Abstract
The Ni(II) and Zn(II) derivatives of Desulfovibrio vulgaris rubredoxin (DvRd) have been studied by NMR spectroscopy to probe the structure at the metal centre. The βCH2 proton pairs from the cysteines that bind the Ni(II) atom have been identified using 1D NOE difference spectra and sequence specifically assigned via NOE correlations to neighbouring protons and by comparison with the published x-ray crystal structure of a Ni(II) derivative of Clostridium pasteurianum rubredoxin. The solution structures of DvRd(Zn) and DvRd(Ni) have been determined and the paramagnetic form refined using pseudocontact shifts. The determination of the magnetic susceptibility anisotropy tensor allowed the contact and pseudocontact contributions to the observed chemical shifts to be obtained. Analysis of the pseudocontact and contact chemical shifts of the cysteine Hβ protons and backbone protons close to the metal center allowed conclusions to be drawn as to the geometry and H-bonding pattern at the metal binding site. The importance of NH-S hydrogen bonds at the metal centre for the delocalization of electron spin density is confirmed for rubredoxins and can be extrapolated to metal centers in Cu proteins: amicyanin, plastocyanin, stellacyanin, azurin and pseudoazurin.
Keywords: NMR, Rubredoxin, [Fe-4S] centre, Paramagnetic protein, nickel
Introduction
Rubredoxin (Rd) belongs to the class of Fe-S proteins containing one Fe atom tetrahedrally coordinated to four cysteinyl sulphur atoms. Rd, isolated from sulphate reducing bacteria, has a molecular weight of ca. 6–7kDa and the metal atom in the native state is high spin Fe3+ (S=5/2). The reduced state has high spin Fe2+ (S=2). The cysteines that bind the metal have a conserved sequence of the type: -CX1X2CG-//-CX1X2CG-. More than 20 rubredoxin structures are to be found in the protein data bank (PDB) including 2 very high resolution structures at (0.7Å) from a P. abyssi mutant and from D. gigas [1,2].
When applying NMR to paramagnetic metalloproteins a number of problems can be encountered that include large hyperfine shifts and extensive line broadening which can result in loss of NMR signals close to the metal centre. This problem is illustrated for the solution structure of the oxidised and reduced forms of CpRd(Fe) where constraints near the metal centre are almost absent resulting in disorder close to the metal [3]. However, compared to tetrahedral Fe(II) or Fe(III) where signal loss is extensive, tetrahedral Ni(II), due to its favourable electronic relaxation properties, allows even the Hβ resonances of the coordinating cysteines to be observed, albeit at low field [4]. Nowadays the presence of a paramagnetic centre can be used to obtain pseudocontact shifts (PCS) and residual dipolar couplings (RDC) that can be combined with traditional NOESY data for structure determination [5,6]. Even if the system under study does not have an inherent metal centre one can be added to take advantage of these data [7,8]. As Rd is a small accessible protein with easy metal replacement a number of studies have used Rd as a model to attempt new structure determination approaches using PCSs and RDCs [9–15]. Also for CpRd(Fe) the relaxation properties and chemical shifts of hyperfine-shifted resonances have given information on the state of hydrogen bonding at and around the metal centre and studies, involving theoretical calculations, have shown a dependence of the redox potential on H-bond strength (essentially distance) [16–21]. The magnetic susceptibility anisotropy tensor (MST) for oxidised and reduced CpRd(Fe) has been determined and it was shown that redox dependant chemical shift changes, for protons further than ca. 11 Å from the Fe atom, were due to changes in the MST and not from structural modifications when going from the oxidised to the reduced state [22]. Also, very recently, using selective isotope labelling and novel techniques for detecting fast relaxing resonances, an almost complete assignment of the 15N and 13C signals from oxidized and reduced CpRd(Fe) has been carried out [23].
The importance of the H-bonding network in Rds has also been illustrated in a study of the Zn(II) forms of Cp and PfRd where it was suggested that the thermostability of PfRd results from a subtle redistribution of H-bonds in the β-sheet sections of the protein and at the metal centre [11]. A study of diamagnetic derivatives of Cp and PfRd [24] has further indicated that the symmetry of H-bonds to the metal-coordinated sulfurs is more closely maintained in the hyperthermophile Pf.
Ni(II) containing enzymes such as urease and hydrogenase are involved in important biochemical processes and as such they have been extensively studied. NiFe hydrogenase and carbon monoxide dehydrogenase both have a tetrahedral Ni(II) centre bound to 4 sulfurs at the active site, a centre relatively rare in biochemistry [25]. In the past, in order to study the mechanistic reaction of these (or of any metal containing) proteins, theoretical models have been used. As metal substitution is easily carried out for Rd, the Ni(II) derivative is a candidate for a model of the active site of these enzymes. There are very few NMR solution structures of Ni(II) containing proteins (PDB: 1ZRR [26], 2DEF [27], and 2GQK [28]) and the only Ni(II) containing Rd structure determined up until now is the x-ray structure of CpRd(Ni). This was resolved at relatively low resolution (2 Å) and therefore no detailed analysis was carried out, however the data indicate that the overall structures of the native Fe and Ni(II) forms are very similar [29].
Other metalloproteins containing Ni(II) studied by NMR include the Ni(II) substituted forms of azurin [30–33], amicyanin [34], pseudoazurin [35], stellacyanin [36], and umecyanin [37]. Using the crystal structure of the Cu and Ni(II) forms of azurin it was possible to calculate the axial and rhombic components of the MST. It was found that the Ni(II) form had a lower anisotropy than Co due to the presence of an extra ligand resulting in pentacoordination.
In order to probe the structure in solution of Ni(II) substituted DvRd, PCSs were obtained and combined with NOESY data. By obtaining the MST for DvRd(Ni) it was possible to predict the PCS contribution to the chemical shifts of nuclei close to the metal centre and subsequently extract their contact shift contribution. This not only allowed additional assignments to be made but it also allowed the delocalization of unpaired electron spin density onto nuclei near the metal center to be estimated. Conclusions could subsequently be drawn as to the geometry of the H-bonding network at the metal centre in this nickel derivative.
Materials and Methods
Protein purification and metal derivative preparation
Unlabeled DvRd was isolated and purified according to Bruschi et al [38]. Isotopic labeling of Rd was carried out using an identical process to that described in Goodfellow et al. [39]. The nickel form of Rd was prepared according to Moura et al. [4]. The NMR samples were prepared by exchange into phosphate buffer (10mM, pH 7.2) containing 5% D2O and by repeated concentration/dilution using a centricon YM3 concentrator (Amicon). Final sample concentrations were 1–2 mM.
NMR spectroscopy
For structure determination, backbone 1H and 15N resonances (for the Zn(II) and Ni(II) forms of DvRd) were assigned using manual methods with data from the following experiments: [1H-15N]-HSQC, 2D-NOESY (mixing time, 150 ms), 2D-TOCSY (mixing time, 70 ms), 15N-edited NOESY-HSQC (mixing time, 150 ms), and 15N-edited TOCSY-HSQC (mixing time, 70 ms). A fast recycle 2D NOESY spectrum with a 20ms mixing time, a 300ms recycle delay and a 80ppm sweep width, was also used for assignment in the case of DvRd(Ni). These spectra were run on either Bruker DRX500 or DRX600 (at NMRFAM) spectrometers using TBI and TXI probes respectively. All spectra were processed and analyzed using either NMRPipe [40], Sparky [41] or XEASY [42] software. Chemical shifts were referenced, either directly or indirectly, to DSS at 0 ppm [43].
1D NOE difference spectra were recorded at 400 and 500MHz using the super-WEFT pulse sequence [44] for water suppression (180-t-90-AQ) with t values and recycle times of ca. 150 ms. Selective saturation of the resonances was made during the delay time t. Difference spectra were obtained by subtracting the off-resonance spectra from the on-resonance spectra [45, 46].
Structure determination
Distance constraints for the DvRd(Zn) structure were obtained from 2D-NOESY and 3D 15N-edited NOESY-HSQC spectra. Structures were generated using the torsion angle dynamics program CYANA [47], followed by manual refinement of the NOE assignments to eliminate consistent violations. The coordinates and experimental constraints have been deposited in the Protein Data Bank (PDB), entry 2QL0, and the chemical shift assignments at BMRB (15374). The structure of paramagnetic DvRd(Ni) was determined using distance constraints from 1D NOE, 2D-NOESY and 3D 15N-edited NOESY-HSQC spectra. Pseudocontact shift restraints were included using the program PSEUDYANA [48] which is based on DYANA 1.5 [49]. The coordinates and experimental constraints for this form have been deposited in the PDB, entry 2KKD, and the chemical shift assignments at BMRB (15375). The minimization parameters used for the DYANA and PSEUDYANA runs are described in the results section.
The programs FANTASIAN [50] and NUMBAT [51] were used to calculate the MST parameters from PCS data. For the initial PCS tensor calculations only shifts from residues further than 8 covalent bonds from the metal atom were used in order to avoid any possible contact shift contributions (including via H-bonds, vide infra). The X-ray structures of the native Fe form of DvRd (8RXN) or the Ni(II) form of CpRd (1R0J) were used in the calculations. PCSs isosurfaces were calculated using the program NUMBAT. PyMOL [52] was used for all manipulation of structures, for the addition of hydrogen atoms when required and for graphical representations.
Results
DvRd(Zn) solution structure
A total of 90% of the 1H resonance assignments of DvRd(Zn) were obtained through standard procedures. From a total of 47 resonances in an [1H-15N]-HSQC spectrum, 44 result from main chain NH groups and 3 from the side chains of N22 (NH2) and W37 (NHε). No resonances were observed for residues M1 and K2 due to fast exchange of the amide group with the solvent under these experimental conditions.
The torsion angle dynamics program CYANA was used to calculate a family of low target function structures. The Zn(II) atom was included in the calculations by covalently linking it to the C6 Sγ atom and introducing 3 constraints to the other cysteine Sγ atoms. The tetrahedral geometry of the centre was achieved by constraining, with upper and lower constraints, the distance between the sulfur atoms of the coordinating cysteines (all with a weighting of 1). This resulted in 12 distance constraints for the Zn(II) center. These limits were calculated based on the X-ray structure of oxidised DvRd(Fe). A final total of 581 distance constraints were used (intra 122; short 147; med 121; long 203). A CYANA calculation from 200 starting structures gave a final family of 20 with an average target function value of 0.020±0.003Å2. The global RMSD values for the family were 0.58±0.18 Å and 1.00±0.19 Å for backbone and heavy atoms, respectively. A comparison of the NMR structure closest to the mean structure and an X-ray structure of oxidsed DvRd(Fe) gave a global backbone RMSD of 1.0 Å (excluding the N-terminus and 2 disordered loops; 18–25,45–47). The RMSD per residue is given as supplementary material.
Assignment of the Hβ cysteine protons in DvRd(Ni)
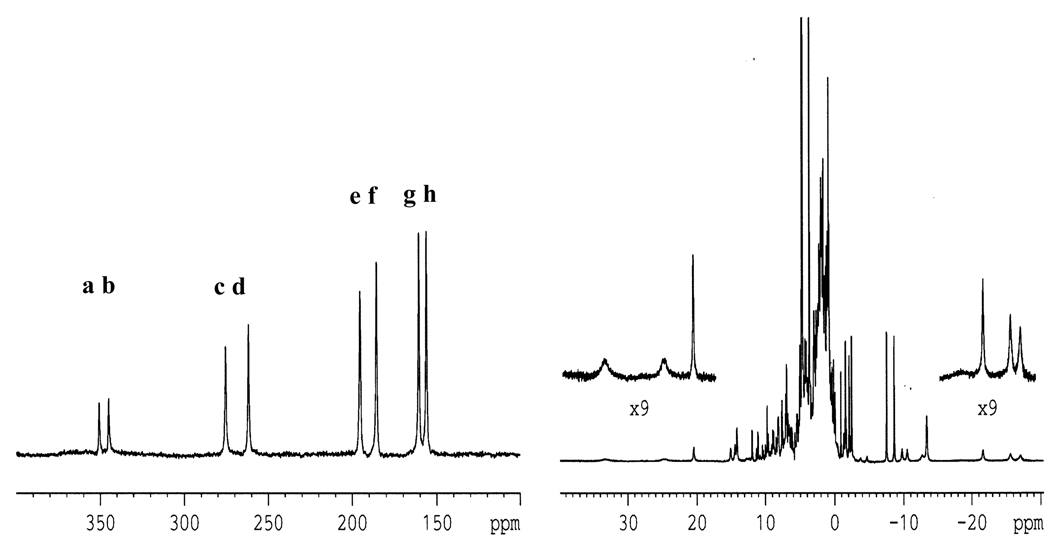
Figure 1 shows the low field 400–100ppm region of the 1H NMR spectra of DvRd(Ni) where 8 non exchangeable, resonances can be seen (a–h). 1D NOE difference spectra were recorded in D2O indicating that the resonances a/c, b/d, e/h and f/g form 4 pairs of neighbouring protons (Table 1). As these peaks are not solvent exchangeable and the cysteine Hβ protons can be expected to display the largest low field shifts due to their contact contribution, they can be assigned to the coordinating cysteines (C6, C9, C39 and C42 in Rd). Similar observations have been made for reduced CpRd(Fe) for samples selectively labelled with [2Hα]Cys or [2Hβ]Cys [19]. A combination of four 1D NOE difference spectra and a 2D NOESY spectrum (20ms) allowed sequence specific assignment for the cysteine C6 (e/h) and C39 (f/g) Hβ protons. As only peaks g and h show NOEs to the Hζ and Hε protons of F49, stereospecific assignment of these peaks to the pro-S protons of C39 and C6 respectively can be made (Table 1). By combining information from a 100ms NOESY spectrum it was also possible to identify another 1D NOE (from the irradiation of peak h) as resulting from the CH3 group of A44. This group is within 3.5 Å of the Hβ of C39. The protons of the a/c and b/d pairs only show one NOE in the region 30-5ppm. This is consistent with their assignment to either C9 or C42 as there are very few protons within 5 Å of these Hβ pairs. In both cases the closest proton to the Hβ pair is the Hα of the same residue and therefore the most intense NOE observed in both spectra was identified as the Hα proton (data not shown). In this case it was impossible to sequentially or stereospecifically assign the Hβ protons since the intensities of both NOEs were similar.
FIGURE 1.
The 1D 1H NMR spectrum of DvRd(Ni) at 302K. The low field contact shifted Hβ protons from the 4 binding cysteines can be observed between 350 and 150 ppm (a–h). Other contact and pseudocontact shifted peaks can be seen outside of the diamagnetic envelope up to +30 and down to −30 ppm. The difference in peak intensity seen for the low field shifted peaks is due to an uneven excitation profile.
TABLE 1.
1H NMR chemical shifts for the ligating cysteine Hβ protons from the Ni(II) and Fe(II) forms of Dv and Cp rubredoxin respectively and published chemical shifts for a number of Ni(II) containing azurin-like proteins. Dihedral angles for the Cα carbon of the cysteines ligating the metal atom in DvRd(Fe), CpRd(Ni) and CpRd(Fe) are taken from the x-ray structures 8RXN, 1R0J and 5RXN respectively. The average NMR chemical shifts for the cysteine Hβ protons in DvRd(Ni) and CpRd(Fe) are included along with the number of HN-S hydrogen bonds each cysteinyl sulfur is involved in. The structural data for the azurin-like proteins are for the native Cu forms and the NMR data for the Ni(II) derivatives.
| Hβ | δobs | δ1/2 | M-Sγ-Cβ-Cα | HN-S H-bondsg |
||
|---|---|---|---|---|---|---|
| DvRd(Ni) | C9/C42 | a(c) | 362 (279) | 321/320 | −94.35/−89.658RXN | 1 |
| C42/C9 | b(d) | 360 (269) | 321/320 | −89.65/−94.35 | 1 | |
| C6 proR | e(h) | 198 (161) | 183 | −172.69 | 2 | |
| C39 proR | f(g) | 188 (167) | 178 | −175.33 | 2 | |
| CpRd(Fe) | C42 proS | a(c) | 251 (233) | 242a | −83.85RXN | 1 |
| C9 proS | b(d) | 244 (233) | 239a | −90.5 | 1 | |
| C6 proR | e(h) | 196 (157) | 178a | −177.7 | 2 | |
| C39 proR | f(g) | 193 (159) | 176a | −178.2 | 2 | |
| CpRd(Ni) | C42 | −84.71R0J | 1h | |||
| C9 | −95.2 | 1 | ||||
| C6 | −167.7 | 2 | ||||
| C39 | −178.8 | 2 | ||||
| UMC | C85 proS | 224(167) | 196b | −169.61X9U | 2 | |
| AZ | C112 proS | 238 (197) | 218c | −161.42AZA | 2 | |
| AZ | C112 proS | 233 (187) | 210d | −171.04AZU | 2 | |
| AZM121Q | C112 proS | 237(178) | 208c | 169.11URI | 2 | |
| AZ | C112 proS | 238(194) | 216j | −172.72CCW | 2 | |
| STC | C87 proS | 197(177) | 187e | −176.21JER - CsSTC | 2 | |
| PA | C78 proS | 297 (274) | 285f | −169.21BQK | 1 | |
| AMI | C93 proS | 254 (296) | 275i | −171.61ID2 | 1 |
DvRd(Ni) solution structure
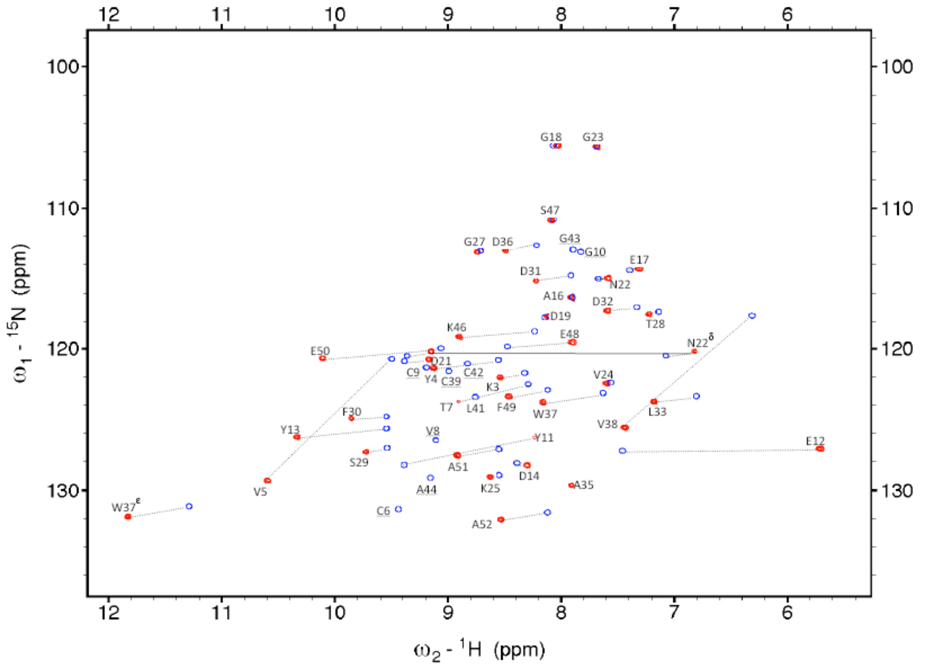
Initial resonance assignment of peaks from residues far from the metallic center was straightforward using previously assigned residues from DvRd(Zn). As expected, for residues close to the metal centre, assignment was more demanding. A combination of a 3D HSQC-NOESY and an HSQC-TOCSY spectrum allowed the assignment of the 2D [1H-15N]-HSQC spectrum of DvRd(Ni). Of 46 possible HN resonances 33 were observed along with 3 resonances from the side chains of residues N22 (NH2) and W37 (NHε). No NH resonances were observed for residues M1, K2, C6-Y11, C39, and V41-A44 in this spectrum (Figure 2). In order to obtain the resonances of nuclei near the metallic centre, 2D NOESY and TOCSY experiments with a mixing times of 20 ms and a recycle time of 300 ms were performed. In this manner a significant number of additional hyperfine shifted resonances were detected and assigned. 1D NOE difference spectra acquired by irradiating the contact shifted cysteine Hβ protons allowed further assignment. Final assignments were obtained after calculation of the MST and prediction of PCSs via the program FANTASIAN. After this process there were only 2 residues (M1 and G10) for which there were no assigned resonances.
FIGURE 2.
The [1H-15N]-HSQC spectra of DvRd(Zn) (blue) and DvRd(Ni) (red) at 296K in phosphate buffer at pH 7.2. Assignments are indicated along with selected hyperfine shifts.
A family of structures was calculated using the program PSEUDYANA [48] which allows for the inclusion of pseudocontact shifts in a torsion angle dynamics protocol. The PCSs of the 1H and 15N nuclei were determined by subtracting the diamagnetic contribution from the total hyperfine shifts, using the diamagnetic DvRd(Zn) analogue. Possible contact shift contributions (via covalent and H-bonds) were avoided by excluding pseudocontact shifts from any nucleus within 8 covalent bonds of the metal, i.e. residues V5-Y11 and V38-A44. A total of 147 1H and 15N pseudocontact shifts were initially used as restraints. The initial MST for the structure calculations was determined using the program FANTASIAN. Atomic coordinates from the X-ray structure of the Fe form of DvRd (8RXN) and the experimental PCSs served as input for this step. The subsequent calculations in PSEUDYANA use experimental PCSs and calculated NMR structures in the minimisation protocol.
The Ni(II) atom was included in the calculations using a series of linker residues placed at the C-terminus with additional constraints between the metal and the cysteine Sγ and Cβ atoms and between all cysteine Sγ atoms. In order to keep the center in a tetrahedral environment these constraints were given a weighting of 20. In addition, experimental constraints from the 2D and 3D NOESY spectra, from the fast recycle 2D NOESY and from the 1D NOE difference experiments were included to give a total of 529 constraints (161 intra, 95 short, 96 medium, 177 long). The experimental PCS were included with a weighting of 5 compared to the NOE constraints. This was due to lower weightings giving poorer definition of the metal atom. From an initial total of 800 conformers 15 gave a lowest target function of 3.65 Å2.This value is rather high, target function values of less than 1.5 Å2 are normally considered as acceptable. However, inspecting the constraint violations indicated that the PCSs for a number of side chain resonances and many 15N resonances were being violated. It has been noted previously that the use of 15N PCSs can be problematic. A study of the use of lanthanide based PCS for structure assignment [53] found that for 2 diamagnetic reference compounds, although backbone 1H chemical shifts did not change between apo-ε186 and ε186(La3+), the 15N shifts varied considerably. Also due to the small size of Rd, a large percentage of the residues are surface exposed with the possibility of motional averaging. Therefore the calculation was repeated using only backbone PCSs and no 15N PCSs giving a total of 66 restraints. PSEUDYANA does not minimize the magnitude of the MST during the structure calculation only its position and orientation. Therefore, after calculating an initial family of structures a new MST was calculated and this tensor was used in a new round of structure calculations [54]. This process was repeated 5 times resulting in an average MST with magnitude of χax −3.85±0.02×10−32 m3 and χrh −0.62±0.02×10−32 m3. The family of structures with a MST closest to this value was subsequently used.
This family of 15 structures with a maximum TF of 1.34 Å2 was obtained from an initial total of 400 conformations. Here there were 3 NOE constraints greater than 0.43 Å. The global RMSD for the family was 0.96±0.22 Å for the backbone and 1.52±0.22 Å for the heavy atoms. The constraints and RMSD per residue are given in supplementary material.
Determination of the magnetic susceptibility tensor in DvRd(Ni)
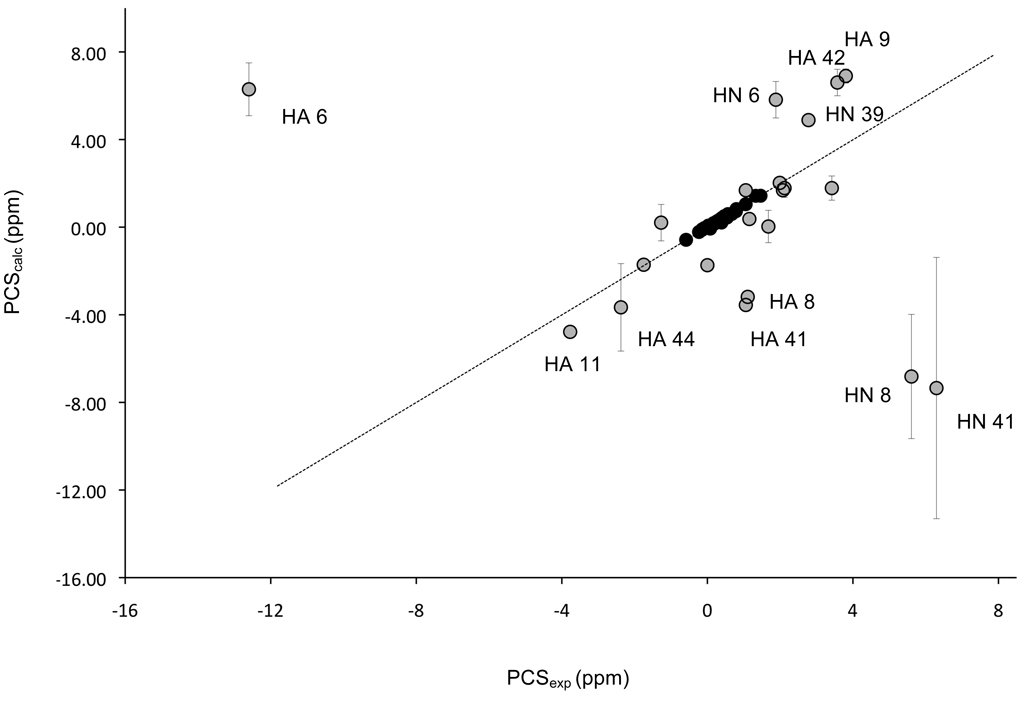
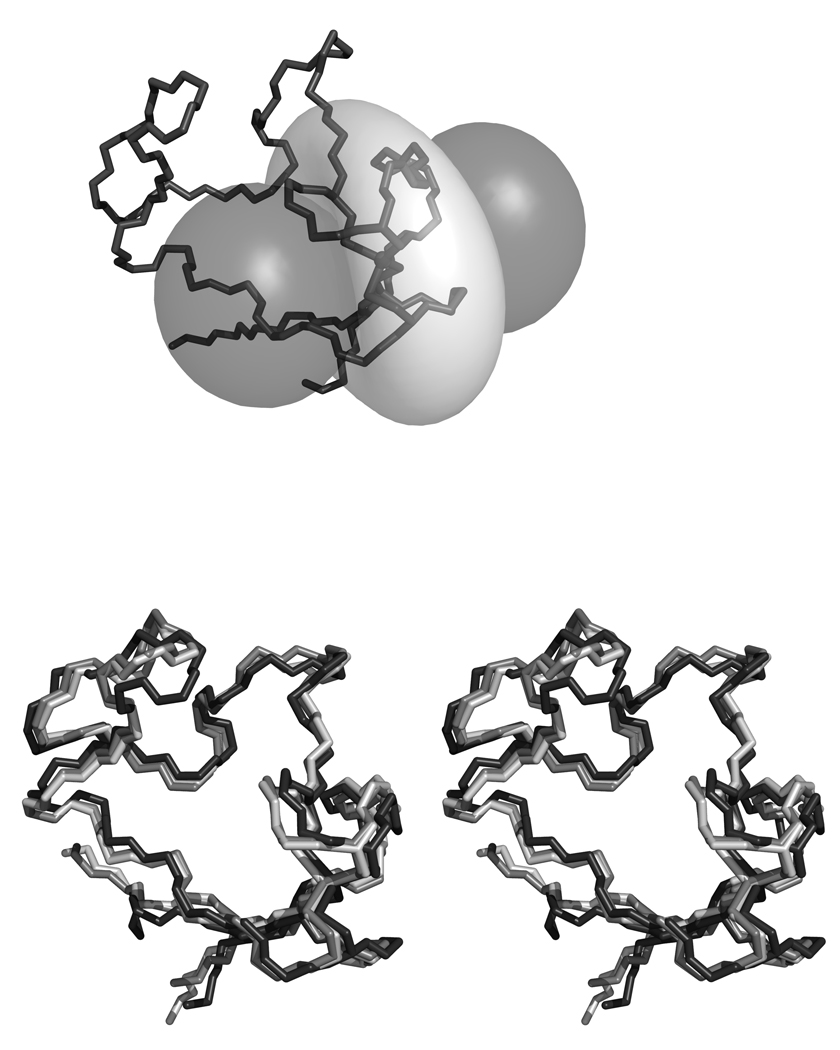
The program NUMBAT was used to calculate the MST parameters for 3 different rubredoxin structures: DvRd(Fe) (8RXN); CpRd(Ni) (1R0J); DvRd(Ni) (2KKD). Experimental PCS data from DvRd(Ni) was used in conjunction with the above atomic coordinates. For these tensor calculations only the reduced set of backbone PCSs, excluding residues further than 8 covalent bonds from the metal atom, were used to avoid any possible contact shift contribution. The 15N resonances were also excluded. The origin of the MST was constrained to the coordinates of the metal atom in the structures used for the calculations. The resulting tensors and their orientations are shown in Table 2. By plotting experimental PCSs and PCS values calculated from the fitted MST for all the PCS used in the calculation and the PCS shifts from nuclei within 8 covalent bonds of the Ni(II) atom, Figure 3, the presence of contact shift contributions to the observed chemical shifts in DvRd(Ni) can be seen (vide infra). A chemical shift pseudocontact shift isosurface at ± 1 ppm for DvRd(Ni) using the MST parameters obtained using the DvRd(Ni) NMR structure is shown in Figure 4a.
TABLE 2.
The MSTs calculated using experimental PCSs from DvRd(Ni) and the coordinates from the DvRd(Ni) NMR structure and the DvRd(Fe) and CpRd(Ni) x-ray structures. The errors presented in the table are standard deviations calculated from 100 sets of randomized (0.1 A standard deviation) atom coordinates via NUMBAT.
| Δχaxa | Δχrha | α | β | γ | |
|---|---|---|---|---|---|
|
DvRd(Fe) – PCS: 8RXN |
−3.61(0.10) | −0.88(0.05) | 148(0.3) | 69(0.6) | 167(1.8) |
|
CpRd(Ni) – PCS: 1R0J |
−3.41(0.08) | −0.92(0.05) | 57(0.5) | 96(0.4) | 59(1.8) |
|
DvRd(Ni) – PCS: 2KKD |
−3.86(0.09) | −0.62(0.07) | 174(0.6) | 121(0.9) | 81(2.9) |
×10−32 m3
FIGURE 3.
A plot of the calculated versus experimental PCSs for DvRd(Ni). The data were obtained using the MST calculated from the mean solution structure and backbone chemical shifts excluding those within 8 covalent bonds of the metal center (black circles). Also shown are the experimental and average calculated PCSs (using the DvRd(Fe), CpRd(Ni) and mean NMR structures) from backbone nuclei within 8 covalent bonds (grey circles). The error bars are the standard deviations for the results from the 3 structures. The equation of the line is y=0.984x-0.005 with an R2 of 0.98
FIGURE 4.
a) A pseudocontact chemical shift isosurface at −1 (dark grey) and +1 (light grey) ppm using the MST parameters obtained from the DvRd(Ni) NMR structure; b) A stereo overlay plot of the backbone and metal centre for the x-ray structures of DvRd(Fe) (dark grey) and CpRd(Ni) (light grey) and the mean NMR structure for DvRd(Ni) (black).
Estimation of Contact shifts in DvRd(Ni)
In order to estimate the contact shift contributions to the observed chemical shifts the diamagnetic and PCS contributions must be factored out ( δfc = δexp - δpsc - δdia ). Using the calculated MSTs from the NMR data from DvRd(Ni) and the coordinates from the DvRd(Ni) NMR structure and the DvRd(Fe) and CpRd(Ni) x-ray structures the PCS contribution to the chemical shift of nuclei close to the metal center could be calculated. These PCS values (see supplementary material) and the chemical shifts from the diamagnetic zinc form of DvRd were subtracted from the observed chemical shifts to estimate the contact shift contribution in DvRd(Ni).
Table 3 shows the average (from the 3 structures) estimated contact shifts (and the standard deviation) for the 1H nuclei within 7 bonds (covalent and/or H-bond) of the Ni(II) atom. The nuclei included in the table and the numbers in parentheses, which indicate the number of bonds removed from the metal centre, assume that the H-bonding pattern at the metal center, Figure 5, is of the standard Rd type [16].
TABLE 3.
The estimated contact shifts for all 1H nuclei within 7 bonds (covalent and/or H-bonds and assuming a standard Rd H-bonding pattern at the metal center [16]) of the Ni(II) atom. The number of bonds that separate each nucleus from the Ni(II) atom is presented in parentheses. ND = not detected. The contact shifts presented (with standard deviations) are average values calculated using the mean NMR solution structure and x-ray structure from CpRd(Ni) and DvRd(Fe).
| covalent | NH-O | NH-S | δcon | SD | covalent | NH-O | NH-S | δcon | SD | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| V5 | Hα (7) | −0.03 | 0.1 | V38 | Hα (7) | 0.39 | 0.1 | ||||
| C6 | HN (5) | −3.94 | 1.2 | C39 | HN (5) | HN (7)* | −2.11 | 0.3 | |||
| Hα (4) | −18.89 | 1.2 | Hα (4) | ND | |||||||
| Hβ (3) | 168.5 | 5.7 | Hβ (3) | 164.0 | 4.9 | ||||||
| 124.0 | 6.3 | 139.09 | 4.3 | ||||||||
| T7 | HN (6) | HN (7) | −1.48 | 0.8 | P40 | ||||||
| Hα (7) | Hα (6) | 0.78 | 0.2 | Hα (7) | Hα (6) | 1.65 | 0.7 | ||||
| V8 | HN (2+7) | 12.43 | 2.8 | V41 | HN (2+7) | 13.64 | 5.7 | ||||
| Hα (7) | Hα (5+6) | 4.61 | 0.3 | Hα (7) | Hα (5+6) | 4.29 | 0.3 | ||||
| C9 | HN (5) | HN (2+7) | ND | C42 | HN (5) | HN (2+7) | ND | ||||
| Hα (4) | Hα (5) | −3.10 | 0.3 | Hα (4) | Hα (5) | −3.03 | 0.6 | ||||
| Hβ (3) | Hβ (6) | 325.8 | 4.8 | Hβ (3) | Hβ (6) | 326.1 | 4.5 | ||||
| 264.5 | 1.4 | 257.4 | 1.2 | ||||||||
| G10 | HN (6) | HN (6) | HN (7+7) | ND | G43 | HN (6) | HN (6) | HN (7+7) | ND | ||
| Hα (7) | Hα (6) | ND | Hα (7) | Hα (6) | 1.64 | 0.6 | |||||
| 0.34 | 0.4 | ||||||||||
| Y11 | HN (2) | ND | A44 | ||||||||
| Hα (5) | 1.01 | 0.2 | Hα (5) | 1.28 | 2.0 | ||||||
| E12 | HN (7) | −0.03 | 0.2 | E50 | HN (7) | 0.39 | 0.1 |
via NH(39)-O(44)
FIGURE 5.
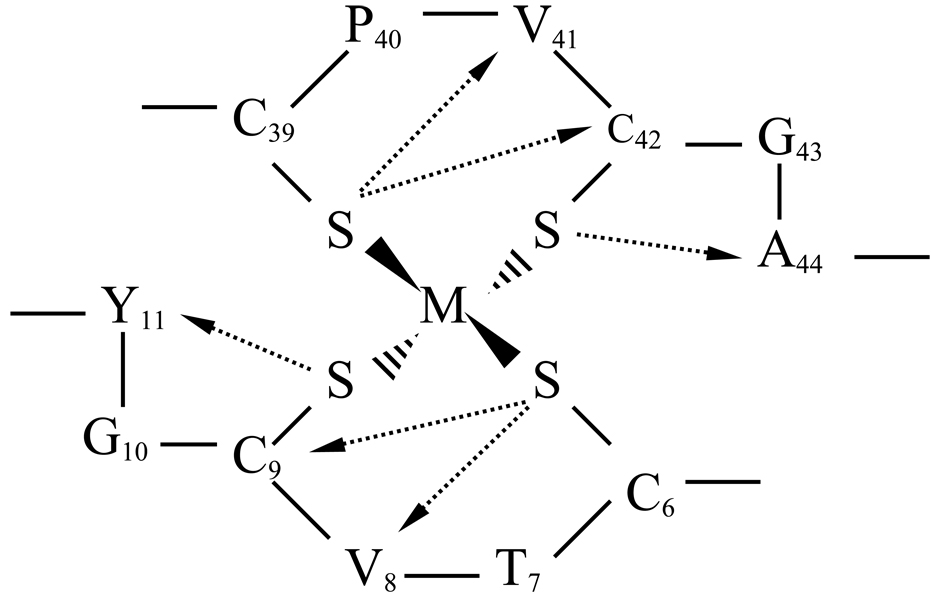
Representation of the NH to Sγ H-bonding network found at the metal center in rubredoxins. Arrows indicate the Sγ to NH direction. Amino acid numbering is for DvRd. Figure adapted from reference [19].
Discussion
The solution structures of DvRd(Zn) and DvRd(Ni)
The backbone conformation of the Zn form of DvRd is very similar to the backbone fold of the x-ray structure of DvRd(Fe), as expected. In fact the global fold of rubredoxins is conserved in almost all organisms: the maximum RMSD for the backbone alignment of the high resolution (0.5–1.5 Å) x-ray structures for Pyrococcus furiosus, Clostridium pasteurianum, Desulfovibrio gigas, Desulfovibrio vulgaris, Pyrococcus abyssi and Desulfovibrio desulfuricans is 1.5 Å2.
The solution structure of the Ni(II) form has a relatively good global backbone RMSD with poorer definition near the paramagnetic Ni(II) centre, especially the C6–C9 region, due to a lack of experimental NOE constraints. The backbone RMSD, excluding the N-terminus, to the x-ray structure of the Fe form is 1.09 Å2. The backbone RMSD of the solution structures of the Zn(II) and Ni(II) forms is 1.6 Å2. A comparison with the published x-ray structure of CpRd(Ni) indicates that the backbone conformation is very similar (bb RMSD, residues 1–52, 1.1 Å2, Figure 4b). The RMSD per residue for these comparisons is shown as supplementary material. Comparison of the geometry at the metal centre is more problematic due to the poorer definition in the NMR structure, however as the sequences of CpRd and DvRd differ only slightly near the binding cysteines: DvRd –CTVC-//-CPVCGA- and CpRd -CTVC-//-CPLCGV-; the backbone geometries would be expected to be similar.
The H-bonding network at the metal center in Rubredoxins is well known and includes a number of NH-Sγ H-bonds (Figure 5). In order to probe the H-bonding in DvRd(Ni), NH to Sγ distances in the family of solution structures were measured. It was found that of the 2 of the 6 NH-Sγ H-bonds present in most Rds, Y11-C9 and V41-C39, had longer distances than normally found: 4.50(0.54) and 3.01(0.29) Å. In order to confirm the presence of these H-bonds an analysis of the contact shift contribution to the observed chemical shifts in DvRd(Ni) was carried out.
Contact shifts in DvRd(Ni)
The estimation of contact shifts requires that the PCS and diamagnetic contributions be known. As Ni(II) has no accessible diamagnetic oxidation state another diamagnetic metal must be used. Zn(II) is a good candidate as it adopts tetrahedral coordination and Zn-S bonds lengths are comparable to Ni-S bond lengths. Figure 3 shows how the calculated PCSs for a number of backbone chemical shifts deviate from their experimental values. The error bars shown in Figure 3 are the standard deviations for the calculated PCSs using the solution structure and the 2 X-ray structures for DvRd(Fe) and CpRd(Ni). The largest standard deviations are seen for HA from residue 44 and the HN for residues 8 and 41. The variations for HN 8 and HN 41 result from structural differences between the solution and x-ray structures. This is most probably due to the poor definition, due to the lack of experimental constraints, in the solution structure for the backbone near the Ni(II) center. In fact the standard deviation for calculated PCSs between the DvRd(Fe) and CpRd(Ni) X-ray structures for these atoms is 0.24 and 0.29 respectively compared to 2.8 and 6.0 for all 3 structures (Table 3). For HA 44 the standard deviation does not change significantly by considering the X-ray structures alone (2.0 compared to 1.7). However, even assuming that the solution structure may not be well defined near the Ni(II) center structural differences alone do not explain the deviations of the calculated PCS from their experimental values. Contact shift contributions to the observed chemical shifts however can be used to explain these deviations.
A number of studies, both experimental and theoretical, have shown the importance of H-bonds (NH-O and NH-S) in Rd and changes in H-bond strength have been found to modulate the redox potential of the active site [16]. The presence of these H-bonds, especially NH-S, at the metal center can be confirmed for DvRd(Ni) in solution by considering the pattern of contact shifts shown in Table 3. Contact shifts result from the presence of unpaired electron spin density at a nucleus and it is assumed that the larger the contact shift the more unpaired electron spin density resides at a nucleus. This proportionality is valid for a single electron in an orbital which is well separated from any other excited level. This spin density can arrive via covalent bonds or via H-bonds. For DvRd(Ni), most of the larger contact shifts (> 2 ppm) in Table 3 can be explained by invoking an H-bonding network near the Ni(II) center that facilitates unpaired electron spin delocalization. Appreciable contact contributions to observed chemical shifts are seen here for protons up to 5 covalent bonds removed from the Ni(II) atom and importantly for atoms further than 5 covalent bonds from Ni(II). For instance, the 1H NH resonances for V8 and V41 are both 8 covalent bonds removed from the Ni(II) atom but have contact shifts of 12.43 and 13.64 ppm respectively. These large contact shifts can be explained by the fact that they are also involved in NH-S H-bonds to Ni-ligating Sγ atoms. Also, for the NH protons of C9 and C42 the unpaired electron spin density from the Ni(II) atom arrives via 5 covalent bonds and via 2 bonds involving an NH-S H-bond (Table 3) resulting in these resonances being undetectable under our experimental conditions. Conversely, the NHs of C6 and C39 are not involved in NH-S H-bonds and only receive unpaired electron spin density via 5 covalent bonds and are therefore detectable.
These contact shift results confirm the V8-C6, C9-C6, C42-C39 and A44-C42 NH-S H-bonds seen in the DvRd(Ni) solution structure. They also confirm that the V41-C39 NH-S H-bond is present as well, something that could not be confirmed using the solution structure alone. The final Y11-C9 NH-S H-bond was not detected in the solution structures, and the Y11 1H NH resonance could not be detected under our experimental conditions. However this fact in combination with the results of Wilkens et al [19] where DFT calculations indicated the nitrogen of Tyr11 as having the largest calculated contact electron density and the shortest NH to S H-bond in a CpRd(Fe) structure and of Lin et al [23] where the 15N resonance of Y11 in oxidised CpRd(Fe) is the most low field shifted of all the 15N hyperfine signals, suggests that the absence of this NH resonance may be due to relaxation broadening or a large hyperfine shift due to unpaired electron spin density arriving via a NH to S H-bond.
The use of NMR, more specifically a combination of experimental chemical shifts from paramagnetic and diamagnetic forms of DvRd in combination with available structures, allows the distribution of unpaired electron spin density to be determined from contact shifts and H-bonding networks to be inferred even in regions close to the metal where there is a lack of experimental constraints.
Analysis of the Hβ shifts from the binding cysteines in DvRd(Ni)
In order to determine the amount of electron spin density present at the Hβ nuclei the average chemical shift of a Hβ proton pair (δ1/2) is often used [55] as is it less sensitive to conformational changes in the Hβ-Cβ-Sγ-M dihedral angle [56]. The δ1/2 values for the Hβ proton pairs in DvRd(Ni) and CpRd(Fe) show (Table 1) that C9 (321/320 ppm) and C42 (320/321 ppm) have much higher electron spin density present at the nucleus than C6 (183 ppm) and C39 (178 ppm). A comparison of experimental NMR data with density functional calculations for the hyperfine shifts in oxidised and reduced CpRd(Fe) showed that NH-S H-bonds are very effective in transferring electron spin density from the metal to other nuclei and that small changes in NH-S H-bond distances create large changes in spin density at the resonating nuclei [18]. Intuitively, as the Sγ atoms for C6 and C39 have two NH atoms H-bonded, it may be expected to have less electron spin density due to these “extra” outlets for spin delocalisation when compared to the Sγ atoms (and attached nuclei) for C9 and C42 which only have one NH-S H-bond each. The experimental δ1/2 values, seen for DvRd(Ni), confirm that the Hβ protons of C9 and C42 have higher unpaired electron spin density compared to C6 and C39 and that the same electron spin delocalization (H-bond) pathway may be active here. This type of pattern is also seen for oxidised and reduced CpRd(Fe) where the 2H hyperfine shifts for Cys9 and Cys42 were seen further downfield (Table 1) than those for C6 and C39 [19].
In general it appears that, for a paramagnetic metal bound to cysteine in Rd, the spin density on the cysteine Hβ nuclei depends, not only on the Hβ-Cβ-Sγ-M dihedral angle, but also on the number of NH-S H-bonds to the Sγ atom : 2 NH-S H-bonds compared to 1 allow more electron spin density to be siphoned off resulting in lower δ1/2 values for the corresponding Hβ protons. NH-S H-bonds are also present in blue copper proteins and their derivatives where one ligating cysteine is present with approximately the same dihedral angle. NMR chemical shift data is available for a number of Ni(II) derivatives along with structural data from the native Cu forms. Table 1 presents the δ1/2 values for the cysteine Hβ protons for Ni(II) forms of azurin [33, 34], pseudoazurin [35], stellacyanin [36], umecyanin [37] and amicyanin [34] where it can be seen that the presence of NH-S H-bonds may be correlated to a decrease in δ1/2 values. Those cysteine Hβ protons whose Sγ atom has 2 H-bonds appear at higher field. It must be remembered however that other factors such as differences in M–S bond strength/length and the presence of axial ligands will also affect chemical shifts and may also play a role in these cases [34, 37].
Conclusions
The large number of NMR studies using diamagnetic and paramagnetic forms of the small Fe-S protein Rubredoxin in order to validate theoretical calculations, test new pulse sequences and probe unfolding pathways confirms Rd as an important metalloprotein model system. In this work the Ni(II) form of this protein was studied not only because it acts as a model for Ni(II) containing enzymes but also because Ni(II), due to its relaxation properties, allows more of the protein to be seen by NMR compared to the native Fe form. Initially solution structures of the Zn(II) and Ni(II) forms of DvRd were determined by NMR. The assignment of the spectra of the paramagnetic Ni(II) form required the use of tailored NMR experiments in conjunction with the MST obtained via PCSs. The structure of the Ni(II) form was subsequently determined using constraints from standard 2D/3D spectra, 1D NOE difference spectra and from PCS data. The structures were found to be very similar to the numerous published Rd structures obtained using NMR and x-ray.
In order to probe the geometry and H-bonding network at the metal center the contact shifts for the observable resonances near the Ni(II) center were determined by subtracting the experimental diamagnetic and calculated dipolar (PCS) shifts from the observed chemical shifts. The subsequent pattern of contact shifts for DvRd(Ni) observed in solution can be explained by invoking a H-bond network similar to that seen in a published low resolution x-ray structure from CpRd(Ni). The results also confirm the importance of the NH-S H-bonds in the distribution of electron spin density in Rd and show how structural information can be obtained from the distribution of contact shifts even when there is a lack of experimental NOE constraints.
Analysis of the contact shift dominated δ1/2 values from the cysteine Hβ protons shows how NH-S H-bonds as well as Hβ-Cβ-Sγ-M dihedral angles are important in unpaired electron spin density delocalization on these atoms. A Sγ atom involved in 2 NH-S H-bonds results in electron spin density being siphoned off and an observed shift for the Hβ protons of C6 and C39 of approximately 150–200 ppm. The presence of 1 NH-S H-bond to a Sγ atom results in more electron spin density at the Hβ protons as is the case for C9 and C42 (shifts of approximately 300–250 ppm). This type of analysis can also be applied to the Hβ protons from the single ligating cysteine ligand found in the Ni(II) forms of azurin and azurin type Cu proteins. Here a similar correlation between the δ1/2 values of the Hβ protons and the number of NH-S H-bonds (for the same Hβ-Cβ-Sγ-M dihedral angles) was found suggesting that these of type H-bonds may also play a role in the distribution/delocalization of electron spin density in these native Cu proteins as well.
Supplementary Material
Acknowledgements
This work was supported by the Fundação para a Ciência e Tecnologia POCI/QUI/57741/2004 (JJGM) and FEDER in Portugal (BD/13879/97, SGN). ICD thanks CICECO for a young researchers grant. Work in Madison USA was supported by NIH grant GM R01 GM58667 (JLM).
Abbreviations used
- NOESY
nuclear Overhauser enhancement spectroscopy
- Rd
rubredoxin
- Dv
Desulfovibrio vulgaris
- Cp
Clostridium pasteurianum
- Pf
Pyrococcus furiosus
- MST
magnetic susceptibility anisotropy tensor
- HSQC
heteronuclear single quantum coherence
- TOCSY
total correlation spectroscopy
- NOE
nuclear Overhauser enhancement
- RDC
residual dipolar coupling
- PCS
pseudocontact shift
- AZ
Azurin
- UMC
umecyanin
- PA
pseudoazurin
- STC
stellacyanin
- AMI
amicyanin
References
- 1.Bonisch H, Schmidt C, Bianco P, Ladenstein R. Acta Crystallographica Section D-Biological Crystallography. 2005;61:990–1004. doi: 10.1107/S090744490501293X. [DOI] [PubMed] [Google Scholar]
- 2.Chen C, Lin Y, Huang Y, Liu M. Biochemical and Biophysical Research Communications. 2006;349:79–90. doi: 10.1016/j.bbrc.2006.07.205. [DOI] [PubMed] [Google Scholar]
- 3.Bertini I, Kurtz D, Eidsness M, Liu G, Luchinat C, Rosato A, Scott R. Journal of Biological Inorganic Chemistry. 1998;3:401–410. [Google Scholar]
- 4.Moura I, Teixeira M, Legall J, Moura J. Journal of Inorganic Biochemistry. 1991;44:127–139. doi: 10.1016/0162-0134(91)84025-5. [DOI] [PubMed] [Google Scholar]
- 5.Bertini I, Luchinat C, Parigi G, Pierattelli R. Chembiochem. 2005;6:1536–1549. doi: 10.1002/cbic.200500124. [DOI] [PubMed] [Google Scholar]
- 6.Otting G. J Biomol NMR. 2008;42:1–9. doi: 10.1007/s10858-008-9256-0. DOI 10.1007/s10858-008-9256-0. [DOI] [PubMed] [Google Scholar]
- 7.Gaponenko V, Sarma SP, Altieri AS, Horita DA, Li J, Byrd RA. Journal of Biomolecular NMR. 2004;28:205–212. doi: 10.1023/B:JNMR.0000013706.09264.36. [DOI] [PubMed] [Google Scholar]
- 8.Gaponenko V, Altieri AS, Li J, Byrd RA. Journal of Biomolecular NMR. 2002;24:143–148. doi: 10.1023/a:1020948529076. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Valafar H, Prestegard J. Journal of Magnetic Resonance. 2005;172:85–90. doi: 10.1016/j.jmr.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Prestegard JH, Bougault CM, Kishore AI. Chemical Reviews. 2004;104:3519–3540. doi: 10.1021/cr030419i. [DOI] [PubMed] [Google Scholar]
- 11.Bougault C, Eidsness M, Prestegard J. Biochemistry. 2003;42:4357–4372. doi: 10.1021/bi027264d. [DOI] [PubMed] [Google Scholar]
- 12.Zartler E, Jenney F, Terrell M, Eidsness M, Adams M, Prestegard J. Biochemistry. 2001;40:7279–7290. doi: 10.1021/bi0026831. [DOI] [PubMed] [Google Scholar]
- 13.Tian F, Valafar H, Prestegard J. Journal of the American Chemical Society. 2001;123:11791–11796. doi: 10.1021/ja011806h. [DOI] [PubMed] [Google Scholar]
- 14.Tian F, Fowler CA, Zartler ER, Jenney FA, Adams MW, Prestegard JH. Journal of Biomolecular NMR. 2000;18:23–31. doi: 10.1023/a:1008384904380. [DOI] [PubMed] [Google Scholar]
- 15.Al-Hashimi H, Valafar H, Terrell M, Zartler E, Eidsness M, Prestegard J. Journal of Magnetic Resonance. 2000;143:402–406. doi: 10.1006/jmre.2000.2049. [DOI] [PubMed] [Google Scholar]
- 16.Lin I, Gebel E, Machonkin T, Westler W, Markley J. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14581–14586. doi: 10.1073/pnas.0505521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin I, Gebel E, Machonkin T, Westler W, Markley J. Journal of the American Chemical Society. 2003;125:1464–1465. doi: 10.1021/ja028710n. [DOI] [PubMed] [Google Scholar]
- 18.Wilkens S, Xia B, Volkman B, Weinhold F, Markley J, Westler W. Journal of Physical Chemistry B. 1998;102:8300–8305. [Google Scholar]
- 19.Wilkens S, Xia B, Weinhold F, Markley J, Westler W. Journal of the American Chemical Society. 1998;120:4806–4814. [Google Scholar]
- 20.Xia B, Wilkens S, Westler W, Markley J. Journal of the American Chemical Society. 1998;120:4893–4894. [Google Scholar]
- 21.Xia B, Westler W, Cheng H, Meyer J, Moulis J, Markley J. Journal of the American Chemical Society. 1995;117:5347–5350. [Google Scholar]
- 22.Volkman B, Wilkens S, Lee A, Xia B, Westler W, Beger R, Markley J. Journal of the American Chemical Society. 1999;121:4677–4683. [Google Scholar]
- 23.Lin IJ, Xia B, King DS, Machonkin TE, Westler WM, Markley JL. Journal of the American Chemical Society. 2009 doi: 10.1021/ja905928x. DOI 10.1021/ja905928x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeMaster D, Minnich M, Parsons P, Anderson J, Hernandez G. Journal of Inorganic Biochemistry. 2006;100:1410–1412. doi: 10.1016/j.jinorgbio.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Harrop T, Mascharak P. Model Complexes of Ni-Containing Enzymes. Weinheim: WILEY-VCH; 2006. [Google Scholar]
- 26.Pochapsky T, Pochapsky S, Ju T, Mo H, Al-Mjeni F, Maroney M. Nature Structural Biology. 2002;9:966–972. doi: 10.1038/nsb863. [DOI] [PubMed] [Google Scholar]
- 27.Dardel F, Ragusa S, Lazennec C, Blanquet S, Meinnel T. Journal of Molecular Biology. 1998;280:501–513. doi: 10.1006/jmbi.1998.1882. [DOI] [PubMed] [Google Scholar]
- 28.Banci L, Bertini I, Calderone V, Ciofi-Baffoni S, Mangani S, Martinelli M, Palumaa P, Wang S. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8595–8600. doi: 10.1073/pnas.0601375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maher M, Cross M, Wilce M, Guss J, Wedd A. Acta Crystallographica Section D-Biological Crystallography. 2004;60:298–303. doi: 10.1107/S090744490302794X. [DOI] [PubMed] [Google Scholar]
- 30.Moratal J, Salgado J, Donaire A, Jimenez H, Castells J, Martinezferrer M. Magnetic Resonance in Chemistry. 1993;31:S41–S46. [Google Scholar]
- 31.Salgado J, Jimenez H, Moratal J, Kroes S, Warmerdam G, Canters G. Biochemistry. 1996;35:1810–1819. doi: 10.1021/bi951748a. [DOI] [PubMed] [Google Scholar]
- 32.Hannan J, Davy S, Moore G, Eady R, Andrew C. Journal of Biological Inorganic Chemistry. 1998;3:282–291. [Google Scholar]
- 33.Donaire A, Salgado J, Moratal J. Biochemistry. 1998;37:8659–8673. doi: 10.1021/bi971974f. [DOI] [PubMed] [Google Scholar]
- 34.Salgado J, Kalverda A, Diederix R, Canters G, Moratal J, Lawler A, Dennison C. Journal of Biological Inorganic Chemistry. 1999;4:457–467. doi: 10.1007/s007750050332. [DOI] [PubMed] [Google Scholar]
- 35.Dennison C, Sato K. Inorganic Chemistry. 2002;41:6662–6672. doi: 10.1021/ic020303p. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez C, Sannazzaro A, Diaz L, Vila A. Inorganica Chimica Acta. 1998;273:367–371. [Google Scholar]
- 37.Dennison C, Harrison M. Journal of the American Chemical Society. 2004;126:2481–2489. doi: 10.1021/ja0375378. [DOI] [PubMed] [Google Scholar]
- 38.Bruschi M, Hatchikian EC, Legall J, Moura JJG, Xavier AV. Biochimica Et Biophysica Acta. 1976;449:275–284. doi: 10.1016/0005-2728(76)90139-0. [DOI] [PubMed] [Google Scholar]
- 39.Goodfellow BJ, Nunes SG, Rusnak F, Moura I, Ascenso C, Moura JJG, Volkman BF, Markley JL. Protein Science. 2002;11:2464–2470. doi: 10.1110/ps.0208802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 41.Goddard T, Kneller D. [Google Scholar]
- 42.Bartels C, Xia T-H, Billeter M, Güntert P, Wüthrich K. J. Biomol. NMR. 1995;5:1–10. doi: 10.1007/BF00417486. [DOI] [PubMed] [Google Scholar]
- 43.Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD. J. Biomol. NMR. 1995;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- 44.Inubushi T, Becker ED. Journal of Magnetic Resonance. 1983;51:128–133. [Google Scholar]
- 45.Macedo AL, Palma PN, Moura I, Legall J, Wray V, Moura JJG. Magnetic Resonance in Chemistry. 1993;31:S59–S67. [Google Scholar]
- 46.Bertini I, Briganti F, Luchinat C, Messori L, Monnanni R, Scozzafava A, Vallini G. Febs Letters. 1991;289:253–256. doi: 10.1016/0014-5793(91)81082-j. [DOI] [PubMed] [Google Scholar]
- 47.Herrmann T, Guntert P, Wuthrich K. Journal of Molecular Biology. 2002;319:209–227. doi: 10.1016/s0022-2836(02)00241-3. [DOI] [PubMed] [Google Scholar]
- 48.Banci L, Bertini I, Cremonini MA, Gori-Savellini G, Luchinat C, Wuthrich K, Guntert P. Journal of Biomolecular NMR. 1998;12:553–557. doi: 10.1023/A:1008388614638. [DOI] [PubMed] [Google Scholar]
- 49.Guntert P, Mumenthaler C, Wuthrich K. Journal of Molecular Biology. 1997;273:283–298. doi: 10.1006/jmbi.1997.1284. [DOI] [PubMed] [Google Scholar]
- 50.Banci L, Bertini I, Bren KL, Cremonini MA, Gray HB, Luchinat C, Turano P. Journal of Biological Inorganic Chemistry. 1996;1:117–126. [Google Scholar]
- 51.Schmitz C, Stanton-Cook MJ, Su XC, Otting G, Huber T. J Biomol NMR. 2008;41:179–189. doi: 10.1007/s10858-008-9249-z. DOI 10.1007/s10858-008-9249-z. [DOI] [PubMed] [Google Scholar]
- 52.DeLano W. 2002 [Google Scholar]
- 53.Pintacuda G, Keniry MA, Huber T, Park AY, Dixon NE, Otting G. Journal of the American Chemical Society. 2004;126:2963–2970. doi: 10.1021/ja039339m. [DOI] [PubMed] [Google Scholar]
- 54.Bertini I, Luchinat C, Parigi G, Pierattelli R. Dalton Transactions. 2008:3782–3790. doi: 10.1039/b719526e. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez CO, Niizeki T, Kohzuma T, Vila AJ. Journal of Biological Inorganic Chemistry. 2003;8:75–82. doi: 10.1007/s00775-002-0390-y. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez CO, Sannazzaro AI, Vila AJ. Biochemistry. 1997;36:10566–10570. doi: 10.1021/bi970504i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.