Abstract
G protein-coupled receptors (GPCRs) are among the most common potential targets for pharmacological design. Synthesized in the endoplasmic reticulum (ER), they interact with endogenous chaperones which assist in folding (or may retain incorrectly folded proteins) and are transferred to the plasma membrane (PM), where they exert their physiological functions. We summarize trafficking of the gonadotropin-releasing hormone receptor (GnRHR) to the plasma membrane. Trafficking of the GnRHR is among the best characterized, due in part to its small size and the consequent ease of making mutants (fewer primers needed and less chance for PCR errors). Special emphasis is placed on therapeutic opportunities presented by drugs that allow misrouted mutants to be routed correctly and restored to function.
Protein Mutations Can Cause Misrouting of Otherwise Functional Proteins
Conformational diseases are disorders of protein misfolding, often due to mutation, compromising protein function [1]. Proteins are monitored by a quality control system (QCS) in the ER which assists in folding and may retain misfolded structures in the ER for their subsequent degradation through the polyubiquitination/proteasome pathway [2, 3]. The etiology of many conformational diseases has now been traced to proteins that are either misfolded immediately after synthesis or have undergone post-translational conformational alterations.
Many conformational diseases associated with misfolding involve membrane-associated proteins [2, 4]. Among these are forms of familial hypercholesterolemia [5], retinitis pigmentosa [6], cystic fibrosis [7], diabetes insipidus [8], and hypogonadotropic hypogonadism (HH) [9] (Table 1). In cystic fibrosis (caused by mutation of the cystic fibrosis transmembrane conductance regulator), the Phe508 deletion mutation is found in nearly 70% of patients; this mutation leads to ER retention and degradation of the cAMP-regulated chloride transmembrane channel [7]. Another example is nephrogenic diabetes insipidus, in which urine is not concentrated due to arginine-vasopressin resistance of the kidney or to defects involving the arginine-vasopressin-responsive aquaporin-2 water channel [8, 10, 11]. When expressed in vitro, most (~70%) AVP V2 receptor mutations exhibit intracellular trapping of the receptor molecules that are then unable to reach the cell membrane [8, 12]. Similarly, loss-of-function mutations of the TSH receptor can cause destabilization of the newly synthesized receptor, preventing its cell surface expression and leading to TSH resistance [13]. Misfolding can result in proteins that retain function but, for reasons of mislocation alone, cease to function normally and result in disease.
Table 1.
Loss of function diseases or abnormalities caused by GPCR misfolding.
| Disease or Abnormality | GPCR | Refs |
|---|---|---|
| Retinitis pigmentosa | Rhodopsin | [6] |
| Nephrogenic diabetes insipidus | V2R | [8, 10, 11] |
| Hypogonadotropic hypogonadism | GnRHR | [18] |
| Familial hypocalciuric hypercalcemia | CaR | [56] |
| Male pseudohermaphroditism | LHR | [57] |
| Hypergonadotropic hypogonadism | ||
| Ovarian dysgenesis | FSHR | [57] |
| Congenital hypothyroidism | TSHR | [13] |
| Hirschsprung’s disease | E-BR | [58] |
| Red head color and fair skin (RHC phenotype and propensity to skin cancer) |
MC1R | [37, 52] |
| Familial glucocorticoid deficiency | MC2R | [59] |
| Obesity | MC3R, MC4R | [60] |
| Resistance to HIV-1 infection | CCR5 | [61] |
V2R: Vasopressin Type-2 receptor; GnRHR: Gonadotropin-releasing hormone receptor; CaR: Calcium-sensing receptor; LHR: Lutropin (luteinizing hormone) receptor; FSHR: Follitropin (follicle-stimulating hormone) receptor; TSHR: Thyrotropin receptor; E-BR: Endothelin-B receptor; MC1R: Melanocortin-1 receptor; MC2R: Melanocortin-2 receptor [or adrenocorticotropin (ACTH) receptor]; MC3R: Melanocortin-3 receptor; MC4R: Melanocortin-4 receptor; CCR5: Chemokine receptor-5.
Origin of Protein Misfolding
Control of protein folding is complex because of the proximity and diversity of proteins and because the steric character of the nascent protein backbone restricts the configurations recognized by a stringent QCS [14–16]. Chaperones of the QCS tend to recognize general “errors,” such as the presentation of a hydrophobic plate in an aqueous environment [15], unpaired cysteines, or immature glycans [12]. Identification of misfolded proteins by the QCS prevents aggregation [3, 16, 17]. If chaperone-assisted protein folding fails, the conformationally defective protein is targeted for degradation. Failure of this process results in disease.
One therapeutic opportunity is based on the misfolding and retention of otherwise functional proteins [2, 18]. In addition, recent attention has focused on the observation that certain GPCRs are normally exported from the ER in a relatively inefficient manner [17, 19–22]. For these, only a fraction of the synthesized protein is transferred to the site of normal function; the rest is retained in the ER and degraded. This naturally occurring “inefficiency” may result in a post-translational modification that reduces the concentration of the receptor protein at the PM [17, 19] and presents a therapeutic opportunity because of the availability of drugs that can alter routing by rescuing misfolded mutants.
Mutations in the human gonadotropin-releasing hormone receptor influence trafficking and lead to disease in humans
Gonadotropin-releasing hormone (GnRH) is a decapeptide produced by neurons in nuclei of the mediobasal and anterior hypothalamus (Figure 1). GnRH travels through the hypothalamic-pituitary portal system, binds to GnRHR in the pituitary gonadotrope and stimulates the synthesis and release of luteinizing hormone and follicle stimulating hormone. These gonadotropins enter the peripheral circulation and stimulate the synthesis and release of sex steroids and maturation of the gametes. Hypogonadotropic hypogonadism (HH) results from several etiologies, one of which is loss-of-function mutations in the GnRH receptor gene (GNRHR) that result in the inability to respond to GnRH, resulting in decreased or apulsatile gonadotropin release and reproductive failure 23. Twenty-one inactivating mutations in human (h)GNRHR have been described as a cause of HH (Figure 2a). Seven homozygous and 12 heterozygous combinations of hGnRHR mutants are expressed by individuals exhibiting either partial or complete forms of HH [18, 23]. Expression of the majority of these hGnRHR mutants in heterologous systems results in cells that neither bind GnRH agonists nor respond to GnRH stimulation by effector activation. Initially, these observations suggested that such mutations were associated with alterations in ligand binding, receptor activation or interaction with coupled effectors (G proteins). However, the majority (90%) of GnRHR mutants, whose function has been examined to date (19 mutants), are trafficking-defective receptors as disclosed by mutational studies and/or response to pharmacological chaperones [24]. Because reproductive failure is not life-threatening, it is likely that many cases (particularly partial HH forms) go undiagnosed, and individual mutants, if severe in phenotype, are not passed to progeny. Such ER-retained mutants frequently show a change in residue charge compared with the wild type (WT) receptor (e.g. the Glu90Lys GnRHR), or gain or loss of either Cys (an amino acid known to form bridges associated with the formation of third order structure of proteins; e.g. the Tyr108Cys and Cys200Tyr GnRHRs) or Pro (an amino acid associated with a forced turn in the protein sequence; e.g. the Pro320Leu GnRHR) (Figure 2a) residues [23].
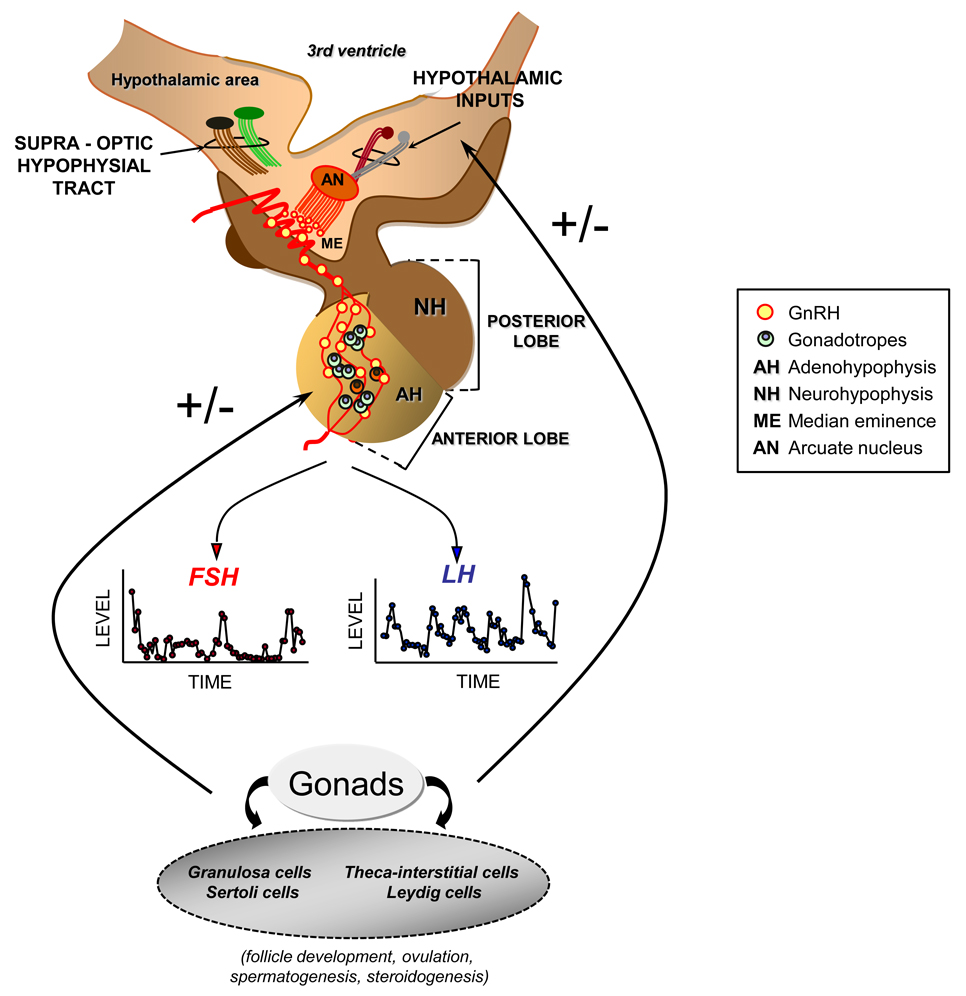
Figure 1.
Functional relations of the hypothalamic–pituitary axis. Gonadotropin-releasing hormone (GnRH) is synthesized and secreted by specialized neurons located mainly in the arcuate nucleus (AN) of the medial basal hypothalamus and the preoptic area of the anterior hypothalamus. GnRH producing neurons project to the median eminence (ME) where they terminate in an extensive plexus of boutons on the primary portal vessel, which delivers GnRH to its target cell, the gonadotrope of the adenohypophysis (AH). The secretion and interaction of GnRH with its cognate receptor occurs in a pulsatile and intermittent manner; such episodic signaling allows the occurrence of distinct rates and patterns of synthesis and pulsatile release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These gonadotrophic hormones are responsible for stimulating the synthesis and secretion of gonadal hormones and for affecting the process of gametogenesis. The characteristics of the pulsatile release of GnRH, LH, and FSH appear to be positively or negatively regulated by several hypothalamic neurotransmitters (e.g., adrenergic and opioidergic regulation), as well as by the gonadal hormone environment.
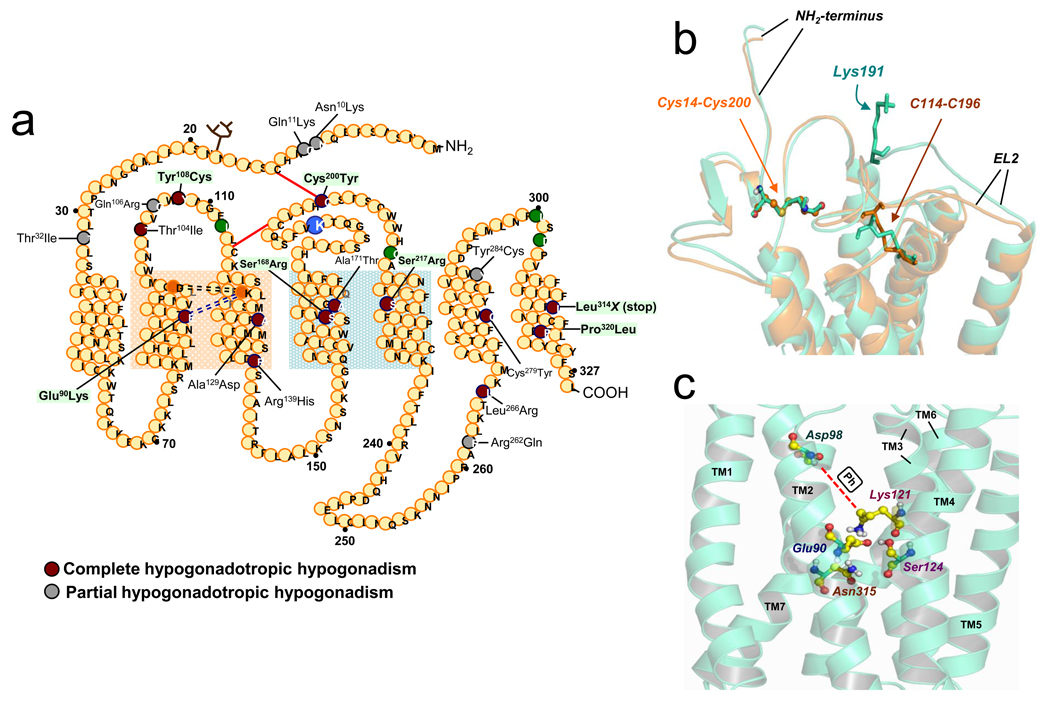
Figure 2.
(a) Sequence of the human gonadotropin-releasing hormone receptor and location of the inactivating mutations identified to date. Circles represent amino acids; those colored dark-red or grey are residues in which the indicated mutation leads to complete and partial HH, respectively; those colored green form a motif of four non-contiguous residues that are required in the human GnRHR for Lys191 to destabilize the formation of the Cys14-Cys200 bridge [29]. The circle corresponding to the Lys residue at position 191 in the second extracellular loop is enlarged and colored blue. The red lines indicate the position of the Cys14-Cys200 and Cys114-Cys196 disulfide bridges; the light purple dashed lines indicate the Glu90-Lys121 (residues in orange circles) salt bridge and the black dashed lines show the association of Asp98 and Lys121 enabled by pharmacoperones. The light orange shadow corresponds to the portion of the receptor where the TMs 2 and 3 are located and that are stabilized by the conserved Glu90-Lys121 salt bridge or the surrogate Asp98-Ph-Lys121 bridge resulting from pharmacoperone action; the light green shadow corresponds to the “zone of death” in TMs 4 and 5, where mutations are completely recalcitrant (Ser168Arg and Ser217Arg) or marginally responsive (Ala171Thr) to pharmacoperones [23]. (b) Superposition of the WT hGnRHR conformation (green structure) and the hGnRHR lacking Lys191 (orange structure), showing the positions of the Cys14–Cys200 and Cys114–Cys196 disulfide bridges (highlighted in both structures). (Reproduced from [31] with permission from the Society for Endocrinology). (c) Close-up showing specific interactions between Glu90 (in TM2), Asn315 (TM7), Lys121 (TM3), and Ser124 (TM3), forming a microdomain that is important for GnRHR stability [25, 32, 53]. Also shown is Asp98 (in TM2), which forms the surrogate Asp98-Lys121 salt bridge (dashed red line) upon pharmacoperone action (shown as “Ph” in the white box) [32]. (Reproduced from [53] with permission from the American Chemical Society).
Structural features of the GnRHR that impact on trafficking to the plasma membrane
Structural features of the hGnRHR explain how mutations in this receptor result in defective intracellular trafficking and cause disease. Because it is among the smallest GPCRs (328 amino acids), creating mutants is technologically easier; therefore, a great deal is known about the structure of this receptor [25]. In primates, GnRHR bears unique structural features including the lack of an intracellular carboxyl-terminal extension, whose presence is associated with differential physiological receptor regulation [25, 26]. In other species (i.e. fish, reptiles, and birds), the presence of this extended tail prolongs the presence of the receptor on the plasma membrane (PM) [27]. Another particular feature of primate GnRHRs is the presence of a lysine residue at position 191, which is located in the second extracellular loop (EL) (Figure 2b); this residue restricts GnRHR PM expression [28]. Non-primate mammals often utilize a less effective Glu191 in this position, whereas rats and mice do not have an orthologous residue (327 amino acids); this results in a higher proportion of the translation product of both rodent receptors localized at the PM compared to the human counterpart [28–30]. The mechanism by which the presence of Lys191 limits the number of hGnRHR molecules potentially exported from the ER to the PM involves formation of the Cys14-Cys200 bridge, which stabilizes the human receptor in a conformation compatible with ER export [29–31]. The spatial alignment necessary for formation of the Cys14-Cys200 bridge is specific because the two Cys residues need to be very close, approximately the size of one water molecule, in order for the bond to form [31]. When the bridge forms, the hGnRHR is recognized by the cell as correctly folded, allowing the receptor to continue trafficking to the PM. The presence of K191 appears to decrease the probability of bridge formation and likely explains why it decreases trafficking to the PM.
Mutagenesis studies identified a motif of four non-contiguous residues at positions 112 (EL1), 208 (EL2), 300, and 302 (EL3) that presumably control the destabilizing role of Lys191 on the association of the NH2-terminus and the EL2 and subsequent formation of the Cys14-Cys200 bridge in the hGnRHR (Figure 2a). In the rat GnRHR (no lysine in position 191), formation of this bridge (Cys14-Cys199; 199 is the orthologous position in the rat to the human 200 position) is not an essential requirement for correct folding, as a mutation in any of these positions does not affect agonist-stimulated intracellular signaling [29]. Human receptors containing the orthologous rat sequence at these sites lack the requirement for the Cys14-Cys200 bridge [29, 30] and are therefore expressed at higher levels at the PM. This difference in folding requirements for the human versus rat receptor may have evolved to allow tighter control of transfer of hGnRH to the PM associated with the increased complexity associated with primate reproduction, in comparison to the rodent.
Other structural features of the GnRH can be associated with trafficking to the PM. In humans, mutation of Gly90Lys precludes formation of a critical Glu90-Lys121 salt bridge that is essential to allow passage of the receptor through the QCS [32] (Figure 2a). The mutation Cys200Tyr breaks the Cys14-Cys200 bridge that is essential in the human, but not rat or mouse GnRHRs, for correct folding and trafficking. Consistent with this, the rat and mouse orthologs of Cys200Tyr have no effect [17, 29]. Mutants Ser168Arg and Ser217 Arg involve a thermodynamically unfavorable exchange that rotates the transmembrane segments (TM) 4 and TM5, moving the EL2, making formation of the Cys14-Cys200 bridge improbable; the mutant never passes the cellular QCS and pharmacoperones do not rescue them [18, 33], so GnRHRs with this mutation do not traffic to the PM and are degraded instead in the ER.
The dominant negative effect
Receptor oligomerization is an important determinant of GPCR function [34–36]. Intracellular association of GPCRs as oligomers can lead to either cell surface targeting or to intracellular retention of the complex (dominant negative effect) [13, 24, 34, 36]. GPCR mutants that do not traffic properly to the PM, when co-expressed with WT receptor, cause intracellular retention of the WT receptor, increasing the effect of the mutation. Dominant negativity is common among GPCRs [12, 13, 18, 33, 34, 36–38]. In some diseases, particularly those with autosomal dominant modes of inheritance, defective PM expression has been attributed to the dominant negative effect of the misfolded receptor on its WT counterpart, which may limit PM expression of the normal receptor resulting in a loss-of-function disease [12, 18, 39]. This is the case in dominant forms of TSH resistance, in which co-transfections of WT and mutant TSH receptors that are poorly expressed at the PM showed reduced functional activity of the WT receptor associated with formation and intracellular retention of hetero-oligomers formed by WT and mutant TSH receptors [13, 40]. Co-expression of the WT receptor with eight naturally occurring loss-of-function hGnRHR mutants led to inhibition of both WT receptor-mediated agonist binding and intracellular signaling in a dose-dependent manner and with specificity for individual mutant cDNA [23, 41]. This effect seems to be due to ER retention of aggregates of wild-type and mutant proteins [24, 33], and does not occur when the mutant receptors are coexpressed with genetically modified WT receptors intrinsically exhibiting high maturation efficiencies (e.g. the hGnRHR-desLys 191) [42]. In this latter scenario, it is possible that the dominant negative effect of the mutants on WT GnRHR function requires intrinsic low PM expression of the WT receptor species that co-evolved with the dominant negative effect.
Pharmacoperone Rescue Drugs
There are several types of compounds that can rescue or stabilize proteins in configurations that allow even (otherwise) misrouted mutants to become correctly routed. The most nonspecific are protein stabilizing compounds (glycerol, trimethylamine N-oxide, 4-phenylbutyric acid, and deuterated water) [43]. These are nonspecific agents and, therefore, can influence different proteins in various cellular compartments leading to undesirable changes. Genetic approaches in which modifications are introduced to an already defective protein have been used to rescue function of conformationally abnormal molecules; these approaches increase expression of molecules rendered unstable by genetic defects, such as the Glu90Lys hGnRHR mutant, whose function may be completely recovered by deleting Lys191 [24, 44].
Pharmacoperones are small molecules that enter cells, bind specifically to misfolded mutant proteins, correct their folding, and allow correct routing [24, 43, 45, 46]. Frequently, such molecules are identified as peptidomimetic antagonists from high throughput screens and may come from diverse chemical classes. Because these are known to interact with receptors, it was the first place we and others started in the search for agents that bind to and stabilize misfolded mutants in the configuration that would pass the quality control system of the cell. In vitro studies show that pharmacoperone rescue applies to an array of human diseases, including cystic fibrosis, hypercholesterolemia, cataracts, phenylketonuria, neurodegenerative diseases (e.g. Alzheimer’s, Parkinson’s, and Huntington’s), cancer, and some GPCR-related diseases such as retinitis pigmentosa, nephrogenic diabetes insipidus, and HH caused by conformationally defective GnRHRs.
When mice with phenylketonuria (caused by mutations in phenylalanine hydroxylase (PH), an enzyme that converts Phe to Tyr) were treated with compounds that enhanced this enzyme’s thermal stability, PH was stabilized in the liver after 12 days of administration, with increased activity and protein levels [47]. In a rat model of cerebral amyloid-β deposition, administration of β-sheet breaker peptides reduced amyloid-β deposition and prevented fibril formation [48]. In humans with nephrogenic diabetes insipidus due to mutant AVP V2 receptors, short-term administration of a nonpeptide vasopressin 1a receptor antagonist to a small cohort of patients decreased both 24-hour urine volume and water intake and concomitantly led to increased urine osmolality [49]. These observations suggest that pharmacoperone drugs can function in vivo.
Treatment of misfolded hGnRHR with pharmacological chaperones
In the human, the Glu90Lys mutation of the hGnRHR leads to complete HH. The first indication that this mutation leads to a misfolded and retained molecule came from studies showing that complete functional rescue of the mutant receptor was achieved by deleting Lys191 [44]. Soon after, it was shown that function of this, and other mutant receptors, was rescued by pharmacoperones from diverse chemical classes: indoles, quinolones, and erythromycin macrolides [50]. In selecting these drugs, we were seeking their binding interaction with the GnRHR, rather than their actions as antagonists. There is no reason that a pharmacoperone must be an antagonist. In fact, rescue of misfolded GPCRs may also be achieved by agonists of the natural ligand [51]. One could even imagine, in principle, that drugs which are neither antagonists nor agonists could then serve as pharmacoperones.
All but three [Ser168Arg, Ser217Arg and L314X(stop); (Figure 2a)] of the 17 hGnRHR mutants tested to date may be completely or partially rescued with pharmacoperones [18, 50]. The Ser168Arg and Ser217Arg GnRHRs have large thermodynamic changes leading to conformational alterations that preclude rescue by pharmacoperones [29, 33]. Even though these two mutants are not rescued, however, their failure to route correctly is still attributable to misfolding.
The dominant negative effect of misfolded mutant receptors may lead to a loss-of-function disease due to defective expression of normally functioning receptors [12, 13, 18, 24, 33, 34, 36–38, 52]. To examine interactions between misfolded mutants that influence receptor function and response to pharmacological rescue, a series of mutant GnRHR pairs associated with compound heterozygous patients showing complete or partial forms of HH was analyzed [39]. Coexpression of each pair of mutants resulted in either an active predominant effect, where the combination of mutants yielded similar responses to agonist stimulation, as did the more active of the two mutants transfected individually (e.g. Gln106Arg/Leu266Arg and Ala171Thr/Gln106Arg mutant GnRHR pairs), an additive effect (e.g. Arg262Gln/Gln106Arg, and Asn10Lys/Gln106Arg, mutant GnRHR pairs), or a dominant negative effect [e.g. Leu314X(stop)/Gln106Arg, Gln106Arg+Ser217Arg/Arg262Gln, and Leu314X(stop)/Arg262Gln mutant GnRHR pairs]. For all combinations, addition of a pharmacoperone increased both agonist binding and effector coupling (Figure 3). These studies suggest that, depending on the genotype, partial or full restoration of receptor function in response to pharmacological chaperones may be achievable goals in patients bearing inactivating mutations in the GnRHR gene.
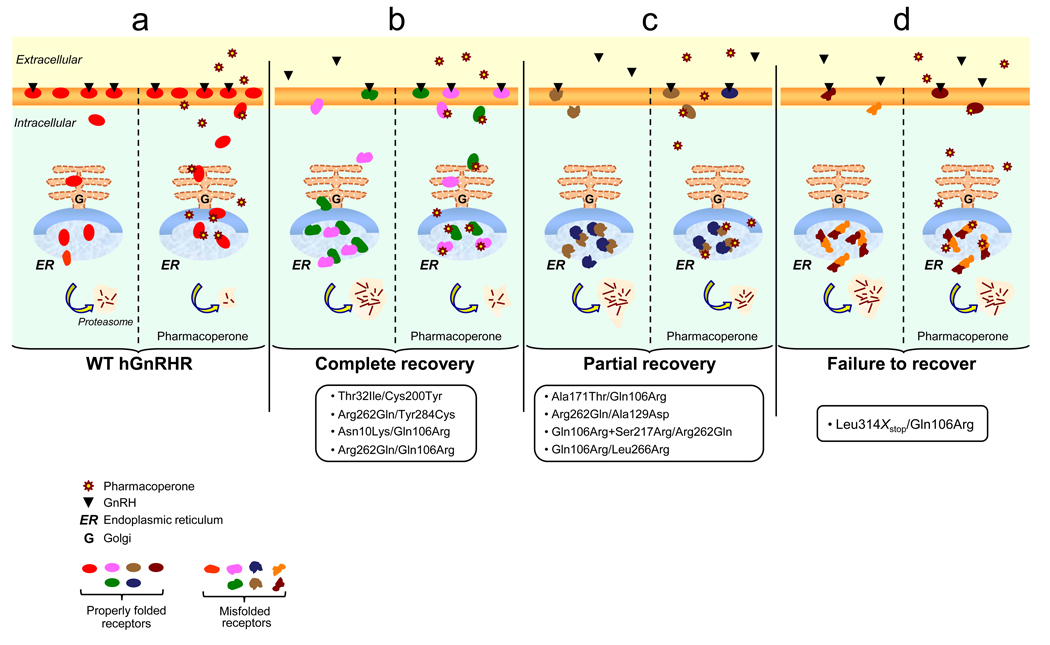
Figure 3.
Prediction of functional response of the (a) WT hGnRHR and (b–d) heterozygous hGnRHR mutants to pharmacological treatment in vivo based on in vitro co-expression studies [39]. (a) In the case of the WT hGnRHR, administration of pharmacoperones would presumably lead to improved function, whereas, for the different naturally occurring heterozygous combinations (b–d) leading to complete or partial HH, the in vitro response would predict complete or partial clinical recovery or nearly complete failure to pharmacological rescue. The extent of clinical responses following pharmacological rescue depends on potential interactions between mutant receptors, including the dominant negative effects imposed by one of the defective heterozygous receptors. For example, in the case of HH patients with hGnRHR genotypes bearing the Ala171Thr, Ala129Asp, Ser217Arg, or L314X(stop) alleles, the dominant effect exerted by these defective receptors may lead to a less than expected clinical response to pharmacoperones. For others, the potential interactions between mutant receptors would not negatively affect or might even favor the outcome to pharmacoperone treatment [39]. In these latter cases, a full clinical response may be an achievable goal. The oval forms represent hGnRHR molecules with a conformation compatible with endoplasmic reticulum (ER) export; the free forms represent conformationally defective receptors whose intracellular traffic to the cell surface plasma membrane is impaired. These misfolded receptors are retained in the ER and eventually degraded through the polyubiquitination/proteasome pathway.
Pharmacoperones may either correct folding of the mutant receptors, allowing the possibility that one or both of the mutants may escape the QCS and traffic to the PM, or interfere with aggregation and degradation of the mutant receptors. The ability of pharmacoperones to rescue mutants coexpressed with WT receptor as well as the WT receptor involved [33] suggest that in vivo use of such compounds could be highly effective in overriding the dominant negative effect of a mutation on the WT, as well as in the rescue of the mutant itself. Identification of the hGnRHR mutants present in patients harboring compound heterozygous expression may be useful to determine whether treatment with pharmacological means will lead to a favorable therapeutic outcome (Figure 3).
Molecular mechanism of action of pharmacoperone rescue of GnRHR mutants: understanding the mechanism by which pharmacoperones work
Glu90, in helix 1, forms a salt bridge with Lys121 32 as observed in computational GnRHR models [25, 32, 53, 54] (Figure 2c); this bridge is lost in the Glu90Lys mutation. It appears highly conserved in the GnRHR of virtually all mammals, fish, birds and reptiles [25]. The ability of pharmacoperones to completely rescue function of this mutant led us to analyze the chemical relation between these drugs and the Glu90-Lys121 bridge. Pharmacoperones appear to act by forming an alternative bridge between Asp98 (a residue located near the extracellular face of TM1) and Lys 121 (Figure 2a and 2c) that may effectively function as a surrogate for the original Glu90-Lys121 bridge disrupted by the Glu→Lys substitution [32]. Asp98 and Lys121 are also points of contact for the receptor´s natural ligand [25], so it is not surprising that pharmacoperone antagonists, as competitors of GnRH, may interact at or near the ligand binding site. This site resides in the lateral plane of the PM, a region bearing a high percentage of hydrophobic residues [53]. In fact, the linear sequences of both Glu90 and Lys121 are hydrophobic regions with a modest number of ionic or polar groups; therefore, the observation of this conserved ionic site could reflect that the pharmacoperones tested were all chosen on the basis of this preferential ion-pair and/or polar interaction with the charged residues. It is not clear why the pharmacoperones tested to date rescue most of the GnRHR mutants, despite the fact that mutations are distributed along the entire coding sequence of the receptor, including, not only the transmembrane helices, but also the intra- and extracellular domains (Figure 2a). This raises the issue of whether, in addition to Cys bridges, there is another “critical core” that, once stabilized, forms a structure that passes the scrutiny of the QCS; for the GnRHR this core might stabilize the orientation of, and relation between, TMs 2 and 3. The fact that pharmacoperones rescue function of receptors bearing mutations in other locations probably reflects the interactive nature of GPCRs (Figure 2c). Alternatively, stabilization of the relation between TMs 2 and 3 (and 7 as well) may reflect the critical requirement of the Glu90-Lys121 bridge for the endogenous chaperone system to recognize the protein as correctly folded. The importance of both the Cys14-Cys200 disulfide and Glu90-Lys121 salt bridges to serve as critical cores of the hGnRHR for being recognized as properly folded by the QCS is further emphasized by the fact that rescued function of mutants lacking Lys191 is enhanced by pharmacoperone treatment.
Conclusion
It is evident that the pharmacoperone rescue approach might apply to an array of diseases resulting from misfolding and defective intracellular trafficking. In addition to mutants and, as noted above for the hGnRHR, it is clear that variable amounts of other WT GPCRs or protein, with minor alterations, are normally retained by the QCS, presumably as a result of misfolding, but they can function properly if they reach the PM. Current evidence suggests that the quality-control machinery in the ER is quite stringent as it prefers to err on the side of rapidly degrading a protein that, when given time, may fold into a functionally active receptor. This offers the therapeutic opportunity of manipulating the ER QCS by pharmacoperone administration to correct not only a misfolding defect leading to disease, but also to improve function by increasing the number of functional membrane WT receptors available to interact with agonists.
One problem in translating this concept from the laboratory bench to clinical practice is that almost all known pharmacoperones for GPCRs are peptidomimetic antagonists of the native ligand, making it necessary to remove the drug after rescue in order to allow the receptor to bind agonist and become activated. Nevertheless, it is possible to imagine pharmacoperones that stabilize the correctly routed form of the receptor and not show any antagonism. In this vein, one pharmacoperone drug has been recently identified that does not seem to compete for the agonist or antagonist binding site [55]. It is possible that this approach may pave the way for the rational design of therapeutic strategies based on pharmacoperones that behave as allosteric modulators and may stabilize misfolded receptors without inhibiting endogenous (or exogenous) agonists.
Acknowledgements
This work was supported by NIH grants: HD-19899, RR-00163, and HD-18185 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. Alfredo Ulloa-Aguirre is recipient of a Research Career Development Award from the Fundación IMSS, Mexico. We thank Jo Ann Binkerd for formatting the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lin JC, Liu HL. Protein conformational diseases: from mechanisms to drug designs. Curr Drug Discov Technol. 2006;3:145–153. doi: 10.2174/157016306778108866. [DOI] [PubMed] [Google Scholar]
- 2.Castro-Fernandez C, et al. Beyond the signal sequence: protein routing in health and disease. Endocr Rev. 2005;26:479–503. doi: 10.1210/er.2004-0010. [DOI] [PubMed] [Google Scholar]
- 3.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 4.Gregersen N. Protein misfolding disorders: pathogenesis and intervention. J Inherit Metab Dis. 2006;29:456–470. doi: 10.1007/s10545-006-0301-4. [DOI] [PubMed] [Google Scholar]
- 5.Beglova N, Blacklow SC. The LDL receptor: how acid pulls the trigger. Trends Biochem Sci. 2005;30:309–317. doi: 10.1016/j.tibs.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Mendes HF, et al. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol Med. 2005;11:177–185. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 8.Bichet DG. Nephrogenic diabetes insipidus. Adv Chronic Kidney Dis. 2006;13:96–104. doi: 10.1053/j.ackd.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Janovick JA, et al. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87:3255–3262. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara TM, Bichet DG. Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol. 2005;16:2836–2846. doi: 10.1681/ASN.2005040371. [DOI] [PubMed] [Google Scholar]
- 11.Hermosilla R, et al. Disease-causing V(2) vasopressin receptors are retained in different compartments of the early secretory pathway. Traffic. 2004;5:993–1005. doi: 10.1111/j.1600-0854.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 12.Conn PM, et al. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59:225–250. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- 13.Calebiro D, et al. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet. 2005;14:2991–3002. doi: 10.1093/hmg/ddi329. [DOI] [PubMed] [Google Scholar]
- 14.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 15.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 16.Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol. 2009;16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 17.Conn PM, et al. Protein folding as posttranslational regulation: evolution of a mechanism for controlled plasma membrane expression of a G protein-coupled receptor. Mol Endocrinol. 2006;20:3035–3041. doi: 10.1210/me.2006-0066. [DOI] [PubMed] [Google Scholar]
- 18.Ulloa-Aguirre A, Conn PM. Targeting of G protein-coupled receptors to the plasma membrane in health and disease. Front Biosci. 2009;14:973–994. doi: 10.2741/3290. [DOI] [PubMed] [Google Scholar]
- 19.Conn PM, et al. 'Effective inefficiency': cellular control of protein trafficking as a mechanism of post-translational regulation. J Endocrinol. 2006;190:13–16. doi: 10.1677/joe.1.06771. [DOI] [PubMed] [Google Scholar]
- 20.Wuller S, et al. Pharmacochaperones post-translationally enhance cell surface expression by increasing conformational stability of wild-type and mutant vasopressin V2 receptors. J Biol Chem. 2004;279:47254–47263. doi: 10.1074/jbc.M408154200. [DOI] [PubMed] [Google Scholar]
- 21.Lu M, et al. Endoplasmic reticulum degradation impedes olfactory G-protein coupled receptor functional expression. BMC Cell Biol. 2004;5:34. doi: 10.1186/1471-2121-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietila EM, et al. Inefficient maturation of the rat luteinizing hormone receptor. A putative way to regulate receptor numbers at the cell surface. J Biol Chem. 2005;280:26622–26629. doi: 10.1074/jbc.M413815200. [DOI] [PubMed] [Google Scholar]
- 23.Ulloa-Aguirre A, et al. Misrouted cell surface GnRH receptors as a disease aetiology for congenital isolated hypogonadotrophic hypogonadism. Hum Reprod Update. 2004;10:177–192. doi: 10.1093/humupd/dmh015. [DOI] [PubMed] [Google Scholar]
- 24.Ulloa-Aguirre A, et al. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 2004;5:821–837. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 25.Millar RP, et al. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- 26.Brothers SP, et al. Conserved mammalian gonadotropin-releasing hormone receptor carboxyl terminal amino acids regulate ligand binding, effector coupling and internalization. Mol Cell Endocrinol. 2002;190:19–27. doi: 10.1016/s0303-7207(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 27.Lin X, et al. Addition of catfish gonadotropin-releasing hormone (GnRH) receptor intracellular carboxyl-terminal tail to rat GnRH receptor alters receptor expression and regulation. Mol Endocrinol. 1998;12:161–171. doi: 10.1210/mend.12.2.0056. [DOI] [PubMed] [Google Scholar]
- 28.Arora KK, et al. Influence of a species-specific extracellular amino acid on expression and function of the human gonadotropin-releasing hormone receptor. Mol Endocrinol. 1999;13:890–896. doi: 10.1210/mend.13.6.0291. [DOI] [PubMed] [Google Scholar]
- 29.Janovick JA, et al. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy. J Biol Chem. 2006;281:8417–8425. doi: 10.1074/jbc.M510601200. [DOI] [PubMed] [Google Scholar]
- 30.Ulloa-Aguirre A, et al. G-protein-coupled receptor trafficking: understanding the chemical basis of health and disease. ACS Chem Biol. 2006;1:631–638. doi: 10.1021/cb600360h. [DOI] [PubMed] [Google Scholar]
- 31.Jardon-Valadez E, et al. Conformational effects of Lys191 in the human GnRHR: mutagenesis and molecular dynamics simulations studies. J Endocrinol. 2009;201:297–307. doi: 10.1677/JOE-08-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janovick JA, et al. Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: the GnRH receptor. Molec Endocrinol. 2009;23:157–168. doi: 10.1210/me.2008-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brothers SP, et al. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol. 2004;18:1787–1797. doi: 10.1210/me.2004-0091. [DOI] [PubMed] [Google Scholar]
- 34.Milligan G. G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 35.Conn PM, et al. Conversion of a gonadotropin-releasing hormone antagonist to an agonist. Nature. 1982;296:653–655. doi: 10.1038/296653a0. [DOI] [PubMed] [Google Scholar]
- 36.Bulenger S, et al. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Beaumont KA, et al. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum Mol Genet. 2007;16:2249–2260. doi: 10.1093/hmg/ddm177. [DOI] [PubMed] [Google Scholar]
- 38.McElvaine AT, Mayo KE. A dominant-negative human growth hormone-releasing hormone (GHRH) receptor splice variant inhibits GHRH binding. Endocrinology. 2006;147:1884–1894. doi: 10.1210/en.2005-1488. [DOI] [PubMed] [Google Scholar]
- 39.Leanos-Miranda A, et al. In vitro coexpression and pharmacological rescue of mutant gonadotropin-releasing hormone receptors causing hypogonadotropic hypogonadism in humans expressing compound heterozygous alleles. J Clin Endocrinol Metab. 2005;90:3001–3008. doi: 10.1210/jc.2004-2071. [DOI] [PubMed] [Google Scholar]
- 40.Persani L, et al. Technology Insight: modern methods to monitor protein-protein interactions reveal functional TSH receptor oligomerization. Nat Clin Pract Endocrinol Metab. 2007;3:180–190. doi: 10.1038/ncpendmet0401. [DOI] [PubMed] [Google Scholar]
- 41.Leanos-Miranda A, et al. Dominant-negative action of disease-causing gonadotropin-releasing hormone receptor (GnRHR) mutants: a trait that potentially coevolved with decreased plasma membrane expression of GnRHR in humans. J Clin Endocrinol Metab. 2003;88:3360–3367. doi: 10.1210/jc.2003-030084. [DOI] [PubMed] [Google Scholar]
- 42.Knollman PE, et al. Parallel regulation of membrane trafficking and dominant-negative effects by misrouted gonadotropin-releasing hormone receptor mutants. J Biol Chem. 2005;280:24506–24514. doi: 10.1074/jbc.M501978200. [DOI] [PubMed] [Google Scholar]
- 43.Bernier V, et al. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004;15:222–228. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Maya-Nunez G, et al. Molecular basis of hypogonadotropic hypogonadism: restoration of mutant (E(90)K) GnRH receptor function by a deletion at a distant site. J Clin Endocrinol Metab. 2002;87:2144–2149. doi: 10.1210/jcem.87.5.8386. [DOI] [PubMed] [Google Scholar]
- 45.Bernier V, et al. Pharmacological chaperone action on G-protein-coupled receptors. Curr Opin Pharmacol. 2004;4:528–533. doi: 10.1016/j.coph.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Conn PM, Janovick JA. Drug development and the cellular quality control system. Trends Pharmacol Sci. 2009;30:228–233. doi: 10.1016/j.tips.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Pey AL, et al. Identification of pharmacological chaperones as potential therapeutic agents to treat phenylketonuria. J Clin Invest. 2008;118:2858–2867. doi: 10.1172/JCI34355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Estrada LD, Soto C. Inhibition of protein misfolding and aggregation by small rationally-designed peptides. Curr Pharm Des. 2006;12:2557–2567. doi: 10.2174/138161206777698792. [DOI] [PubMed] [Google Scholar]
- 49.Bernier V, et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 2006;17:232–243. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 50.Janovick JA, et al. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther. 2003;305:608–614. doi: 10.1124/jpet.102.048454. [DOI] [PubMed] [Google Scholar]
- 51.Leskela TT, et al. Opioid receptor pharmacological chaperones act by binding and stabilizing newly synthesized receptors in the endoplasmic reticulum. J Biol Chem. 2007;282:23171–23183. doi: 10.1074/jbc.M610896200. [DOI] [PubMed] [Google Scholar]
- 52.Beaumont KA, et al. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet. 2005;14:2145–2154. doi: 10.1093/hmg/ddi219. [DOI] [PubMed] [Google Scholar]
- 53.Jardon-Valadez E, et al. Modeling and molecular dynamics simulation of the human gonadotropin-releasing hormone receptor in a lipid bilayer. J Phys Chem B. 2008;112:10704–10713. doi: 10.1021/jp800544x. [DOI] [PubMed] [Google Scholar]
- 54.Soderhall JA, et al. Antagonist and agonist binding models of the human gonadotropin-releasing hormone receptor. Biochem Biophys Res Commun. 2005;333:568–582. doi: 10.1016/j.bbrc.2005.05.142. [DOI] [PubMed] [Google Scholar]
- 55.Janovick JA, et al. Increased plasma membrane expression of human follicle-stimulating hormone receptor by a small molecule thienopyr(im)idine. Mol Cell Endocrinol. 2009;298:84–88. doi: 10.1016/j.mce.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y, Breitwieser GE. Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J Biol Chem. 2007;282:9517–9525. doi: 10.1074/jbc.M609045200. [DOI] [PubMed] [Google Scholar]
- 57.Huhtaniemi IT, Themmen AP. Mutations in human gonadotropin and gonadotropin-receptor genes. Endocrine. 2005;26:207–217. doi: 10.1385/ENDO:26:3:207. [DOI] [PubMed] [Google Scholar]
- 58.Amiel J, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- 59.Clark AJ, et al. Inherited ACTH insensitivity illuminates the mechanisms of ACTH action. Trends Endocrinol Metab. 2005;16:451–457. doi: 10.1016/j.tem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Tao YX. Molecular mechanisms of the neural melanocortin receptor dysfunction in severe early onset obesity. Mol Cell Endocrinol. 2005;239:1–14. doi: 10.1016/j.mce.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Reiche EM, et al. Genetic polymorphisms in the chemokine and chemokine receptors: impact on clinical course and therapy of the human immunodeficiency virus type 1 infection (HIV-1) Curr Med Chem. 2007;14:1325–1334. doi: 10.2174/092986707780597934. [DOI] [PubMed] [Google Scholar]