Abstract
The study of Drosophila, and other genetically tractable insects, has expanded our understanding of innate immunity and more recently antiviral innate mechanisms. The Drosophila antiviral program includes inflammatory signaling cascades as well as antiviral RNA silencing and autophagy. This review will highlight the recent discoveries in antiviral immunity in insects and will reveal some of the lessons learned.
Introduction
Innate immunity is the first and most ancient line of defense against pathogens. Many insights into innate immunity have been obtained from studies in Drosophila [1-3]. Drosophila have conserved developmental and cell biological processes making then a fruitful model to study mammalian development and disease [4]. Moreover, many of the classic signal transduction systems, including those involved in immunity, were identified first in Drosophila using forward genetic screens. As illustrated by studies of Toll and autophagy, Drosophila is a powerful model system for studying mammalian innate immunity [2,5-7]. Moreover, insects are infected by more than 20 groups of viruses comprising 12 viral families that are quite diverse in their replication strategies, tropism and pathogenesis. Many of these viruses also infect humans, or serve as models for related human pathogens [8]. The study of these viruses in a genetically powerful system such as Drosophila can give us both virus-specific as well as more general insights into pathogenesis and innate immunity. Insects clearly respond to viral infections by initiating an antiviral program that includes the small RNA silencing pathways, and a number of signaling cascades that converge on effector mechanisms including autophagy. Because several of these pathways are conserved, many of these new findings will likely play fundamental roles in innate immunity in vertebrates.
RNA interference is a potent antiviral pathway in Drosophila
RNA interference (RNAi) is one of several small RNA-dependent silencing pathways that control gene expression in a sequence-specific manner (reviewed in [9]). RNA silencing plays roles both in the control of endogenous gene expression, as well as antiviral roles in plants and invertebrates. In contrast, while mammals have maintained RNA silencing pathways to control endogenous gene expression, it is thought that they have lost antiviral silencing activities and instead, have evolved the pan-antiviral interferon response pathway. Interestingly, both strategies are triggered by viral dsRNA. RNA silencing is initiated by the RNaseIII-like enzyme Dicer, which generates a 21-23nt RNA duplex from a larger dsRNA precursor molecule [10,11]. The small interfering RNA duplex (siRNA) is incorporated into the effector complex, the RNA-induced silencing complex (RISC), where one strand is preferentially retained and guides RISC to cleave the complimentary sequence on the mRNA target, in this case a viral RNA species [12-15]. Mutants in the core siRNA machinery (Dcr-2, r2d2, AGO2) display increased sensitivity to infection by several RNA viruses, including Flock House virus (FHV), Drosophila C virus (DCV), Cricket Paralysis virus (CrPV), Sindbis virus (SINV), Vesicular Stomatitis virus (VSV), Drosophila X virus (DXV), West Nile virus (WNV), and Rift Valley Fever virus (RVFV) [16-22](Sabin and Cherry, unpublished results). In addition, studies of mosquito vectors have demonstrated that the RNAi pathway restricts arboviruses including O'nyong-nyong virus, SINV, and Dengue virus [23-26].
Mechanism of vsiRNA biogenesis
The activity of Dcr-2 on viral dsRNAs leads to the production of virus-derived siRNAs (vsiRNAs) [17,25,27] (Figure 1). However, the identity of the viral RNA precursor that is recognized and processed by Dcr-2 has only recently been queried. To date, two groups have deep sequenced the small RNAs present in FHV-infected tissue culture cells [27,28]. Both studies found that FHV vsiRNAs derived from the (+) and (-) RNA strands were present in approximately equal ratios. Furthermore, the majority of RNA1-derived vsiRNAs were clustered in ∼500 nucleotide region at the extreme 5′ terminus. This suggests that the mechanism of vsiRNA biogenesis is through Dcr-2 cleavage of dsRNA formed during replication initiation. Moreover, the FHV-encoded RNAi suppressor B2 was found to interact with the FHV replication complex and may therefore attenuate the ability of Dcr-2 to access to the dsRNA replication intermediates [27]. Accordingly, the authors found that the distribution of FHV vsiRNAs changes in the presence of B2; the vsiRNAs are no longer skewed towards the 5′ terminal region [27]. These data suggest that an important factor in Dcr-2 processing of viral triggers is the accessibility of the precursor RNA molecule. It will therefore be critical to determine whether this proposed mechanism can be generalized to other viruses. For instance, SINV-derived vsiRNAs sequenced from infected mosquitoes are not skewed toward the 5′ terminus, but instead are equally distributed across the genome [25]. However, it is unclear whether the disparities between FHV and SINV vsiRNA distributions are due to differences between the viruses or differences between host species. Lastly, whether other structures such as hairpins formed within a single stranded viral RNA molecule are targeted by the RNAi pathway in Drosophila, as they are in plants, has yet to be determined [29].
Figure 1. RNA interference restricts virus infection on a cellular and organismal level.
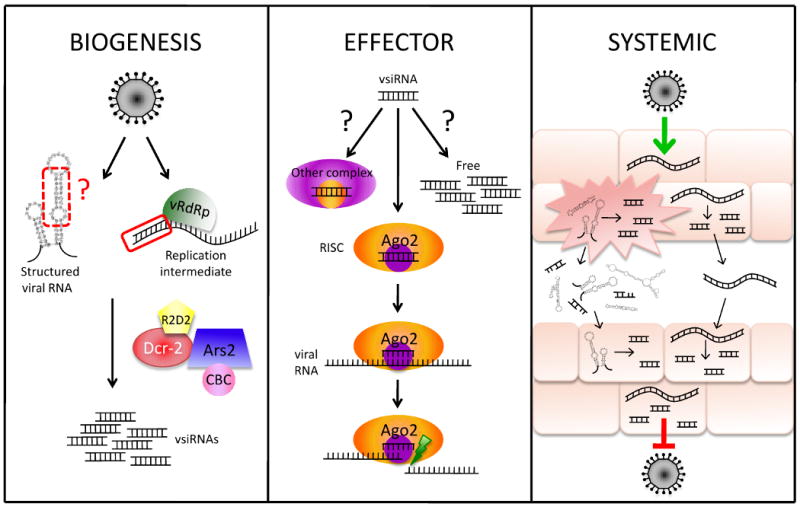
The biogenesis of virus-derived siRNAs (vsiRNAs) is mediated by Dcr-2, which recognizes dsRNA produced by the viral RNA-dependent RNA polymerase (vRdRp) or structured regions of single stranded viral RNA (left panel). Dcr-2 complexes with R2D2 and Ars2, which is required for efficient dsRNA processing and interacts with the nuclear cap binding complex (CBC) (left panel). Following vsiRNA biogenesis, Ago2 mediates the effector step of antiviral silencing through RISC-mediated cleavage of viral RNA (middle panel). The vsiRNAs not bound to Ago2 remain stabilized, either in another complex or free in the cytoplasm. A potential model for systemic antiviral RNAi is depicted at right. Viral RNA produced during infection is released extracellularly, either by controlled export or by lysis of the infected cell. The RNA is then internalized and processed by uninfected cells, protecting them from subsequent infection.
Effector mechanism of antiviral silencing
Robust antiviral RNAi requires not only vsiRNA biogenesis by Dcr-2, but also the core effector of siRNA RISC, Ago2 [19] (Figure 1). Consistent with a direct effector role of Ago2 in antiviral silencing, vsiRNAs are specifically bound to Ago2 in infected cells [27,28]. However, a large proportion of total vsiRNAs in infected cells are not bound to Ago2 [27,28] but are stable, suggesting that they are either unbound or in another complex. This finding then raises questions regarding which vsiRNAs reflect the active pool, and whether viral sequences are targeted by Ago2-RISC. To address this question, reporters bearing FHV sequences were generated by Flynt and colleagues, and the silencing of the reporters was assessed in chronically FHV-infected S2 cells [28]. Surprisingly, the FHV reporters were not silenced, suggesting that there must be complex regulation or sorting of the active vsiRNAs. However, these experiments were performed in the context of a persistent infection, which may not faithfully recapitulate an acute FHV infection. Moreover, it is possible that the reason chronic FHV infections can persist is due to the fact that RISC-mediated virus silencing is impaired. Finally, the artificial reporter constructs may not present the target RNA in the same context as it would be displayed during an infection. Additional studies of the effector step of antiviral RNAi are necessary to resolve these issues.
Finally, insect viruses and the RNAi pathway appear to be coevolving. The core antiviral RNAi genes Dcr-2, r2d2, and AGO2 are amongst the most rapidly evolving genes in the Drosophila genome [30]. Furthermore, several insect viruses encode suppressors of RNAi, suggesting that the pathway is exerting significant pressure on the viruses [17,19]. Since these suppressors antagonize the cellular RNAi machinery, it is possible that the normal functions of the cellular pathways are also impaired during infection. Recently, this question was addressed using a panel of transgenic fly lines carrying viral suppressors from plant and insect viruses [31,32]. To varying degrees, the suppressors enhanced viral replication and impaired canonical siRNA silencing. Importantly, transposon silencing by endogenous siRNAs was also defective in the presence of some suppressors [31]. Therefore, it is possible that dysregulation of cellular gene expression may contribute to viral pathogenesis.
Ars2 is a novel component of antiviral RNAi
Although the existing evidence clearly implicates Dcr-2, r2d2 and AGO2 as the core antiviral RNAi machinery in Drosophila, one important question that remains is whether additional cellular factors are involved in the antiviral RNAi response. We recently identified Ars2 and its binding partners Cbp20 and Cbp80 as novel components of the Drosophila antiviral arsenal [21] (Figure 1). Ars2 is a single-copy gene present in organisms ranging from plants to mammals. The plant homolog, SERRATE, has been implicated in RNA silencing in Arabidopsis [33,34]. Depletion of Ars2 from cells and adult flies renders them highly susceptible to infection with several RNA viruses, including VSV, DCV, SINV and FHV. Ars2 was also shown to play a role in several modes of RNA silencing in Drosophila, including the miRNA, esiRNA and siRNA pathways. Mechanistic studies placed Ars2 at an upstream step in these silencing pathways; in particular, Ars2 was required for efficient Dcr-2-mediated processing of long dsRNAs. The precise role of Ars2 in virus restriction has not yet been fully elucidated, although the protein likely functions in vsiRNA biogenesis as it both functionally and biochemically interacts with Dcr-2. Ars2 could, therefore, be functioning as a substrate recognition factor for Dcr-2; although the enzyme can act alone in vitro, it remains an open question whether the Dcr-2 requires specificity factors to direct it to particular RNAs in vivo.
Systemic antiviral silencing
In plants, the antiviral RNAi response has both a cell-intrinsic component and systemic component; vsiRNAs are amplified by the action of an RNA-dependent RNA polymerase, spreading the signal throughout the plant. A recent study supports a role for the systemic spread of antiviral silencing in Drosophila [35] (Figure 1). Infection of flies with virus-specific dsRNA induced immunity in wildtype flies, but was unable to confer immunity to mutants in the previously described dsRNA uptake pathway [36,37]. Plant systemic RNAi-based immunity depends on the spread of siRNAs; however the Drosophila dsRNA uptake pathway is length-dependent and does not internalize 21bp siRNAs [36]. Therefore, the as yet unidentified spreading intermediate must be a longer viral dsRNA or hairpins that have been released extracellularly.
Vago: Intersection of antiviral RNAi and signaling
While Dcr-2 clearly plays an antiviral role through the RNA silencing pathway, recent evidence suggests that Dcr-2 may also trigger a downstream antiviral signaling cascade upon binding and recognition of viral dsRNA [38]. Dcr-2 belongs to the DExD/H-box helicase family, as do the mammalian RIG-I-like receptors, which sense and respond to cytoplasmic viral RNA. Virus infection in Drosophila initiates a specific transcriptional response, including the induction of Vago, a recently-identified antiviral molecule that is required to restrict viral replication in flies [38]. Vago expression is dependent upon Dcr-2, suggesting that Dcr-2-driven signaling contributes to the induction of a specific set of antiviral effectors during infection. Whether other signaling pathways are activated by or modulate the antiviral siRNA pathway has yet to be elucidated.
The antiviral Jak-STAT pathway
Interestingly, Vago was one of a number of genes originally identified as induced by RNA virus infection of adult flies. Amongst this gene-set were three genes (vir-1, CG12780 and CG9080) revealed to be dependent upon the Jak-STAT signaling pathway [39]. In mammals the Jak-STAT pathway is a major component of the innate immune response to many viruses, including Dengue, most notably via the interferon response [40-42]. As in mammals, it was found that the Drosophila Jak-STAT pathway, likely through this induced transcriptional program, restricts RNA virus infection in Drosophila [39] (Figure 2). This activity is conserved across insects where a recent study in mosquitoes demonstrates that the Jak-STAT pathway restricts infection of Dengue, a medically important arbovirus [43]. Interestingly, this study identified a number of genes upregulated in response to Dengue infection and also dependent on the Jak-STAT pathway. Two of these genes, DVFR1 and DVFR2, have demonstrated antiviral properties. However, whether these Jak-STAT responsive genes are sufficient to explain the antiviral activity, or if other effectors are involved, remains unclear. Furthermore, in Drosophila it has been shown that the pathway is induced non-autonomously leading to the question of how viruses are sensed by flies [39].
Figure 2. Antiviral Innate Immune Signaling in Insects.
Four pathways including three classical immune signaling pathways (Toll, Imd, Jak-STAT) are responsive to infection by different viruses (blue text) based on studies in both Drosophila and mosquitoes (italics). The Toll pathway, responsive to fungi and gram-positive bacteria, has been found to be antiviral in response to infection by Drosophila X and Dengue viruses. The Imd pathway, responsive to gram-negative bacteria, is antiviral in response to infection with Sindbis and Cricket Paralysis viruses. The Jak-STAT pathway restricts infection by Drosophila C and Dengue viruses. Some downstream effectors were induced by infection (red text, italicized for studies in mosquitoes). In addition, Drosophila recognize VSV via the glycoprotein VSV-G (the PAMP), through an unidentified PRR which leads to attenuation of nutrient signaling, likely at the level of PI3K. Repression of this pathway results in the induction of antiviral autophagy which attenuates VSV replication.
NFκB pathways also restrict viral infection
In mammals, viruses are recognized by pattern recognition receptors (PRRs), such as Toll-like receptors, which activate antiviral signaling programs. In Drosophila there are two well-characterized PRR pathways which recognize pathogen-associated molecular patterns (PAMPs) on invading bacteria or fungi: the Toll and Imd pathways. The Drosophila Toll pathway shares similarities with the Interleukin-1 and Toll-like receptor signaling systems found in vertebrates, while the Imd pathway shares homology with the tumor necrosis factor pathway; however each pathway converges on different NFκB transcription factors which induce an antimicrobial program (Figure 2). Studies have indicated that viral infection by either DXV or Dengue induces genes involved in both the Jak-STAT and Toll pathways [26,44,45]. The Toll pathway is both activated by and restricts Dengue infection of mosquitoes [45]. Additionally, a study using DXV infection of Drosophila found that either induction or attenuation of the Toll pathway led to increased viral replication [44]. In contrast, DCV infection of flies is insensitive to Toll pathway inhibition [46]. Altogether these data suggest that the Toll pathway plays a role in the antiviral response to some viruses; however, there seem to be unique antiviral responses to different viral pathogens.
In support of this, two studies found that the Imd pathway, rather than the Toll pathway, was important to control Sindbis and Cricket Paralysis virus infection (Figure 2)[47,48]. The Imd pathway and restricted infection of both viruses, although only Sindbis infection led to activation of the Imd pathway as measured by canonical antimicrobial peptide gene expression. Additionally, a tissue culture study of a Sindbis-related virus, Semliki Forest virus (SFV), found that infection of mosquito cells did not induce the Toll, Imd, or Jak-STAT pathways [49]. However, induction of the Imd or Jak-STAT pathways prior to infection attenuated viral replication, supporting an antiviral role for these pathways. This in vitro data also suggests that viral activation of the innate immune signaling pathways may not be direct, but instead may require cell extrinsic PRRs to initiate signaling. This is consistent with the observation that the Jak-STAT pathway is induced non-autonomously in adult flies [39] and that cell culture-based RNAi screens for factors that impact Dengue or Influenza replication did not reveal any role for the Toll, Imd or Jak-STAT pathways [50,51]
Antiviral autophagy and nutrient signaling
While some of the antiviral signaling pathways have been identified, the effector mechanisms that directly restrict viral infection in Drosophila have been elusive. In a search for cell-autonomous pathways that directly inhibit viral infection, we identified an antiviral role for autophagy against Vesicular Stomatitis Virus (VSV) in Drosophila [5]. Autophagy is a cell-intrinsic mechanism that degrades cytoplasmic contents. Upon infection of either cells or flies we found that autophagy is activated and controls viral replication. This program is activated via the attenuation of the PI3K/Akt pathway that normally controls autophagy in response to nutrient signaling. Importantly, the PAMP was identified as the viral glycoprotein, which suggests that the PRR is likely an extracellular receptor such as a TLR. How viral recognition is coupled to the repression of Akt signaling has yet to be identified.
The inhibition of nutrient signaling may play two roles during infection. First, our data suggest that this inhibition leads to the activation of autophagy. Second, we also recently showed that attenuation of Akt signaling allows for the reallocation of resources from growth to immune defense against bacterial and fungal pathogens [52]. Activation of the Toll pathway via infection with these microbes led to the repression of Akt signaling and the inhibition of fat storage. Whether reallocation from nutrient storage to innate immune defense is also essential to combat viral infection has yet to be elucidated.
Conclusions
These recent studies highlight the role of RNA silencing and innate immune signaling in antiviral defense in insects. While there is a clear requirement for innate immune signaling, little is known about the virus specific mediators that restrict viral replication. These effector mechanisms are only beginning to be unraveled. Thus far, only a small number of unique virally induced molecules have been identified [39,45,53]. Of these Vago, DVRF1 and DVRF2 have been shown to have antiviral properties, with Vago mediating cross-talk with the antiviral RNAi response, while DVFR1 and DVFR2 are antiviral mediators downstream of the Jak-STAT pathway [38,43]. In contrast, no virus-specific effectors have been identified downstream of the Toll or Imd pathways. Autophagy is another effector mechanism identified that directly inhibits viral replication of VSV; whether this pathway is broadly antiviral remains unclear [5].
Another important outstanding question is how viruses are recognized by the host. The RNA silencing pathway must recognize a dsRNA, but the identity of this substrate and how it is targeted has yet to be fully elucidated. Additionally, it is not known whether cellular PRRs recognize the invading viruses directly (similar to the direct interaction of bacterial DAP-type peptidoglycans with the Imd PPR PGRP-LC), or whether recognition is indirect (Toll is activated by an endogenous cytokine, spaetzle). In the case of VSV infection, we found that the viral glycoprotein VSV-G is sufficient to activate an antiviral program, suggesting that a PRR directly recognizes this PAMP [5]. For the other viral pathogens, and other signaling pathways, the viral PAMPs have yet to be identified. Further studies are required to determine how each of these viral pathogens is sensed and how the appropriate antiviral program is initiated.
It is also apparent that the known antiviral pathways cannot account for the entire innate antiviral arsenal because loss-of-function mutations in each of these pathways have only a modest impact on survival and viral replication. Additionally, colonization of the host by other pathogens can also impact viral susceptibility and host defense, as Wolbachia infected flies are resistant to DCV infection [54,55] and mosquitoes depleted of their bacterial flora are more susceptible to Dengue [45]. Further studies are required to identify additional players and pathways in insect innate antiviral defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 2.Pinheiro VB, Ellar DJ. How to kill a mocking bug? Cell Microbiol. 2006;8:545–557. doi: 10.1111/j.1462-5822.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- 3.Schneider D. Using Drosophila as a model insect. Nat Rev Genet. 2000;1:218–226. doi: 10.1038/35042080. [DOI] [PubMed] [Google Scholar]
- 4.Veraksa A, Del Campo M, McGinnis W. Developmental patterning genes and their conserved functions: from model organisms to humans. Mol Genet Metab. 2000;69:85–100. doi: 10.1006/mgme.2000.2963. [DOI] [PubMed] [Google Scholar]
- 5.Shelly S, Lukinova N, Bambina S, Berman A, Cherry S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherry S, Kunte A, Wang H, Coyne C, Rawson RB, Perrimon N. COPI activity coupled with fatty acid biosynthesis is required for viral replication. PLoS Pathog. 2006;2:e102. doi: 10.1371/journal.ppat.0020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royet J, Reichhart JM, Hoffmann JA. Sensing and signaling during infection in Drosophila. Curr Opin Immunol. 2005;17:11–17. doi: 10.1016/j.coi.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Friesen PD, Miller LK. Insect Viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Vol. 1 Lippincott Williams & Wilkins; 2001. pp. 599–628. [Google Scholar]
- 9.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 11.Jaskiewicz L, Filipowicz W. Role of Dicer in posttranscriptional RNA silencing. Curr Top Microbiol Immunol. 2008;320:77–97. doi: 10.1007/978-3-540-75157-1_4. [DOI] [PubMed] [Google Scholar]
- 12.Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115:209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 15.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 16.Zambon RA, Vakharia VN, Wu LP. RNAi is an antiviral immune response against a dsRNA virus in Drosophila melanogaster. Cell Microbiol. 2006;8:880–889. doi: 10.1111/j.1462-5822.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Li WX, Ding SW. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- 18.Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler JL. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol. 2006;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- 19.van Rij RP, Saleh MC, Berry B, Foo C, Houk A, Antoniewski C, Andino R. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20:2985–2995. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, Ding SW. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabin LR, Zhou R, Gruber JJ, Lukinova N, Bambina S, Berman A, Lau CK, Thompson CB, Cherry S. Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell. 2009;138:340–351. doi: 10.1016/j.cell.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chotkowski HL, Ciota AT, Jia Y, Puig-Basagoiti F, Kramer LD, Shi PY, Glaser RL. West Nile virus infection of Drosophila melanogaster induces a protective RNAi response. Virology. 2008;377:197–206. doi: 10.1016/j.virol.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci U S A. 2004;101:17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell CL, Keene KM, Brackney DE, Olson KE, Blair CD, Wilusz J, Foy BD. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microbiol. 2008;8:47. doi: 10.1186/1471-2180-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myles KM, Wiley MR, Morazzani EM, Adelman ZN. Alphavirus-derived small RNAs modulate pathogenesis in disease vector mosquitoes. Proc Natl Acad Sci U S A. 2008;105:19938–19943. doi: 10.1073/pnas.0803408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Vargas I, Scott JC, Poole-Smith BK, Franz AW, Barbosa-Solomieu V, Wilusz J, Olson KE, Blair CD. Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito's RNA interference pathway. PLoS Pathog. 2009;5:e1000299. doi: 10.1371/journal.ppat.1000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aliyari R, Wu Q, Li HW, Wang XH, Li F, Green LD, Han CS, Li WX, Ding SW. Mechanism of induction and suppression of antiviral immunity directed by virus-derived small RNAs in Drosophila. Cell Host Microbe. 2008;4:387–397. doi: 10.1016/j.chom.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynt A, Liu N, Martin R, Lai EC. Dicing of viral replication intermediates during silencing of latent Drosophila viruses. Proc Natl Acad Sci U S A. 2009;106:5270–5275. doi: 10.1073/pnas.0813412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obbard DJ, Jiggins FM, Halligan DL, Little TJ. Natural selection drives extremely rapid evolution in antiviral RNAi genes. Curr Biol. 2006;16:580–585. doi: 10.1016/j.cub.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 31.Berry B, Deddouche S, Kirschner D, Imler JL, Antoniewski C. Viral suppressors of RNA silencing hinder exogenous and endogenous small RNA pathways in Drosophila. PLoS One. 2009;4:e5866. doi: 10.1371/journal.pone.0005866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou YT, Tam B, Linay F, Lai EC. Transgenic inhibitors of RNA interference in Drosophila. Fly (Austin) 2007;1:311–316. doi: 10.4161/fly.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L, Liu Z, Lu F, Dong A, Huang H. SERRATE is a novel nuclear regulator in primary microRNA processing in Arabidopsis. Plant J. 2006;47:841–850. doi: 10.1111/j.1365-313X.2006.02835.x. [DOI] [PubMed] [Google Scholar]
- 34.Lobbes D, Rallapalli G, Schmidt DD, Martin C, Clarke J. SERRATE: a new player on the plant microRNA scene. EMBO Rep. 2006;7:1052–1058. doi: 10.1038/sj.embor.7400806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleh MC, Tassetto M, van Rij RP, Goic B, Gausson V, Berry B, Jacquier C, Antoniewski C, Andino R. Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature. 2009;458:346–350. doi: 10.1038/nature07712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saleh MC, van Rij RP, Hekele A, Gillis A, Foley E, O'Farrell PH, Andino R. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol. 2006;8:793–802. doi: 10.1038/ncbl439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulvila J, Parikka M, Kleino A, Sormunen R, Ezekowitz RA, Kocks C, Ramet M. Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 cells. J Biol Chem. 2006;281:14370–14375. doi: 10.1074/jbc.M513868200. [DOI] [PubMed] [Google Scholar]
- 38.Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, Dostert C, Antoniewski C, Hoffmann JA, Imler JL. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat Immunol. 2008;9:1425–1432. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- 39.Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, Imler JL. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- 40.Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 41.Ho LJ, Hung LF, Weng CY, Wu WL, Chou P, Lin YL, Chang DM, Tai TY, Lai JH. Dengue virus type 2 antagonizes IFN-alpha but not IFN-gamma antiviral effect via down-regulating Tyk2-STAT signaling in the human dendritic cell. J Immunol. 2005;174:8163–8172. doi: 10.4049/jimmunol.174.12.8163. [DOI] [PubMed] [Google Scholar]
- 42.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HWt. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003;299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 43.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci U S A. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabatier L, Jouanguy E, Dostert C, Zachary D, Dimarcq JL, Bulet P, Imler JL. Pherokine-2 and -3. Eur J Biochem. 2003;270:3398–3407. doi: 10.1046/j.1432-1033.2003.03725.x. [DOI] [PubMed] [Google Scholar]
- 47.Avadhanula V, Weasner BP, Hardy GG, Kumar JP, Hardy RW. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009;5:e1000582. doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costa A, Jan E, Sarnow P, Schneider D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS One. 2009;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fragkoudis R, Chi Y, Siu RW, Barry G, Attarzadeh-Yazdi G, Merits A, Nash AA, Fazakerley JK, Kohl A. Semliki Forest virus strongly reduces mosquito host defence signaling. Insect Mol Biol. 2008;17:647–656. doi: 10.1111/j.1365-2583.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao L, Sakurai A, Watanabe T, Sorensen E, Nidom CA, Newton MA, Ahlquist P, Kawaoka Y. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature. 2008;454:890–893. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sessions OM, Barrows NJ, Souza-Neto JA, Robinson TJ, Hershey CL, Rodgers MA, Ramirez JL, Dimopoulos G, Yang PL, Pearson JL, et al. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diangelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 55.Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:e2. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]



