Abstract
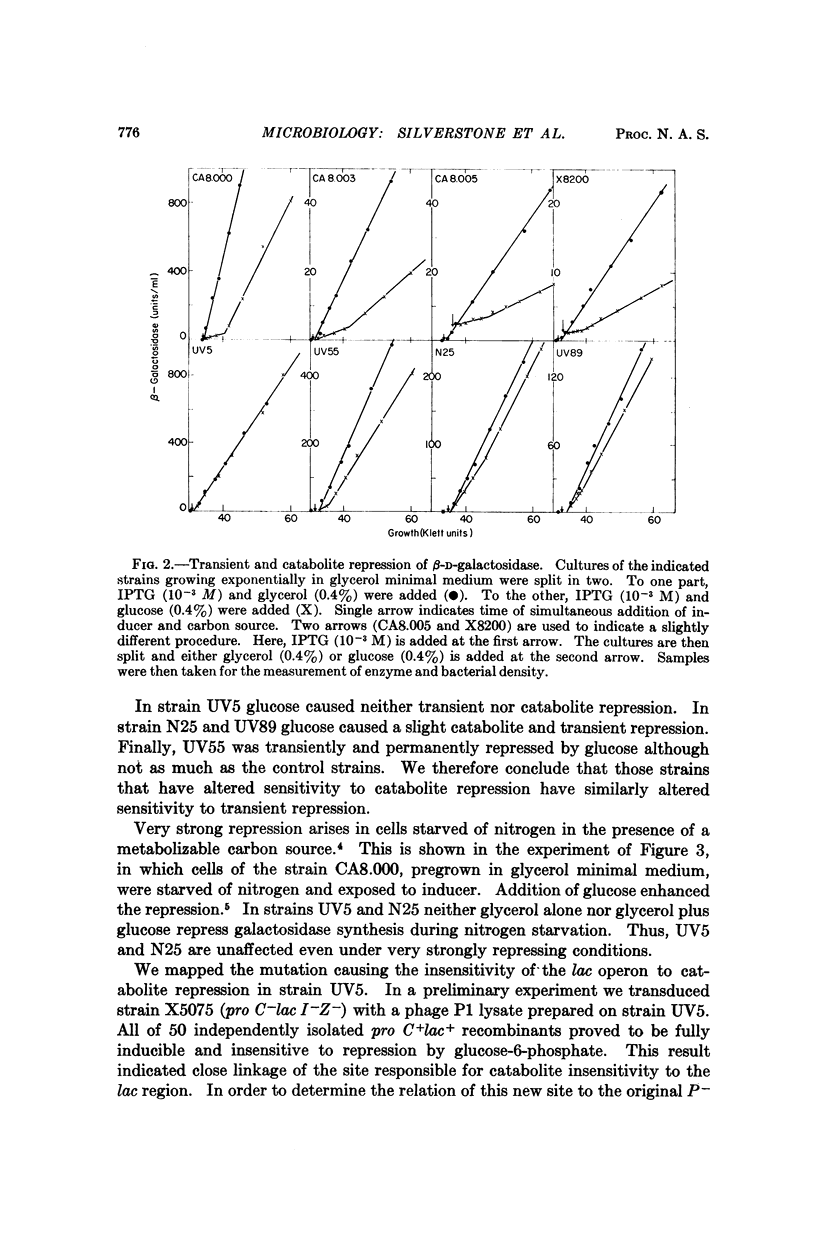
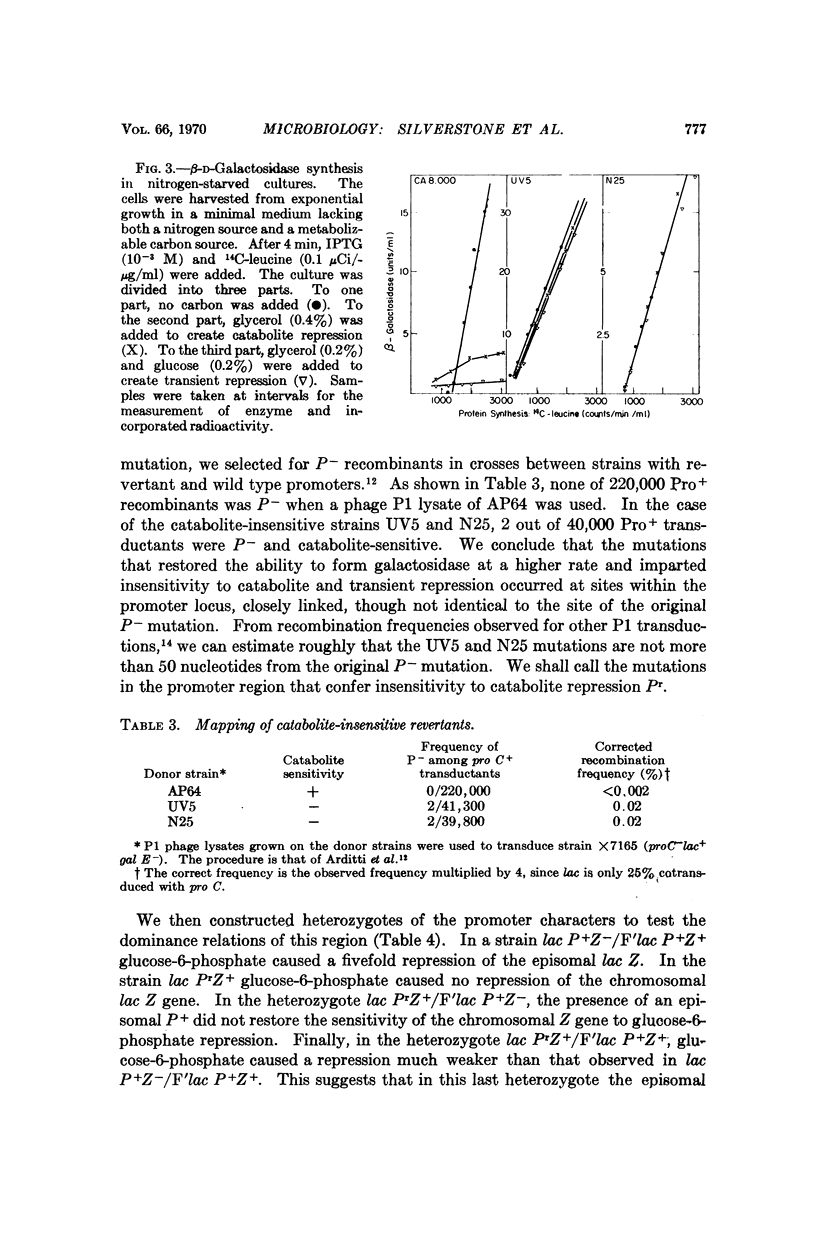
The maximum rate of expression of the lac operon is severely reduced in lac promoter mutants. Revertants of these mutations which produce higher levels of enzyme were isolated. Some of these revertants had lost sensitivity to catabolite repression and transient repression. The mutations responsible for these losses took place at sites very close to the original promoter mutations. From these results we conclude that the promoter itself is the target site for both catabolite and transient repression of the lac operon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arditti R. R., Scaife J. G., Beckwith J. R. The nature of mutants in the lac promoter region. J Mol Biol. 1968 Dec;38(3):421–426. doi: 10.1016/0022-2836(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Chambers D. A., Zubay G. The stimulatory effect of cyclic adenosine 3'5'-monophosphate on DNA-directed synthesis of beta-galactosidase in a cell-free system. Proc Natl Acad Sci U S A. 1969 May;63(1):118–122. doi: 10.1073/pnas.63.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasin L. A., Magasanik B. Induction and repression of the histidine-degrading enzymes of Bacillus subtilis. J Biol Chem. 1968 Oct 10;243(19):5165–5178. [PubMed] [Google Scholar]
- Friedman S. B., Margolin P. Evidence for an altered operator specificity: catabolite repression control of the leucine operon in Salmonella typhimurium. J Bacteriol. 1968 Jun;95(6):2263–2269. doi: 10.1128/jb.95.6.2263-2269.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen K., Miller J. H., Scaife J., Beckwith J. New controlling element in the Lac operon of E. coli. Nature. 1968 Mar 2;217(5131):825–827. doi: 10.1038/217825a0. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. The step sensitive to catabolite repression and its reversal by 3'-5' cyclic AMP during induced synthesis of beta-galactosidase in E. coli. Biochem Biophys Res Commun. 1969 Jul 7;36(1):84–92. doi: 10.1016/0006-291x(69)90653-6. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Meiss H. K., Brill W. J., Magasanik B. Genetic control of histidine degradation in Salmonella typhimurium, strain LT-2. J Biol Chem. 1969 Oct 10;244(19):5382–5391. [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. The role of the lac promotor locus in the regulation of beta-galactosidase synthesis by cyclic 3',5'-adenosine monophosphate. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1336–1342. doi: 10.1073/pnas.61.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., De Crombrugghe B., Pastan I. Cyclic AMP regulates catabolite and transient repression in E. coli. Nature. 1969 Aug 23;223(5208):810–812. doi: 10.1038/223810a0. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Magasanik B., Reznikoff W. S., Miller J. H., Beckwith J. R. Catabolite sensitive site of the lac operon. Nature. 1969 Mar 15;221(5185):1012–1014. doi: 10.1038/2211012b0. [DOI] [PubMed] [Google Scholar]
- Tyler B., Loomis W. F., Jr, Magasanik B. Transient repression of the lac operon. J Bacteriol. 1967 Dec;94(6):2001–2011. doi: 10.1128/jb.94.6.2001-2011.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Magasanik B. Molecular basis of transient repression of beta-galactosidase in Escherichia coli. J Bacteriol. 1969 Feb;97(2):550–556. doi: 10.1128/jb.97.2.550-556.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Magasanik B. Physiological basis of transient repression of catabolic enzymes in Escherichia coli. J Bacteriol. 1970 May;102(2):411–422. doi: 10.1128/jb.102.2.411-422.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Wishnow R., Loomis W. F., Jr, Magasanik B. Catabolite repression gene of Escherichia coli. J Bacteriol. 1969 Nov;100(2):809–816. doi: 10.1128/jb.100.2.809-816.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C., CARLTON B. C., GUEST J. R., HELINSKI D. R., HENNING U. ON THE COLINEARITY OF GENE STRUCTURE AND PROTEIN STRUCTURE. Proc Natl Acad Sci U S A. 1964 Feb;51:266–272. doi: 10.1073/pnas.51.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Varmus H. E., Perlman R. L., Pastan I. H. Stimulation of lac mRNA synthesis by cyclic AMP in cell free extracts of Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 12;38(5):894–901. doi: 10.1016/0006-291x(70)90805-3. [DOI] [PubMed] [Google Scholar]



