroff AR, Wilding GE, Gollnick SO. Enhanced systemic immune reactivity to a basal cell carcinoma associated antigen following photodynamic therapy. Clin Cancer Res. 2009;15:4460–6. doi: 10.1158/1078-0432.CCR-09-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thong PS, Olivo M, Kho KW, et al. Immune response against angiosarcoma following lower fluence rate clinical photodynamic therapy. J Environ Pathol Toxicol Oncol. 2008;27:35–42. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i1.40. [DOI] [PubMed] [Google Scholar]
- 93.Granville DJ, Levy JG, Hunt DWC. Photodynamic treatment with benzoporphyrin derivative monoacid ring A produces protein tyrosine phosphorylation events and DNA fragmentation in murine P815 cells. Photochem Photobiol. 1998;67:358–62. [PubMed] [Google Scholar]
- 94.Hunt DW, Levy JG. Immunomodulatory aspects of photodynamic therapy. Expert Opin Investig Drugs. 1998;7:57–64. doi: 10.1517/13543784.7.1.57. [DOI] [PubMed] [Google Scholar]
- 95.Friedberg JS, Mick R, Stevenson JP, et al. Phase II trial of pleural photodynamic therapy and surgery for patients with non-small-cell lung cancer with pleural spread. J Clin Oncol. 2004;22:2192–201. doi: 10.1200/JCO.2004.07.097. [DOI] [PubMed] [Google Scholar]


