Abstract
Stress and emotional brain networks foster eating behaviors that may lead to obesity. The neural networks underlying the complex interactions among stressors, body, brain and food intake are now better understood. Stressors, by activating a neural stress-response network, bias cognition toward increased emotional activity and degraded executive function. This causes formed habits to be used rather than a cognitive appraisal of responses. Stress also induces secretion of both glucocorticoids, which increases motivation for food, and insulin, which promotes food intake and obesity. Pleasurable feeding then reduces activity in the stress-response network, reinforcing the feeding habit. These effects of stressors emphasize the importance of teaching mental reappraisal techniques to restore responses from habitual to thoughtful, thus battling stress-induced obesity.
We find ourselves in the middle of a severe epidemic of obesity that affects not only adults but also has recently permeated younger generations [1–6]. An understanding of the underlying physiological causes of this growing problem is required for its solution.
The physiology of feeding behavior has been studied for many years, generally providing rodents or other experimental animals with standard lab chow. These studies using a single bland food, perforce concentrated on the hypothalamic and brainstem regulation of energy balance. The emergence of leptin as a key fat hormone that stimulates secretion of the anorexigenic and sympathetic-stimulatory neuropeptides and inhibits secretion of the orexigenic and parasympathetic-stimulatory neuropeptides was of critical importance in understanding homeostatic regulation of energy balance [1, 7– 10]. Moreover, findings about other hormonal signals that acutely affect feeding, such as ghrelin and other gut peptides activated by fasting or feeding, added to our knowledge about regulators of energy balance [9, 10]. However, it has become glaringly obvious that voluntary behaviors, stimulated by external or internal challenges or pleasurable feelings, memories and habits can override the basic homeostatic controls of energy balance [11–17].
The increased amount of perceived stress experienced by individuals in modern society affects feeding behavior [18–20]. In fact, a recent study showed that sadness favored eating of high fat/sweet, hedonically rewarding foods, whereas intake during a happy state favored dried fruit [21]. The basis for this behavior and others that lead to obesity are slowly becoming understood. They include cortical and subcortical pathways that involve learning and memory of reward and pleasure, as well as habit formation and decreased cognitive control. Elevated stress hormones and palatable food intake and the consequent accretion of fat may serve as feedback signals that reduce perceived stress [22], thus reinforcing stress-induced feeding behavior.
This review focuses on emotional and regulatory brain networks and how stress and glucocorticoid (GC) secretion foster behaviors that may lead to obesity. From large numbers of recently available structural and functional magnetic resonance imaging (MRI, fMRI) studies on people, and the relationships between stress and feeding found in selected animal studies, the role of stress on the brain and resulting behaviors is becoming rationalized. Together, these studies suggest that programs promoting learned increases in use of the executive brain during periods of stress may reduce stress-induced eating and resultant obesity.
Stress and Food Intake
People usually change their eating behaviors when they perceive themselves to be stressed, or are under persistent external inter-personal, financial or other strains. Although about 20% of people do not change feeding behaviors during stressful periods, the majority do; roughly 40% or more increase and 40% or less decrease caloric intake when stressed [14, 23–25]. In prospective studies, it appears that those who initially are at the upper range of normal, or are overweight, are generally more inclined to weight when stressed, whereas those who are of normal- or under-weight do not [2, 24, 26]. It seems possible that the difference between the gainers and the losers may be a consequence of higher insulin concentrations in people with higher body mass index.
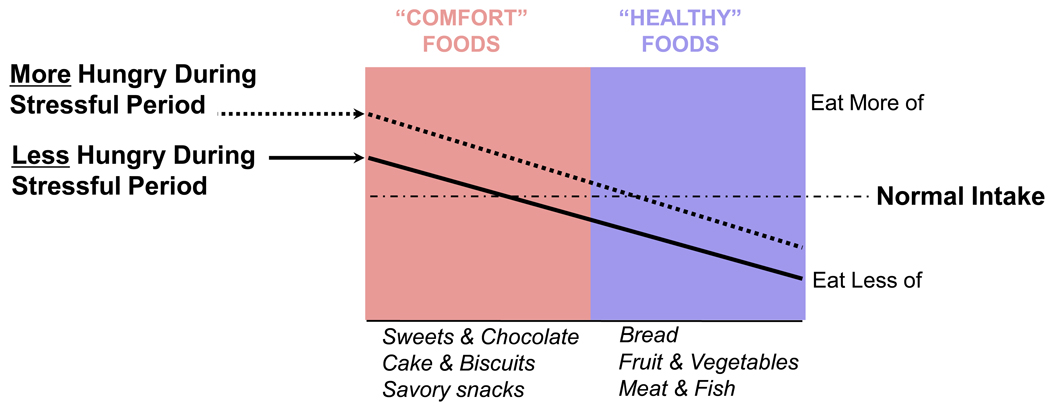
In both people and animals, a shift toward choosing more pleasurable or palatable calories occurs whether or not total caloric intake increases with stress [14, 19, 22, 27– 29]. With choice [30, 31], the foods eaten during times of stress typically favor those with increased fat and/or sugar content (Figure 1). Under controlled lab circumstances, acute physical or emotional distress induces increased intake of ‘comfort’ foods in humans and animals [21, 29–34], even when they are not hungry and have no homeostatic need for calories [31, 35].
Figure 1.
During stressful period, there is a change in what kind of food is eaten, independent of hyperphagia or hypophagia. There is a shift in food intake toward ‘comfort foods’ that is independent of whether total caloric intake increases (dashed line) or decreases (solid line) from normal intake (horizontal dot-dashed line). Student intake was retrospectively interrogated during periods of no stress (normal) or exam stress; the type of foods eaten and the amounts of foods were compared during the two periods. Whether or not food intake increased or decreased, the kind of food ingested shifted toward the more palatable sorts (from [14], with permission).
Stress-induced feeding is also observed in normal weight women who consciously monitor their food intake to remain slim exhibiting ‘dietary restraint’ (although this may reflect ‘emotional’ eating [14]) [36, 37]; such restraint may, through the mental effort it takes, itself serve as a chronic stressor [38]. Stress-induced feeding in women who practice dietary restraint may represent an ironic example that is observed when mental control is challenged by increased mental load [39]. Disinhibition of dietary restraint or possibly emotional eating is likely to occur after a stressor or in the presence of palatable foods in a social setting [37, 38]. It has also been suggested that stress-induced eating is similar to the effects of stress on relapse to drug addiction [40, 41]; indeed, the same brain networks that include both initial liking and learned motivation regulate these behaviors [42, 43].
The emotional nervous system
Studies using imaging techniques in humans have become invaluable in shedding light on the integration of emotion into behavior. Experimental animals cannot be asked how something feels except through their behaviors, and those behaviors may not be correctly interpreted or elicited in inappropriate contexts. Integration of emotion into ongoing behavior is essential to provide the motivation to perform the behaviors. This is shown by many neuroscience studies on animals, in which essentials (food and drink) are rationed, and then supplied as rewards that induce the animals to perform desired tasks.
In the last decade, it has become increasingly clear that the integrated activity of brain networks, rather than single sites, determines coherent and integrated behavioral (muscular), autonomic and endocrine responses to what is happening at that moment. Moreover it is certain that ‘what’s going on’, both outside and inside the body, perceived by the primary senses (vision, audition, touch, taste, smell) and by interoceptors (stretch, pain, temperature, chemicals and hormones) determines the basis for how one feels and the various responses that may result.
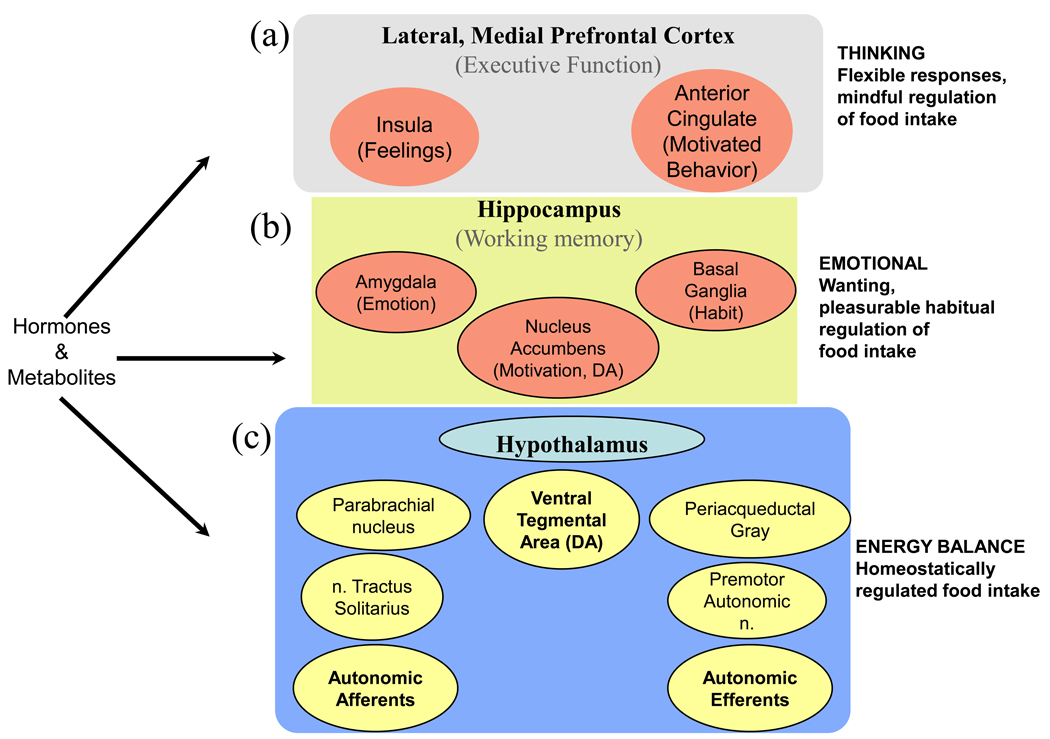
A simple sketch of the anatomical parts of brain involved in stress and feeding behavior, based on imaging studies (Figure 2) shows that the entire brain is involved. Although complex, this figure represents only some of the components in the networks of interactions between stress and obesity. At the cortical level, the emotional brain is embedded in the anterior insula that provides ‘feelings’ resulting from integration of exteroceptive and interoceptive inputs [44] and ‘motivated behavior’ resulting from integrated output from the anterior cingulate cortex [45–48]. It is proposed that an instantaneous replica of the momentary integrated information coming from inside and outside the body is copied into the anterior insula and gives rise to conscious feelings [46, 48]. The anterior insula and anterior cingulate communicate rapidly in large brained mammals (whales, humans, elephants, great apes) through specialized neurons, and the anterior cingulate serves as the consciously-motivated output in response to stimuli, based on how the ‘self’ feels [46, 49, 50]. It is very important to understand that at each organizational level (spinal, medullary, pontine, midbrain, limbic and thalamo-cortical), there is communication between the sensory inputs and motor outputs. Moreover, there is also vertical, reciprocal communication from the cortex to each of the lower levels of brain. Thus, cognitive components of the emotional nervous system can alter ongoing activity at each of the subcortical components.
Figure 2.
Brain structures engaged in feeding behaviors. (a) At the cortical level, the emotional brain is embedded in the anterior insula that provides ‘feelings’ and in the anterior cingulate cortex that governs ‘motivated behavior‘. (b) The limbic brain is responsible for emotional (amygdala), motivational (nucleus accumbens), and habitual (basal ganglia) responses, while (c) the brainstem, containing the hypothalamus, brainstem and spinal cord, regulates energy balance. Afferent inputs to the brain and to emotional and cortical structures are shown on the left, and efferent outputs from cortical and sub-cortical habitual and emotional structures are shown on the right. Horizontal interactions between afferent and efferent components exist at each level, and structures are also bidirectionally connected vertically. While the hypothalamic neurons are sufficient to regulate energy intake, components in the limbic brain and frontal cortex can override the basic maintenance of energy balance and result in either an underweight or overweight phenotype. The cell groups shown in yellow represent the brainstem and spinal cord portion of the brain that is engaged with the homeostatic maintenance of energy balance. The cell groups shown in orange can cause either an increase or decrease in food intake. DA, drug addiction.
The components essential for homeostatic regulation of energy balance are shown in Figure 2. Neurons in the hypothalamus, brainstem and afferent nerves are well studded with leptin, insulin and other hormonal receptors [51–53]. These subcortical sites are sufficient to regulate adequate food intake to sustain energy stores [54–56]. However, components in the limbic brain and frontal cortex (Figure 2) can override the basic maintenance of energy balance and result in either an underweight or overweight phenotype [13].
Positron emission tomography (PET) studies and fMRI analysis found that the amygdalae specifically respond to both positive and negative alerting stimuli in humans [57]. The nuclei accumbens, innervated by dopaminergic neurons from the ventral tegmental area in the brainstem, provide motivation to accomplish a behavior, either at the automatic, habitual level, through the basal ganglia [58], or consciously and with forethought, through the prefrontal cortex [12, 13, 59]. When a verbal instruction about how to deal with emotion-provoking stimuli (reappraise) is given to subjects before the stimuli, the prefrontal response increases and the amygdalar response decreases and may be entirely inhibited [57, 60, 61]. Such verbal instruction, or chemical manipulations of the prefrontal cortex in rodents, activates the infralimbic prefrontal cortex, which features prominently in extinction behavior, and can inhibit amygdalar and accumbal as well as adrenocortical activity [62–64]. This highly complex region is where cognitive control of feelings and motivation is regulated [65, 66]; it is activated in individuals with restriction eating disorders (Box 1) who are shown pictures of palatable foods [67]. Simplistically, at the unconscious level, the amygdalae are the sites at which emotions arise and the nuclei accumbens provide motivation to carry out habitual behaviors appropriate to those emotions, whereas the cognitive, executive control of emotions and drive is heavily regulated by prefrontal cortical structures [68].
Box 1. Restriction Eating Disorders
Restriction eating disorders are reviewed in [100–103]. Anorexia Nervosa (AN) is excessive and habitual regulation of restricted food intake, while Bulimia Nervosa is restricted food intake with frequent loss of control and binge eating followed by purging [104]. Many people who do not meet criteria for an eating disorder, do restrict their food intake with the goal of weight stability, and some of these restricted individuals eat more than they feel they should, during disinhibition of restriction. There is some disagreement about what tests best detect restricted eaters and restricted eaters with disinhibition [105]. However, restricted eaters, like patients with AN, appear to engage the prefrontal cortex when shown highly palatable foods, while disinhibited restrictors engage more amygdalar activity [106].
Stress, glucocorticoids, corticotropin-releasing factor (CRF) and the emotional nervous system
Threatening and cognitively meaningful stimuli activate the emotional nervous system in humans and lab animals [22, 69]; the emotional brain and other prefrontal cortical outputs determine to a large extent what behavioral output (e.g., fight, flight or freezing) will be chosen [22]. Stress-induced elevations in glucocorticoid (GC) secretion appear to intensify emotions and motivation [69, 70]. An overview of the effects of elevating GC on a variety of rat behaviors drawn from different studies suggests strongly that this stress hormone increases wanting, or motivation. It is clear that context and training, as well as conditions for testing the animal [71], are essential for a given effect; however, in all cases, increasing GC increases the amplitude of the behavioral effect, perhaps through actions at the ventral basal ganglia in rats, and possibly in human [72].
Stressors that provoke hypothalamo-pituitary-adrenal (HPA) GC secretion also recruit a central stress-response system, mediated by corticotrophic releasing factor (CRF) neurons in the amygdala, that appears to bias normal responses toward net increased output of activity from the limbic brain that contains the amygdala and nucleus accumbens ([73], Figure 2). Elevated GC are required to stimulate CRF synthesis and secretion from the central nucleus of the amygdala and other limbic sites, and axons from these CRF neurons innervate much of the limbic and cortical brain, where CRF receptors are found. They also innervate and affect activity in the brainstem monoaminergic (noradrenergic, serotoninergic and dopaminergic) neurons, which are responsible for alerting not only the brain to cause discriminative and motivated behaviors, but also the hindbrain and premotor autonomic neurons [69, 74, 75]. GCs act additionally on the monoaminergic neurons to increase their amine synthesis and secretion [69, 75]. The resulting activation of this limbic stress-response network ensures that the HPA response to renewed stressors remains normal or facilitated, and overrides GC-induced inhibition of CRF synthesis and secretion in the hypothalamic motor neurons of the HPA axis. This may explain the apparent insensitivity to exogenous dexamethasone or other steroid feedback tests in chronically stressed individuals (see [69, 74, 75]), and certainly prepares the stressed organism to respond to future insults.
GC receptors are also heavily expressed in the executive brain of the prefrontal cortices, and chronic stressors activate norepinephrine secretion over these sites where GC implants inhibit stress responses (reviewed in [63]); the executive brain has the potential to control activity in limbic brain when it is engaged [65], although in stressed rodents, and perhaps man, executive dysfunction and prefrontal cortical remodeling occurs [76, 77].
GC, Food Intake and Insulin - ‘Comfort Foods’
GC infusions increase caloric intake in both humans and rats [70, 78]. Interestingly, with only chow available, adrenalectomized rats treated with various levels of corticosterone do not reliably eat more chow, but if sucrose and/or fat is available, the rats increase intake of these foods in proportion to the circulating GC concentrations [70]. It is important to note that, as GCs increase, insulin secretion also increases, as is well-known from the strong association of Cushing’s syndrome with type 2 diabetes [79]. In fact, when adrenalectomized rats are made diabetic with streptozotocin, they increase chow intake in proportion to GC levels, but no longer increase sucrose intake above the amounts observed in the absence of steroid [70]. Thus, in the presence of pancreatic insulin, the combination of rising GCs and insulin drives the intake of pleasurable fat/sugar, whereas, in the absence of insulin, increasing GCs drives intake of low fat/sugar, bland rat chow.
Treating corticosterone-injected, diabetic, adrenalectomized rats with insulin restores fat and/or sucrose intake and reduces chow feeding [80, 81]. Thus, it appears that insulin plays a profound role in food selection, while GCs determine the motivation for selecting these foods, perhaps through their actions on dopamine secretion in the nucleus accumbens [82, 83]. When insulin is injected into the brains of intact rats, it is clear that it acts both in the hypothalamus to decrease food intake, and at the ventral tegmental area to decrease dopaminergic activity and associated food intake behaviors [84]. These apparently discrepant sets of results may be resolved by the fact that our studies have moved rats from a condition of no insulin to low-normal concentrations, whereas injecting or infusing insulin into the cerebrospinal fluid must cause very high concentrations of insulin in the brain. Insulin-mediated actions are found throughout the brain and on afferent vagal nerves [85], and it appears that insulin has other effects than just on the hypothalamus and dopaminergic cell groups to determine what is eaten, with important sequelae for energy balance and stress responsiveness.
In the absence of corticosterone, insulin concentrations are low, food intake is reduced and rats contain lower fat stores than normal. However, adrenalectomized rats ingest ~ 30–40% of the amount of lard or sucrose eaten by intact rats. Remarkably, when available, additional calories from sucrose restore adrenalectomized rats to normal, not only in terms of fat stores, but also in terms of hypothalamic CRF expression and expression of the rate-limiting enzyme for catecholamine synthesis in the upper brainstem (Table 1); this suggests that energy stores are critically important for normal activity in the central stress-response network [86]. Eating ‘comfort foods’ also alters stress responsiveness in intact rats. Under both acute and repeated restraint stress, CRF expression and ACTH secretion is reduced when rats are allowed to eat fat or sucrose in addition to chow [30, 31, 87]. Although increased ingestion of palatable foods during and after stressors may simply reflect a pleasurable activity that reduces the discomfort of stress, signals from eating these foods also reduce activity in the central stress response network through reducing CRF hyperactivity. It appears likely that stress-induced activation of the emotional brain is reduced in animals and people with available palatable foods and plentiful energy stores [88].
Table 1.
Drinking 30% Sucrose ad lib has similar restorative effects to supplying replacement corticosterone in adrenalectomized rats, compared to shamadrenalectomized controls.
| Adrenalectomy | Adrenalectomy + 30% sucrose |
Adrenalectomy + corticosterone |
|
|---|---|---|---|
| In the CNS: | |||
| CRF in the CeA | Decreased | Normal | Normal |
| CRF in the PVN | Increased | Normal | Normal |
| DBH in the LC* | Decreased | Normal | Normal |
| In the Periphery: | |||
| Insulin | Decreased | Normal | Normal |
| Fat depot weight | Decreased | Normal | Normal |
DBH = norepinephrine-synthesizing enzyme, dopamine B-hydroxylase, in the brainstem locus coeruleus
GC, CRF, learning, memory and habit
Acting at least in the amygdala, hippocampus, insula, anterior cingulate, and other areas of the prefrontal cortex (Figure 2), norepinephrine, GC and CRF are critical for learning and remembering, particularly following emotional events with negative valence [89–91]. Thus, when stress promotes GC-induced, insulin-delineated palatable food intake, memory is laid down for future recall of this coupling. An association is almost certainly made between ‘feeling stressed’ and ‘feeling better’ after indulging in ‘comfort foods’. This may be a critical link between stressors and eating-induced obesity.
Stressors promote more habitual behaviors at the expense of cognitive, goal-directed actions in humans [92, 93]. Learned associations, when reinforced through synaptic plasticity, may turn into habits that are expressed through activity in the basal ganglia with little conscious recognition of the habit [58, 94]. It is a small step to take, from cooling off emotional feelings induced by an intense, unmanageable stressor via eating rewarding foods, to instead using these foods to produce the same effect during lower intensity stimuli, such as ongoing low-grade stress, tiredness, or repeated, small upsetting events.
The obvious problems with the habitual use of food to reduce feelings of stress are two-fold: First, emotional ‘comfort feeding’ when used repeatedly results in primarily abdominal obesity, because of the greater sensitivity of abdominal adipose tissue to the combined signals of insulin and GC [95]. Second, and perhaps more importantly, it may serve in some individuals to relieve the stress-induced mental discomfort to the extent that conscious thought about how to cope with the stressor does not occur. Once stress-induced feeding become habitual, the problem-solver, executive part of the prefrontal cortex may no longer be actively engaged in the outcome; ‘comfort food’ intake may become a reflex. However, it is clear that conscious use of the prefrontal cortices can, with work, abrogate bad habits. This fact forms the basis for meditational and mindfulness exercises used by many [96, 97].
Stress-induced obesity: a cultural paradox
Given that feeding is essential for life, and that energy stores are required for both finding food and for cognition and planning to allow escape or travel to sites with more food (flight) or warring with neighbors for food (fight), it is not surprising that neural networks that subserve feeding and stress responses appeared in early life forms [98]. The impetus provided by GCs, and the bias that insulin provides to pursue more pleasurable foods that, in excess, can be stored by the same hormones, is a reasonable solution to caloric scarcity. During human evolution, food was scarce, and life-threatening stressors were frequent; GCs were probably frequently elevated and insulin was relatively low, except when feeding. It is likely that during those times there was little to no obesity.
In our current conditions of plentiful, palatable and easily accessible food, together with the proliferation of social stressors, there is increased stressor-associated non-homeostatic feeding. This causes obesity and associated hypersecretion of insulin. However, eating highly palatable foods also appears to decrease the feeling of stress, and this may reinforce subsequent eating of pleasurable foods when the emotional self feels uncomfortable. It is probably not a healthy thing to do, and may decrease longevity; reducing caloric intake by ~30% below normal extends the lifespan and reduces disease in monkeys and other mammals [99]. Thus, to extend our health and lifespan, it makes sense to markedly reduce our total food intake, and particularly our intake of snack and prepared foods that are high in palatable calories. With that said, however, relieving an occasional intense feeling of stress by eating something pleasurable does not cause obesity; however, habitual relief of life’s discomforts using this means inevitably leads to obesity.
It seems of critical importance then, with respect to the current obesity epidemic, to deliberately increase training of our cognitive, executive prefrontal brains to overcome emotional, habitual responses, using techniques like mindfulness and meditation: to become, be and remain aware of those habits that, although acquired easily, strongly reinforce stress-induced eating. Such individual practices, or even public health programs that were centered on focused mindfulness training might modify some of the stress-induced eating habits that contribute to the current epidemic of obesity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Flier JS, et al. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116(2):337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 2.Brunner EJ, Chandola T, Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II Study. Am J Epidemiol. 2007;165(7):828–837. doi: 10.1093/aje/kwk058. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson J, et al. Obesity from cradle to grave. Int J Obes Relat Metab Disord. 2003;27(6):722–727. doi: 10.1038/sj.ijo.0802278. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Flegal KM. High Body Mass Index for Age Among US Children and Adolescents, 2003–2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, et al. Prevalence of Overweight and Obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Trust for America's Health. Washington, D.C.: Trust for America's Health and Robert Wood Johnson Foundation; 2009. F as in Fat: How Obesity Policies Are Failing in America. [Google Scholar]
- 7.Schwartz MW, et al. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 8.Woods SC, et al. Food intake and the regulation of body weight. Annu Rev Psychol. 2000;51:255–277. doi: 10.1146/annurev.psych.51.1.255. [DOI] [PubMed] [Google Scholar]
- 9.Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 2004;25(3):473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutrition, Metabolism and Cardiovascular Diseases. 2008;18(2):158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol. 2003;284:R882–R892. doi: 10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- 12.Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R9–R19. doi: 10.1152/ajpregu.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng H, et al. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obesity. 2009;33 S2:S8–S13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Phys Behavior. 2006;89:51–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Myers MG, Jr, et al. The Geometry of Leptin Action in the Brain: More Complicated Than a Simple ARC. Cell Metabolism. 2009;9(2):117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley AE, et al. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 17.Steptoe A, et al. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology. 2007;32(1):56–64. doi: 10.1016/j.psyneuen.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Lattimore PJ, Maxwell L. Cognitive load, stress, and disinhibited eating. Eating Behav. 2004;(5):315–324. doi: 10.1016/j.eatbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor DB, et al. Effects of daily hassles and eating style on eating behavior. Health Psychol. 2008;27(1) Suppl:S20–S31. doi: 10.1037/0278-6133.27.1.S20. [DOI] [PubMed] [Google Scholar]
- 20.Wallis DJ, Hetherington MM. Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite. 2009;52(2):355–362. doi: 10.1016/j.appet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Garg N, Wansink B, Inman J. The influence of incidental affect on consumers' food intake. J Marketing. 2007;71:194–206. [Google Scholar]
- 22.Pecoraro N, et al. From Malthus to motive: How the HPA axis engineers the phenotype, yoking needs to wants. Prog Neurobiol. 2006;79(5–6):247–340. doi: 10.1016/j.pneurobio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Block JP, et al. Psychosocial Stress and Change in Weight Among US Adults. Am. J. Epidemiol. 2009;170(2):181–192. doi: 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serlachius A, Hamer M, Wardle J. Stress and weight change in university students in the United Kingdom. Physiology & Behavior. 2007;92(4):548–553. doi: 10.1016/j.physbeh.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Anderson SE, et al. Association of Depression and Anxiety Disorders With Weight Change in a Prospective Community-Based Study of Children Followed Up Into Adulthood. Arch Pediatr Adolesc Med. 2006;160(3):285–291. doi: 10.1001/archpedi.160.3.285. [DOI] [PubMed] [Google Scholar]
- 27.Kandiah J, et al. Stress influences appetite and comfort food preferences in college women. Nutrition Research. 2006;26(3):118–123. [Google Scholar]
- 28.Newman E, O'Connor DB, Conner M. Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendo. 2007 32;:125–132. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Zellner DA, et al. Food selection changes under stress. Physiology & Behavior. 2006;87(4):789–793. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 30.la Fleur SE, et al. Choice of lard, but not total lard calories, damps ACTH responses to restraint. Endocrinology. 2005;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- 31.Foster MT, et al. Palatable Foods, Stress, and Energy Stores Sculpt Corticotropin-Releasing Factor, Adrenocorticotropin, and Corticosterone Concentrations after Restraint. Endocrinology. 2009;150(5):2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epel E, et al. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinol. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 33.Gluck ME, et al. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med. 2004;66:876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- 34.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom Med. 2000;62:853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Rutters F, et al. Acute Stress-related Changes in Eating in the Absence of Hunger. 2009 Jan;17(1):72–77. doi: 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- 36.Savage JS, Hoffman L, Birch LL. Dieting, restraint, and disinhibition predict women's weight change over 6 y. Am J Clin Nutr. 2009;90(1):33–40. doi: 10.3945/ajcn.2008.26558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Rutters F, et al. Hyperactivity of the HPA axis is related to dietary restraint in normal weight women. Physiology & Behavior. 2009;96(2):315–319. doi: 10.1016/j.physbeh.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Wegner DM. How to Think, Say, or Do Precisely the Worst Thing for Any Occasion. Science. 2009;325(5936):48–50. doi: 10.1126/science.1167346. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, et al. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Phil Trans R Soc B. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berridge KC. 'Liking' and 'wanting' food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97(5):537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. 2008;11(4):423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Geday J, Gjedde A. Attention, emotion, and deactivation of default activity in inferior medial prefrontal cortex. Brain and Cognition. 2009;69(2):344–352. doi: 10.1016/j.bandc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 2009 Jan;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 47.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 48.Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- 49.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 50.Watson KK, Jones TK, Allman JM. Dendritic architecture of the von Economo neurons. Neuroscience. 2006;141(3):1107–1112. doi: 10.1016/j.neuroscience.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 51.Elmquist JK, et al. Indentifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 52.Figlewicz DP. Insulin, food intake, and reward. Semin Clin Neuropsychiatry. 2003;8(2):82–93. doi: 10.1053/scnp.2003.50012. [DOI] [PubMed] [Google Scholar]
- 53.berthoud h-r. Vagal and hormonal gut–brain communication: from satiation to satisfaction. Neurogastroenterology & Motility. 2008;20 s1:64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powley TL, et al. The role of hypothalamus in energy balance. In: Morgane PJ, Panksepp J, editors. Handbook of the Hypothalamus. New York: Dekker; 1980. pp. 211–298. [Google Scholar]
- 55.Levin BE, Routh VH, Dunn-Meynell AA. Glucosensing neurons in the central nervous system. In: Berthoud H-R, Seeley RJ, editors. Neural and Metabolic Control of Macronutrient Intake. Boca Raton: CRC Press; 2000. pp. 325–337. [Google Scholar]
- 56.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Frontiers in Neuroendocrinology. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- 57.Costafreda SG, et al. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 58.Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- 59.Sgoifo A, et al. The inevitable link between heart and behavior: New insights from biomedical research and implications for clinical practice. Neuroscience & Biobehavioral Reviews. 2009;33(2):61–62. doi: 10.1016/j.neubiorev.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Golden PR, et al. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008 Mar 15;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 62.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning and Memory. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radley JJ, Gosselink KL, Sawchenko PE. A Discrete GABAergic Relay Mediates Medial Prefrontal Cortical Inhibition of the Neuroendocrine Stress Response. J. Neurosci. 2009;29(22):7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pecoraro N, de Jong H, Dallman MF. An unexpected reduction in sucrose concentration activates the HPA axis on successive post shift days without attenuation by discriminative contextual stimuli. Physiology & Behavior. 2009;96(4–5):651–661. doi: 10.1016/j.physbeh.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 65.Gross JJ. Handbook of emotion regulation. First ed. New York, London: The Guilford Press; 2007. p. 654. [Google Scholar]
- 66.Buckley MJ, et al. Dissociable Components of Rule-Guided Behavior Depend on Distinct Medial and Prefrontal Regions. Science. 2009;325(5936):52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- 67.Schienle A, et al. Binge-Eating Disorder: Reward Sensitivity and Brain Activation to Images of Food. Biological Psychiatry. 2009 Apr 15;65(8):654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 68.Alonso-Alonso M, Pascual-Leone A. The Right Brain Hypothesis for Obesity. JAMA. 2007;297(16):1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- 69.Dallman MF, et al. Chapter 4: Glucocorticoids, chronic stress, and obesity. Prog Brain Res. 2006;153:75–105. doi: 10.1016/S0079-6123(06)53004-3. [DOI] [PubMed] [Google Scholar]
- 70.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19(4):275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Roozendaal B, et al. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. PNAS. 2006;103(17):6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith KS, et al. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196(2):155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heimer L, Van Hoesen GW. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neuroscience & Biobehavioral Reviews, The Limbic Brain: Structure and Function. 2006;30(2):126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 2005;28(12):629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 75.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fales CL, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tataranni PA, et al. Effects of glucocorticoid on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 79.Miller WL, Tyrrell JB. Chapter 12, Endocrinology and Metabolism. In: Felig P, Baxter JD, Frohman LA, editors. The Adrenal Cortex. 3rd ed. McGraw-Hill, Inc.; 1995. pp. 555–712. [Google Scholar]
- 80.Warne JP, et al. Disengaging insulin from corticosterone: roles of each on energy intake and disposition. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1366–R1375. doi: 10.1152/ajpregu.91016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.la Fleur SE, et al. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004;145:2174–2185. doi: 10.1210/en.2003-1359. [DOI] [PubMed] [Google Scholar]
- 82.Adzic M, et al. Acute or chronic stress induce cell compartment-specific phosphorylation of glucocorticoid receptor and alter its transcriptional activity in Wistar rat brain. J Endocrinol. 2009;202(1):87–97. doi: 10.1677/JOE-08-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barrot M, et al. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- 84.Figlewicz DP, et al. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R388–R394. doi: 10.1152/ajpregu.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warne JP, et al. Hepatic branch vagotomy, like insulin replacement, promotes voluntary lard intake in streptozotocin-diabetic rats. Endocrinology. 2007;148(7):3288–3298. doi: 10.1210/en.2007-0003. [DOI] [PubMed] [Google Scholar]
- 86.Dallman MF, et al. Glucocorticoids, the etiology of obesity and the metabolic syndrome. Curr Alzheimer Res. 2007;4(2):199–204. doi: 10.2174/156720507780362236. [DOI] [PubMed] [Google Scholar]
- 87.Pecoraro N, et al. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 88.Dallman M, Pecoraro N, la Fleur S. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19(4):275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 90.de Quervain DJ-F, et al. Glucocorticoids and the regulation of memory in health and disease. Frontiers in Neuroendocrinology. 2009 Aug;30(3):358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 91.Roozendaal B, Schelling G, McGaugh JL. Corticotropin-Releasing Factor in the Basolateral Amygdala Enhances Memory Consolidation via an Interaction with the {beta}-Adrenoceptor-cAMP Pathway: Dependence on Glucocorticoid Receptor Activation. J. Neurosci. 2008;28(26):6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwabe L, et al. Modulation of spatial and stimulus-response learning strategies by exogenous cortisol in healthy young women. Psychoneuroendocrinology. 2009;34(3):358–366. doi: 10.1016/j.psyneuen.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 93.Schwabe L, Wolf OT. Stress Prompts Habit Behavior in Humans. J. Neurosci. 2009;29(22):7191–7198. doi: 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Graybiel AM. Habits, rituals, and the evaluative brain. Ann Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 95.Dallman MF, et al. Chronic stress & obesity: a new view of comfort food. Proc Soc Nat Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Creswell JD, et al. Neural Correlates of Dispositional Mindfulness During Affect Labeling. Psychosom Med. 2007;69(6):560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- 97.Lieberman MD, et al. Putting Feelings Into Words: Affect Labeling Disrupts Amygdala Activity in Response to Affective Stimuli. Psychological Science. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 98.Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol. 2005;26(3–4):103–108. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 99.Colman RJ, et al. Caloric restriction delays disease onset and mortality in Rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herpertz-Dahlmann B. Adolescent Eating Disorders: Definitions, Symptomatology, Epidemiology and Comorbidity. Child and Adolescent Psychiatric Clinics of North America Eating Disorders and Obesity. 2009;18(1):31–47. doi: 10.1016/j.chc.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 101.Fernndez-Aranda F, et al. Impulse control disorders in women with eating disorders. Psychiatry Research. 2008;157(1–3):147–157. doi: 10.1016/j.psychres.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 102.Van den Eynde F, Treasure J. Neuroimaging in Eating Disorders and Obesity: Implications for Research. Child and Adolescent Psychiatric Clinics of North America Eating Disorders and Obesity. 2009;18(1):95–115. doi: 10.1016/j.chc.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 103.Mathes WF, et al. The biology of binge eating. Appetite. 2009;52(3):545–553. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiology & Behavior Purdue University Ingestive Behavior Research Center Symposium. Influences on Eating and Body Weight over the Lifespan: Childhood and Adolescence. 2008;94(1):121–135. doi: 10.1016/j.physbeh.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yeomans MR, Coughlan E. Mood-induced eating: interactive effects of restraint and tendency to overeat. Appetite. 2009 Apr;52(2):290–298. doi: 10.1016/j.appet.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 106.DelParigi A, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007;31(3):440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]