Abstract
The zebrafish may represent an excellent compromise between system complexity and practical simplicity for behavioral brain research. It may be particularly appropriate for large scale screening studies whose aim is to identify mutants with altered phenotypes or novel compounds with particular efficacy. For example, the zebrafish may have utility in the analysis of the biological mechanisms of learning and memory. Although learning and memory have been extensively studied and hundreds of underlying molecular mechanisms have been identified, this number may represent only the fraction of genes involved in these complex brain functions. Thus large scale mutagenesis screens may have utility. In order for such screens to succeed, appropriate screening paradigms must be developed. The first step in this research is the characterization of learning and memory capabilities of zebrafish and the development of automatable tasks. Here we show that zebrafish is capable of latent learning, i.e. can acquire memory of their environment after being allowed to explore it. For example, we found experimental zebrafish that experienced an open left tunnel or an open right tunnel of a maze during the unrewarded exploration phase of the test to show the appropriate side bias during a probe trial when they had to swim to a group of conspecifics (the reward). Given that exploration of the maze does not require the presence of the experimenter and the probe trial, during which the subjects are video-recorded and their memory is tested, is short, we argue that the paradigm has utility in high throughput screening.
Keywords: exploratory behavior, forward genetics, latent learning, high throughput screening, zebrafish, zebra danio
INTRODUCTION
The zebrafish has been one of the favored model organisms of embryology [48] and, due to the numerous and extensive developmental studies using genetic approaches conducted with this species, by now zebrafish is one of the most frequently employed animal model organisms of genetics too [27]. As a result of the accumulated genetic knowledge and the large number of genetics tools developed for this species, the zebrafish has been gaining popularity in behavioral brain research as well [42]. Research using genetic methods has been successful in the analysis of the biological mechanisms of brain function [13]. Thus, zebrafish with its strong genetics toolset may also have utility in behavioral brain research in addition to the classical laboratory rodents, mice and rats, and to the lower order organisms such as the nematode or the fruit fly.
Furthermore, when comparing these evolutionarily highly diverse laboratory study species, the zebrafish appears to enjoy some advantages. Although it is a vertebrate that possesses a complex brain whose major anatomical layout [47, 38] and neurochemistry [12, 22, 34, 35] are similar to those of mammals including our own species, it is almost as easy to keep in the laboratory and is also almost as prolific as non-vertebrate laboratory organisms. Thus, the zebrafish appears to strike an optimal compromise between system complexity (brain function in this case) and practical simplicity (pragmatic considerations including cost). Particularly useful this species may be when one considers that a small zebrafish rack (150 cm tall, 120 cm wide, 30 cm deep) can house as much as 4000 fully mature adult zebrafish. This feat is accomplishable due to the small size (maximum length is 4 cm) and social nature (shoaling) of zebrafish. Shoaling is a typical aggregation behavior whereby conspecifics stay close to each other, usually within an inter-individual distance of 3-4 body lengths, and form a group (e.g. 32, 33). If one also considers that mutagenesis methods, including chemical (ethyl-nitroso-urea, ENU) [29], viral vector mediated (insertional) [2], and other (e.g. transposon induced gene breaking) mutagenesis techniques [44] have been worked out for the zebrafish, it becomes clear that this species is an excellent choice for forward genetics [10, 3].
But what is the merit of conducting random mutagenesis with the need to screen thousands of mutants for their phenotypical alterations in learning and memory when we already know a couple of hundreds of molecular players [45] involved in these processes? Perhaps instead, one should conduct a thorough analysis of the already known molecular players using, for example, reverse genetics. Indeed, numerous laboratories have taken this latter route and seminal studies have been published on the functional characterization of what is believed to be the key players of learning and memory (e.g. ref [26]). However, according to some estimates as many as 40-50 % of all genes in the genome of vertebrate species are expressed in the brain of these species [15], which represents a staggering number (about 12-15 thousand genes) compared to the couple of hundred genes with already proven roles in learning and memory. A systematic analysis of the potential involvement of the rest of the genome is thus of high relevance. Discovery of potentially large number of genes involved in learning and memory may be possible using large scale forward genetic screens, but only if proper screening tools, i.e. behavioral testing paradigms are available [25]. The goal of the current paper is to advance our knowledge in this direction by testing zebrafish in a learning task that is easy to administer and one which may be employed for large scale screening.
Although the zebrafish is relatively new in the study of learning and memory as compared to other classical laboratory model organisms [43], by now several studies have shown that this small vertebrate is also capable of performing well in a range of learning tasks. For example, zebrafish showed acquisition of a one trial avoidance learning task [6], performed well in olfactory conditioning [11], shuttle box active appetitive conditioning [36], place conditioning [17], appetitive choice discrimination [5], active avoidance conditioning [51], alternation memory task [50], and most recently in a plus-maze non-spatial and spatial associative learning task [1, 42]. While these results clearly demonstrate the cognitive and mnemonic capabilities of zebrafish, the tasks have not been used in high throughput screening because they often required extensive and labor intensive training. The possibility of automation, and thus high throughput, however, also appears to be within reach for zebrafish as demonstrated by two recent studies [28, 36]. In the current paper, we introduce a simple learning task based upon spatial exploration, which we argue may also be made high throughput. In this task zebrafish are repeatedly exposed to a maze (figure 1) with particular tunnels (left side, right side or both sides) open. This training phase does not require monitoring of behavioral activity and thus can be performed without the presence of the experimenter. The training phase is followed by a short probe trial during which the swim tunnel (spatial bias) of the fish is quantified. We call this task a “latent learning” paradigm [8, 46] because the training phase of the test involves no experimenter controlled delivery of reinforcers. Here, we show that zebrafish develop a significant spatial bias in this task and argue that the paradigm will be appropriate for high throughput screening.
Figure 1.
The maze. The start box, the reward chamber and the left and right tunnels are indicated. The numbers show the dimensions of the maze in cm. The walls of the maze were 10 cm tall and the maze was filled to 5 cm water depth. Note that the removable guillotine doors leading to the reward chamber are also indicated (thick dotted lines).
METHODS
Animals and Housing
Wild-type short-fin zebrafish were obtained from a local pet store (Big Al's Aquarium Services Inc., Mississauga, ON, Canada) and were bred in the Vivarium (University of Toronto Mississauga). Subjects used in the current study were from the first filial generation raised and housed in the same vivarium room under identical conditions. The experimental fish were housed (5 fish per tank) in 3 liter transparent acrylic tanks (a trapezoid tank with a bottom measuring 22 × 9 cm, top measuring 26 × 9 cm and height 15 cm) that were part of a zebrafish rack system (Aquaneering Inc, San Diego CA, USA) with multistage filtration that contained a mechanical filter, a fluidized glass bed biological filter, and an activated carbon filter, as well as a fluorescent UV light sterilizing unit. In addition to the experimental fish, we also used a group of fish that served as a social stimulus (stimulus fish). These stimulus fish came from the same population as our experimental fish and thus were of the same size. The stimulus fish were also kept in a manner identical to the housing and feeding conditions of the experimental fish. Every day 10% of the water was replaced with fresh system water (deionized water supplemented with 60mg/l Instant Ocean Sea Salt [Big Al's Pet Store, Mississauga, Ontario, CA]) in the zebrafish racks housing the experimental and stimulus fish. The water temperature was maintained at 27° C by a thermostat controlled heater. Illumination was provided by fluorescent light tubes from the ceiling of the room with lights turned on at 0800h and off at 2000h. Fish were fed a mixture of ground freeze-dried krill and flake food (Tetramin Tropical Flakes, Tetra USA) twice a day at approximately 10:00 am and 4:00 pm. The fish were young sexually mature adults, between 4 and 6 months old, at the time of the experiment. The gender ratio of the population used in the experiment was approximately 50-50%.
Test Apparatus
The maze (figure 1) consisted of a square start box where the fish were placed for a 2 min acclimation period at the beginning of each training trial and the probe trial. From the start box a connecting tunnel led to a 4 way intersection from where the fish could turn left, right, or swim straight or turn back. The left, right, and straight forward tunnels led to the reward chamber, which contained stimulus fish only during the probe trial. The maze was 10 cm high and was filled with system water of the same salt composition and temperature as in the holding tanks to half the height of the maze. The maze was constructed from 2 mm thin transparent Plexiglas allowing the fish to see all parts of the maze, including the reward chamber, from all locations in the maze. The start box and the reward chamber had floor drains to allow removal of water from the maze. The three entrances to the reward chamber were equipped with transparent Plexiglas guillotine doors that could be operated from a distance via thin nylon strings attached to them. Importantly, the central gate was closed for the duration of training and only the left and/or right gate was open depending on experimental condition.
Procedure
Training
Five experimental zebrafish groups were used. Fish were assigned to these groups randomly. Group 1 was the ‘both tunnels open group’ (n=10). In this group, all fish were repeatedly exposed to the maze with both the right and left tunnels open (guillotine doors raised). Group 2: was the ‘left tunnel open’ group (n=10). In this group fish were repeatedly exposed to the maze with left tunnel open (the guillotine door raised on the left but kept lowered on the right side). Group 3 was the ‘right tunnel open’ group (n=10) in which fish were repeatedly exposed to the maze with right tunnel open (right side guillotine door raised but left side kept lowered). Group 4 was the ‘other tank trained’ group (n=10) in which fish received the same handling procedure associated with training in the above 3 groups but these fish were exposed only to a rectangular tank similar in total volume and water depth to the maze (50 × 30 × 5 cm length × width × height). Group 5 was the ‘naïve’ group (n = 10) in which fish received no training or handling before the probe trial.
Fish of the 5 groups were exposed to their corresponding experimental condition in a randomized order in the same experimental room during the middle of the light phase of their light cycle once a day for 16 days (except the naïve fish, which were not trained), the training trials. Each trial lasted for 50 minutes and the 10 fish of each group were exposed at a time to the maze during each trial.
Probe trial
At the conclusion of training, all fish received a probe trial in a randomized order with respect to their group designation. The experimenter was blind to the group designation of the fish. Each fish was tested singly and once. During the probe trial 5 zebrafish (stimulus fish) were present in the reward chamber. The tunnel directly leading to the reward chamber was blocked. The experimental fish were released from the start box after the acclimation period by raising a transparent guillotine door remotely as in the training trials, but now the guillotine doors to the left and right tunnels were open and the experimental fish was allowed to explore the maze for 10 minutes. Given the strong shoaling response shown in zebrafish [41, 32, 33] and the demonstrated rewarding aspect of the sight of conspecifics [1], we expected all experimental subjects to try to swim close to the reward chamber. Furthermore, we expected the left arm open and the right arm open trained fish to choose their swim path according to their prior experience.
The behavior of the fish was recorded using a Canon mini DV camcorder (Optura 30, Canon Corporation, Japan). The recordings were later replayed and quantified using the Noldus Observer Color Pro 5.0 event recording software (Noldus Info Tech., Wageningen, The Netherlands). The variables measured included the time spent in the left and the right tunnel leading to the reward chamber, the first path taken (the number of fish choosing the left or right tunnel), the latency to leave the start box, the latency to enter the reward chamber measured after the fish left the start box, the number of entries to the reward chamber, and the duration of time spent in the reward chamber.
Statistical analysis
Data were analyzed using SPSS (version 14) written for the PC. Univariate Analysis of Variance (ANOVA) was performed to investigate group differences (the effect of training condition). In case of a significant main effect (p < = 0.05), Tukey Honestly Significant Difference (HSD) post hoc multiple comparison test was performed. These parametric statistical procedures are insensitive to the violation of variance inhomogeneity and/or normality of distribution criteria when the groups compared have equal or similar sample sizes. Although our sample size was 10 for each experimental group, we performed logarithm transformation, as noted in the results section, where significant inhomogeneity of variances was found and performed the statistical analysis after this scale transformation. In addition, to analyze the effect of training condition on the number of fish choosing the left versus the right tunnel, we performed a binomial test.
RESULTS
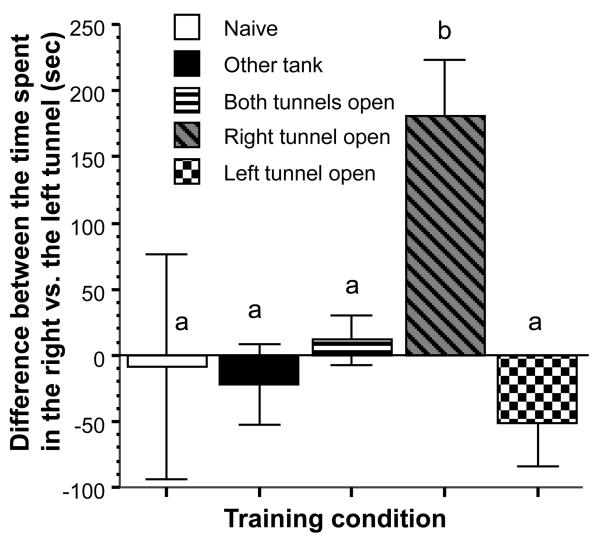
Zebrafish are social fish that swim in shoals in nature [19] as well as in captivity [41, 32, 33]. The fact that we ran 10 fish at a time during each training trial, made the novel test environment less aversive and allowed the fish to properly habituate to this environment. As a result, experimental fish actively explored the maze throughout training and signs of fear, e.g. freezing, erratic movement or jumping, [4,37, 23] were not observed. Similarly, fear responses were absent during the probe trial by which time the experimental fish that were probed singly in the maze had been to this same maze environment 16 times. Fish in the right tunnel open and left tunnel open groups appeared to choose the respective route during the probe trial as predicted. In order to quantify the route bias we calculated the difference between the time spent in the right and the left tunnel (figure 2). ANOVA demonstrated a significant training condition effect (F(4, 45) = 3.702, p < 0.05) and the Tukey HSD post hoc multiple comparison test confirmed that fish in the right tunnel open group significantly (p < 0.05) differed from fish in the left tunnel open group and also from fish in all other groups, but the other groups did not differ from each other. One sample one tailed t-tests confirmed that the difference score for the right tunnel open group was significantly above chance (chance = 0 sec; t = 4.326, df = 9, p < 0.001) whereas the difference score for the left tunnel open group was not statistically below random chance but the p value bordered significance (t = −1.570, df = 9, p = 0.075). The other groups were statistically indistinguishable from random chance (|t| < 0.103, df = 9, p > 0.45).
Figure 2.
The difference between the time spent in the right vs. the left tunnel of the maze during the probe trial is training condition dependent. Mean ± SEM are shown. Sample sizes (n) = 10 for each training condition (different shading and pattern of graphs as indicated by the legend). Note that bars that share at least one letter designation are not significantly (p > 0.05) different from each other (Tukey HSD post hoc multiple comparison test).
In addition to the amount of time the fish spent in the right vs. the left tunnels leading to the reward chamber during the probe trial, we have also analyzed the first path they took, i.e. whether they swam into the left or the right tunnel the first time they made a choice. We counted the number of fish taking the right vs. the left tunnel (figure 3) and analyzed the results using the binomial test. This test confirmed that significantly (p = 0.05) more fish chose the right tunnel from the right tunnel open group and significantly more fish chose the left tunnel from the left tunnel open group as compared to random chance (50%). Conversely, fish that were not trained (naïve), or those that were trained in a regular rectangular tank, or in the maze with both tunnels open did not significantly (p > 0.05) differ from chance. In summary, the above results suggest that in the probe trial fish swam to the reward chamber using the path they were allowed to explore during training or chose randomly if the training did not include a side bias.
Figure 3.
The number of fish choosing the right tunnel the first time they encounter this choice point during the probe trial is dependent upon their training condition. Random chance level, i.e. 5 out of 10 fish, is shown by the broken line. The asterisk indicates significant (p = 0.05) departure from random chance (binomial test).
Figure 4 depicts our results for the latency to leave the start box. This measure reflects the motivation of the experimental fish to explore as opposed to the tendency to stay in one place due to handling induced fear. ANOVA revealed a significant training condition effect (F(4, 45) = 3.896, p < 0.01, logarithm transformed data) and Tukey HSD post hoc analysis showed that fish in the both tunnels open and left tunnel open groups significantly (p < 0.05) differed from fish that were trained in the regular rectangular tank but the other groups did not differ from each other. These results suggest that all the fish that were trained in the maze, irrespective of their group designation, exited the start box within a period of time that did not significantly differ among them.
Figure 4.
The latency to leave the start box during the probe trial is affected by the training the experimental fish received. Mean ± SEM are shown. Sample sizes (n) = 10 for each training condition (different shading and pattern of graphs as indicated by the legend). Note that bars that share at least one letter designation are not significantly (p > 0.05) different from each other (Tukey HSD post hoc multiple comparison test), i.e. only the group (‘Other tank’) marked by ‘b’ is different from the groups marked by ‘a’ (‘Both tunnels open’ and ‘Left tunnel open`) but the other differences are non-significant.
A somewhat similar finding was obtained for the latency to enter the reward chamber (figure 5). This latency measures the period of time between the time point when the experimental fish exited the start box and the time point when it entered the reward chamber. Figure 5 demonstrates that all fish that were trained in the maze (irrespective of under what conditions), i.e. fish in the left tunnel open, the right tunnel open and the both tunnels open groups, reached the reward chamber the fastest. ANOVA showed a significant training condition effect F(4, 45) = 4.922, p < 0.01 (logarithm transformed data) and Tukey HSD post hoc multiple comparisons revealed that only fish in the naïve condition differed significantly (p < 0.05) from those fish that were trained in the maze while differences between any other groups were non-significant.
Figure 5.
Latency to enter the reward chamber after leaving the start box during the probe trial is affected by the training condition of zebrafish. Mean ± SEM are shown. Sample sizes (n) = 10 for each training condition (different shading and pattern of graphs as indicated by the legend). Note that bars that share at least one letter designation are not significantly (p > 0.05) different from each other (Tukey HSD post hoc multiple comparison test), for example, the group marked by ‘ab’ (‘Other tank’) does not differ from any other group.
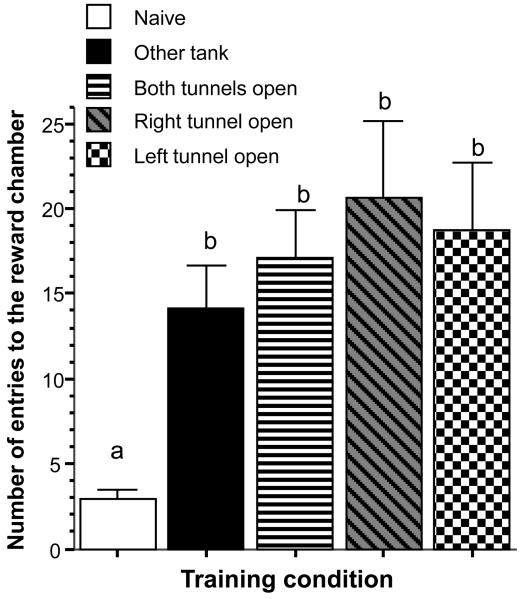
The number of entries to the reward chamber may represent a compromise: on the one hand it may reflect the motivation to explore the environment and to join a shoal (leading to higher values), and on the other it may also reflect novelty or experimenter handling induced fear (leading to lower values). Figure 6 shows our results for this measure. ANOVA showed a significant training condition effect (F(4, 45) = 10.615, p < 0.001, logarithm transformed data) and Tukey HSD demonstrated that fish in the naïve condition significantly (p < 0.05) differed from all other fish but the other groups did not differ from each other.
Figure 6.
Number of entries to the reward chamber during the probe trial is affected by the training condition. Mean ± SEM are shown. Sample sizes (n) = 10 for each training condition (different shading and pattern of graphs as indicated by the legend). Note that bars that share at least one letter designation are not significantly (p > 0.05) different from each other (Tukey HSD post hoc multiple comparison test).
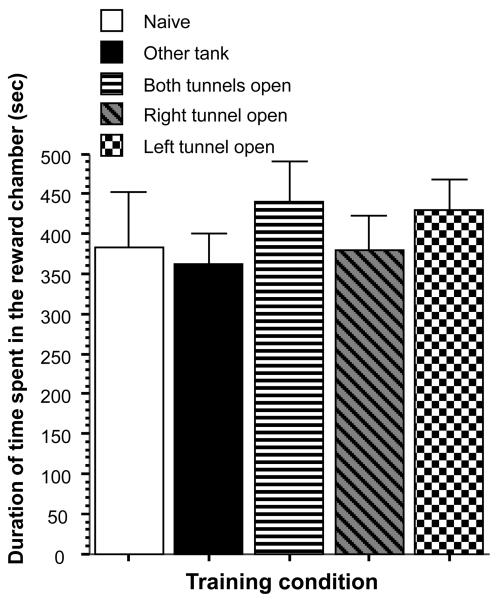
Last, we also measured the duration of time our experimental fish spent in the reward chamber (figure 7), which we argue is the measure of their shoaling tendency, i.e. their motivation to stay close to conspecifics. ANOVA revealed no significant differences among training groups (F(4, 45) = 0.472, p > 0.75).
Figure 7.
Duration of time spent in the reward chamber is unaffected by training condition. Mean ± SEM are shown. Sample sizes (n) = 10 for each training condition (different shading and pattern of graphs as indicated by the legend).
DISCUSSION
The question this study attempted to answer was whether fish exposed to the maze without any reward could learn about the maze and could behave differently as a result of acquiring memory of the maze in a subsequent test, the probe trial. Our results suggest that the answer to this question is yes. Zebrafish exposed to a particular tunnel open during the free exploration (training) phase of the maze showed a side preference in the probe trial that corresponded to this prior exploratory experience. The prior experience (exploration) dependent side bias of our experimental zebrafish during the probe trial is evident from the difference between the times the fish spent in the right versus the left tunnel and also from the number of fish choosing the right versus the left tunnel when they swam to the choice point the first time. Briefly, fish that were trained with the right tunnel open tended to prefer swimming via the right tunnel route and fish that were trained with the left tunnel open tended to prefer the left tunnel, whereas those that were trained with both tunnels chose randomly.
We call this learning latent learning as it resembles the well known phenomenon termed “latent inhibition” [30]. In the latter study, prior experience with an unreinforced stimulus altered the ability of the subject to associate this stimulus with reinforcement. In our case, prior experience with the unreinforced maze environment altered the ability of our experimental zebrafish to navigate the maze environment and led to a side bias. In other words, learning, i.e. modification of behavioral responses as a result of prior experience, has occurred without any experimenter controlled delivery of reinforcement. This type of learning may seem paradoxical from the viewpoint of classical associative learning paradigms in which an unconditioned stimulus (US or reinforcer) must accompany (must be temporally contiguous and contingent with) the conditioned stimulus (CS, a previously neutral stimulus) in order for the association between US and CS to be acquired [9]. However, despite the apparent paradox, latent learning is not principally different from classical conditioning, at least as far as the presence of reinforcement is concerned.
Numerous behavior genetic studies have shown that novelty induced exploration is adaptive and intermediate levels of exploratory activity has been selected for during the evolutionary past of a range of species including mammalian (e.g. the mouse [14]) and fish species (e.g. Paradise fish [24]). In other words, being curious is adaptive as it may allow the individual to discover food, mates, escape routes, hiding places, etc. Indeed, preference for novelty has been demonstrated in a range of paradigms including T-maze spontaneous alternation tasks [21], object recognition tasks [18], and novel place recognition tasks [16]. Briefly, novelty itself acts as a reinforcer and thus exposing zebrafish to the novel maze environment is not principally different from other associative learning tasks that deploy particular experimentally controlled reinforcers such as food or electric shock.
Although the side bias of our experimental zebrafish did correspond to their training condition, an asymmetric response pattern is apparent on figure 2. Briefly, while the right tunnel trained fish showed a robust right bias, the left tunnel trained fish showed only a marginal left side bias (but note that the first choice of the fish, figure 3 showed an equally strong response). This apparent asymmetry may be due to random stochastic variation, i.e. sampling error in our relatively modest sample size study. Alternatively, it may reflect a true lateralization of behavioral responses, a working hypothesis whose validity will be ascertained in the future. Notably, zebrafish have been found to preferentially respond to the stimulus on their right [31] and we also found the right tunnel response to be more robust.
Our results also showed that in addition to learning about the maze environment, our experimental fish have also been affected by the training procedure itself, i.e. procedural learning has also occurred. This is best demonstrated by the robust differences between the naïve, non-trained non-handled, and the other zebrafish. For example, fish in the former group showed a dramatically reduced frequency of visits to the reward chamber compared to all other fish (figure 6). This result is interesting especially if one considers that all fish spent about the same length of time in the reward chamber, suggesting that the motivation of the naïve fish to shoal (stay close to conspecifics) was not altered. It appears that the motivation to explore the maze, i.e. the ability to leave and rejoin the group, was reduced in the naïve fish. This reduction we argue is likely to be the result of enhanced fear, i.e. lack of habituation to the handling procedure. This conclusion is plausible considering that fish exposed to another tank, and thus to the same handling procedure as the maze trained fish were exposed to, did not reduce the frequency of entries to the reward chamber. Thus, it appears that the novelty of the maze environment alone does not explain the behavior of the naïve fish. In summary, our results demonstrate that both the maze environment and also some aspects of the training procedure were learned and remembered by zebrafish.
Zebrafish may learn numerous aspects of their maze environment. They also may employ different strategies to navigate the maze. For example, they may utilize spatial learning (relational memory), single cue-based learning (landmark;), ego-centric learning [40, 20, 49], or perhaps a combination of any of these strategies. To answer these questions a series of follow up studies will be conducted. It is notable, however, that the guillotine doors blocking access to the reward chamber during training were positioned right at the entrance to the reward chamber and far away from the choice point of the four way intersection in front of the start box (figure 1). This arrangement arguably biased the navigation strategy of experimental fish towards spatial (relational) learning as the fish had to make several maneuvers before they could get to the reward chamber and thus a single associative cue (extra- or intra-maze landmark) or a simple ego-centric (turn left or turn right) response could not be easily associated with accessing the reward chamber. Furthermore, other studies have shown that cyprinids, e.g. the goldfish [39] as well as the zebrafish [42], are capable of solving spatial learning tasks. Thus, it is possible that our experimental zebrafish have also developed a spatial map and navigated through the maze based upon their memory of the constellation of extra- and intra-maze cues, a hypothesis that will be tested in the future.
The last point to discuss is the applicability of the current work to future forward genetic and drug screens. Such screens require the testing of a large number of subjects and therefore they must employ simple and fast, i.e. high throughput, phenotypical tests. The current paradigm was not fast; training of 50 fish took 16 days, for example. However, it must be noted that this training did not require the presence of an experimenter and could, in principle, be run in a massively parallel manner: to increase throughput all is needed is to set up multiple identical mazes. It is also important to realize that the probe test, the only phase during which the behavior of the experimental subject was recorded and analyzed, was very short. Quantification of behavior during this phase was done manually [7], i.e. by observing the behavior of the fish, but the behavioral measures recorded, i.e. parameters of the location and swim path patterns of the fish, have been shown to be easy to quantify in zebrafish using computer automated methods such as video-tracking [22]. Briefly, it appears that the current latent learning task has the characteristics that will make it potentially useful for high-throughput screening.
In summary, the current as well as other already published promising zebrafish learning tasks may have utility in high-throughput mutation and drug screens. Such paradigms will facilitate the discovery of novel molecular mechanisms of vertebrate learning and memory and thus will contribute to a more complete understanding of the biology of these complex brain functions.
Acknowledgments
Supported by NIH/NIAAA (USA) and NSERC (Canada) grants to RG and by the University of Oviedo (Spain) visiting grant to LMG-L. We would like to thank Ryan Hoffman for conducting pilot studies and constructing the maze.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Al-Imari L, Gerlai R. Conspecifics as reward in associative learning tasks for zebrafish (Danio rerio) Behav. Brain Res. 2008;189:216–19. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam A, Burgess S, Golling G, Chen W, Sun Z, Townsend K, Farrington S, Haldi M, Hopkins N. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 1999;13:2713–24. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amsterdam A, Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–8. doi: 10.1016/j.tig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Bass SL, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav. Brain Res. 2008;186:107–17. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 5.Bilotta J, Risner ML, Davis EC, Haggbloom SJ. Assessing appetitive choice discrimination learning in zebrafish. Zebrafish. 2005;2:259–68. doi: 10.1089/zeb.2005.2.259. [DOI] [PubMed] [Google Scholar]
- 6.Blank M, Guerim LD, Cordeiro RF, Vianna MR. A one-trial inhibitory avoidance task to zebrafish: rapid acquisition of an NMDA-dependent long-term memory. Neurobiol Learn Mem. 2009;92:529–34. doi: 10.1016/j.nlm.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Blaser R, Gerlai R. Behavioral phenotyping in Zebrafish: Comparison of three behavioral quantification methods. Behavior Research Methods. 2006;38:456–69. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- 8.Blodgett HC. The effect of the introduction of reward upon the maze performance of rats. Univ Calif Publ Psychol. 1929;4:113–34. [Google Scholar]
- 9.Bouton ME, Moody EW. Memory processes in classical conditioning. Neuroscience and Biobehavioral Reviews. 2004;28:663–74. doi: 10.1016/j.neubiorev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Burgess HA, Granato M. The neurogenetic frontier--lessons from misbehaving zebrafish. Brief Funct Genomic Proteomic. 2008;7:474–82. doi: 10.1093/bfgp/eln039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braubach OR, Wood HD, Gadbois S, Fine A, Croll RP. Olfactory conditioning in the zebrafish (Danio rerio) Behav Brain Res. 2009;198:190–8. doi: 10.1016/j.bbr.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee D, Gerlai R. High Precision Liquid Chromatography Analysis of Dopaminergic and Serotoninergic Responses to Acute Alcohol Exposure in Zebrafish. Behav. Brain Res. 2009;200:208–213. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crusio WE, Gerlai R, editors. Handbook of Molecular-Genetic Techniques for Brain and Behavior Research. Elsevier; Amsterdam: 1999. p. 964. [Google Scholar]
- 14.Crusio WE, van Abeelen JH. The genetic architecture of behavioural responses to novelty in mice. Heredity. 1986;56:55–63. doi: 10.1038/hdy.1986.8. [DOI] [PubMed] [Google Scholar]
- 15.Díaz E. From microarrays to mechanisms of brain development and function. Biochem Biophys Res Commun. 2009;385:129–31. doi: 10.1016/j.bbrc.2009.05.057. [DOI] [PubMed] [Google Scholar]
- 16.Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 17.Eddins D, Petro A, Williams P, Cerutti DT, Levin ED. Nicotine effects on learning in zebrafish: the role of dopaminergic systems. Psychopharmacology. 2009;202:103–9. doi: 10.1007/s00213-008-1287-4. [DOI] [PubMed] [Google Scholar]
- 18.Ennaceur A, Aggleton JP. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res. 1997;88:181–93. doi: 10.1016/s0166-4328(97)02297-3. [DOI] [PubMed] [Google Scholar]
- 19.Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- 20.Gerlai R. Behavioral tests of hippocampal function: Simple paradigms, complex problems. Behav. Brain Res. 2001;125:269–77. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- 21.Gerlai R. A new continuous alternation task in T-maze detects hippocampal dysfunction in mice: A strain comparison and lesion study. Behavioral Brain Research. 1998;95:91–101. doi: 10.1016/s0166-4328(97)00214-3. [DOI] [PubMed] [Google Scholar]
- 22.Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R. Acute and Chronic alcohol dose: Population differences in behavior and neurochemistry of zebrafish. Genes, Brain and Behavior. 2009;8:586–99. doi: 10.1111/j.1601-183X.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlai R, Fernandes Y, Pereira T. Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav. Brain Res. 2009;201:318–24. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerlai R, Crusio WE, Csányi V. Inheritance of species specific behaviors in the paradise fish (Macropodus opercularis): A diallel study. Behavior Genetics. 1990;20:487–98. doi: 10.1007/BF01067715. [DOI] [PubMed] [Google Scholar]
- 25.Gerlai R. Phenomics: Fiction or the Future? Trends Neurosci. 2002;25:506–9. doi: 10.1016/s0166-2236(02)02250-6. [DOI] [PubMed] [Google Scholar]
- 26.Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–10. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 27.Grunwald DJ, Eisen JS. Timeline: Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 28.Hicks C, Sorocco D, Levin M. Automated analysis of behavior: a computer-controlled system for drug screening and the investigation of learning. J Neurobiol. 2006;66:977–90. doi: 10.1002/neu.20290. [DOI] [PubMed] [Google Scholar]
- 29.Knapik EW. ENU mutagenesis in zebrafish--from genes to complex diseases. Mamm Genome. 2000;11:511–9. doi: 10.1007/s003350010098. [DOI] [PubMed] [Google Scholar]
- 30.Lubow RE. Latent inhibition. Psychol Bull. 1973;79:398–407. doi: 10.1037/h0034425. [DOI] [PubMed] [Google Scholar]
- 31.Miklósi A, Andrew RJ, Gasparini S. Role of right hemifield in visual control of approach to target in zebrafish. Behav Brain Res. 2001;122:57–65. doi: 10.1016/s0166-4328(01)00167-x. [DOI] [PubMed] [Google Scholar]
- 32.Miller N, Gerlai R. Oscillations in Shoal Cohesion in Zebrafish (Danio rerio) Behav. Brain Res. 2008;193:148–51. doi: 10.1016/j.bbr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller N, Gerlai R. Quantification of Shoaling Behaviour in Zebrafish (Danio rerio) Behav. Brain Res. 2007;184:157–66. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Mueller T, Vernier P, Wullimann MF. The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio. Brain Res. 2004;1011:156–69. doi: 10.1016/j.brainres.2004.02.073. [DOI] [PubMed] [Google Scholar]
- 35.Panula P, Sallinen V, Sundvik M, Kolehmainen J, Torkko V, Tiitula A, Moshnyakov M, Podlasz P. Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish. 2006;3:235–47. doi: 10.1089/zeb.2006.3.235. [DOI] [PubMed] [Google Scholar]
- 36.Pather S, Gerlai R. Shuttle box learning in zebrafish Behav Brain Res. 2009;196:323–27. doi: 10.1016/j.bbr.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parra KV, Adrian JC, Jr, Gerlai R. The synthetic substance hypoxanthine 3-N-oxide elicits alarm reactions in zebrafish (Danio rerio) Behav Brain Res. 2009;205:336–41. doi: 10.1016/j.bbr.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salas C, Broglio C, Durán E, Gómez A, Ocaña FM, Jiménez-Moya F, Rodríguez F. Neuropsychology of learning and memory in teleost fish. Zebrafish. 2006;3:157–71. doi: 10.1089/zeb.2006.3.157. [DOI] [PubMed] [Google Scholar]
- 39.Salas C, Rodríguez F, Vargas JP, Durán E, Torres B. Spatial learning and memory deficits after telencephalic ablation in goldfish trained in place and turn maze procedures. Behav Neurosci. 1996;110:965–80. doi: 10.1037//0735-7044.110.5.965. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro ML, Eichenbaum H. Hippocampus as a memory map: synaptic plasticity and memory encoding by hippocampal neurons. Hippocampus. 1999;9:365–84. doi: 10.1002/(SICI)1098-1063(1999)9:4<365::AID-HIPO4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 41.Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sison M, Gerlai R. Associative learning in zebrafish (Danio rerio) in the plus maze. Behav Brain res. 2009 doi: 10.1016/j.bbr.2009.09.043. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes influencing vertebrate behavior: zebrafish making headway. Lab Anim. 2006;35:33–9. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- 44.Sivasubbu S, Balciunas D, Davidson AE, Pickart MA, Hermanson SB, Wangensteen KJ, Wolbrink DC, Ekker SC. Gene-breaking transposon mutagenesis reveals an essential role for histone H2afza in zebrafish larval development. Mech Dev. 2006;123:513–29. doi: 10.1016/j.mod.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Sweatt JD. 2nd edition Elsevier; Amsterdam: 2010. Mechanisms of memory; p. 400. [Google Scholar]
- 46.Tolman EC, Honzik CH. Introduction and removal of reward, and maze performance in rats. Univ Calif Publ Psychol. 1930;4:257–75. [Google Scholar]
- 47.Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003;2:268–81. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 48.Vascotto SG, Beckham Y, Kelly GM. The zebrafish's swim to fame as an experimental model in biology. Biochem Cell Biol. 1997;75:479–85. [PubMed] [Google Scholar]
- 49.Waller D, Lippa Y. Landmarks as beacons and associative cues: their role in route learning. Mem Cognit. 2007;35:910–24. doi: 10.3758/bf03193465. [DOI] [PubMed] [Google Scholar]
- 50.Williams FE, White D, Messer WS., Jr A simple spatial alternation task for assessing memory function in zebrafish. Behavioural Processes. 2002;58:125–32. doi: 10.1016/s0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 51.Xu X, Scott-Scheiern T, Kempker L, Simons K. Active avoidance conditioning in zebrafish (Danio rerio) Neurobiol Learn Mem. 2007;87:72–7. doi: 10.1016/j.nlm.2006.06.002. [DOI] [PubMed] [Google Scholar]