Abstract
Background & Aims
Helicobacter pylori infection increases gastric Treg response, which may contribute to H pylori immune escape. We hypothesize that H pylori directs Treg skewing by way of dendritic cells and thus inhibits Th17 immunity.
Methods
Two-photon microscopy was used to locate dendritic cells in gastric lamina propria of mice. The induction of Th17 and Treg responses by bacteria-pulsed murine bone marrow–derived dendritic cells was analyzed by cytokine production and stimulation of T cell proliferation. The effect of VacA, CagA, TGF-β, and IL-10 on Th17/Treg balance was assessed. The in vivo significance of Tregs on the H pylori–specific Th17 response and H pylori density was determined using anti-CD25 neutralizing antibodies to deplete Tregs in mice.
Results
We showed that mucosal CD11c+ dendritic cells are located near the surface of normal gastric epithelium and their number increased after H pylori infection. Study of the direct interaction of dendritic cells with H pylori revealed a Treg-skewed response. The Treg skewing was independent of H pylori VacA and CagA and dependent on TGF-β and IL-10. In vivo Treg skewing by adoptive transfer of H pylori–pulsed DCs reduces the ratio of gastric IL-17/Foxp3 mRNA expressions. The depletion of CD25+ Tregs results in early reduction of H pylori density, which is correlated with enhanced peripheral H pylori–specific Th17, but not Th1, response.
Conclusions
Overall, our study indicates that H pylori alters the DC-polarized Th17/Treg balance towards a Treg-biased response, which suppresses the effective induction of H pylori–specific Th17 immunity.
Introduction
Helicobacter pylori is a highly adaptive gram-negative bacterium that colonizes the human stomach and contributes to diseases such as gastric ulcers and cancers. Infected individuals generate a vigorous systemic and mucosal humoral response1 but fail to eradicate the offending organism and thus are susceptible to mucosal damage.2 Several H pylori immune evasion mechanisms have been proposed (e.g., antigenic and phase variation, modulation of adhesion molecules, and immune inhibition by VacA protein and lipopolysaccharides).3
Emerging evidence suggests that H pylori may induce a regulatory T cell (Treg) response against helper T cell immunity, which leads to H pylori persistence. H pylori–specific CD4+CD25+ Tregs have been shown to suppress memory T cell responses to H pylori in infected individuals.4–5 Expression of the Treg marker Foxp3 messenger RNA (mRNA) is higher in the gastric tissue of H pylori–infected persons compared with uninfected controls.6–8 Moreover, Harris et al9 reported the decreased gastric pathology seen in infected children compared to adults was correlated with increased levels of gastric Foxp3 expression in infected children. These data establish an association between H pylori infection and the induction of Tregs, which, by counteracting the host’s immune surveillance, may lead to H pylori persistence.
Developmentally related Foxp3+ Tregs and interleukin (IL)-17+ helper T (Th17) cells have been implicated in host tolerance of or defense against microbial pathogens, particularly at mucosal surfaces.10 Ivanov et al11 reported that specific microbiota direct Th17 cell differentiation in the small intestine mucosa, and further, the absence of Th17 cell–inducing bacteria was accompanied by an increase in Foxp3+ Tregs in the lamina propria. Thus, the regulation of Th17/Treg balance may influence intestinal immunity and tolerance, dictating the outcome of luminal bacterial infection. We hypothesize that H pylori tips the Th17/Treg balance toward a Treg-skewed response resulting in a suboptimal Th17 response and failure to eradicate the offending pathogen. This hypothesis is consistent with evidence of the importance of Th17 in vaccine-induced immunity against H pylori.12
As professional antigen presenting cells with the unique ability to sample luminal bacteria, DCs maintain tolerance to commensals and initiate intestinal immunity against pathogens.13 DCs reside just beneath the surface epithelium in the normal small intestine; however, their mucosal distribution in normal gastric tissue has not been shown. We and others have demonstrated CD11c+ DC recruitment in the lamina propria of mouse stomach in both acute and chronic gastritis after H pylori infection,14–16 implicating a role for these DCs in the mucosal response against H pylori. Moreover, we and others have shown that H pylori–pulsed DCs in vivo induce H pylori–specific adaptive immune responses.17 Our current goals are to examine the influence of H pylori on the induction of Th17/Treg differentiation by DCs and to study the significance of the Th17/Treg balance in determining bacterial density.
We show that H pylori persistence may depend on a Th17/Treg balance mediated by DCs. DCs found near the surface epithelium of mouse stomach are prime candidates for interacting with luminal H pylori. We show that bone marrow-derived DCs pulsed with H pylori fail to induce a robust Th17 response and skew the response toward Treg differentiation by way of a VacA/CagA-independent, TGF-β/IL-10–dependent mechanism. This Treg-skewed response in the gastric mucosa was shown by the adoptive transfer of H pylori–pulsed DCs into infected mice. Treg depletion enhanced the H pylori–specific Th17 response, which correlated with decreased bacterial density. No correlation was observed between bacterial density and the H pylori–specific Th1 response. Our results suggest that H pylori evades immune clearance by influencing DC-induced Th17/Treg differentiation toward a Treg-biased response that inhibits Th17 immunity.
Materials and Methods
Two-photon microscopy
CD11c-YFP mice were injected intravenously with 655 nm Q-dot nanocrystals (20 μl diluted in to PBS to 200 μL total volume, Invitrogen, Carlsbad, CA). Stomachs were removed and glued to plastic cover slips with VetBond, placed in Dulbecco modified eagle’s medium (DMEM), and imaged at room temperature using a custom-built two-photon microscope.18 Each sample was excited at 890 nm. Fluorescence emission was separated into blue, green, and red signals using dichoic mirrors at 480 and 560 nm and collected simultaneously with three bialkali photomultiplier tubes.
Comparison of DC density in uninfected versus infected mice by two-photon microscopy
DCs isolation procedure from mouse stomach
(For additional Materials and Methods, see Supplementary Methods)
Results
DCs are found near the surface of the gastric mucosa
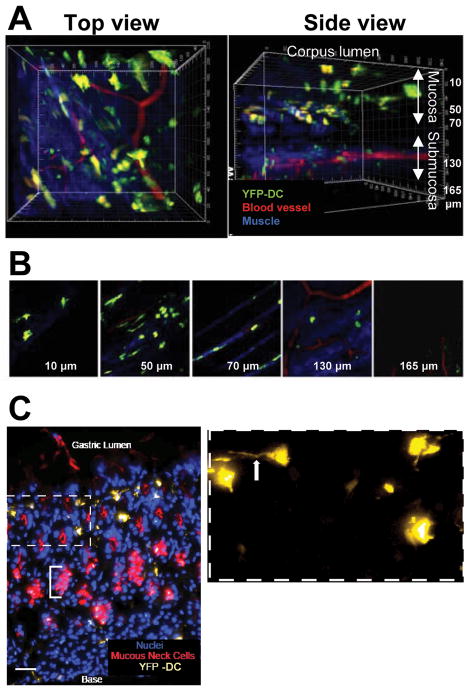
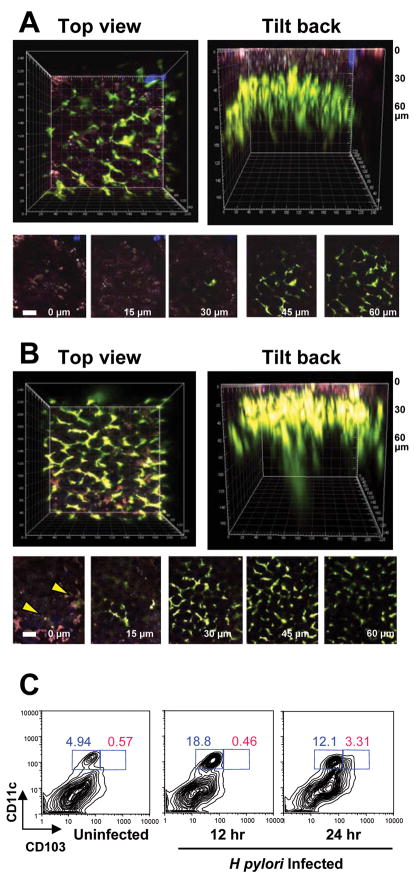
DCs play a key role in mediating adaptive immunity against pathogens by priming antigen-specific helper T cell responses. Maria Rescigno et al19 showed that DCs traverse the epithelial tight junction and sample luminal bacteria in the intestine. The presence of DCs near the gastric mucosal surface would suggest similar bacteria sampling in the gastric lumen. Two-photon microscopy20 revealed the presence of many CD11c+ DCs near the luminal surface of the normal stomach of transgenic pCD11c-YFP mice21–22 (i.e., mice in which DCs express YFP) (Figure 1A). DCs were also seen in the submucosal layer (Figure 1B). Figure 1C shows a well-demarcated gastric architecture with nuclei and mucous neck cells. Cytoplasmic projections of DCs extend between surface epithelial cells. The proximity of DCs to surface epithelium and the presence of DC projections indicate that DCs, along with the gastric epithelium, are likely the first line of host immune responders to H pylori infection. Moreover, within 24 h of H pylori infection, CD11c+ DCs in the gastric lamina propria increased in number and moved closer to the epithelial surface with cytoplasmic extensions seen at the luminal surface (Figure 2A and B). FACS analysis of isolated gastric lamina propria cells after H pylori infection showed a 3-fold increase in the number of CD11c+ DCs 12 h after H pylori infection and the emergence of CD103+ DC subset at 24 h after infection (Figure 2C). The CD11c+ CD103+ mucosal subsets have been shown to induce Treg responses.23–24 These data support the role of DCs in pathogen recognition during H pylori infection and provide the rationale for studying the role of DCs in mediating an H pylori-induced Treg response.
Figure 1. Dendritic cells are found near the surface of the gastric mucosa.
(A) A representative volume of the corpus of the stomach (220 × 240 × 177.5 μm, acquired in 71 2.5-μm steps) rendered in top, and side views imaged by two-photon microscopy. (B) Panels show single image planes taken from the z-stack at indicated depths. DCs (green) appear throughout the tissue. Large blood vessels (red) are visible above the muscle layer (blue striations); smaller vessels are visible at deeper levels. The blue likely represents a second harmonic generation signal emanating from collagen; it appears in several distinct strata from 50 μm to >130 μm. (C) Fresh-frozen section of a CD11c-YFP stomach shows corpus mucosa from the base of a gastric unit (i.e., lamina propria) to the lumen. Mucous neck cells, which are located immediately below the isthmal stem cell compartment, are visualized with GS-II lectin (pseudocolored red). YFP-tagged DCs (pseudocolored yellow) are visible in a region starting above the mucous neck cell zone and extending to the epithelial cells lining the surface. Bracket denotes the neck cell zone in one gastric unit. Inset: Higher magnification reveals dendritic extensions of the YFP-positive cells (arrow). Note: DCs correspond to the cells 10 μm below the surface in (B). Nuclei are counterstained with Hoechst (blue). Bar = 20 μm.
Figure 2. Increased gastric surface CD11c+ DCs during H pylori infection.
DC 3D distribution in uninfected and H pylori infected CD11c-YFP mice were determined by two-photon microscopy. (A) In uninfected mice, a distinctive network of CD11c-YFP cells (green) was found ~30–40 μm below the luminal surface of the stomach corpus (identified by red-purple autofluorescence). In two separate experiments, we did not observe transepithelial DC extensions in uninfected mice. (B) In mice infected with H pylori 24 h previously, the number of CD11c-YFP cells appeared to increase and DCs were observed closer to the epithelium (identified by autofluorescence). We found DC extensions that appeared to probe the epithelial layer and in several cases, DC extended all the way though the epithelial layer to the mucosal surface as a billowing projection (yellow arrows). The image depth is shown in the bottom left corner of A and B. Scale bar equals 40 μm. (C) Increased numbers of CD11c+ DCs in mouse stomach after H pylori infection. Single cell prep of stomach from uninfected C57BL/6 mice or from mice infected with H pylori for 12 and 24 h were dual-labeled with PE-conjugated anti-mouse CD11c and APC-conjugated anti-mouse CD103 antibodies and analyzed by FACS. H pylori infection increased the number of CD11c+ DCs 12 h after infection with the emergence of CD103+ subset 24 h after infection.
Bone marrow–derived DCs maintain a low Th17:Treg ratio with H pylori stimulation
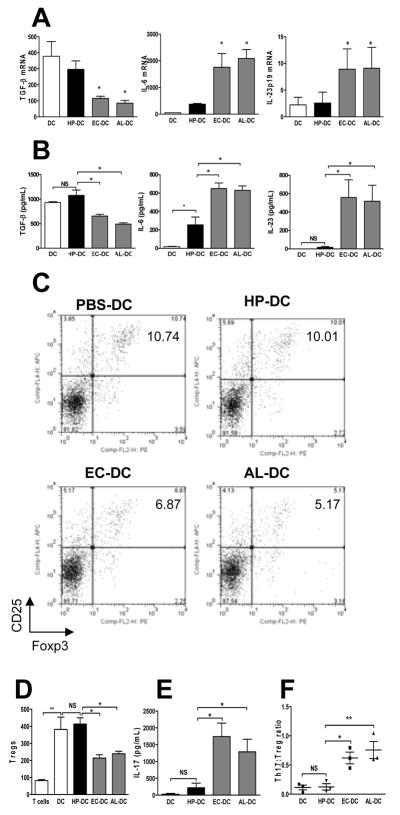
Because DCs congregate near the surface of the gastric epithelium (Figure 1 and 2) and are capable of sampling luminal microbes in the intestine,25 the characterization of the DC response to H pylori may offer insight into how H pylori evades host immunity. Gut microbes have been shown to influence the Th17/Treg balance in the intestine.11 We hypothesized that the Th17/Treg balance in response to H pylori may be different from other gram-negative gut microbes. The Th17 and Treg responses induced by murine bone marrow–derived DCs were compared after stimulation with H pylori, Escherichia coli, or Acinetobacter lwoffii. E coli, which commonly induces a proinflammatory DC phenotype, served as a positive control. A lwoffii was used because, like H pylori, it causes gastritis, but unlike H pylori, it fails to establish chronic infection in mice.26 We noted that unstimulated DCs (PBS-DC) produced high levels of TGF-β (Figure 3A and B), which is critical for the induction of Treg and Th17 responses; the latter promoted by IL-6 and IL-23. PBS-DC were found to induce Tregs in vitro (Figure 3C and D), a finding consistent with the known role of immature DCs in the maintenance of mucosal tolerance.13 H pylori–pulsed DCs (HP-DC) produced significantly lower levels of IL-6 and IL-23 compared with DCs pulsed with E coli (EC-DC) or A lwoffii (AL-DC) (Figure 3A and B). Note that TGF-β production, which was decreased in EC-DC and AL-DC but not HP-DC, correlated with the ability of DCs to induce Tregs. Compared with Treg induction in PBS-DC, Treg induction was reduced in EC-DC and AL-DC but not HP-DC (Figure 3C and D). This is consistent with evidence that TGF-β at high concentrations favors Treg priming, whereas at low concentrations, TGF-β synergizes with IL-6, which favors Th17 cell differentiation.27 IL-17 production was higher in EC-DC and AL-DC compared with HP-DC and PBS-DC (Figure 3E). Thus, the IL-17:Treg ratio of HP-DC was significantly lower than the IL-17:Treg ratio of EC-DC or AL-DC (Figure 3F). These data suggest that the host response to H pylori is characterized by a low Th17:Treg ratio, which favors bacterial persistence.
Figure 3. Bone marrow–derived dendritic cells (DCs) maintain a low Th17:Treg ratio with H pylori stimulation.
Bone marrow–derived DCs were pulsed with live H pylori, E coli, or A lwoffii for 18 h (denoted HP-DC, EC-DC, and AL-DC, MOI 1:100) and DC cytokine PCR-array was used to determine mRNA expressions of DC immunomodulatory genes (see Materials and Methods). Supernatants were collected and protein expression was measured by ELISA. (A) Comparison of TGF-β, IL-6, and IL-23p19 mRNA expressions of DCs using quantitative PCR. (B) Release of TGF-β, IL-6, and IL-23 by DCs was measured by ELISA. Messenger RNA and protein cytokine expressions indicate that lower levels of IL-6 and IL-23 were expressed by HP-DC compared with EC-DC and AL-DC. TGF-β production by DCs was similar in PBS-DC and HP-DC but reduced in EC-DC and AL-DC. A 72-h mixed leukocyte reaction using DCs and naive syngeneic splenocytes further assessed DC function in Treg and Th17 induction. (C) The percentage of CD4+CD25+Foxp3+ Tregs in the total number of CD4+ T cells was determined using FACS-generated dot plots. (D) The relative numbers of CD4+CD25+Foxp3+ Tregs from three separate experiments. (E) IL-17 production measured by ELISA. (F) The Th17:Treg ratio was calculated by dividing the number of IL-17 produced CD4+ cells by the number of Foxp3+ CD4+ cells measured using intracellular FACS staining. The Th17:Treg ratio was lower in HP-DC than in EC-DC and AL-DC (n = 3).
Treg skewed response of HP-DC is independent of H pylori VacA or CagA
To investigate the role of H pylori bacterial factors in determining the Th17:Treg ratio, we assessed the role of VacA and CagA during HP-DC induction of Treg and Th17 cells. VacA has antiinflammatory properties,3 whereas CagA has been associated with gastritis.28 The degree of Treg and Th17 induction in VacA and CagA mutants was similar to that in the wild-type H pylori strain, indicating that the low Th17:Treg ratio of HP-DC was not due to VacA or CagA activity (Supplementary Figure 1).
Neutralization of TGF-β and IL-10 increases the Th17:Treg ratio of HP-DC
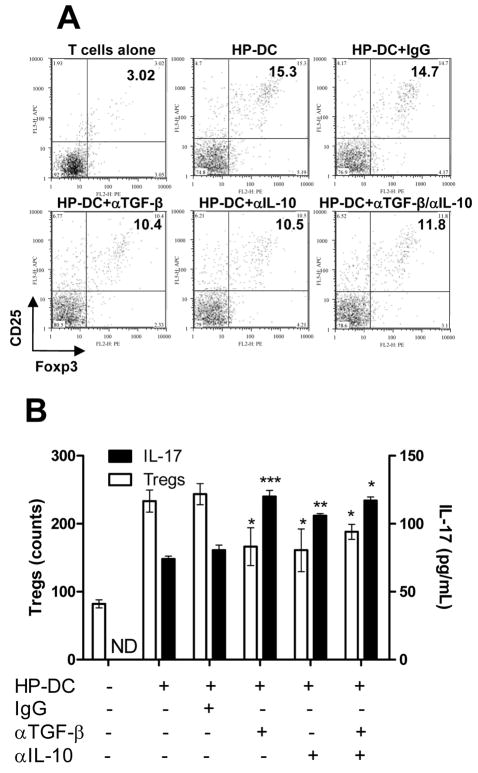
Because TGF-β is required for both Treg and Th17 induction, we studied the effect of TGF-β neutralization on the Th17/Treg balance. Our data showed that TGF-β neutralization decreased Treg induction by HP-DC but increased Th17 induction (Figure 4A and B). Neutralization of IL-10 produced similar results, indicating possible Treg inhibition of Th17 induction. The combined effect of neutralizing TGF-β and IL-10 antibodies is similar to the effect of individual antibodies. These data suggest that both TGF-β and IL-10 influence the Th17/Treg balance in the host. There appears to be a negative correlation between Th17 and Treg generation (Figure 4B).
Figure 4. Neutralization of TGF-β and IL-10 decreased Treg induction and increased Th17 induction by HP-DC.
Syngeneic naïve splenocytes cocultured for 72 h with HP-DC in the presence of PBS, control IgG, or neutralizing anti–TGF-β or anti–IL-10 antibodies separately or combined were stained for CD4+CD25+Foxp3+ Tregs. (A) Dot plot representation of CD25 and Foxp3 dual-labeled CD4+ T cells (gated on CD4+ cells). (B) Relative cell count of CD4+CD25+Foxp3+ Tregs and the Th17 response. IL-17 concentration was measured by ELISA. Blockade of TGF-β, IL-10, or combined TGF-β and IL-10 significantly reduced Treg priming and enhanced IL-17 release. (n = 3, P <.05 compared to IgG group).
Induction of H pylori–specific Treg response in vivo by adoptive transfer of H pylori SS1–pulsed DCs
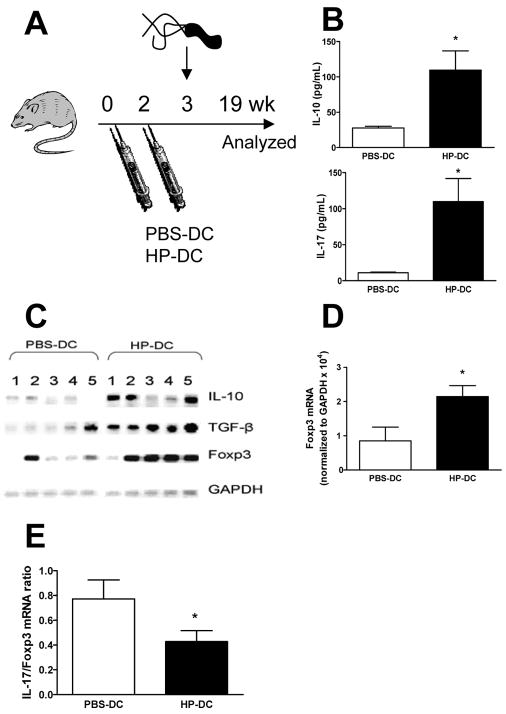
In vitro data showed that both unstimulated DCs and HP-DC induce a high number of Tregs. It is unclear whether HP-DC simply fail to recognize H pylori and thus induce a nonspecific Treg response similar to that induced by PBS-DC or if HP-DC prime an H pylori–specific regulatory response. We examined the effect of PBS-DC versus HP-DC in vivo and measured the H pylori–specific Treg response in the periphery and the stomach. Adoptive transfer with PBS-DC or HP-DC primed an in vivo adaptive response in mice; establishment of chronic H pylori infection followed (Figure 5A). Measurement of the peripheral induction of the H pylori–specific Treg response (indicated by IL-10+ production) showed that CD4+ T cells from the spleens of HP-DC transferred mice produced significantly more H pylori–specific IL-10 than PBS-DC transferred mice (Figure 5B). We speculate that the increased number of H pylori–specific Tregs in HP-DC transferred mice home to the H pylori–infected gastric mucosa, resulting in higher expression of Foxp3 as well as other Treg markers in the stomach. In fact, Foxp3, IL-10, and TGF-β expression were significantly higher in HP-DC transferred mice compared with PBS-DC transferred mice (Figure 5C). Gastric quantitative PCR analyses revealed an increase in Foxp3 mRNA expression and a decrease in IL-17/Foxp3 mRNA ratio in HP-DC transferred compared with PBS-DC transferred mice (Figure 5D and E). No significant difference in H pylori colonization or gastritis was detected (Supplementary Figure 2), possibly due to the concurrent increase in H pylori–specific immunity induced by HP-DCs.17 These data indicate that HP-DC induces a Treg-skewed response. We found increased peripheral H pylori–specific Th17 and Th1 responses in HP-DC compared to the responses in PBS-DC transferred mice (Figure 5B and Supplementary Figure 2E). Thus, HP-DC primed H pylori–specific helper T cell immunity could not promote H pylori sterilization in the stomach likely due to the concurrent induction of a strong Treg response.
Figure 5. Induction of H pylori–specific Treg response in vivo by adoptive transfer of HP-DC.
C57BL/6 mice (n = 15 per group) were adoptively IP transferred with 106 PBS-DC or HP-DC on days 0 and 14 followed by H pylori SS1 oral challenge (108 CFU/mL) on day 21. Mice were sacrificed and analyzed after 19 wk. (A) Schematic representation of the animal study. (B) MACS-sorted splenic CD4+ T cells were stimulated ex vivo for 48 h in the presence of DCs and H pylori sonicate to examine H pylori–specific release of IL-10 and IL-17. (C) The mRNA expressions of IL-10, TGF-β, Foxp3, and the housekeeping gene GAPDH (glyceraldehyde 3-phosphate dehydrogenase) were compared using RT-PCR. (D) Quantitative PCR of stomach Foxp3 mRNA expressions. Increased expressions of stomach Foxp3 was observed in HP-DC mice compared to PBS-DC mice (n = 8 mice). (E) The IL17:Fopx3 mRNA expression ratio was calculated by dividing the mRNA expressions of gastric IL-17 by the mRNA expressions of gastric Foxp3. The Th17:Treg ratio was lower in HP-DC mice compared to PBS-DC mice ( P <.05 compared to PBS-DC group).
Treg depletion in HP-DC transferred mice enhances H pylori-specific Th17 response
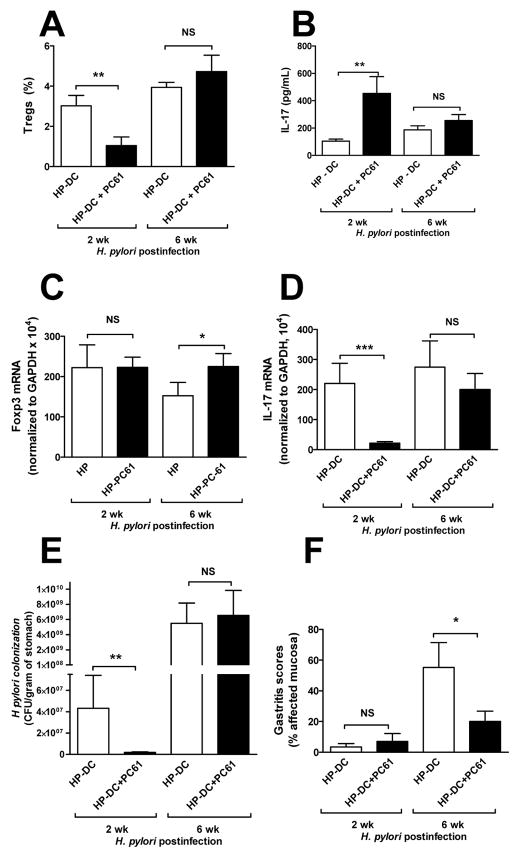
To confirm that HP-DC–induced Tregs are capable of inhibiting Th17 differentiation, the effect of CD4+CD25+ Treg depletion using anti-CD25 antibody (PC61) on Th17 induction was studied. Treatment with CD25 neutralizing antibodies using PC61 has been shown also to reduce CD4+ Foxp3+ Tregs.29 Similar to the experimental design shown in Figure 5A, HP-DC transferred mice treated with PBS or PC61 were infected with H pylori for 2 wk and then analyzed before gastritis developed. H pylori infection of such short duration eliminated the influence of inflammation on H pylori density. PC61 treatment significantly reduced the number of Tregs (Figure 6A). HP-DC transferred mice with Treg depletion showed a higher H pylori–specific Th17 response compared with controls (i.e., mice without Treg depletion) (Figure 6B). In the gastric mucosa, the Foxp3 mRNA level was similar between PC61 naïve and PC61-treated mice, while a lower level of IL-17 mRNA was observed in PC61-treated mice likely due to a lower density of H pylori in these mice (Figure 6F). There is no significant gastritis at this early phase of H pylori infection (Figure 6E). These data suggest that Treg skewing by HP-DC inhibits Th17 immunity, which appears to be important in suppressing H pylori colonization.
Figure 6. Treg depletion in HP-DC transferred mice enhances H pylori–specific Th17 response and is correlated with lower level of bacterial colonization.
C57BL/6 mice (n = 10 per group) were injected IP with PBS or PC61 (anti-CD25 monoclonal antibody, 1 mg per mouse) on day 0. Mice were then immunized with HP-DC (106 cells per injection) on days 0 and 14. Mice were orally challenged with H pylori SS1 (108 organisms) three times in 1 wk starting on day 21. Mice were sacrificed on day 35 (2 wk postinfection) or given another IP injection of PC61 on day 30 and then sacrificed on day 65 (6 wk postinfection). (A) Percentages of CD4+CD25+ Tregs from splenocytes were determined by FACS. Treatment with PC61 reduced the percentage of Tregs in mouse spleen. (B) CD4+ T cells isolated from mouse spleens using MACS MicroBeads were stimulated with PBS or H pylori sonicate in the presence of unstimulated DCs. IL-17 concentrations were measured by ELISA. PC61 treatment significantly increased the production of H pylori–specific IL-17 by CD4+ T cells (n = 10 mice per group, * P <.05). (C-D) Gastric Foxp3 and IL-17 mRNA expressions are shown. (E) H pylori colonization was lower in PC61-treated mice. (F) Gastritis score was determined in a blinded fashion by Dr. Eaton.44
Further, to assess the effect of Treg depletion during chronic H pylori infection, mice were also examined 6 wk after infection. We discovered that PC61 loses its effectiveness after 1 month despite giving an additional dose at 1 month (similar levels of splenic Tregs measured at 6 wk post infection, Figure 6A). The 6 wk data reveal a decrease in gastritis score (Figure 6F) and an increase in gastric Foxp3 expression (Figure 6C) in the PC61-treated mice. With no difference in H pylori density, the reduced gastritis suggest H pylori tolerance in the PC61 treated mice possibly related to a decrease antigenic load30 seen at 2 wk postinfection.
Th17, not Th1, response during acute H pylori infection is correlated with a lower level of H pylori colonization in HP-DC transferred mice
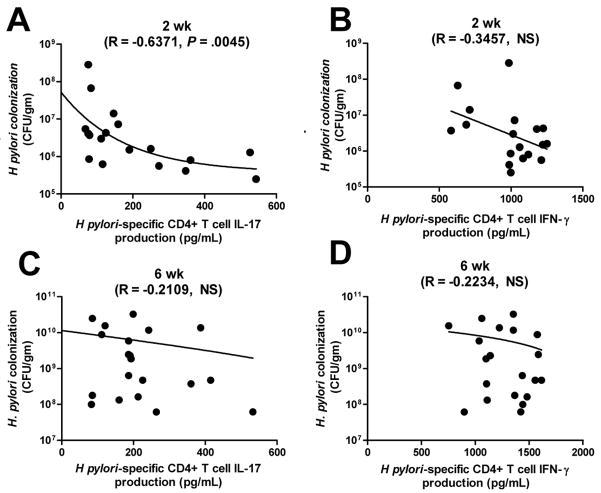
Because a reduction in H pylori colonization was associated with Treg depletion, the relative importance of Th1 versus Th17 immunity in lowering H pylori density was determined by correlating H pylori density with H pylori–specific IFN-γ or IL-17 production. A statistically significant negative correlation exists between IL-17, but not IFN-γ, production and H pylori density at 2 wk postinfection (Figure 7A and B). Treg number is also correlated inversely with H pylori–specific IL-17, but not IFN-γ, production (Supplementary Figure 3). This correlation does not exist at 6 wk postinfection. Because neutrophils are critical downstream cellular targets of Th17 in controlling extracelullar bacterial infection,12 an enhanced Th17 response during acute H pylori infection may augment the bacterial killing of H pylori. Overall, our results demonstrate a mechanism of immune escape by H pylori through the induction of Tregs, which hinders Th17 immunity, an important requirement of bacterial clearance.
Figure 7. Th17, not Th1, production is correlated with lower level of H pylori colonization in HP-DC transferred mice 2 wk postinfection.
H pylori colonization was determined by quantitative PCR of H pylori–specific 16s rRNA and then correlated with H pylori–specific IFN-γ or IL-17 production. (A-B) Two wk postinfection. (C-D) Six wk postinfection. Correlations were compared. H pylori-specific IL-17 negatively correlated with H pylori colonization at 2 wk postinfection, not 6 wk postinfection.
Discussion
There is growing evidence that H pylori–infected persons have a higher Treg response than noninfected persons; a characteristic that may contribute to immune evasion. Our observation of DCs near the surface epithelium of the gastric mucosa and their recruitment during H pylori infection is consistent with the hypothesis that DCs are critical immune mediators in the host response to H pylori infection. HP-DC induced a lower Th17/Treg response compared with EC-DC and AL-DC. Deletion of VacA and CagA did not alter Treg skewing but neutralization of TGF-β and IL-10 resulted in a shift toward a Th17 response. Further, a Th17, not Th1, response in the absence of Tregs was inversely correlated with bacterial colonization during acute H pylori infection, but not during chronic infection. Our data indicate that H pylori modulates the DC Th17/Treg differentiation pathway, which results in a Treg-skewed response. Th17, which is important for anti H pylori–immunity during acute infection, is thus suppressed and H pylori persists.
Two-photon and confocal microscopy were used to localize DCs near the surface epithelium of normal intestine and also revealed their processes that extend into the epithelial layer. Despite the failure of previous histological staining for CD11c+ DCs in normal gastric mucosa,31–32 evidence of a similar localization of DCs in gastric tissue has been regarded as critical. In this light, gastric epithelium is regarded to be the immune-sensing mechanism in the host response to H pylori. Using two-photon microscopy we showed that gastric CD11c+ DCs reside near the surface epithelium with extending processes similar to those observed in the intestine. Mice infected with H pylori were shown to recruit CD11c+ DCs to the surface epithelium with DC extensions seen on the epithelial surface. This suggests that DCs, in addition to epithelial cells, can potentially interact directly with live H pylori and propagate downstream signals to initiate a host response against the bacteria. Previous studies using conventional immunohistochemistry did not demonstrate the presence of DCs; however, two-photon imaging followed by frozen sections reveals them using CD11c-YFP mice. The discrepancy between our two-photon findings and data using immunohistochemistry could be explained by the increased sensitivity of the promoter-based approach. To our knowledge, this is the first evidence of resident gastric DCs with transepithelial extensions capable of sampling luminal contents.
As professional antigen-presenting cells, DCs orchestrate critical adaptive helper T cell responses to microbes at mucosal surfaces.13 Emerging evidence that the regulation of the Th17/Treg balance is critical for mucosal immunity,11 and reports of increased gastric expression of Foxp3 in H pylori–infected patients6, 9 raise the question: Does a high Treg response in the gastric mucosa of an H pylori–infected host give the bacteria a survival advantage by skewing the Th17/Treg balance away from Th17? Our data indicate H pylori directs a Treg-skewed DC-induced helper T cell differentiation, in contrast to the Th17-skewed response seen with proinflammatory bacteria, E coli. The H pylori–associated Treg skewing was not altered by the deletion of H pylori VacA or CagA genes. Inhibition of Treg induction by anti–TGF-β or anti–IL-10 antibodies resulted in increased Th17 induction, which is likely the result of reduced Treg inhibition of Th17 differentiation.33 Thus, the increased Treg induction in H pylori infected–hosts forces an imbalance of the Th17/Treg axis, which may lead to ineffective bacterial eradication.
Several groups have used in vitro cultures to characterize DC profiles and functions after H pylori stimulation. These include increased production of IL-12, IL-10, and IL-23, upregulation of MHC class II, CD80, CD83, and CD86 costimulatory molecules, and promotion of NK cells and Th1-effector responses.34–37 DCs isolated from H felis infected stomach also showed increased costimulatory molecules.16 We and Otsu et al previously reported H pylori-pulsed DCs were able to augment anti-H pylori immunity, but neither study was able to completely eradicate H pylori.17, 38 In this study, HP-DC induce both H pylori-specific Treg and Th1/Th17 responses in vivo but the net results is a Treg skewed response; this is a possible explanation for the inability of DC vaccine to completely eradicate H pylori.
One question remains: Does Th17 confer vaccine-induced resistance to H pylori colonization? It is well established that cellular rather than humoral immunity is required to eradicate H pylori infection according to studies showing that successful vaccination against H pylori can be achieved in mice deficient in antibodies or Th2 responses.39 As to which specific cellular response is important for eradicating H pylori, studies of IFN-γ null mice revealed mixed results, although the requirement of IL-12p40 was clear.40–41 However, because IL-12p40 is also a subunit of IL-23, a Th17-promoting cytokine, the critical immune component needed to successfully vaccinate against H pylori can be either Th1 or Th17. Our vaccine model showed an inverse correlation between the H pylori–specific Th17, not Th1, response and H pylori colonization, evidence that the Th17 response is more important than the Th1 response. This is consistent with evidence that vaccine-induced immunity is associated with an early rise in the level of IL-17 rather than IFN-γ.42–43 We speculate that IFN-γ, while not contributing directly to H pylori eradication, is responsible for disease pathology observed with chronic infection. Thus, we propose that the role of Treg induction by H pylori is to restrict Th17 development, in addition to exerting a suppressive effect on other immune cells. Inhibition of Th17 immunity may allow chronic persistence of the bacteria.
In summary, induction of Tregs by H pylori may prove to be a major adaptation to evade host immunity. We provide evidence that H pylori is able to stimulate DCs to prime Tregs. The functional significance of an H pylori–induced Treg response is the restriction of Th17 priming, which we speculate is the important immune component that limits H pylori colonization. This mechanism may also explain the negative correlation that exists between H pylori infection and the incidence of inflammatory bowel disease, a condition in which IL-17 may play a significant role; further studies will examine a causal relationship.
Supplementary Material
Acknowledgments
Grant support: This study was supported by grants from the National Institutes of Health (1 KO8 DK0669907-01 to JYK, R01 DK079798-01 to JCM) and the Foundation of Digestive Health and Nutrition.
Abbreviations
- AL-DC
dendritic cells pulsed with Acinetobacter lwoffii
- DC
dendritic cell
- EC-DC
dendritic cells pulsed with Escherichia coli
- HP-DC
dendritic cells pulsed with Helicobacter pylori
- IL
interleukin
- IFN
interferon
- IP
intraperitoneal(ly)
- PBS-DC
dendritic cells pulsed with phosphate-buffered saline
- PT-PCR
reverse transcriptase polymerase chain reaction
- TGF
transforming growth factor
- Th17 cells
IL-17+ helper T cells
- Treg
regulatory T cell
Footnotes
Financial Disclosures: No conflicts of interest exist.
Writing Assistance: None.
Author’s Contribution:
John Y. Kao - study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; technical, or material support; study supervision.
Min Zhang - study concept and design; acquisition of data; analysis and interpretation of data; statistical analysis; technical, or material support; study supervision.
Mark J. Miller - acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical and material support.
Jason C. Mills - acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical and material support.
Baomei Wang - acquisition of data; analysis and interpretation of data; technical support.
Maochang Liu - acquisition of data; analysis and interpretation of data; technical support.
Kathyn A. Eaton - analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical and material support.
Weiping Zou - critical revision of the manuscript for important intellectual content; technical and material support.
Bradford E. Berndt - acquisition of data; analysis and interpretation of data; technical support.
Tyler S. Cole - acquisition of data; analysis and interpretation of data.
Tomomi Takeuchi - acquisition of data; analysis and interpretation of data.
Stephanie Y. Owyang - acquisition of data; analysis and interpretation of data.
Jay Luther - acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perez-Perez GI, Dworkin BM, Chodos JE, et al. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109:11–7. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- 2.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 3.Cooke CL, Huff JL, Solnick JV. The role of genome diversity and immune evasion in persistent infection with Helicobacter pylori. FEMS Immunol Med Microbiol. 2005;45:11–23. doi: 10.1016/j.femsim.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Lundgren A, Suri-Payer E, Enarsson K, et al. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71:1755–62. doi: 10.1128/IAI.71.4.1755-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghavan S, Suri-Payer E, Holmgren J. Antigen-specific in vitro suppression of murine Helicobacter pylori-reactive immunopathological T cells by CD4CD25 regulatory T cells. Scand J Immunol. 2004;60:82–8. doi: 10.1111/j.0300-9475.2004.01447.x. [DOI] [PubMed] [Google Scholar]
- 6.Rad R, Brenner L, Bauer S, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–37. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Lundgren A, Stromberg E, Sjoling A, et al. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73:523–31. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandulski A, Wex T, Kuester D, et al. Naturally occurring regulatory T cells (CD4+, CD25high, FOXP3+) in the antrum and cardia are associated with higher H. pylori colonization and increased gene expression of TGF-beta1. Helicobacter. 2008;13:295–303. doi: 10.1111/j.1523-5378.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- 9.Harris PR, Wright SW, Serrano C, et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134:491–9. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–49. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLyria ES, Redline RW, Blanchard TG. Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology. 2009;136:247–56. doi: 10.1053/j.gastro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–46. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao JY, Pierzchala A, Rathinavelu S, et al. Somatostatin inhibits dendritic cell responsiveness to Helicobacter pylori. Regul Pept. 2006;134:23–9. doi: 10.1016/j.regpep.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Kao JY, Rathinavelu S, Eaton KA, et al. Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. Am J Physiol Gastrointest Liver Physiol. 2006;291:G73–81. doi: 10.1152/ajpgi.00139.2005. [DOI] [PubMed] [Google Scholar]
- 16.DDrakes ML, Czinn SJ, Blanchard TG. Regulation of murine dendritic cell immune responses by Helicobacter felis antigen. Infect Immun. 2006;74:4624–33. doi: 10.1128/IAI.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, Berndt BE, Eaton KA, et al. Helicobacter pylori-pulsed dendritic cells induce H. pylori-specific immunity in mice. Helicobacter. 2008;13:200–8. doi: 10.1111/j.1523-5378.2008.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoshi T, Zinselmeyer BH, Konjufca V, et al. Bacterial entry to the splenic white pulp initiates antigen presentation to CD8+ T cells. Immunity. 2008;29:476–86. doi: 10.1016/j.immuni.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 20.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–6. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 21.Lindquist RL, Shakhar G, Dudziak D, et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–50. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 22.Miller MJ, Safrina O, Parker I, et al. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–56. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annacker O, Coombes JL, Malmstrom V, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–61. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 26.Zavros Y, Rieder G, Ferguson A, et al. Gastritis and hypergastrinemia due to Acinetobacter lwoffii in mice. Infect Immun. 2002;70:2630–9. doi: 10.1128/IAI.70.5.2630-2639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jonge R, Kusters JG, Timmer MS, et al. The role of Helicobacter pylori virulence factors in interleukin production by monocytic cells. FEMS Microbiol Lett. 2001;196:235–8. doi: 10.1111/j.1574-6968.2001.tb10570.x. [DOI] [PubMed] [Google Scholar]
- 29.Couper KN, Blount DG, de Souza JB, et al. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J Immunol. 2007;178:4136–46. doi: 10.4049/jimmunol.178.7.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop GA, Sun J, Sheil AG, et al. High-dose/activation-associated tolerance: a mechanism for allograft tolerance. Transplantation. 1997;64:1377–82. doi: 10.1097/00007890-199711270-00001. [DOI] [PubMed] [Google Scholar]
- 31.Dixon MF. Histological responses to Helicobacter pylori infection: gastritis, atrophy and preneoplasia. Baillieres Clin Gastroenterol. 1995;9:467–86. doi: 10.1016/0950-3528(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 32.Sarsfield P, Jones DB, Wotherspoon AC, et al. A study of accessory cells in the acquired lymphoid tissue of helicobacter gastritis. J Pathol. 1996;180:18–25. doi: 10.1002/(SICI)1096-9896(199609)180:1<18::AID-PATH624>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhry A, Rudra D, Treuting P, et al. CD4+ Regulatory T Cells Control TH17 Responses in a Stat3-Dependent Manner. Science. 2009 doi: 10.1126/science.1172702. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guiney DG, Hasegawa P, Cole SP. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect Immun. 2003;71:4163–6. doi: 10.1128/IAI.71.7.4163-4166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kranzer K, Eckhardt A, Aigner M, et al. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect Immun. 2004;72:4416–23. doi: 10.1128/IAI.72.8.4416-4423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hafsi N, Voland P, Schwendy S, et al. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J Immunol. 2004;173:1249–57. doi: 10.4049/jimmunol.173.2.1249. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell P, Germain C, Fiori PL, et al. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect Immun. 2007;75:810–9. doi: 10.1128/IAI.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otsu S, Gotoh K, Yamashiro T, et al. Transfer of antigen-pulsed dendritic cells induces specific T-Cell proliferation and a therapeutic effect against long-term Helicobacter pylori infection in mice. Infect Immun. 2006;74:984–93. doi: 10.1128/IAI.74.2.984-993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garhart CA, Nedrud JG, Heinzel FP, et al. Vaccine-induced protection against Helicobacter pylori in mice lacking both antibodies and interleukin-4. Infect Immun. 2003;71:3628–33. doi: 10.1128/IAI.71.6.3628-3633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garhart CA, Heinzel FP, Czinn SJ, et al. Vaccine-induced reduction of Helicobacter pylori colonization in mice is interleukin-12 dependent but gamma interferon and inducible nitric oxide synthase independent. Infect Immun. 2003;71:910–21. doi: 10.1128/IAI.71.2.910-921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akhiani AA, Pappo J, Kabok Z, et al. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J Immunol. 2002;169:6977–84. doi: 10.4049/jimmunol.169.12.6977. [DOI] [PubMed] [Google Scholar]
- 42.Velin D, Favre L, Bernasconi E, et al. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology. 2009;136:2237–2246. e1. doi: 10.1053/j.gastro.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 43.Delyria ES, Redline RW, Blanchard TG. Vaccination of Mice Against Hpylori Induces a Strong Th-17 Response and Immunity That Is Neutrophil Dependent. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eaton KA, Danon SJ, Krakowka S, et al. A reproducible scoring system for quantification of histologic lesions of inflammatory disease in mouse gastric epithelium. Comp Med. 2007;57:57–65. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.