Abstract
The planum temporale (PT) is the bank of tissue that lies posterior to Heschl’s gyrus and is considered a key brain region involved in language and speech in the human brain. In the human brain, both the surface area and grey matter volume of the PT is larger in the left compared to right hemisphere in approximately 2/3rds of individuals, particularly among right-handed individuals. Here we examined whether chimpanzees show asymmetries in the PT for grey matter volume and surface area in a sample of 103 chimpanzees from magnetic resonance images. The results indicated that, overall, the chimpanzees showed population-level leftward asymmetries for both surface area and grey matter volumes. Furthermore, chimpanzees that prefer to gesture with their right-handed had significantly greater leftward grey matter asymmetries compared to ambiguously- and left-handed apes. When compared to previously published data in humans, the direction and magnitude of PT grey matter asymmetries were similar between humans and apes; however, for the surface area measures, the human showed more pronounced leftward asymmetries. These results suggest that leftward asymmetries in the PT were present in the common ancestor of chimpanzees and humans.
Keywords: chimpanzees, planum temporale, brain asymmetry, handedness, gestural communication
Hemispheric specialization refers to the differential representation of perceptual, motor and cognitive processes by the left and right cerebral hemispheres. Clinical and more recent functional imaging approaches have clearly demonstrated that there many functional asymmetries in the brain, including a number regions associated with the comprehension and production of language [1-5]. The two halves of the brain are also not symmetrical. There are a number of well documented neuroanatomical asymmetries in the human brain [6, 7]. Probably no other brain asymmetry has received more attention than the planum temporale (PT). The PT is the flat bank of tissue that lies posterior to Heschl’s gyrus in the superior temporal lobe. In one of the first systematic studies of asymmetries in this region, Geschwind and LeMay [8] quantified the PT on the left and right hemispheres in a sample of 100 post-mortem brains and found that the left side was larger than the right in 65% of the cases. Since that time, a number of subsequent studies in post-mortem specimens and more recently from structural magnetic resonance imaging (MRI) and voxel-based morphometry (VBM) have confirmed the leftward asymmetry in the PT surface and grey matter volume [9, 10]. The significance of the leftward asymmetry in the PT is that it overlaps with Wernicke’s area, a key brain region involved language comprehension and other complex functions [11-13]. Thus, the leftward PT asymmetry is thought to be an anatomical marker of cerebral lateralization for language, though some studies have failed to show an association between PT asymmetry and language lateralization [14-16]. From a clinical standpoint, though individual difference in PT asymmetry exists in neurologically intact populations, studies suggest that reversed or bilateral symmetry in this region are observed more often in individuals with neurodevelopmental disorders, learning disabilities [17-20] and some psychiatric conditions, notably schizophrenia [21].
There is also some evidence of sex and handedness effects on PT asymmetry in humans and this is consistent with the notion that the cerebral organization between genders and handedness groups differs with respect to language functions [22-26]. The question of sex and handedness effects on PT asymmetry are not without controversy with some authors reporting significant differences [27] while others have found the opposite effect or no difference [25]. Regarding handedness, Foundas and colleagues [28, 29] as well as Steinmetz et al. [30] have shown that right-hand individuals show a greater leftward asymmetry in the PT compared to left-handed individuals but not all studies have revealed this same results [11].
Because the PT asymmetry is presumably associated with language functions [15], there has been considerable comparative interest in neuroanatomical asymmetries in the posterior region of the temporal lobe, particularly in primates. Anatomically, there have been a significant number of indirect and direct measures of the PT in both postmortem and more recently in vivo specimens. Many studies in Old and New world monkeys have more indirectly assessed asymmetries in the PT by quantifying the length of the Sylvian fissure (SF). This is because Heschl’s gyrus, the main landmark used to demark the anterior border of the PT in the human brain, cannot be unambiguously visualized from the external morphology or from in vivo scans in Old and New World monkeys but can in great apes. For SF length, Yeni-Komshian and Benson [31] compared a sample of humans, chimpanzees and monkeys and reported leftward asymmetries in humans and chimpanzees but not monkeys. Falk and colleagues [32] initially reported a leftward asymmetry in SF from endocasts of rhesus monkeys but could not replicate this result in a subsequent study in a larger sample of subjects [33]. Heilbronner and Holloway [34] reported population-level level leftward asymmetries in SF length in two species of macaques and one species of marmoset but reported no asymmetries in squirrel monkeys. Gilissen [35] measured the SF from endocasts in a number of Old and New World monkeys and reported a significant leftward asymmetry in the SF in capuchin monkeys but not in spider monkeys. In chimpanzees, and to a lesser extent other great apes, leftward asymmetries have been reported in the PT in both cadaver [36, 37] and in vivo specimens [38-40]. For instance, Gannon et al. (1998) measured the surface area of the PT in 18 post-mortem specimens and found that 17 showed a leftward asymmetry. Cantalupo et al. (2003) measured the surface area of the PT in 28 great apes from MRI scans and similarly found significant leftward asymmetries.
One aim of this study was to further examine asymmetries in the PT of chimpanzees and to assess what effect, if any, sex and handedness have on this anatomical region. Specifically, Hopkins and Cantalupo (2004) failed to find significant sex or handedness effects on asymmetries in the surface area of the PT in chimpanzees; however, this previous study only quantified handedness for behavioral actions that were non-communicative in function. Because the PT is thought to be involved in language and communication, in this study we examined whether handedness for manual gestures was associated with asymmetries in the PT. For comparison to the measures of handedness for manual gestures, the PT asymmetries were also compared in these same chimpanzees when their handedness was classified on the basis of a task requiring coordinated bimanual actions and therefore was not communicative in function. If PT asymmetries are associated with handedness for communicative behaviors, as hypothesized, then significant handedness effects should be evident for manual gestures but not coordinated bimanual actions.
A second aim of this study was to quantify, not only the surface area corresponding to the PT, but also the grey matter volume of the left and right PT in chimpanzees. In humans, both the surface area and grey matter volume of the PT are larger in the left compared to right hemisphere. Whether this is the case in chimpanzees has yet to be tested because in all studies to date, measures of the PT were limited to the surface area. The goal in this study was to expand the scope of measurement of asymmetry in the PT beyond the surface area and to include grey matter volume. In addition, with measures of asymmetry of the PT for both the surface area and grey matter volume, this allowed us to evaluate whether handedness was better associated with variation in PT asymmetry for one or both of these measures.
Finally, there is some theoretical debate about the direction and magnitude of behavioral and brain asymmetries between human and nonhuman primates. For example, there is growing body of evidence for population-level behavioral asymmetries in vertebrates [41, 42] including for measures of handedness in nonhuman primates [43-46]. In captive chimpanzees, there is evidence of population-level handedness for several measures including throwing, coordinated bimanual actions and gestural communication [47]. In wild chimpanzees, there is evidence of population-level handedness for several tool using behaviors including termite fishing [48], ant-dipping [49, 50], wadge dipping and to a lesser extent, nut-cracking [51, 52]. In all of these reports, the proportion of right-to-left handed individuals (or left-to-right in the case of termite fishing) is about 2:1 whereas reports of human handedness put the ratio at 8:1 or 9:1 [53, 54]. To what extent biological or socio-cultural factors explain these observations are not clear because of differences in the measurement and assessment of handedness between human and nonhuman primates as well as cultural differences (i.e., chimpanzees do not have teachers putting pencils in their right hands, to give just one example). A potential and arguably better approach would be to compare human and nonhuman primates on brain asymmetries because presumably these types of measures would be objective and consistent across species. The PT is an ideal brain region for this type of analysis because identical landmarks and procedures can be used to quantify this region in both chimpanzees and humans. Thus, the third aim of this study was to compare the PT area and grey matter asymmetry measures in chimpanzees with previously reported data in the human literature that is comparable to the methods and procedures employed in this study. This allowed for a more direct comparative analysis of PT asymmetry between humans and their closest living relative, the chimpanzee.
Methods
Subjects
Magnetic resonance images were obtained from a sample of 103 chimpanzees including 41 males and 62 females. The subjects ranged in age from 6 to 51 years (Mean = 24.736, s.d. =11. 91). All the chimpanzees were members of a captive colony housed at Yerkes National Primate Research Center (YNPRC) in Atlanta, Georgia. All procedures used with the chimpanzees were approved by the Institutional Animal Care and Use Committee of Emory University. Shown in Table 1 are the handedness distributions and the number of male and female apes scanned post-mortem and in vivo in this study.
Table 1.
Sex and Handedness Compositions within the Sub-samples of Chimpanzees for each Scanner Type
| Handedness | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Gesture | Tube | ||||||
| Scanner | #Females | #Males | #L | #A | #R | #L | #A | #R |
| 3.0T | 33 | 15 | 9 | 10 | 29 | 17 | 9 | 22 |
| 1.5T | 21 | 13 | 3 | 6 | 23 | 11 | 5 | 17 |
| Cadaver | 8 | 13 | 3 | 6 | 5 | 7 | 1 | 13 |
| Total | 62 | 41 | 15 | 22 | 57 | 35 | 16 | 53 |
Note. For the handedness measures, the total sample size is less than 103 because behavioral data were not available in all subjects.
Image Collection and Procedure
Magnetic resonance images (MRI) were obtained from cadaver specimens and in vivo. The cadaver specimens (n = 21) were stored in a solution of water and 10% formaldehyde for intervals ranging from 1 week to 5 years and were scanned with a 4.7 or 7.0 Tesla magnet (Bruker, BioSpec). All of the post-mortem brain scans were of apes’ that died from natural causes and, in absolutely no cases, was euthanasia used in this study. For the in vivo scans (n =82), subjects were first immobilized by ketamine injection (10 mg/kg) and subsequently anaesthetized with propofol (40–60 mg/(kg/h)) following standard procedures at the YNPRC. Subjects were then transported to the MRI facility. The subjects remained anaesthetized for the duration of the scans as well as the time needed to transport them between their home cage and the imaging facility (total time ~ 2 h). Subjects scanned in vivo were placed in the scanner chamber in a supine position with their head fitted inside the human-head coil. Scan duration ranged between 40 and 60 min as a function of brain size. A portion of the subjects were scanned using a 1.5 Tesla scanner (Phillips, Model 51) while the remaining chimpanzees were scanned using a 3.0 Tesla scanner (Siemens Trio, Siemens Medical Solutions USA, Inc., Malvern, Pennsylvania, USA) at the YNPRC.
For all chimpanzees scanned in vivo using the 1.5 T machine (n = 34), T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition =19.0 ms, echo time =8.5 ms, number of signals averaged =8, and a 256 × 256 matrix). For the 21 postmortem scans, T2-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 22.0 s, echo time = 78.0 ms, number of signals averaged = 8-12, and a 256 × 192 matrix reconstructed to 256 × 256). For the chimpanzees scanned using the 3.0T scanner (n = 48), T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition= 2300 ms, echo time=4.4 ms, number of signals averaged=3, matrix size =320 × 320).
After completing MRI procedures, the subjects scanned in vivo were returned to the YNPRC and temporarily housed in a single cage for 6–12 h to allow the effects of the anesthesia to wear off, after which they were returned to their home cage. The archived MRI data were transferred to a PC running Analyze 7.0 (Mayo Clinic, Mayo Foundation, Rochester, Minnesota, USA) software for post-image processing.
Image Segmentation and Region of Interest
Prior to measurement, the raw T1-weighted MRI scans were aligned in the axial, coronal and sagittal planes along the AC-PC line. The aligned MRI scans were then segmented into grey, white and CSF tissue using FSL (Analysis Group, FMRIB, Oxford, UK) [55, 56] (see Figure 1). Both the surface area and grey matter volumes of the PT were quantified in the coronal plane and prior to data collection, two raters blind to the hemisphere and handedness of the chimpanzees independently measured the PT in 10 specimens. Inter-rater correlations between the two tracers were positive and significant for both the right (r = .91, p< .01) and left hemispheres (r = .97, p < .01).
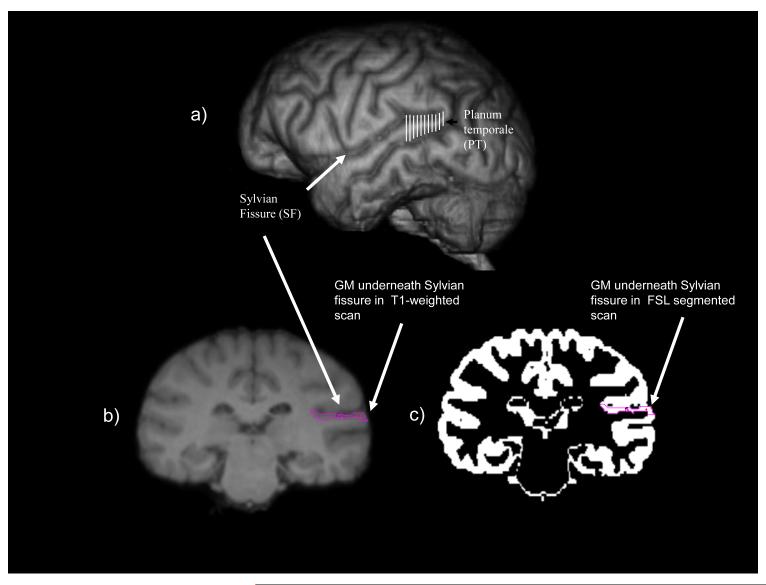
Figure 1.
a) 3-D reconstruction of a chimpanzee brain with the region corresponding to the planum temporale etched in white b) coronal view of a single 1 mm slice of an in vivo T1- weighted MRI with the sylvian fissure as well as the grey matter (GM) underneath the fissure indicated in purple. c) coronal view of the same single 1 mm slice presented on the T1-weighted image but this image displays the segmented grey matter with the corresponding area underneath the sylvian fissure indicated in purple. For all slices in which the SF was present, using a freehand tool, a line was drawn along the inferior edge of the SF from the most lateral to medial portion of the sulcus, corresponding to the most medial visible grey matter. All the grey matter forming the inferior bank of the gyrus was then traced along the lateral-medial axis.
The surface area of the PT was measured following the procedures described by Cantalupo et al. [38]. The PT was traced in the coronal plane and the anterior border was defined by the most frontal slice showing Heschl’s gyrus (HG). The posterior border was defined by the most caudal slice showing the Sylvian fissure. Once the anterior and posterior border were delineated, the depth of SF (i.e., width of PT) on each slice was measured from the superolateral margin of the superior temporal gyrus. Depth measures were taken up to the lateral ridge of HG in all the slices where HG was present (normally, HG was no longer present in slices proximal to the posterior border of PT). PT area was measured to the closest 0.1 mm using a mouse-driven computer-guided cursor available in ANALYZE 7.0. Following a well established procedure in the human literature [11], an estimate of the PT surface areas (in mm2) was computed as the sum of the cumulative PT depth measures for each slice within a hemisphere multiplied by the slice thickness.
The individual segmented GM volumes were imported into ANALYZE at a resolution of 1 mm and placed in the same stereological space as the T1-weighted MRI scan (see Figure 1). The side-by-side display of the T1-weighted and segmented image allowed for the clear delineation of the inferior border of GM for the PT region. For this measurement, using a mouse driven pointer, the raters drew a line from the most lateral portion of the sylvian fissure to the most medial point, keeping the line on the most ventral edge of the fissure. The raters then followed the grey matter to its most inferior, medial edge then followed the grey matter boundary to the lateral edge of the brain. This was repeated on each 1 mm slice moving posteriorly until the sylvian fissure fell out of view. Thus, a GM area was generated for each 1 mm slice and these areas were traced on all images in which the SF was present. If the posterior region of the SF bifurcated, the descending was followed to its end point. The individual GM areas were then summed across all slices to create GM volumes independently for the left and right hemispheres, respectively.
Handedness Measures
Manual Communicative Gestures
At the onset of each trial, an experimenter would approach the subject’s home cage and center themselves in front of the subject at a distance of approximately 1.0 to 1.5 meters. If the subject was not already positioned in front of the experimenter at the onset of the trial, the subject would immediately move towards the front of the cage when the experimenter arrived with the food. The experimenter then spoke the chimpanzee’s name and offered a piece of food until the subject produced a manual gesture. Only responses in which the chimpanzees’ unimanually extended the digit(s) through the cage mesh to request the food were considered a response. Other possible manual responses such as cage banging or clapping were not counted as a gesture. Two-handed gestures, although rare, were not scored as were gestures that were produced by the subject prior to the experimenter arriving in front of the subject’s home cage. Subjects were tested over a 15-day period, and a minimum of 30 responses were collected from each subject [57]. The number of trials administered on a given day varied with the subject’s motivation and availability for testing. Subjects were tested in both the indoor and outdoor sections of their home enclosures, and none of the subjects were separated from their groups or cagemates during testing. Hand use was recorded as right or left for each response.
Coordinated Bimanual Task (TUBE)
The second handedness measure was a task requiring bimanual coordinated actions, referred to as the TUBE task [58]. For the TUBE task, peanut butter is smeared on the inside edges of poly-vinyl-chloride (PVC) tubes approximately 15 cm in length and 2.5 cm in diameter. Peanut butter is smeared on both ends of the PVC pipe and is placed far enough down the tube such that the subjects cannot lick the contents completely off with their mouths but rather must use one hand to hold the tube and the other hand to remove the substrate. The PVC tubes were handed to the subjects in their home cages and a focal sampling technique was used to collect individual data from each subject. The hand of the finger used to extract the peanut butter was recorded as either right or left by the experimenter. Each time the subjects reached into the tube with their finger, extracted peanut butter and brought it to their mouth, the hand used was recorded as left or right.
Data Analysis
For both the PT area (Area_AQ) and GM volume (GM_AQ), asymmetry quotients (AQ) were derived following the formula [AQ = (R – L) / ((R + L) *.5)] where R and L represents the right and left hemispheres area or volume measures. Positive AQ values indicated a rightward asymmetry and negative values indicated a leftward asymmetry. For each handedness task, binomial z-scores were calculated for each subject based on the total frequency of left and right hand use. Subjects with z-scores greater than or equal to 1.96 or −1.96 were classified as right-handed and left-handed while all other subjects were classified as ambiguously-handed (z > −1.96 and z < 1.96). Using this criteria, for the manual gesture task, there were 15 left-handed, 22 ambiguously- handed and 57 right-handed individuals, respectively. For the TUBE task, there were 35 left-handed, 16 ambiguously-handed and 53 right-handed individuals.
Results
Descriptive Statistics
The left and right PT surface areas and grey matter volumes for each scanner type are shown in Table 2. In the initial analyses, we compared the left and right PT areas and GM volumes between sexes (male, female) and the scanner type (1.5, 3.0 and cadaver) using a mixed model ANOVA. Hemisphere was the repeated measure while sex and scanner types were the between group factors. For the PT surface area, a significant main effect for hemisphere was found F(1, 99) = 28.36, p < .001 with the left PT area significantly larger than the right (see Table 2). No other significant main effects or interactions were found. For the grey matter volumes, a significant main effect for hemisphere was also found F(1, 97) = 48. 32, p < .001 with left hemisphere volumes significantly larger than the right (see Table 1). No other significant main effects or interactions were found, though the main effect for scanner approached conventional levels of statistical significance F(2, 97) = 2.57, p < .09. We next considered the association between the Area _AQ and GM_AQ scores with age. The GM_AQ and Area_AQ values were significantly positively correlated with each other (r = .503, p < .001) and neither measure was associated with variation in age.
Table 2.
Mean Surface Areas and Grey Matter Volumes ( +/− s.e.) for the Left and Right Hemispheres for each Scanner Type
| PT Surface Area | PT GM Volume | |||||||
|---|---|---|---|---|---|---|---|---|
| Scanner | R | L | %Difference | t | R | L | %Difference | t |
| 3.0T | 305.97 | 341.18 | 5.66 | −4.68** | 483.04 | 533.22 | 5.16 | −3.97** |
| (11.21) | (11.93) | (18.27) | (20.90) | |||||
| 1.5T | 298.77 | 333.82 | 5.52 | −4.17** | 407.49 | 500.69 | 10.41 | −6.14 ** |
| (12.53) | (13.33) | (19.87) | (22.74) | |||||
| Cadaver | 287.29 | 303.43 | 2.59 | −1.82 + | 417.03 | 460.21 | 3.90 | −2.55 * |
| (13.84) | (14.72) | (25.07) | (28.68) | |||||
| Overall | 298.61 | 329.16 | 4.96 | −6.36*** | 438.57 | 500.32 | 6.63 | −7.21 *** |
| (6.96) | (7.54) | (11.81) | (13.33) | |||||
Values in parentheses are standard errors. All values are in mm3.
p < .001
p < .01
p < .05, + p < .10
Sex and Handedness Effects
We next considered the effect of hand preference for the TUBE and manual gesture tasks on the Area_AQ and GM_AQ values. For these analyses, the Area_AQ and GM_AQ values served as dependent variables in two separate MANOVAs. For each analysis, sex (male, female) and handedness (right, ambiguous, left) served as between group factors. For manual gestures, the MANOVA revealed a significant main effect for handedness F(4, 166) = 2.67, p < .04. Subsequent univariate F-tests indicated that there was a significant main effect for handedness for the GM_AQ values F(2, 86) = 4.63, p < .005 but not the Area_AQ values F(2, 86) = 0.72, n.s. The mean Area_AQ and GM_AQ values for right-, ambiguously- and left-handed chimpanzees are shown in Figure 2. Post-hoc analysis indicted that right-handed chimpanzees had greater leftward asymmetries in grey matter compared to the ambiguously- and left-handed chimpanzees. We also compared the grey matter volumes for the left and right PT within each handedness group. Right-handed t(56)=−6.86, p < .01 and ambiguously-handed t(21)=−2.83, p < .01 chimpanzees had larger left volumes compared to the right but no significant difference was found in left-handed apes t(14)=−0.66, n.s. No other significant main or interactions were found. The MANOVA analysis for the TUBE task failed to reveal any significant main effects or interactions.
Figure 2.
Mean grey matter volume (+/− s.e.) for the left and right PT left-, ambiguously-and right-handed chimpanzees when measured on the manual gesture task.
Discussion
Two main results emerged from this study. First, chimpanzees show significant population-level leftward asymmetries in the planum temporale when measuring both the surface area and the grey matter volumes. Second, chimpanzees that prefer to gesture with the right hand show significantly greater leftward asymmetries in the grey matter volume of the PT compared to left-handed individuals. Hand preferences for non-communicative functions were not associated with asymmetries in either the surface area or grey matter volumes of the PT.
Comparison to Human PT Asymmetries and Previous Findings in Chimpanzees
To what extent the pattern of asymmetry in the PT reported here are similar or different than those a) previously reported in chimpanzees and b) previously reported in humans is a fundamental question that has really never been addressed in the literature in here we attempt make these comparisons.
With respect to the population-level leftward asymmetries in the surface area of the PT in chimpanzees, the results reported here are consistent with previous reports in cadaver specimens and from in vivo measurement [36-38], despite the fact that the approaches and work was conducted in different laboratories (see Table 3). To compare the chimpanzee and human findings for the surface area measures, we adopted the values reported by Shapleske et al. (1999) in their review article on PT asymmetries in humans. In order to facilitate direct comparisons, we classified out subjects as being left-biased, right-biased or having no bias using the same AQ cut-points employed by Shapleske et al. (1999). Those apes with AQ values less than −.024 or greater than +.024 were classified as being left- or right-biased. All other were classified as having no bias. In the meta-analysis conducted by Shapleske et al. (1999), surface area measures of the PT were derived from more than 500 human subjects from 22 different studies and the percentage of individuals showing a leftward asymmetry was 78.34, a value nearly identical to those found when considering all the published data on chimpanzees (see Table 3). We also calculated the percentage difference in the mean surface area of the left and right PT for the chimpanzees and human data reported by Shapelske et al. (199). For both species, the left PT was, on average, ~ 13% larger than the right though, it must emphasized, we can be much more confident of the estimates of this effect size in humans due to the substantially larger sample size compared to chimpanzees.
Table 3.
Summary of PT Surface Area Asymmetries in Chimpanzees and Humans
| %L | %A | %R | N | %Difference | |
|---|---|---|---|---|---|
| Chimpanzees | |||||
| Gannon et al. [36] | 94 | 0 | 6 | 18 | 24.8 |
| Gilissen [37] | 70 | 10 | 20 | 10 | 8.0 |
| This study, 3.0T | 77 | 6 | 17 | 48 | 5.7 |
| This study, 1.5T | 68 | 12 | 21 | 34 | 5.5 |
| This study, Cadaver | 65 | 17 | 17 | 21 | 2.7 |
| Average (all samples) | 78 | 7 | 15 | 129 | 12.6 |
| Humans | |||||
| Shapleske et al. [11] | 78 | 8 | 14 | 667 | 13.2 |
%L = percent left-biased, %A = percent with no bias, %R = percent right-biased. N = sample size; % Difference = percentage difference in the PT area of the right and left hemispheres. Positive values indicate left hemisphere bias and negative values indicate right side bias.
Comparing the asymmetry in PT grey matter is more difficult because there are fewer studies that have quantified this region in humans, and as far as we know, the results reported here are the only available data in chimpanzees. For the comparison in the distribution of asymmetries, we used the data recently reported by Knaus et al. (2006) because they reported raw data on the percentage of subjects with leftward asymmetries in PT grey matter using the same cut-points as Shapelske et al. (1999). Knaus et al. (2006) reported that 71% of the subjects showed a leftward asymmetry, 4% showed no bias and 25% had a rightward bias (see Table 4). Within the chimpanzee sample, the results were comparable with 69% of the apes showing a leftward bias, 11% with no bias and 20% with a rightward bias. This pattern of results was consistent for the sub-samples of apes that were scanned with different magnets and protocols (see Table 4). At least 8 studies in humans have reported mean grey matter volumes for the left and right PT (in non-clinical samples) and, on average, the left grey matter volume is 8.9% larger than the right (see Table 4). For the chimpanzees, the left grey matter PT volume was 6.5% larger than the right, a value lower than the average for humans but within range of the values reported in these studies. In summary, for grey matter volume, the proportion of subjects showing a leftward asymmetry is comparable between humans and chimpanzees.
Table 4.
Summary of PT Grey Matter Volumetric Asymmetries in Chimpanzees and Humans
| %L | %A | %R | N | %Difference | |
|---|---|---|---|---|---|
| Chimpanzees | |||||
| This study, 3.0T | 67 | 10 | 23 | 48 | 5.2 |
| This study, 1.5T | 76 | 9 | 15 | 34 | 10.4 |
| This study, Cadaver | 61 | 17 | 22 | 21 | 2.1 |
| Average | 69 | 11 | 20 | 103 | 6.5 |
| Humans | |||||
| Knaus et al. [72] | 71 | 4 | 25 | 48 | NP |
| Kasai et al. [73] | NP | NP | NP | 22 | 13.5 |
| Knaus et al. [27] | NP | NP | NP | 24 | 10.6 |
| Hirayasu et al. [74] | NP | NP | NP | 22 | 12.1 |
| Barta et al. [75] | NP | NP | NP | 32 | −2.4 |
| Kwon et al. [76] | NP | NP | NP | 16 | 16.1 |
| Frangou et al. [77] | NP | NP | NP | 39 | 7.9 |
| Frangou et al. [78] | NP | NP | NP | 17 | 3.4 |
| McCarley et al. [79] | NP | NP | NP | 18 | 14.6 |
| Average | 190 | 9.7 |
NP = data not provided. %L = percent left-biased, %A = percent with no bias, %R = percent right-biased. N = sample size; % Difference = percentage difference in the PT grey matter volume of the right and left hemispheres. Positive values indicate left hemisphere bias and negative values indicate right side bias.
Handedness Effects
Handedness for manual gestures but not the TUBE task was associated with asymmetries in the grey matter of the PT. Neither handedness measure was associated with PT asymmetries in surface area. There are two significant points to be made regarding these findings. First, the type of handedness measure in chimpanzees has a significant impact on whether or not associations are found with brain asymmetries. Increasingly, the evidence suggests that communicative behaviors seem to be associated with asymmetries in the homologs to the human language centers whereas other types of handedness measures do not. For example, hand preferences for gestures are associated with morphological asymmetries in the inferior frontal gyrus [59] and now the planum temporale (this study). In contrast, handedness for the TUBE task, bimanual feeding and other non-communicative functions appear to be associated with other brain areas, notably the motor-hand area of the precentral gyrus [39, 60, 61]. Whether different types of handedness measures in humans are also associated with asymmetries in different brain regions remains an untested hypothesis but should be considered in future studies. It is of note that Steinmetz et al. (1991) found that self-reported handedness was not associated with asymmetries in the surface area of the PT whereas performance measures did, which we believe reinforces our view that different measures of manual asymmetry may be an important variable to consider when looking for brain-behavior associations. Second, as stated above, the association between handedness and PT asymmetries has not always revealed consistent results in humans and the majority of these studies have measured the surface area of the PT. Based on the results reported here, it appears that grey matter volumes of the region may better predict functional asymmetries (in the form of handedness) compared to surface area measure and this might explain some of the inconsistencies in the human literature [see 62].
One cautionary note from this study was the use of images obtained from different scanners and that some images were obtained in vivo and some from postmortem materials. Though no significant differences in asymmetries were found for either the surface area or grey matter between the different scanners or between the in vivo and post-mortem scans, this is a very important factor, particularly for the grey matter measures because the strength of the magnet would obviously influence the contrast of grey and white matter in the images, as was found in this report (see Table 1). For this reason, we have avoided attempting to compare the absolute grey matter volumes between the humans and chimpanzees because this is impossible without use of identical scanning protocols. Related to this limitation is the selection of the human samples that we compared to our chimpanzee results. We did not attempt to characterize all studies of the PT in humans but rather selected those that have adopted similar approaches and used reference data from several review articles. Small methodological differences did exist between the human studies we used for comparison to the chimpanzees and we cannot fully rule out the possibility that this may have influenced the results but we believe it unlikely.
The functional significance of leftward asymmetries in the PT in chimpanzees is not readily apparent but warrants further investigation. The most obvious potential parallel might be in the processing of species-specific vocalizations and this has been investigated in monkeys and apes. For example, lesions to the posterior temporal lobe induce transient deficits in the discrimination of two types of “coo” calls in Japanese macaque monkeys [63]. More recently, Poremba et al. [64] conducted a positron emission tomography (PET) study in macaque monkeys and assessed asymmetries in the temporal lobe in the perception of species-specific calls. Poremba et al. found rightward lateralized activity in the posterior temporal lobe regions and leftward activation in the temporal pole in the processing of species-specific vocalization, a finding somewhat at odds with the lesion results found in Japanese macaques [see also 65]. Taglialatela et al. [66] also conducted a PET examining the processing of two different classes of vocalizations in chimpanzees and found significant and pronounced rightward asymmetries in the posterior temporal lobe, findings also at odds with those reported in the lesion studies of Japanese macaques but more similar to those reported in the PET study by Poremba et al. (2004). These results certainly suggest that the PT region may be involved in the processing species-specific vocalizations in macaques and chimpanzees but they do not directly implicate the left PT in the processing of these sounds. This might be attributable to what acoustic features the monkeys and apes attend to in these tasks (i.e, emotive versus reference) but nonetheless, the role of the left PT remains unclear.
Lastly, the leftward asymmetries in grey matter reported here are consistent with a recent report of leftward asymmetries in the cytoarchitectonic region of the Tpt in chimpanzees. Spocter et al. [67] cytoarchitectonically quantified Brodmann’s area 22 in a sample of 12 chimpanzee post-mortem brains and found that a significant majority of the showed a leftward asymmetry BA22 volume and neuron count. Moreover, though not significant at conventional levels of significance due to the small sample size, Spocter et al. found a negative association between BA22 asymmetries and handedness for manual gestures (r = −.50) with chimpanzees who preferred to gesture with right hand much more leftward than those that did not, a result consistent with those reported in this study. Thus, for the PT, the patterns of asymmetry are consistent at the macro and microstructural levels of analysis. There remain some questions are regarding potential lateralization in connectivity within the PT and this has remained relatively uninvestigated but there is at least one report of a lack of asymmetry in minicolumns within Tpt [68]; however, this study had very few chimpanzee subjects (n = 7) and none of these apes had been behaviorally characterized, so additional studies are needed on this topic [see also 69].
In summary, the results of this study indicate that chimpanzees show a population-level leftward asymmetry in the surface area and grey matter volume of the planum temporale. These results add to a growing body of evidence demonstrating both functional and neuroanatomical asymmetries in nonhuman animals [41, 70]. Given the consistency in results on PT asymmetries across laboratories, materials, methods, and species, it is highly unlikely that these results can be attributed to experimenter or sampling bias, as suggested by some [71]. Rather the results suggest that asymmetries in the PT were present in the common ancestor of human and chimpanzees and sub-served some communicative functions. After the human-ape split, we would suggest that further elaboration of the size and connectivity of PT occurred in the human brain in response to selection for emergent perceptual and cognitive processes necessary to support the evolution of language and speech.
Acknowledgment
This research was supported in part by NIH grants NS-36605, NS-42867, HD-38051, and HD-56232. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study. We are grateful to the helpful assistance of the entire veterinary staff at the Yerkes Center for their assistance in collection of the MRI scans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Annett M. Handedness and brain asymmetry: The right shift theory. Psychology Press; Hove: 2002. [Google Scholar]
- 2.Bradshaw JL, Rogers LJ. The evolution of lateral asymmetries, language, tool use, and intellect. Academic Press, Inc.; San Diego: 1993. [Google Scholar]
- 3.Corballis MC. The lopsided brain: Evolution of the generative mind. Oxford University Press; New York: 1992. [Google Scholar]
- 4.Corballis MC. From hand to mouth: The origins of language. Princeton University Press; Princeton, NJ: 2002. [Google Scholar]
- 5.Davidson RJ. Cerebral Asymmetry, Emotion and Affective Style. In: Davidson RJ, Hugdahl K, editors. Brain Asymmetry. MIT Press; Cambridge, MA: 1995. pp. 361–387. [Google Scholar]
- 6.Toga AW, Thompson M. Mapping Brain Asymmetry. Nature. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 7.Galaburda AM. Anatomic basis of cerebral dominance. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. MIT Press; Cambridge, MA: 1995. pp. 51–70. [Google Scholar]
- 8.Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:837186–837187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 9.Good CD, et al. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal human brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 10.Watkins KE, et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cerebral Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- 11.Shapleske J, et al. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Research Reviews. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 12.Eckert MA, et al. Uncoupled leftward asymmetries for planum morphology and functional language processing. Brain and Language. 2006;98:102–111. doi: 10.1016/j.bandl.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pekkola J, et al. Attention to Visual Speech Gestures Enhances Hemodynamic Activity in the left Planum Temporale. Human Brain Mapping. 2006;27:471–477. doi: 10.1002/hbm.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorsaint-Pierre R, et al. Asymmetries of the planum temporale and Heschl’s gyrus: relationship to language lateralization. Brain. 2006;129(5):1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- 15.Josse G, et al. Left planum temporale: an anatomical marker of left hemispheric specialization for language comprehension. Cognitive Brain Research. 2003;18:1–14. doi: 10.1016/j.cogbrainres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Keller SS, et al. Broca’s area: Nomenclature, anatomy, typology and asymmetry. Brain and Language. 2009;109:29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Hugdahl K, et al. Significant relation between MR measures of planum temporale area and dichotic processing of syllables in dyslexic children. Neuropsychologia. 2003;41:666–675. doi: 10.1016/s0028-3932(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 18.Heiervang E, et al. Planum temporale, planum parietale and dichotic listening in dyslexia. Neuropsychologia. 2000;38:1704–1713. doi: 10.1016/s0028-3932(00)00085-3. [DOI] [PubMed] [Google Scholar]
- 19.Rumsey JM, et al. A magnetic resonance imaging study of planum temporale asymmetry in men with developmental dyslexia. Archives of Neurology. 1997;54:1481–1489. doi: 10.1001/archneur.1997.00550240035010. [DOI] [PubMed] [Google Scholar]
- 20.Rojas DC, et al. Smaller left hemisphere planum temporale in adults with autistic disorder. Neuroscience Letters. 2002;328:237–240. doi: 10.1016/s0304-3940(02)00521-9. [DOI] [PubMed] [Google Scholar]
- 21.Sommer I, Ramsey N, Kahn R. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: Meta-analysis. British Journal of Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- 22.Sommer IEC, et al. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127:1845–1852. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- 23.Witelson SF, Kigar DL. Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men. Journal of Comparative Neurology. 1992;323(3):326–340. doi: 10.1002/cne.903230303. [DOI] [PubMed] [Google Scholar]
- 24.Hatta T. Handedness and the brain: A review of brain-imaging techniques. Magnetic Resonance in Medical Science. 2006;6(2):99–112. doi: 10.2463/mrms.6.99. [DOI] [PubMed] [Google Scholar]
- 25.Beaton AA. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: A review of the evidence. Brain and Language. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- 26.Kulynych JJ, et al. Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl’s gyrus and the planum temporale. Cerebral Cortex. 1994;4:107–118. doi: 10.1093/cercor/4.2.107. [DOI] [PubMed] [Google Scholar]
- 27.Knaus TA, et al. Sex-linked differences in the anatomy of the persi-sylvian language cortex: A volumetric MRI study of gray matter volumes. Neuropsychology. 2004;18(4):738–747. doi: 10.1037/0894-4105.18.4.738. [DOI] [PubMed] [Google Scholar]
- 28.Foundas A, Leonard C, Heilman K. Morphological cerebral asymmetries and handedness: The pars triangularis and planum temporale. Archives of Neurology. 1995;52:501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- 29.Foundas AL, Leonard CM, Hanna-Pladdy B. Variability in the anatomy of the planum temporale and posterior ascending ramus: Do right- and left handers differ? Brain and Language. 2002;83:403–424. doi: 10.1016/s0093-934x(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 30.Steinmetz H, et al. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Annals of Neurology. 1991;29:315–319. doi: 10.1002/ana.410290314. [DOI] [PubMed] [Google Scholar]
- 31.Yeni-Komshian G, Benson D. Anatomical study of cerebral asymmetry in the temporal lobe of humans, chimpanzees and monkeys. Science. 1976;192:387–389. doi: 10.1126/science.816005. [DOI] [PubMed] [Google Scholar]
- 32.Falk D, et al. Advanced computer-graphics technology reveals cortical asymmetry in endocasts of rhesus-monkeys. Folia Primatologica. 1986;46:98–103. doi: 10.1159/000156242. [DOI] [PubMed] [Google Scholar]
- 33.Falk D, et al. Cortical asymmetries in the frontal lobe of rhesus monkeys (Macaca mulatta) Brain Research. 1990;512:40–45. doi: 10.1016/0006-8993(90)91167-f. [DOI] [PubMed] [Google Scholar]
- 34.Heilbronner PL, Holloway RL. Anatomical brain asymmetries in New World and Old World monkeys. Stages of temporal lobe development in primate evolution. American Journal of Physical Anthropology. 1988;76:39–48. doi: 10.1002/ajpa.1330760105. [DOI] [PubMed] [Google Scholar]
- 35.Gilissen E. The neocortical sulci of the capuchin monkey (Cebus): evidence for asymmetry in the sylvian sulcus and comparison with other primates. Comptes Rendus de l’Academie de Sciences Paris, Series III. 1992;314:165–170. [Google Scholar]
- 36.Gannon PJ, et al. Asymmetry of chimpanzee Planum Temporale: Humanlike pattern of Wernicke’s language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- 37.Gilissen E. Structural symmetries and asymmetries in human and chimpanzee brains. In: Falk D, Gibson KR, editors. Evolutionary anatomy of the primate cerebral cortex. Cambridge University; Cambridge: 2001. pp. 187–215. [Google Scholar]
- 38.Cantalupo C, Pilcher D, Hopkins WD. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia. 2003;41:1975–1981. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- 39.Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor but not with homologous language areas. Behavioral Neuroscience. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hopkins WD, et al. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- 41.Rogers LJ, Andrew JR. Comparative vertebrate lateralization. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- 42.Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behavioral and Brain Sciences. 2005;28:574. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- 43.Hook-Costigan MA, Rogers LJ. Hand preferences in New World primates. International Journal of Comparative Psychology. 1997;9:173–207. [Google Scholar]
- 44.Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychological Bulletin. 2006;132(4):538–559. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. [Google Scholar]
- 46.Hopkins WD, Wallis J, editors. American Socieity of Primatology. Vol. 5. Elsevier; Oxford: 2007. Evolution of hemispheric specialization in primates. [Google Scholar]
- 47.Hopkins WD. Hemispheric specialization in chimpanzees: Evolution of hand and brain. In: Shackelford T, Keenan JP, Platek SM, editors. Evolutionary Cognitive Neuroscience. MIT Press; Boston: 2007. pp. 99–120. [Google Scholar]
- 48.Lonsdorf EV, Hopkins WD. Wild chimpanzees show population level handedness for tool use. Proceedings of the National Academy of Sciences. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humle T, Matsuzawa T. Laterality in hand use across four tool use behaviors among the wild chimpanzees of Bossou, Guinea, West Africa. American Journal of Primatology. doi: 10.1002/ajp.20616. in press. [DOI] [PubMed] [Google Scholar]
- 50.Marchant LF, McGrew WC. Ant fishing by wild chimpanzees is not lateralised Primates. 2007;48:22–26. doi: 10.1007/s10329-006-0020-3. [DOI] [PubMed] [Google Scholar]
- 51.Biro D, Sousa C, Matsuzawa T. Ontogeny and cultural propagation of tool use by wild chimpanzees at Bossou, Guinea: Case studies in nut cracking and leaf folding. In: Matsuzawa T, Tomonaga T, Tanaka M, editors. Cognitive development of chimpanzees. Springer; New York: 2006. pp. 476–507. [Google Scholar]
- 52.Boesch C. Handedness in wild chimpanzees. International Journal of Primatology. 1991;6:541–558. [Google Scholar]
- 53.Perelle IB, Ehrman L. An international study of human handedness : The data. Behavior Genetics. 1994;24:217–227. doi: 10.1007/BF01067189. [DOI] [PubMed] [Google Scholar]
- 54.Raymond M, Pontier D. Is there geographical variation in human handedness? Laterality. 2004;9:35–51. doi: 10.1080/13576500244000274. [DOI] [PubMed] [Google Scholar]
- 55.Smith SM, et al. Advances in functional and structural MR image analysis and implementation of FSL. NeuroImage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Brady M, Smith SM. Segmentation of the brain MR images through hidden Markov random filed model and expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 57.Hopkins WD, et al. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes) Psychological Science. 2005;16(6):487–493. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- 59.Taglialatela JP, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. NeuroReport. 2006;17(9):923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dadda M, Cantalupo C, Hopkins WD. Further evidence of an association between handedness and neuroanatomical asymmetries in the primary cortex of chimpanzees (Pan troglodytes) Neuropsychologia. 2006;44:2482–2486. doi: 10.1016/j.neuropsychologia.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sherwood CC, et al. Histological asymmetries of primary motor cortex predicts handedness in chimpanzees (Pan troglodytes) Journal of Comparative Neurology. 2007;503:525–537. doi: 10.1002/cne.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zetzsche T, et al. In-vivo analysis of human planum temporale (PT): does the definition of PT borders influence the resulys with regard to cerebral asymmetry and correlation with handedness? Psychiatry Research: Neuroimaging Section. 2001;107:99–115. doi: 10.1016/s0925-4927(01)00087-7. [DOI] [PubMed] [Google Scholar]
- 63.Heffner HE, Heffner RS. Temporal lobe lesions and perception of species-specific vocalizations by macaques. Science. 1984;226:75–76. doi: 10.1126/science.6474192. [DOI] [PubMed] [Google Scholar]
- 64.Poremba A, et al. Species-specific calls evoke asymmetric activity in the monkey’s temporal poles. Nature. 2004;427:448–451. doi: 10.1038/nature02268. [DOI] [PubMed] [Google Scholar]
- 65.Gil-da-Costa R, et al. Species-specific calls activate homologs of Broca’s and Wernicke’s areas in the macaque. Nature Neuroscience. 2006;9(8):1064–1070. doi: 10.1038/nn1741. [DOI] [PubMed] [Google Scholar]
- 66.Taglialatela JP, et al. Visualizing vocal perception in the chimpanzee brain. Cerebral Cortex. doi: 10.1093/cercor/bhn157. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spocter MA, et al. Society for Neuroscience. Illinois; Chicago: 2009. Wernicke’s area homolog in chimpanzees (Pan troglodytes): Probabilstic mapping, asymmetry and comparison with humans. [Google Scholar]
- 68.Buxhoeveden DP, et al. Morphological differences between minicolumns in human and nonhuman primate cortex. American Journal of Physical Anthropology. 2001;115(4):361–371. doi: 10.1002/ajpa.1092. [DOI] [PubMed] [Google Scholar]
- 69.Rilling JK, et al. The evolution of the arcuate fasciculus revealed with comparative DTI. Nature Neuroscience. 2008;11:426–428. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- 70.Taglialatela JP. Functional and structural asymmetries for auditory perception and vocal production in nonhuman primates. In: Hopkins WD, editor. Evolution of hemispheric specialization in primates. Academic Press; London: 2007. [Google Scholar]
- 71.Crow T. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom): A reply to Rogers’ review of The Speciation of Modern Homo Sapiens. Laterality: Asymmetries of Body, Brain and Cognition. 2004;9:233–242. [Google Scholar]
- 72.Knaus TA, et al. Variability in perisylvian brain anatomy in healthy adults. Brain and Language. 2006;97:219–232. doi: 10.1016/j.bandl.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 73.Kasai K, et al. Progressive decrease of left heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia. Archive of Genetic Psychiatry. 2003;60:766–755. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirayasu Y, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia. Archives of General Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barta P, et al. Planum temporale asymmetry reversal in schizophrenia: Replication and relationship to gray matter abnormalities. American Journal of Psychiatry. 1997;154:661–667. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- 76.Kwon JS, et al. Left planum temporale reduction in schizophrenia. Archives of General Psychiatry. 1999;56:142–148. doi: 10.1001/archpsyc.56.2.142. [DOI] [PubMed] [Google Scholar]
- 77.Frangou S, et al. The Maudsley Family Study. 4. Normal planum temporale asymmetry in familial schizophrenia. A volumetric MRI study. The British Journal of Psychiatry. 1997;170:328–333. doi: 10.1192/bjp.170.4.328. [DOI] [PubMed] [Google Scholar]
- 78.Frangou S, et al. Small planum temporale volume in down’s syndrome: a volumetric MRI study. The American Journal of Psychiatry. 1997;154:1424–1429. doi: 10.1176/ajp.154.10.1424. [DOI] [PubMed] [Google Scholar]
- 79.McCarley RW, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Archives of General Psychiatry. 2002;59(4):321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]