Abstract
Developmental exposure of rats to the organophosphate (OP) pesticides leads to altered neurobehavioral function in juvenile and young adult stages. The current study was conducted to determine whether effects of neonatal parathion exposure on cognitive performance persist in older adult and aged rats, and the relationship of behavioral changes to underlying cholinergic and serotonergic mechanisms. We administered parathion to rat pups on postnatal days 1–4, at doses spanning the threshold for the initial signs of systemic toxicity and for barely-detectable cholinesterase inhibition (0.1 or 0.2 mg/kg/day). Beginning at 14 months of age and continuing until 19 months, the rats were trained in the 16-arm radial maze. Controls showed the normal sex difference in this spatial learning and memory task, with the males committing significantly fewer working memory errors than females. Neonatal parathion exposure eliminated the sex difference primarily by causing impairment in males. In association with the effects on cognitive performance, neonatal parathion exposure elicited widespread abnormalities in indices of serotonergic and cholinergic synaptic function, characterized by upregulation of 5HT2 receptors and the 5HT transporter, deficits in choline acetyltransferase activity and nicotinic cholinergic receptors, and increases in hemicholinium-3 binding to the presynaptic choline transporter. Within-animal correlations between behavior and neurochemistry indicated a specific correlation between working memory performance and hippocampal hemicholinium-3 binding; parathion exposure destroyed this relationship. Like the behavioral effects, males showed greater effects of parathion on neurochemical parameters. This study demonstrates the sex-selective, long-term behavioral alterations caused by otherwise nontoxic neonatal exposure to parathion, with effects persisting into the beginning of senescence.
Keywords: Parathion, memory, organophosphates, radial-arm maze, aging, acetylcholine, serotonin
Introduction
Over the past decade, the developmental neurotoxicity and neurobehavioral teratogenicity or organophosphate pesticides (OPs) have been well-explored (reviews, [4,6,13,28,35,36]). Originally, it was thought that OPs produced their effects on brain development secondarily to their ability to inhibit cholinesterase, the mechanism that underlies their systemic toxicity [28]. However, it is increasingly clear that these agents act as developmental neurotoxicants through a family of mechanisms, some of which operate at exposures below the threshold for cholinesterase inhibition [35,36]; consequently the individual OPs may differ in their net effects on brain development and their consequent impact on behavioral performance. In a series of studies with toxicodynamically equivalent exposures in neonatal rats, we showed that chlorpyrifos, diazinon and parathion (PRT) all elicit behavioral abnormalities in association with adverse effects on acetylcholine (ACh) and serotonin (5HT) circuits, but that the underlying defects and behavioral outcomes differ among the three OPs [33,35–38,43,48,53,58,59]. In particular, PRT exposure did not elicit the cognitive impairment noted with the other two OPs, as evaluated in the radial-arm maze in adolescence and young adulthood, although, it did share adverse effects on indices of ACh synaptic function [38,59],
Recently, we have pursued longitudinal assessments of the effects of developmental PRT exposure that extend into later stages [38,41,43]. From adolescence (postnatal day PN30) to young adulthood (PN60, PN100) to full adulthood (5 months of age), both ACh and 5HT systems showed progressive changes in indices of synaptic activity and function rather than reflecting a defect that simply continued unchanged from the initial, neonatal injury. This implies that the net effect of PRT exposure is a function both of the direct action of the OP on neurodevelopmental, as well as its effects on the subsequent trajectory of synaptic development, superimposed on any late-occurring adaptive changes over the lifespan. In the current study, we extended these evaluations to encompass the effects of early-life PRT exposure on synaptic function and behavior in aging. Using the same cohort of animals as those explored in our earlier evaluations, we tested littermates with radial-arm maze training begun at 14 months of age, with testing continued into the start of senescence. The rats were trained initially over a period of two months and then underwent subsequent additional training and retesting at 16 months age and at 19 months of age.
After the end of behavioral testing, we conducted neurochemical evaluations at 20 months of age, at the beginning of senescence. At 20 months of age, rats already show senescent dysregulation of 5HT synaptic function [40,45,46,52] without the neurodegeneration, synaptic dysmorphology and neuronal loss that are present in very old rats, at the extreme of the life span and that can obscure defects that are associated with synaptic dysfunction [23]. We performed evaluations of multiple indices of ACh and 5HT synaptic function in brain regions containing projections and cell bodies for each transmitter. For 5HT systems, we measured three 5HT synaptic proteins known to be highly affected by developmental exposure to organophosphates [1–3,43,48,49,53], the 5HT1A and 5HT2 receptors, and the presynaptic 5HT transporter (5HTT). The two receptors play major roles in 5HT-related mental disorders, particularly depression [5,16,63,64], and the transporter, which regulates the synaptic concentration of 5HT, is the primary target for antidepressant drugs [21,26,27]. Similarly, for ACh systems, we focused on three markers of ACh synaptic function that are targeted by developmental exposure to PRT and that contribute to ACh-related behavioral impairment by OPs [38,42,44,59]: activity of choline acetyltransferase (ChAT), cell membrane binding of hemicholinium-3 (HC3) to the presynaptic high-affinity choline transporter, and the concentration of α4β2 nicotinic ACh receptors (nAChRs). ChAT is the enzyme that synthesizes ACh, and because it is a constitutive component of ACh nerve terminals, its activity provides an index of the development of ACh projections [11,17,25,30,32,39]. Although HC3 binding to the choline transporter is also a constituent of ACh nerve terminals, its expression is directly responsive to neuronal activity [19,34], so that comparative effects on HC3 binding and ChAT enables the characterization of both the development of innervation and presynaptic activity. Last, the α4β2 nAChR is a key player in the ability of ACh systems to release other neurotransmitters involved in reward, cognition and mood [8,9,12,14,31], and is also the most abundant nAChR subtype in the mammalian brain [15,18,60,61].
Methods
Animal treatments
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Institutional Animal Care and Use Committee and in accordance with all federal and state guidelines. Timed-pregnant Sprague-Dawley rats (Charles River, Raleigh, NC, USA) were housed in breeding cages, with a 12-hr light/dark cycle and free access to food and water. On the day of birth, all pups were randomized and redistributed to the dams with a litter size of 10 to maintain a standard nutritional status. Randomization within their respective treatment groups was repeated at intervals of several days. In addition, dams were rotated among litters to distribute any maternal caretaking differences randomly across litters and treatment groups. Offspring were weaned on PN21. Because of its poor water solubility, PRT (Chem Service, West Chester, PA, USA) was dissolved in dimethylsulfoxide to provide consistent absorption [62] and was injected subcutaneously in a volume of 1 ml/kg once daily on PN1–4. Control animals received equivalent injections of dimethylsulfoxide vehicle, which does not itself produce developmental toxicity [55,62]. These PRT doses were chosen because they straddle the threshold for barely-detectable cholinesterase inhibition and the first signs of reduced weight gain or impaired viability [42,53]. Brain cholinesterase inhibition 24 hr after the last dose of 0.1 mg/kg parathion is reduced 5–10%, well below the 70% threshold necessary for signs of cholinergic hyperstimulation [10]. We chose to dose the animals on PN1-4 because this is a period of peak sensitivity to organophosphates and one with extensive documentation of neurochemical and behavioral outcomes [20,33,35,36,58,59].
Behavior
Rats (N=10–12/sex/treatment) began training in the radial-arm maze at 14 months of age. During the training, rats had ad libitum access to water, with daily feeding being given after testing; the amount of food provided maintained a lean, healthy body weight with a target of approximately 85% of the free-feeding weight in age-matched animals. The wood maze was painted black and was at an elevation of 30 cm above the floor, in a room with numerous visual cues. The central platform had a diameter of 50 cm, with and 16 arms (10 × 60 cm) projecting radially outward. Each arm contained a food cup 2 cm from the distal end. Rats were given 2 shaping sessions in which they were placed individually in a large, opaque cylinder on the platform of the maze, given food reinforcement (halves of sugar coated cereal, Froot Loops®, Kellogg’s, Battle Creek, MI, USA), and allowed 10 min to eat. The rats were then trained in the radial-arm maze. The same 12 arms were baited for each rat once at the beginning of each session to assess working memory, while the other four arms were always left unbaited to test reference memory in subsequent sessions. The pattern of baited and unbaited arms was consistent throughout testing for each rat but differed among rats. Each trial began by placing the rat in an opaque cylinder on the central platform for 10 s to allow for orientation and thus avoid introducing bias as to which arm would be entered first. The rat was then allowed to enter any arm. The session lasted for up to 10 min, or until all 12 baited arms were entered. Arms were baited only once and a repeated entry into a baited arm was counted as a working memory error, whereas entrance into an unbaited arm was recorded as a reference memory error. Latency (seconds per arm entry) was calculated as the total session time in seconds divided by the total number of arms entered. The animals were trained for 18 sessions in the maze, twice per week, alternating males and females, and cleaning the maze in between animals with a damp paper towel. In addition to the typical 18 sessions of acquisition the rats underwent two additional six-session blocks of training with the first at 17 months of age and the second at 19 months of age.
Behavioral testing was completed at 19 months of age, after which the animals were allowed to remain in the home cage until 20 months of age while being maintained under the same dietary conditions as those used during the maze-performance phase. At that point, animals were decapitated and brain regions were dissected: the cerebellum (including flocculi) was removed and the forebrain was separated from the hindbrain by a cut rostral to the thalamus; the forebrain was then subdivided into frontal/parietal cortex, temporal occipital cortex, hippocampus and striatum, whereas the hindbrain was divided into midbrain and brainstem. All regions were flash-frozen in liquid nitrogen, and maintained at −45°C until assayed.
Neurochemistry
Determinations were made in 8 animals per sex for each treatment group. All of the assays used in this study have appeared in previous papers [3,29,30,37,42,49,50], so only brief descriptions will be provided here. For ACh synaptic markers, aliquots of tissue homogenates were assayed for ChAT using 50 μM [14C]acetyl-coenzyme A (specific activity 6.7 mCi/mmol; PerkinElmer Life Sciences, Boston, MA, USA) as a substrate and activity was determined as the amount of labeled ACh produced relative to tissue protein. For measurements of HC3 binding, aliquots of the cell membrane fraction were incubated with 2 nM [3H]HC3 (specific activity, 125 Ci/mmol; PerkinElmer) with or without 10 μM unlabeled HC3 (Sigma Chemical Co., St. Louis, MO, USA) to displace specific binding. Determinations of nAChR binding were carried out in another aliquot, each assay containing 1 nM [3H]cytisine (specific activity 35 Ci/mmol; PerkinElmer) with or without 10 μM nicotine (Sigma) to displace specific binding. Binding was calculated relative to the membrane protein concentration.
For 5HT markers, we used 1 nM [3H]8-hydroxy-2-(di-n-propylamino)tetralin (135 Ci/mmol, PerkinElmer) as the ligand for the 5HT1A receptor, and 0.4 nM [3H]ketanserin (63 Ci/mmol, PerkinElmer) for the 5HT2 receptor. Binding to the presynaptic 5HTT site was evaluated with 85 pM [3H]paroxetine (19.4 Ci/mmol, PerkinElmer). Specific binding was displaced by addition of 100 μM 5HT for the 5HT1A receptor and the 5HTT site, and by 10 μM methylsergide (Sandoz Pharmaceuticals, E. Hanover, NJ, USA) for the 5HT2 receptor.
Data Analysis
Data were compiled as means and standard errors. In the analysis of the behavioral data the choice accuracy measures of working and reference memory over the course of 18 sessions of acquisition and two six-session blocks of continued training were assessed by the analysis of variance (ANOVA) for between subjects factors (PRT exposure and sex) and repeated measures (memory error type and six-session block of trials). The Statview and Supernova statistical software packages (SAS, Carey, NC) were used for the analyses. Further analyses were conducted to follow-up interactions to determine specific effects in each sex during the different phases of behavioral training. Response latency (seconds per arm entry) was analyzed in a similar fashion. The log of response latency was taken before the analysis to normalize the distribution of the data, which is typically skewed for response latency data.
We evaluated multiple neurochemical measures that were all related to ACh or 5HT synapses, so the initial comparisons were conducted by a global ANOVA (data log-transformed because of heterogeneous variance among regions and measures) incorporating all the variables and measurements so as to avoid an increased probability of type 1 errors that might otherwise result from multiple tests of the same data set. The variables in the global test were treatment (control, PRT 0.1 mg/kg, PRT 0.2 mg/kg), brain region, sex and measure (nAChR binding, ChAT, HC3 binding for ACh synapses; 5HT1A receptor, 5HT2 receptor and 5HTT binding for 5HT synapses), with the latter considered a repeated measure, since all three determinations were derived from the same sample. Where we identified interactions of treatment with the other variables, data were then subdivided for lower-order ANOVAs to evaluate treatments that differed from the corresponding control. Where permitted by the interaction terms, individual groups that differed from control were identified with Fisher’s Protected Least Significant Difference Test.
Significance for all tests was assumed at the level of p < 0.05. For interactions at p < 0.1, we also examined whether lower-order main effects were detectable after subdivision of the interactive variables [54]. The criterion for interaction terms was not used to assign significance to the effects but rather to identify interactive variables requiring subdivision for lower-order tests of main effects of PRT, the variable of chief interest [54]. Relationships for within-animal neurochemical and behavioral variables were carried out by linear regression analysis.
To enable visual comparison of the effects on multiple neurochemical markers across different brain regions, these variables are presented as the percent change from control but statistical evaluations were conducted on the original data. Control values are given in Table 1.
Table 1.
Control Values for Neurochemical Variables
| frontal/parietal cortex | temporal/occipital cortex | hippocampus | striatum | midbrain | brainstem | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| male | female | male | female | male | female | male | female | male | female | male | female | |
| ChATa | 0.98±0.02 | 0.95±0.03 | 0.60±0.02 | 0.66±0.04 | 0.96±0.03 | 0.98±0.03 | 1.71±0.06 | 1.76±0.05 | 0.64±0.02 | 0.66±0.03 | 1.40±0.02 | 1.23±0.01 |
| HC3b | 16.7±0.7 | 17.3±0.6 | 10.6±0.4 | 10.8±0.5 | 21.4±0.4 | 20.1±0.7 | 37±2 | 41±2 | 9.1±0.5 | 9.5±0.4 | 8.1±0.4 | 8.7±0.5 |
| nAChRb | 44±1 | 44±2 | 51±3 | 45±2 | 27±1 | 24±1 | 60±2 | 59±3 | 58±2 | 55±2 | 24±1 | 23±1 |
| 5HT1ARb | 65±3 | 64±4 | 26±1 | 29±1 | ||||||||
| 5HT2Rb | 133±3 | 133±2 | 19.9±0.6 | 19.4±0.8 | ||||||||
| 5HTTb | 521±13 | 558±17 | 544±16 | 601±16 | ||||||||
nmol/min per mg protein
fmol per mg protein
Results
Behavior
The overall analysis of working and reference memory errors over the 18 sessions of acquisition (three blocks of 6 sessions) and two retest blocks of six sessions was analyzed. The main effect of PTN was not significant (p=0.15) with the controls averaging 12.4±0.8 errors per session, the 0.1 mg/kg PTN group 13.2±0.6 and the 0.2 mg/kg PTN group 13.5±0.7. However there was an interaction of PRT treatment x sex (F(2,47)=2.65, p<0.09), which triggered lower-order testing of the main effect of sex separately in each treatment group. In controls, we saw the normal sex difference, characterized by significantly (p<0.05) fewer errors for males than females. PRT evoked cognitive impairment in males, characterized by significant increases in error rates (Figure 1). In contrast, females were not significantly affected. We also found differential effects of PRT with regard to working vs. reference memory errors (error type x PRT x sex, p<0.08) as well as differential effects of PRT over the different test session blocks (session block x PRT x sex, p<0.06) that were then followed by corresponding lower-order tests of PRT effects in each sex for working and reference memory errors, as described below.
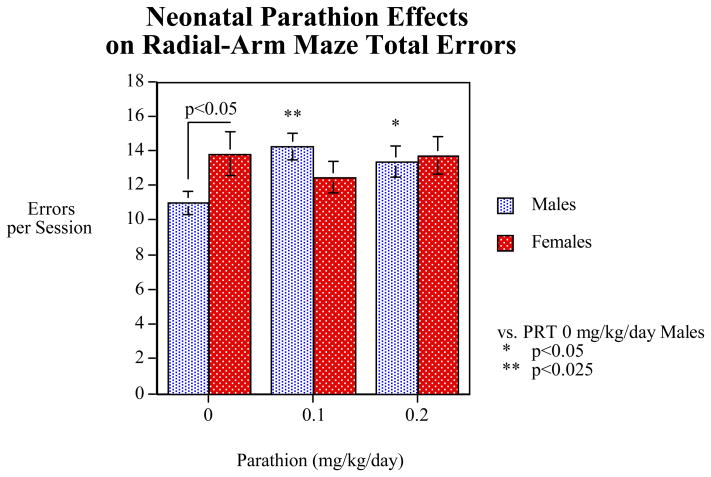
Figure 1.
Effects of neonatal PRT exposure on radial-arm maze accuracy averaged over all phases of testing, shown as mean ± SE of total working + reference memory errors. Control rats showed the normal, significant (p<0.05) sex difference for spatial memory tasks, with males having fewer errors. In males, PRT caused significant increases in errors, erasing the sex differences.
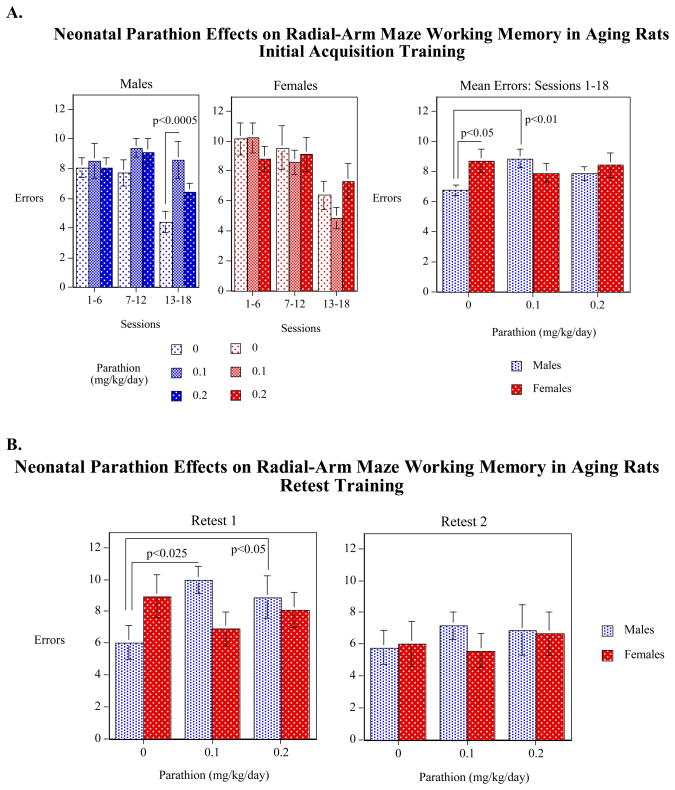
For working memory errors taken as a whole (over the entire 18 sessions of training), we found a significant (p<0.05) effect of sex in the vehicle-treated controls, with males committing fewer errors than females (Figure 2A). This effect was eliminated by neonatal PRT exposure. Comparisons of the PRT effects on memory within each sex showed that, although females were unaffected, for males, the 0.1 mg/kg/day dose significantly increased working memory errors relative to controls. Further, the three-way interaction of PRT x Sex x Session Block was significant (p<0.05) and subdivision of the data revealed that the PRT effect was most evident in the final block (sessions 13–18, p<0.0005). Controls showed substantial improvement with training, as evidenced by a decrease in errors in the last sessions as compared to earlier sessions; in contrast, PRT-dosed males did not show any improvement in performance over the 18 sessions. We then examined whether, with extensive training, the PRT group could overcome the deficits in performance. During the first retest period there was still a significant PRT-induced impairment in males whereas females remained unaffected (Figure 2B). With even further training, 6 additional sessions at 19 months of age, the PRT group finally performed as well as the controls.
Figure 2.
Effects of neonatal PRT exposure on working memory performance during acquisition (A) and subsequent retests (B), shown as mean ± SE. Averaged over the 18 sessions of acquisition training, controls showed a significant (p<0.05) sex difference, with males committing fewer errors than females. In males, PRT caused significant increases in errors, erasing the sex differences. PRT effects present during acquisition training (A) persisted into the first retest period but disappeared with additional training in the second retest (B).
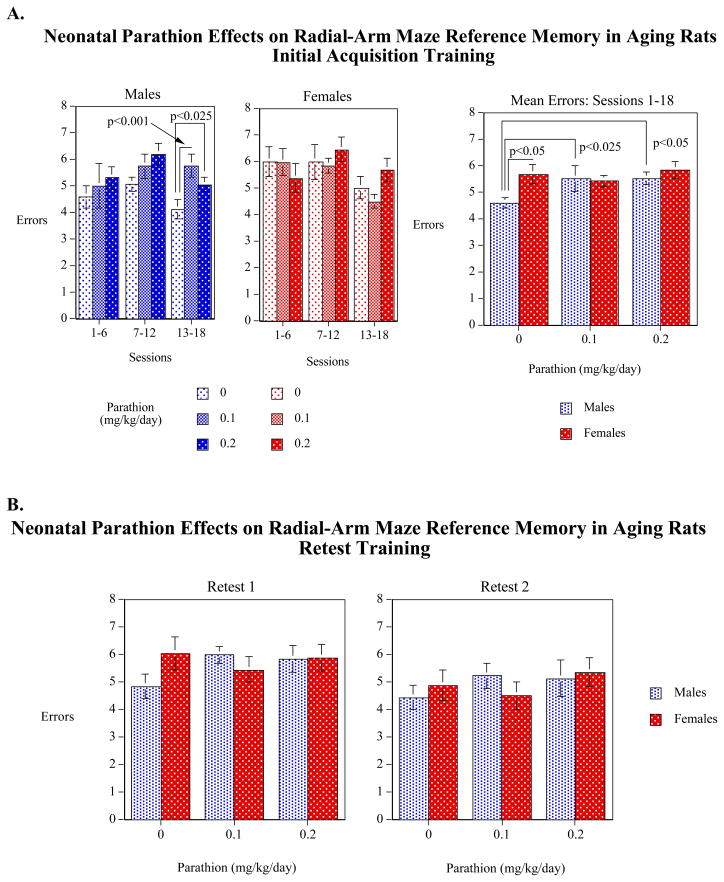
With reference memory, again averaged over the 18 sessions of acquisition, there was a significant sex effect in the vehicle-treated controls with the males making significantly (p<0.05) fewer reference memory errors than control females (Figure 3A). In males, PRT exposure caused significant impairments at either dose. Again, the differences were most prominent in the last sessions (sessions 13–18), reflecting the improvement in controls, and lack of improvement in the PRT-exposed animals. As before, no differences were seen in females. For reference memory errors, extensive training again enabled the animals to learn the task, as no PRT effects were seen during the two retests (Figure 3B).
Figure 3.
Effects of neonatal PRT exposure on reference memory performance during acquisition (A) and subsequent retests (B), shown as mean ± SE. Averaged over the 18 sessions of acquisition training, controls showed a significant (p<0.05) sex difference, with males committing fewer errors than females. In males, parathion caused significant increases in errors, erasing the sex differences. Parathion effects present during acquisition training (A) disappeared with additional training in the retest periods (B).
We did not observe any significant effects on response latency during either acquisition or the retests, indicating that the PRT effects on memory performance did not reflect more general behavioral or motivational debilitation. During acquisition training the latency means were Control=1.29±0.04, PRT 0.1 mg/kg/day =1.27±0.04 PRT 0.2 mg/kg/day=1.26±0.04 log sec/arm entry. During the retest sessions there were similarly no significant PRT associated differences in latency.
Neurochemistry
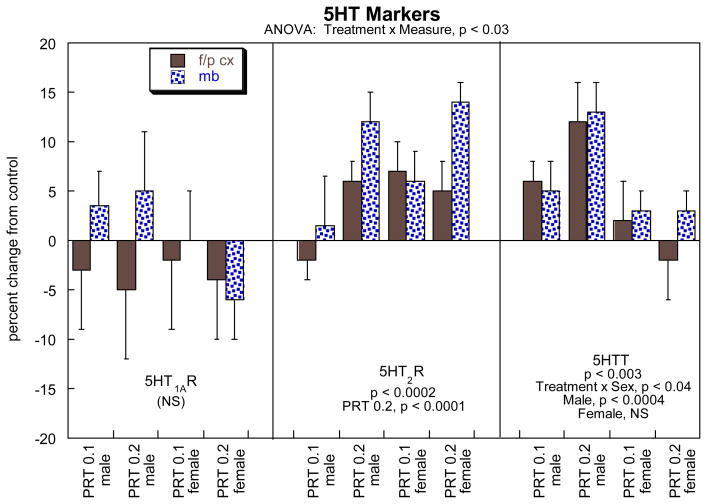
For 5HT markers, the global ANOVA indicated a significant treatment × measure interaction and accordingly, we subdivided the data into the three individual binding measurements and then reexamined the results for lower-order effects of PRT treatment (Figure 4). There were no significant effects on 5HT1A receptors but a significant increase for 5HT2 receptors as well as for the 5HTT site. The latter showed sex-selectivity, with significant differences in males but not females.
Figure 4.
Effects of neonatal PRT exposure on 5HT synaptic markers. Data represent means ± SE, presented as the percent change from control values (Table 1). The result of the global ANOVA is shown at the top of the panel, with lower-order tests shown within the panel for each measure. Because there were no interactions of treatment × region, individual tests for each region were not conducted and only main effects are shown. Abbreviations: f/p cx, frontal/parietal cortex; mb, midbrain; NS, not significant.
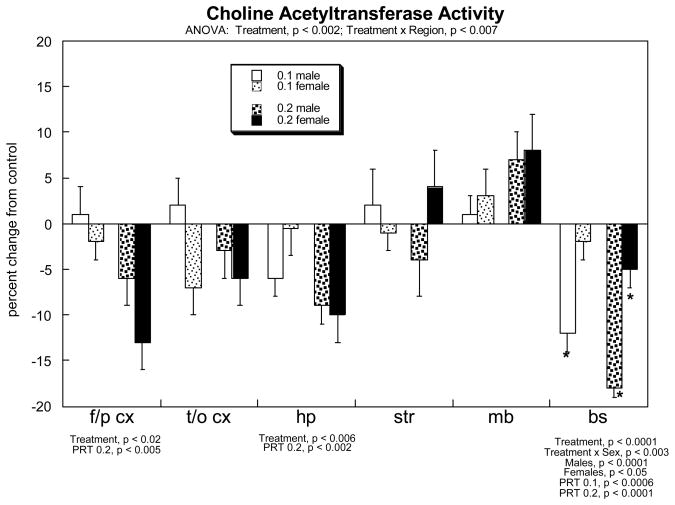
For ACh markers, the global statistical test showed interactions of treatment × measure (p<0.002), treatment × measure × sex (p<0.04) and treatment × measure × region (p<0.06). Accordingly, we subdivided the data into the three measures (i.e. the strongest interaction) and then performed lower-order evaluations for each in order to evaluate main treatment effects and interactions with the remaining variables of region and sex. For ChAT, the multivariate ANOVA indicated a significant overall reduction caused by neonatal PRT exposure (main treatment effect, p<0.002) and a treatment × region interaction (p<0.007); therefore, the results were subdivided into the six different regions (Figure 5). PRT elicited significant reductions in ChAT in the frontal/parietal cortex, hippocampus and brainstem; in the latter region, there was a preferential effect on males.
Figure 5.
Effects of neonatal PRT exposure on brain ChAT activity. Data represent means ± SE, presented as the percent change from control values (see Table 1). The significant results of the multivariate ANOVA are shown at the top of the panel, with lower-order tests for each region shown below the panel. Where there was no interaction of treatment × sex, only main effects are shown; in the brainstem, which did show a treatment × sex interaction, asterisks denote individual values that differ from the corresponding control. Abbreviations: f/p cx, frontal/parietal cortex; t/o cx, temporal/occipital cortex; hp, hippocampus; str, striatum; mb, midbrain; bs, brainstem.
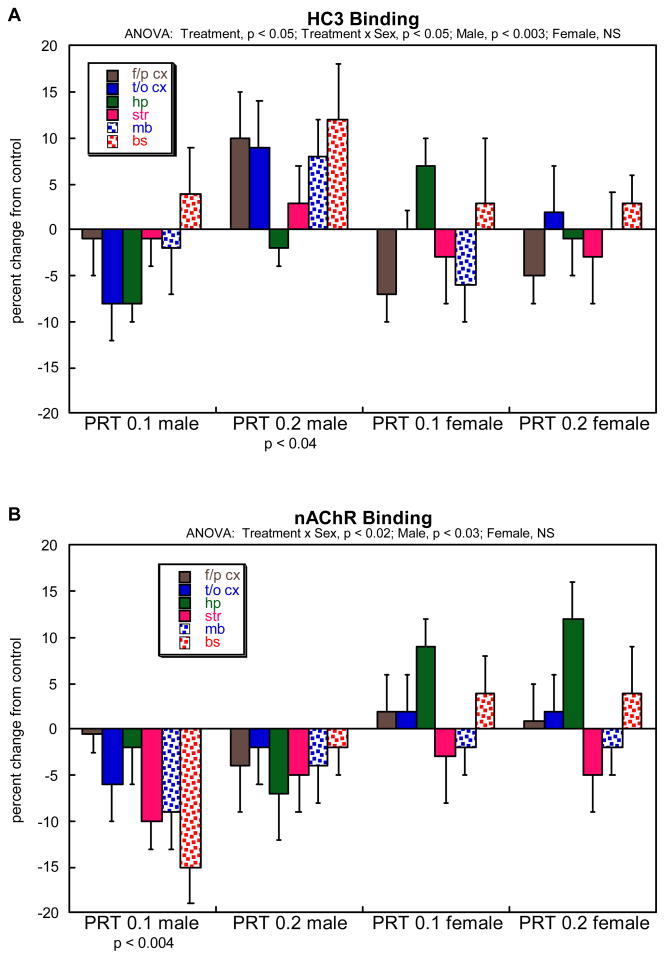
For HC3 binding, multivariate ANOVA indicated a significant main effect of treatment (p<0.05), reflecting a net overall increase caused by neonatal PRT exposure, along with a significant (p<0.05) treatment × sex interaction. Accordingly, the data were subdivided by sex and reexamined for lower-order treatment effects (Figure 6A). Overall, the effects of PRT were significant in males but not females, reflecting a net increase in the group receiving the higher dose of PRT; although the lower dose produced a trend toward reduced values compared to control, a contrast that was highly significant when compared to the increase seen at the high dose (p<0.007). Although this relationship was seen in every individual region, we did not test the regions separately because there was no treatment × region interaction. For nAChR binding, ANOVA also indicated a treatment × sex interaction (p<0.02), so the same subdivisions were examined for treatment effects (Figure 6B). Again, effects were limited to males, in this case reflecting an overall decrease caused by neonatal PRT exposure, with individual significance in the group receiving the lower dose of PRT; however, the lack of significance for the high dose group should be viewed with caution, since these values were not statistically distinguishable from the significant differences in the low-dose PRT group.
Figure 6.
Effects of neonatal parathion exposure on (A) HC3 binding to the presynaptic, high-affinity choline transporter, and (B) nAChR binding. Data represent means ± SE, presented as the percent change from control values (Table 1). The results of multivariate ANOVA are shown at the top of each panel; because of the treatment × sex interactions, values were subdivided into the individual treatments for males and females before performing lower-order tests, shown below the panel. Because there were no interactions of treatment × region, individual tests for each region were not conducted and only main effects are shown. Abbreviations: f/p cx, frontal/parietal cortex; t/o cx, temporal/occipital cortex; hp, hippocampus; str, striatum; mb, midbrain; bs, brainstem; NS, not significant.
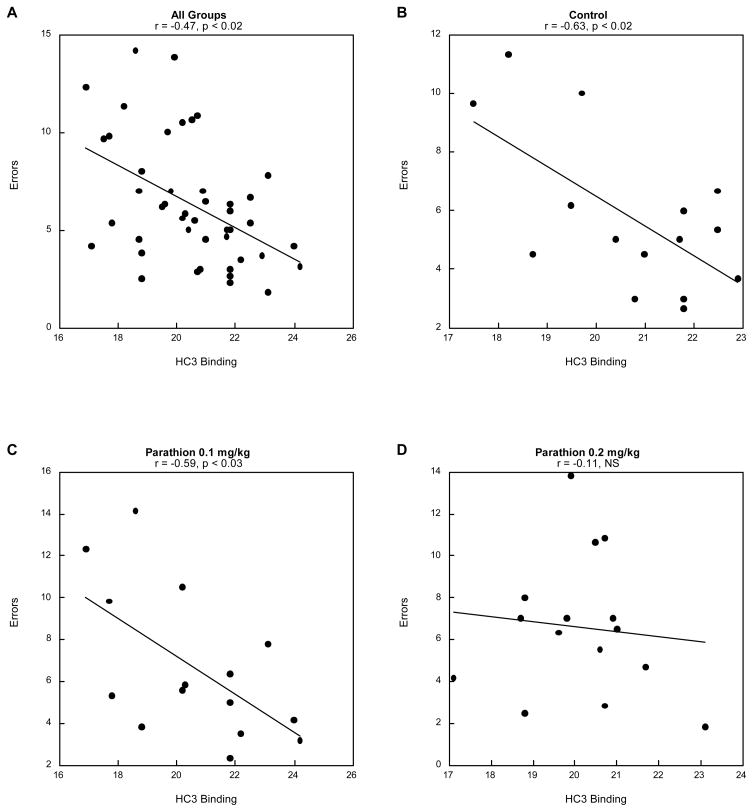
We then evaluated the relationship of neurochemical variables to performance in the radial-arm maze, concentrating on sessions 13–18, where the effects of PRT were most evident. First, we performed a global test of all measures for ACh and 5HT systems as multiple regressors (independent variables) vs. working memory errors (dependent variable). For 5HT systems, there was no significant correlation in either of the two brain regions (data not shown). However, for the three ACh measures, there was a significant regression relationship, restricted to the hippocampus: r = -0.47, p<0.02; not significant in other regions (not shown). Subdividing the results for the three separate variables revealed that the entire correlation reflected the relationship to HC3 binding (Figure 7A); there was no significant relationship of behavioral performance to nAChR binding or ChAT activity (not shown). We then evaluated the correlation of HC3 binding and working memory errors for each treatment group. Controls showed a strong negative correlation (Figure 7B), as did the animals exposed to the low dose of PRT (Figure 7C) but the relationship was lost at the higher dose (Figure 7D).
Figure 7.
Correlation between hippocampal HC3 binding and mean working memory errors for sessions 13–18: (A) all treatment groups, (B) controls, (C) parathion 0.1 mg/kg and (D) parathion 0.2 mg/kg).
Discussion
In our earlier work with neonatal exposure to chlorpyrifos or diazinon, we consistently found cognitive impairment in adolescence and young adulthood, in association with deficient ACh synaptic function [20,35,36,58]. In contrast, neonatal exposure PRT produced no discernible impairment of visuospatial performance during young adulthood, while nevertheless affecting other behaviors, including greater risk taking behavior and hyperactivity [59]. The PRT-induced cognitive impairment did emerge, but only with the progression to later adulthood and senescence, suggesting an unveiling of subtle neural impairments with the progressive neural decline with aging.
The effects of the other OPs typically involved the dissipation of normally-occurring sex differences in behavior [1,20,58], precisely the effect found for PRT in the older animals in the current study. The PRT-induced impairment seen during the initial acquisition in older adults and during the first retest phase were eventually overcome when the affected rats were given even more training, indicating that the impairment was not one of inability to learn the task. It just took much more training for the affected animals to learn the task.
Visuospatial memory is highly dependent on the integrity of ACh pathways and our results for indices of cholinergic synaptic function are entirely consonant with the behavioral findings. We identified a defect in presynaptic ACh innervation (decreased ChAT) in regions where ACh plays key roles in cognition, reward and other essential behaviors. These changes were associated with a rise in HC3 binding, indicative of compensatory increases in presynaptic ACh activity, akin to that found in Alzheimer’s Disease [7,47]. Ordinarily, there should also be cholinergic receptor upregulation to compensate for the loss of input but in fact, we found decreases in nAChRs, effects that would exacerbate deficient ACh innervation. Indeed, these defects were generally more evident in males, reinforcing their relationship to the behavioral outcome of selective cognitive PRT-induced impairment in males. Finally, we did a within-animal correlation of working memory errors with HC3 binding, the index of presynaptic activity, focusing on the hippocampus, a region where ACh projections are known to provide critical control of cognitive performance. In control animals, there was the expected negative correlation: the higher the presynaptic ACh activity, the fewer the number of errors. With PRT exposure, there was a progressive loss of the input-output relationship, that is, a loss of the dependence of cognitive performance on ACh input.
The current finding is quite similar to that reported previously for effects of neonatal chlorpyrifos, exposure, where cognitive impairment is similarly associated with the specific loss of ACh function [20,35,36]; the difference is that the effects of chlorpyrifos are evident in adolescence and young adulthood but with PRT, they only emerge later on, as seen here. Indeed, comparison of the neurochemical effects of neonatal PRT exposure evaluated here in aging animals, with the effects seen earlier in life again point to a change in the trajectory of development rather than an ongoing effect from the point of injury. In young adulthood through 6 months of age, neonatal PRT exposure elicits deficits in ChAT, HC3 binding and nAChR binding with sex-selectivity for males [38,41] but the effect on HC3 binding clearly changes over time, since the aging animals showed increases instead of decreases. It is thus evident that the effects of neonatal PRT exposure reflect interactions with events occurring during aging of the brain, so that the net effects differ in intensity at the various life stages, continuing into senescence. This conclusion is reinforced by our findings for 5HT systems. In our earlier work with chlorpyrifos, we found long-term upregulation of 5HT synaptic markers, reflecting deficits in 5HT neurotransmission resulting from “miswiring” of 5HT circuits [1,3,43,49,51,59]. With PRT, similar effects were apparent in adolescence, but waned in young adulthood, only to reemerge by 6 months of age [43]. However, the fact that values for the specific proteins as shown in these studies and the current one continue to shift in terms of intensity and regional distribution between young adulthood, 6 months, and 20 months, again indicates that PRT alters the developmental trajectory of 5HT synaptic function; since 20 months is the beginning of senescence, this suggests that early neonatal PRT exposure may alter the course of the age-related decline in 5HT systems.
The connection between sex-selective effects of neonatal OP exposure and late-emerging deficits in behavior and synaptic function may be connected. As shown here, after the initial injury, the subsequent effects reflect a continuum of outcomes that shift with age and that involve an intensification of sex differences. Many aspects of neuronal plasticity are promoted by estrogen receptor activation [56,57], so that the female brain displays greater recovery capabilities after injury as well as resistance to decline in senescence due to greater neuroplasticity [22,24,56] resulting in smaller persistent effects in females than those seen in males. If this holds true for the long-term effects of early-life exposures to developmental neurotoxicants, then males may show greater impairment in aging, effects that could contribute to the eventual emergence and symptomatology of neurodegenerative disorders. In general, our studies with developmental exposure to OP pesticides show that the persisting neurochemical and neurobehavioral impairments are more pronounced in males than females. This may be due to greater neuroplasticity in females accommodating for the developmental neurotoxic insult. In more modest cases, such as the neurotoxic insult caused by developmental low-dose PRT exposure, it takes the additional neural challenge of aging to unveil the functional impairment from subtle neural damage during dearly postnatal exposure. In this case too, the males were at greater risk for long-term functional damage from early life exposure.
This line of research continues to demonstrate long-term cognitive impairment after developmental exposure to OP pesticides, notably extending the results to show that further impairments emerge well after young adulthood and at the beginning of senescence. The continuing paring away of neural reserve with aging may unveil persisting neurotoxic effects which may be accommodated at younger but not in older individuals. Many of the OPs are being phased out, and while eliminating future exposures of pregnant women and children are clearly helpful to future generations, for those already exposed, the consequences may become more evident as aging proceeds. Because of their short lifespan, rats provide a useful model with which to pursue the issue of how long-lasting impairments from developmental neurotoxic exposure emerge with aging.
Acknowledgments
Research supported by the Duke University Superfund Basic Research Center (NIH ES10356).
Footnotes
TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston, WV), Frost Brown Todd (Charleston, WV), Weiner & Weltchek (Lutherville, MD), Frommer Lawrence Haug (Washington, DC), Carter Law (Peoria, IL), Corneille Law (Madison, WI), Angelos Law (Baltimore, MD), Kopff, Nardelli & Dopf (New York, NY).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environmental Health Perspectives. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aluigi MG, Angelini C, Falugi C, Fossa R, Genever P, Gallus L, Layer PG, Prestipino G, Rakonczay Z, Sgro M, Thielecke H, Trombino S. Interaction between organophosphate compounds and cholinergic functions during development. Chem Biol Interact. 2005;157–158:305–316. doi: 10.1016/j.cbi.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin-1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 6.Bellinger DC. Children’s cognitive health: the influence of environmental chemical exposures. Altern Ther Health Med. 2007;13:S140–S144. [PubMed] [Google Scholar]
- 7.Bissette G, Seidler FJ, Nemeroff CB, Slotkin TA. High affinity choline transporter status in Alzheimer’s Disease tissue from rapid autopsy. Ann NY Acad Sci. 1996;777:197–204. doi: 10.1111/j.1749-6632.1996.tb34419.x. [DOI] [PubMed] [Google Scholar]
- 8.Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human a4b2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- 10.Clegg DJ, van Gemert M. Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. Journal of Toxicology & Environmental Health Part B: Critical Reviews. 1999;2:211–55. doi: 10.1080/109374099281179. [DOI] [PubMed] [Google Scholar]
- 11.Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Dev Brain Res. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 12.Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- 13.Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, Johnson C, Fenster L, Barr DB. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102:228–236. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 14.Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RAJ. Upregulation of surface a4 b2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999;19:4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of a4 and b2 subunits and is upregulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- 16.Fujita M, Charney DS, Innis RB. Imaging serotonergic neurotransmission in depression: hippocampal pathophysiology may mirror global brain alterations. Biol Psychiat. 2000;48:801–812. doi: 10.1016/s0006-3223(00)00960-4. [DOI] [PubMed] [Google Scholar]
- 17.Happe HK, Murrin LC. High-affinity choline transport regulation by drug administration during postnatal development. J Neurochem. 1992;58:2053–2059. doi: 10.1111/j.1471-4159.1992.tb10946.x. [DOI] [PubMed] [Google Scholar]
- 18.Happe HK, Peters JL, Bergman DA, Murrin LC. Localization of nicotinic cholinergic receptors in rat brain: autoradiographic studies with [3H]cytisine. Neuroscience. 1994;62:929–44. doi: 10.1016/0306-4522(94)90484-7. [DOI] [PubMed] [Google Scholar]
- 19.Klemm N, Kuhar MJ. Post-mortem changes in high affinity choline uptake. J Neurochem. 1979;32:1487–1494. doi: 10.1111/j.1471-4159.1979.tb11089.x. [DOI] [PubMed] [Google Scholar]
- 20.Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Developmental Brain Research. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 21.Maes M, Meltzer H. The serotonin hypothesis of major depression. In: Bloom FE, Kupfer DJ, Bunney BS, Ciaranello RD, Davis KL, Koob GF, Meltzer HY, Schuster CR, Shader RI, Watson SJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 933–944. [Google Scholar]
- 22.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 23.Meister B, Johnson H, Ulfhake B. Increased expression of serotonin transporter messenger RNA in raphe neurons of the aged rat. Mol Brain Res. 1995;33:87–96. doi: 10.1016/0169-328x(95)00110-e. [DOI] [PubMed] [Google Scholar]
- 24.Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: implications for synaptic patterning. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Monnet-Tschudi F, Zurich MG, Schilter B, Costa LG, Honegger P. Maturation-dependent effects of chlorpyrifos and parathion and their oxygen analogs on acetylcholinesterase and neuronal and glial markers in aggregating brain cell cultures. Toxicol Appl Pharmacol. 2000;165:175–183. doi: 10.1006/taap.2000.8934. [DOI] [PubMed] [Google Scholar]
- 26.Nemeroff CB. The neurobiology of depression. Sci Am. 1998;278(6):42–49. doi: 10.1038/scientificamerican0698-42. [DOI] [PubMed] [Google Scholar]
- 27.Nutt DJ. The neuropharmacology of serotonin and noradrenaline in depression. Int Clin Psychopharmacol. 2002;17:S1–S12. doi: 10.1097/00004850-200206001-00002. [DOI] [PubMed] [Google Scholar]
- 28.Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- 29.Qiao D, Seidler FJ, Abreu-Villaca Y, Tate CA, Cousins MM, Slotkin TA. Chlorpyrifos exposure during neurulation: cholinergic synaptic dysfunction and cellular alterations in brain regions at adolescence and adulthood. Dev Brain Res. 2004;148:43–52. doi: 10.1016/j.devbrainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ Health Perspect. 2003;111:536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- 32.Richardson JR, Chambers JE. Effects of repeated oral postnatal exposure to chlorpyrifos on cholinergic neurochemistry in developing rats. Toxicol Sci. 2005;84:352–359. doi: 10.1093/toxsci/kfi081. [DOI] [PubMed] [Google Scholar]
- 33.Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: Later effects on emotional response. Brain Research Bulletin. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon JR, Atweh S, Kuhar MJ. Sodium-dependent high affinity choline uptake: a regulatory step in the synthesis of acetylcholine. J Neurochem. 1976;26:909–922. doi: 10.1111/j.1471-4159.1976.tb06472.x. [DOI] [PubMed] [Google Scholar]
- 35.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- 37.Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect. 2008;116:340–348. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slotkin TA, Bodwell BE, Ryde IT, Levin ED, Seidler FJ. Exposure of neonatal rats to parathion elicits sex-selective impairment of acetylcholine systems in brain regions during adolescence and adulthood. Environmental Health Perspectives. 2008;116:1308–1314. doi: 10.1289/ehp.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- 40.Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Serotonergic cell signaling in an animal model of aging and depression: olfactory bulbectomy elicits different adaptations in brain regions of young adult versus aging rats. Neuropsychopharmacology. 2005;30:52–57. doi: 10.1038/sj.npp.1300569. [DOI] [PubMed] [Google Scholar]
- 41.Slotkin TA, Lassiter TL, Ryde IT, Wrench N, Levin ED, Seidler FJ. Consumption of a high-fat diet in adulthood ameliorates the effects of neonatal parathion exposure on acetylcholine systems in rat brain regions. Environmental Health Perspectives. 2009;117:916–922. doi: 10.1289/ehp.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: Effects on brain development are separable from systemic toxicity. Environmental Health Perspectives. 2006;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slotkin TA, Levin ED, Seidler FJ. Developmental neurotoxicity of parathion: Progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicology and Teratology. 2009;31:11–17. doi: 10.1016/j.ntt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ Health Perspect. 2007;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slotkin TA, McCook EC, Ritchie JC, Carroll BJ, Seidler FJ. Serotonin transporter expression in rat brain regions and blood platelets: aging and glucocorticoid effects. Biol Psychiat. 1997;41:172–183. doi: 10.1016/S0006-3223(96)00215-6. [DOI] [PubMed] [Google Scholar]
- 46.Slotkin TA, Miller DB, Fumagalli F, McCook EC, Zhang J, Bissette G, Seidler FJ. Modeling geriatric depression in animals: biochemical and behavioral effects of olfactory bulbectomy in young versus aged rats. J Pharmacol Exp Ther. 1999;289:334–345. [PubMed] [Google Scholar]
- 47.Slotkin TA, Nemeroff CB, Bissette G, Seidler FJ. Overexpression of the high affinity choline transporter in cortical regions affected by Alzheimer’s Disease: evidence from rapid autopsy studies. J Clin Invest. 1994;94:696–702. doi: 10.1172/JCI117387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slotkin TA, Ryde IT, Levin ED, Seidler FJ. Developmental neurotoxicity of low-dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res Bull. 2008;75:640–647. doi: 10.1016/j.brainresbull.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Slotkin TA, Seidler FJ. Developmental exposure to terbutaline and chlorpyrifos, separately or sequentially, elicits presynaptic serotonergic hyperactivity in juvenile and adolescent rats. Brain Res Bull. 2007;73:301–309. doi: 10.1016/j.brainresbull.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reprod Toxicol. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Slotkin TA, Seidler FJ, Ali SF. Cellular determinants of reduced adaptability of the aging brain: neurotransmitter utilization and cell signaling responses after MDMA lesions. Brain Res. 2000;879:163–173. doi: 10.1016/s0006-8993(00)02767-0. [DOI] [PubMed] [Google Scholar]
- 53.Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: Disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environmental Health Perspectives. 2006;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snedecor GW, Cochran WG. Statistical Methods. 6. Iowa State University Press; Ames, Iowa: 1967. p. 593. [Google Scholar]
- 55.Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 57.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide diazinon. Neurotoxicology and Teratology. 2008;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D, Wells C, Perraut C, Seidler FJ, Slotkin TA, Levin ED. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Research Bulletin. 2008;77:404–11. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whiting P, Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci. 1987;84:595–599. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whiting PR, Lindstrom J. Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J Neurosci. 1988;8:3395–3404. doi: 10.1523/JNEUROSCI.08-09-03395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- 63.Yatham LN, Liddle PF, Dennie J, Shiah IS, Adam MJ, Lane CJ, Lam RW, Ruth TJ. Decrease in brain serotonin-2 receptor binding in patients with major depression following desipramine treatment: a positron emission tomography study with fluorine-18-labeled setoperone. Arch Gen Psychiat. 1999;56:705–711. doi: 10.1001/archpsyc.56.8.705. [DOI] [PubMed] [Google Scholar]
- 64.Yatham LN, Liddle PF, Shiah IS, Scarrow G, Lam RW, Adam MJ, Zis AP, Ruth TJ. Brain serotonin-2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiat. 2000;57:850–858. doi: 10.1001/archpsyc.57.9.850. [DOI] [PubMed] [Google Scholar]