Abstract
The development of targeted therapies with true specificity for cancer relies upon exploiting differences between cancerous and normal cells. Genetic and genomic alterations including somatic mutations, translocations, and amplifications have served as recent examples of how such differences can be exploited as effective drug targets. Small molecule inhibitors and monoclonal antibodies directed against the protein products of these genetic anomalies have led to cancer therapies with high specificity and relatively low toxicity. Recently, our group and others have demonstrated that somatic mutations in the PIK3CA gene occur at high frequency in breast and other cancers. Moreover, the majority of mutations occur at three hotspots, making these ideal targets for therapeutic development. Here we review the literature on PIK3CA mutations in cancer, as well as existing data on PIK3CA inhibitors and inhibitors of downstream effectors for potential use as targeted cancer therapeutics.
Keywords: PIK3CA, mutation, oncogene, PI3 kinase, AKT, mTOR
INTRODUCTION
Insults to the genome are a hallmark of cancer and a driving force in carcinogenesis. These genomic alterations are an obvious difference between normal and cancerous cells, which provide an opportunity to exploit them as potential targets for therapeutics. Recently, successful clinical trials of imatinib, gefitinib/erlotinib, and trastuzumab, which are specific for BCR-ABL translocations [1], epidermal growth factor receptor (EGFR) mutations [2, 3], and HER2/neu amplifications [4–6] respectively, have illustrated the ability to develop drugs that target genetic abnormalities and opened the possibility for future streamlined therapies based on the genomic landscape of an individual’s cancer.
The phosphatidylinositol 3-kinase (PI3K) p110α catalytic subunit, PIK3CA, is one the most highly mutated oncogenes in human cancers, and high mutational frequencies of PIK3CA have been reported in colorectal [7], breast [8] and liver cancers [9] while lower rates of mutation have been described in many other human malignancies including ovarian [10, 11], lung [7, 9], gastric [7, 9, 12, 13], and brain cancers [7, 9, 14–21]. While a wide variety of PIK3CA mutations have been found, the vast majority of mutations occur in three hotspots, E542K, E545K, and H1047R, which will be the focus of this review (Figure 1). E542K and E545K are located within exon 9 in the helical domain of PIK3CA whereas H1047R is encoded by exon 20 within the kinase domain. Studying the effects of these mutations in colorectal cells [22–24], breast epithelial cells [25, 26], and chicken embryos/fibroblasts [27, 28] have illustrated a direct connection between these mutations and carcinogenesis. Through crystallographic and biochemical methods, it has been determined that the probable mechanism for the oncogenicity of the E545K mutation is the disruption of an inhibitory charge-charge interaction between PIK3CA and the N-terminal SH2 domain of the p85 regulatory subunit [29] (Figure 1). Additionally, it has been previously proposed that the oncogenic mechanism of the E542K mutation is a change in interaction with the p85 regulatory subunit, while the H1047R mutation increases binding affinity of PIK3CA for the negatively charged phosphatidylinositol substrate [30]. PIK3CA mutations have also been associated with paclitaxel resistance in breast epithelial cells [25], and PI3K signaling in general has been linked with resistance to a number of other cancer therapies. Clinically, the presence of PIK3CA mutations has been linked to both favorable [31, 32] and unfavorable [33, 34] patient prognosis, and it has also been reported that exon 9 mutations have a less favorable prognosis than exon 20 mutations in breast cancer [35]. The reasons for these conflicting data are not clear, but likely reflect limited sample sizes and difference in treatment regimens between the various studies.
Figure 1.
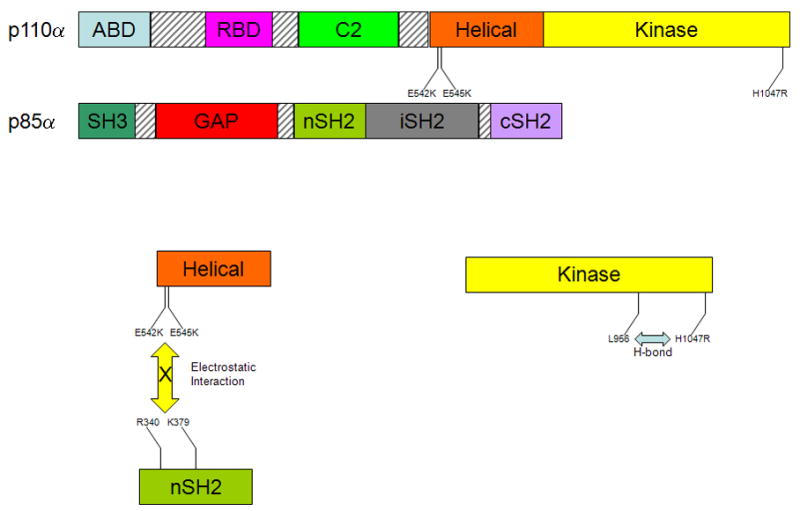
A representation of the domains of the PI3K subunits p110α and p85α. The p110α catalytic subunit has 5 domains including adaptor-binding domain (ABD), the Ras-binding domain (RBD), a calcium binding domain (C2), a helical domain and a kinase domain. The p85α regulatory subunit contains 5 domains as well, which include a Src homology 3 domain (SH3), a GTPase activating protein domain (GAP), an N-terminal Src homology 2 domain (nSH2), an inter- Src homology 2 domain (iSH2), and a C-terminal Src homology 2 domain (cSH2). The exon 9 hotspot mutations, E542K and E545K, occur in the helical domain of the catalytic subunit p110α, and the charge reversal caused by these mutations inhibits electrostatic interactions between those amino acids on the p110α helical domain and R340 and K379 on the nSH2 domain of p85α. The exon 20 hotspot mutation, H1047R, is in the kinase domain of p110α, and this mutation has been proposed to form a hydrogen bond with L956 of p110α, which in turn leads to catalytic activity of p110α.
TARGETING PIK3CA MUTATIONS
With the recent therapeutic successes of imatinib, erlotinib/gefitinib and trastuzumab, finding additional targeted therapies for high frequency oncogenic somatic genomic alterations is of great importance and interest. PIK3CA somatic mutations would be ideal for targeting due to their high rate of occurrence and the fact that 80% to 90% of these mutations are in one of three recurrent hotspot sequences. Below, we review several classes of targeted compounds that may have clinical utility for the treatment of cancers harboring PIK3CA mutations.
PI3K Inhibitors
The most direct method of targeting cancers that have PIK3CA mutations would be to develop inhibitors that have high specificity for mutant PIK3CA but not its wild type counterpart. The ability to create mutation specific small molecule inhibitors is exemplified by erlotinib and gefitinib, which were initially developed as EGFR inhibitors but were found to be most effective in patients whose tumors contained specific EGFR mutations [36, 37]. This finding is attributed to oncogene addiction [36], which is the phenomenon whereby cancer cells become dependent on growth signals from aberrantly activated pathways by mutated oncogenes, and therefore removal of these signals leads to decreased cellular growth and apoptosis [38]. Although historical evidence suggests that mutant specific inhibitors may be feasible, there are currently no PI3K inhibitors with specificity for even the p110α isoform, which initially suggested that the development of compounds specific for mutant PIK3CA would not be possible. However, recent structural data demonstrate that rational design of such inhibitors is feasible [39], and therefore the emergence of targeted mutant PIK3CA therapies is likely to be imminent.
Prior to the discovery of somatic PIK3CA mutations in human cancers, the PI3K enzyme was already recognized as being an important molecule in mediating carcinogenesis. As such, inhibitors of PI3K were developed with the hope that a therapeutic window could be achieved. The earliest and best characterized PI3K inhibitors are wortmannin and LY294002. Both of these compounds have been shown to be effective antitumor agents in in vitro cell culture models, as well as in in vivo animal models [40–42]. LY294002 has been shown to inhibit both in vitro PI3K activity and phosphorylation of downstream effectors of PIK3CA in breast epithelial [25] and colorectal cancer cells [24]. However, due to their poor pharmacological properties and marked cytotoxicity, LY294002 and wortmannin do not have clinical utility as reviewed by Workman [43]. Additionally, they are not ideal for specifically targeting PIK3CA mutants because they can inhibit other kinases of the PI-3 kinase-like kinase (PIKK) family, and several other kinases such as the mammalian target of rapamycin (mTOR) [44–48]. Recently, a number of groups have reported developing PIK3CA selective inhibitors [49–55], and some have demonstrated efficacy in vitro and in vivo [49–51, 53, 54, 56]. Within the past year, the PI3K inhibitors XL147 (Exilixis), BEZ235 (Norvartis) and GDC-0941 (Genentech) have entered early phase clinical trials, and many more of these compounds will soon follow. Given that mutant PIK3CA results in constitutively active PI3K activity, it will be of interest to determine if the presence of PIK3CA mutations will allow for the selection of patients with a high likelihood of response.
Buttressed against these exciting developments, specific targeting of PIK3CA may be problematic, as PIK3CA is involved in a number of signaling pathways associated with normal cellular function, such as insulin signaling [55, 57]. This may result in PIK3CA inhibitors that are prohibitively cytotoxic, thus limiting their clinical benefit. To illustrate this point, some PIK3CA inhibitors have been shown to abrogate the effects of insulin in mice [55]. Similarly, PIK3CA deficient mice have recently been shown to have an increased rate of heart failure in response to cardiac stress [58]. Thus, the possibility of a significant side effect profile has led to the development of compounds designed to target downstream effectors within the PIK3CA pathway with the hope that this may be a more effective strategy to target cancers containing PIK3CA mutations.
AKT Inhibitors
AKT, also known as protein kinase B, is a serine threonine kinase directly downstream of PI3K, and dysregulation of AKT is commonly associated with many different cancers [59]. More specifically, constitutive activation of AKT has been associated with PIK3CA mutations in several in vitro cell models [23–27]. Therefore, AKT inhibitors may prove to be useful in targeting cancers with PIK3CA mutations.
AKT was identified as an oncogene over two decades ago [60], and multiple AKT inhibitors have been developed and used successfully to inhibit AKT activation and cellular growth in in vitro and in vivo tumor models [61–88]. Perifosine is the most studied AKT inhibitor, proving efficacious at inhibiting the growth of various cancer cell types [85]. However as a single agent, Perifosine has not shown therapeutic benefit in several phase II trials. However, Perifosine may have some therapeutic potential when used in combination with radiation or other standard cytotoxic agents, as evidenced by in vitro studies [89–91]. Another AKT inhibitor, Miltefosine, has shown efficacy in clinical trials for the topical treatment of cutaneous lymphoma and breast cancer skin metastases [92–95]. Recently, it was discovered that API-2, a compound that had shown some efficacy in early phase trials but was abandoned due to excessive toxicity [96, 97], is an AKT inhibitor [82]. This finding opens the door to investigation of specific AKT inhibition at lower doses than those used previously, thus potentially avoiding the detrimental side effects. Currently, there are multiple ongoing early phase clinical trials of AKT inhibitors that should ascertain the effectiveness of this class of agents.
Additional studies need to be performed on the relationship between PIK3CA mutations and AKT as there are three distinct isoforms (AKT1, AKT2, and AKT3) and each may have different effects on tumor growth and/or cytotoxicity. To date, PIK3CA mutations have been most closely linked to AKT1 in in vitro cell models [23, 25–27], with a single study examining all three isoforms revealing that AKT1 may be most affected by PIK3CA mutations [24]. However, isoform specific functions have been delineated through various laboratory studies. For example, RNA interference (RNAi) mediated gene knockdown of AKT1 in breast cancer cell lines has been shown to increase cell motility [98], and AKT1 knockout mice are small in size and infertile [99]. In addition, AKT2 knockout mice develop diabetes mellitus [100], while AKT3 knockout mice exhibit abnormal brain development [101]. Selective AKT1 and 2 inhibitors have been developed and have shown some promise in vitro [77]. Further elucidation of the relative effects of inhibiting different AKT isoforms in cancers harboring PIK3CA mutations will be required, as the potential for significant toxicities remains an obstacle for using AKT inhibitors as effective targeted therapies for these cancers.
mTOR Inhibitors
mTOR is a downstream effector of PIK3CA and is very important for many cellular processes, including cell proliferation [102] and angiogenesis [103]. The mTOR pathway has been shown to be activated by PIK3CA mutations in both chicken embryo fibroblasts [27] and colorectal cancer cells [23]. Therefore, blocking the mTOR pathway may prove to be an effective strategy for targeting aberrant growth signaling in cancers with PIK3CA mutations.
Of the compounds that could potentially target cancers bearing PIK3CA mutations, mTOR inhibitors are the most mature in terms of their development and clinical use. Rapamycin, an mTOR inhibitor, was initially developed in the early 1970’s as an antifungal agent [104] and was later FDA approved as an immunosuppressive therapy [105]. Rapamycin and its analogs have been used with variable success to treat a multitude of different cancers [106–115]. The best evidence of response thus far is in the treatment of advanced renal cancers for which temsirolimus [116], a rapamycin prodrug, has been FDA approved. Identifying biomarkers that predict for response to mTOR inhibitors is of great importance for the development of these agents, as response to mTOR inhibitors has been greatly variable. Theoretically, PIK3CA mutations may lend themselves as predictive biomarkers for response to mTOR inhibitors. For example, it has been shown that either rapamycin or its analogs can impede the transformation of chicken embryo fibroblasts expressing PIK3CA mutations [27], inhibit tumor growth induced by PIK3CA mutations in chicken embryos [28], and reduce the formation of abnormal human breast epithelial cell acini induced by mutant PIK3CA overexpression using a three-dimensional basement membrane morphogenesis assay [25].
Despite the clinical potential of mTOR inhibitors, significant hurdles for their further development still exist. mTOR actually forms two complexes within cells, mTORC1 and mTORC2 [117]. mTORC1 is known to mediate a negative feedback loop with PI3K/AKT signaling, and therefore inhibiting mTOR pharmacologically causes a paradoxical upregulation of PI3K/AKT growth promoting signaling [118]. Additionally, mTORC2 directly phosphorylates AKT [119] but is only rarely inhibited by rapamycin and its analogs in a cell/tissue type dependent manner [120]. Since the mTOR complexes are on both ends of the PI3K/AKT signaling pathway and mTORC2 inhibition is limited, more studies need to be completed to elucidate the mechanisms of sensitivity/resistance to mTOR inhibitors and to develop biomarkers to predict the efficacy of mTOR inhibition. Other effective strategies for targeting this complex signaling pathway in the future may include combining current mTOR inhibitors with either newer PI3K/AKT inhibitors or more traditional therapies, such as chemotherapy and endocrine therapy. In addition, it is possible that the development of new inhibitors capable of blocking mTORC2, or alternatively both mTORC1 and mTORC2, could provide a more effective therapeutic regimen.
Possibilities of Novel Dual Inhibition
Due to the genetic instability of most human cancers, it can be expected that any targeted therapy when used as a single agent will ultimately succumb to drug resistance. However, the development of targeted therapies with minimal side effects would hopefully enable the combinatorial use of multiple drugs with non-overlapping toxicities, to effectively treat and eradicate the disease. Recently, it was demonstrated that the combination of PIK3CA and mTOR inhibitors could prevent the increase in AKT signaling caused by mTOR inhibition alone [121]. A single molecule, PI-103, was found to effectively inhibit both PIK3CA and mTOR, and its efficacy has been shown in glioma, ovarian cancer, and breast cancer in in vitro and in vivo models [121, 122]. This class of molecules, capable of inhibiting multiple targets within the PIK3CA pathway, may prove to be an effective strategy for targeting cancer cells containing PIK3CA mutations.
In addition, combination therapies with existing anti-neoplastic drugs are also being explored. For example, PIK3CA mutations have been positively correlated with increased expression of both estrogen receptor alpha (ERα) and HER2/neu in the NCI 60 panel of cancer cell lines [123] as well breast tumor samples [124]. Hormonal therapies and trastuzumab are currently used to target ERα and HER2/neu respectively. However, it has been shown that increased AKT signaling is associated with resistance to both of these therapies [125–128], and it is therefore possible that PIK3CA mutations may actually confer resistance to these drugs. If this hypothesis proves to be true, then combining either of these therapies with a PIK3CA, AKT, or mTOR inhibitor may provide an effective strategy for abrogating drug resistance.
CONCLUSIONS
Due to their high frequency in many cancers, PIK3CA mutations are a prime candidate for targeted therapeutics. However given the fact that these mutations have only recently been accurately characterized, much work lies ahead to fully elucidate their biological and clinical significance. The process of discovery for developing targeted PIK3CA therapies remains in its infancy. However, the wealth of potential targets along the PIK3CA pathway is certainly enticing from a developmental therapeutics viewpoint, and the opportunity exists to significantly impact cancer care with future success in this arena.
Acknowledgments
This work was supported by The Flight Attendant’s Medical Research Institute (FAMRI), NIH/NCI CA109274, the Summer Running Fund, The Susan G. Komen for the Cure Foundation, Mary Kay Ash Charitable Foundation and the Avon Foundation. J.P.G. is a recipient of a Department of Defense Breast Cancer Research Program Predoctoral Fellowship Award W81XWH-06-1-0325. D.P.C. is supported on an NIH Institutional Training Grant T32 CA09071 and an American Cancer Society Young Investigator Award.
Abbreviations
- EGFR
epidermal growth factor receptor
- PI3K
phosphatidylinositol 3-kinase
- mTOR
mammalian target of rapamycin
- PIKK
PI-3 kinase-like kinase
- RNAi
RNA interference
- ERα
estrogen receptor alpha
Footnotes
J.P.G. and D.P.C. declare no conflict of interests. B.H.P. receives support from GlaxoSmithKline under the terms regulated by The Johns Hopkins University policies on conflicts of interest.
References
- 1.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ, Durrant S, Schwarer A, Joske D, Seymour J, Grigg A, Ma D, Arthur C, Bradstock K, Joshua D, Louwagie A, Martiat P, Straetmans N, Bosly A, Shustik C, Lipton J, Forrest D, Walker I, Roy DC, Rubinger M, Bence-Bruckler I, Kovacs M, Turner AR, Birgens H, Bjerrum O, Facon T, Harousseau JL, Tulliez M, Guerci A, Blaise D, Maloisel F, Michallet M, Hossfeld D, Mertelsmann R, Andreesen R, Nerl C, Freund M, Gattermann N, Hoeffken K, Ehninger G, Deininger M, Ottmann O, Peschel C, Fruehauf S, Neubauer A, Le Coutre P, Aulitzky W, Fanin R, Rosti G, Mandelli F, Morra E, Carella A, Lazzarino M, Petrini M, Ferrini PR, Nobile F, Liso V, Ferrara F, Rizzoli V, Fioritoni G, Martinelli G, Ossenkoppele G, Browett P, Gedde-Dahl T, Tangen JM, Dahl I, Odriozola J, Boluda JCH, Steegmann JL, Canizo C, Sureda A, Diaz J, Granena A, Fernandez MN, Stenke L, Paul C, Bjoreman M, Malm C, Wadenvik H, Nilsson PG, Turesson I, Hess U, Solenthaler M, Russel N, Mufti G, Cavenagh J, Clark RE, Green AR, Holyoake TL, Lucas GS, Smith G, Milligan DW, Rule SJ, Burnett AK, Moroose R, Wetzler M, Bearden J, Brown R, Lobell M, Cataland S, Rabinowitz I, Meisenberg B, Gabrilove J, Thompson K, Graziano S, Emanuel P, Gross H, Cobb P, Bhatia R, Dakhil S, Irwin D, Issell B, Pavletic S, Kuebler P, Layhe E, Butera P, Glass J, Moore J, Grant B, Niell H, Herzig R, Burris H, Peterson B, Powell B, Kalaycio M, Stirewalt D, Samlowski W, Berman E, Limentani S, Seay T, Shea T, Akard L, Becker P, DeVine S, Hart R, Veith R, Wade J, Brunvand M, Silver R, Kalman L, Strickland D, Shurafa M, Bashey A, Shadduck R, Cooper S, Safah H, Rubenstein M, Collins R, Keller A, Stone R, Tallman M, Stevens D, Pecora A, Agha M, Holmes H, Rowe J, Schiffer CA, Buyse M, Cornelissen J. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, Leiberman G, Slamon DJ. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clinical Breast Cancer. 2005;6:240–246. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 7.Samuels Y, Wang ZH, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell DM, Riggins GJ, Willson JKV, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554–554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 8.Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, Konishi H, Karakas B, Blair BG, Lin C, Peters BA, Velculescu VE, Park BH. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 9.Lee JW, Soung YH, Kim SY, Lee HW, Park WS, Nam SW, Kim SH, Lee JY, Yoo NJ, Lee SH. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005;24:1477–1480. doi: 10.1038/sj.onc.1208304. [DOI] [PubMed] [Google Scholar]
- 10.Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, Boyd J. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11:2875–2878. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- 11.Campbell IG, Russell SE, Choong DYH, Montgomery KG, Ciavarella ML, Hooi CSF, Cristiano BE, Pearson RB, Phillips WA. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 12.Velho S, Oliveira C, Ferreira A, Ferreira AC, Suriano G, Schwartz S, Duval A, Carneiro F, Machado JC, Hamelin R, Seruca R. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur J Cancer. 2005;41:1649–1654. doi: 10.1016/j.ejca.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Li VSW, Wong CW, Chan TL, Chan ASW, Zhao W, Chu KM, So S, Chen X, Yuen ST, Leung SY. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005;5 doi: 10.1186/1471-2407-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kita D, Yonekawa Y, Weller M, Ohgaki H. PIK3CA alterations in primary (de novo) and secondary glioblastomas. Acta Neuropathol. 2007;113:295–302. doi: 10.1007/s00401-006-0186-1. [DOI] [PubMed] [Google Scholar]
- 15.Gallia GL, Rand V, Siu IM, Eberhart CG, James CD, Marie SKN, Oba-Shinjo SM, Carlotti CG, Caballero OL, Simpson AJG, Brock MV, Massion PP, Carson BS, Riggins GJ. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res. 2006;4:709–714. doi: 10.1158/1541-7786.MCR-06-0172. [DOI] [PubMed] [Google Scholar]
- 16.Dam V, Morgan BT, Mazanek P, Hogarty MD. Mutations in PIK3CA are infrequent in neuroblastoma. BMC Cancer. 2006;6 doi: 10.1186/1471-2407-6-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang JCS, Chung NYF, Chan NHL, Poon WS, Thomas T, Ng HK. Rare mutation of PIK3CA in meningiomas. Acta Neuropathol. 2006;111:284–285. doi: 10.1007/s00401-005-0021-0. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann C, Devermann L, Gehlhaar C, Holtkamp N, von Deimling A. PIK3CA mutations in oligodendroglial tumours. Neuropathol Appl Neurobiol. 2006;32:209–212. doi: 10.1111/j.1365-2990.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann C, Bartels G, Gehlhaar C, Holtkamp N, von Deimling A. PIK3CA mutations in glioblastoma multiforme. Acta Neuropathol. 2005;109:639–642. doi: 10.1007/s00401-005-1000-1. [DOI] [PubMed] [Google Scholar]
- 20.Mueller W, Mizoguchi M, Silen E, D’Amore K, Nutt CL, Louis DN. Mutations of the PIK3CA gene are rare in human glioblastoma. Acta Neuropathol. 2005;109:654–655. doi: 10.1007/s00401-005-1001-0. [DOI] [PubMed] [Google Scholar]
- 21.Broderick DK, Di CH, Parrett TJ, Samuels YR, Cummins JM, McLendon RE, Fults DW, Velculescu VE, Bigner DD, Yan H. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res. 2004;64:5048–5050. doi: 10.1158/0008-5472.CAN-04-1170. [DOI] [PubMed] [Google Scholar]
- 22.Guo XN, Rajput A, Rose R, Hauser J, Beko A, Kuropatwinski K, Levea C, Hoffman RM, Brattain NG, Wang J. Mutant PIK3CA-bearing colon cancer cells display increased metastasis in an orthotopic model. Cancer Res. 2007;67:5851–5858. doi: 10.1158/0008-5472.CAN-07-0049. [DOI] [PubMed] [Google Scholar]
- 23.Ikenoue T, Kanai F, Hikiba Y, Obata T, Tanaka Y, Imamura J, Ohta M, Jazag A, Guleng B, Tateishi K, Asaoka Y, Matsumura M, Kawabe T, Omata M. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 2005;65:4562–4567. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 24.Samuels Y, Diaz LA, Schmidt-Kittler O, Cummins JM, DeLong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, Pearline RV, Cantley LC, Brugge JS. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 26.Zhao JJ, Liu ZN, Wang L, Shin E, Loda MF, Roberts TM. The oncogenic properties of mutant p110 alpha and p110 beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci U S A. 2005;102:18443–18448. doi: 10.1073/pnas.0508988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang SY, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bader AG, Kang SY, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci U S A. 2006;103:1475–1479. doi: 10.1073/pnas.0510857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y, Schneidman-Duhovny D, Wolfson HJ, Backer JM, Williams RL. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science. 2007;317:239–242. doi: 10.1126/science.1135394. [DOI] [PubMed] [Google Scholar]
- 30.Kang S, Bader AG, Zhao L, Vogt PK. Mutated PI 3-kinases - Cancer targets on a silver platter. Cell Cycle. 2005;4:578–581. doi: 10.4161/cc.4.4.1586. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama N, Miyoshi Y, Taguchi T, Tamaki Y, Monden M, Noguchi S. Clinicopathologic analysis of breast cancers with PIK3CA mutations in Japanese women. Clin Cancer Res. 2007;13:408–414. doi: 10.1158/1078-0432.CCR-06-0267. [DOI] [PubMed] [Google Scholar]
- 32.Pérez-Tenorio G, Alkhori L, Olsson B, Waltersson MA, Nordenskjöld B, Rutqvist LE, Skoog L, Stål O. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- 33.Li SY, Rong MN, Grieu F, Iacopetta B. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006;96:91–95. doi: 10.1007/s10549-005-9048-0. [DOI] [PubMed] [Google Scholar]
- 34.Kato S, Iida S, Higuchi T, Ishikawa T, Takagi Y, Yasuno M, Enomoto M, Uetake H, Sugihara K. PIK3CA mutation is predictive of poor survival in patients with colorectal cancer. International Journal of Cancer. 2007;121:1771–1778. doi: 10.1002/ijc.22890. [DOI] [PubMed] [Google Scholar]
- 35.Barbareschi M, Buttitta F, Felicioni L, Cotrupi S, Barassi F, Del Grammastro M, Ferro A, Dalla Palma P, Galligioni E, Marchetti A. Different Prognostic Roles of Mutations in the Helical and Kinase Domains of the PIK3CA Gene in Breast Carcinomas. Clin Cancer Res. 2007;13:6064–6069. doi: 10.1158/1078-0432.CCR-07-0266. [DOI] [PubMed] [Google Scholar]
- 36.Davies M, Hennessy B, Mills GB. Point mutations of protein kinases ion and individualised cancer therapy. Expert Opin Pharmacother. 2006;7:2243–2261. doi: 10.1517/14656566.7.16.2243. [DOI] [PubMed] [Google Scholar]
- 37.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinstein IB. Cancer: Addiction to oncogenes - The Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 39.Huang CH, Mandelker D, Schmidt-Kittler O, Samuels Y, Velculescu VE, Kinzler KW, Vogelstein B, Gabelli SB, Amzel LM. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science. 2007;318:1744–8. doi: 10.1126/science.1150799. [DOI] [PubMed] [Google Scholar]
- 40.Schultz RM, Merriman RL, Andis SL, Bonjouklian R, Grindey GB, Rutherford PG, Gallegos A, Massey K, Powis G. In-Vitro and in-Vivo Antitumor-Activity of the Phosphatidylinositol-3-Kinase Inhibitor, Wortmannin. Anticancer Res. 1995;15:1135–1139. [PubMed] [Google Scholar]
- 41.Semba S, Itoh N, Ito M, Harada M, Yamakawa M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer cells. Clin Cancer Res. 2002;8:1957–1963. [PubMed] [Google Scholar]
- 42.Hu LM, Zaloudek C, Mills GB, Gray J, Jaffe RB. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002) Clin Cancer Res. 2000;6:880–886. [PubMed] [Google Scholar]
- 43.Workman P. Inhibiting the phosphoinositide 3-kinase pathway for cancer treatment. Biochem Soc Trans. 2004;32:393–396. doi: 10.1042/bst0320393. [DOI] [PubMed] [Google Scholar]
- 44.Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF, Waterfield MD. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, Abraham RT. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58:4375–4382. [PubMed] [Google Scholar]
- 46.Banin S, Moyal L, Shieh SY, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATN in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 47.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. Embo J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 48.Hartley KO, Gell D, Smith GCM, Zhang H, Divecha N, Connelly MA, Admon A, Leesmiller SP, Anderson CW, Jackson SP. DNA-Dependent Protein-Kinase Catalytic Subunit - a Relative of Phosphatidylinositol 3-Kinase and the Ataxia-Telangiectasia Gene-Product. Cell. 1995;82:849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- 49.Hayakawa M, Kawaguchi KI, Kaizawa H, Tomonobu K, Ohishi T, Yamano M, Okada M, Ohta M, Tsukamoto S, Raynaud FI, Parker P, Workman P, Waterfield MD. Synthesis and biological evaluation of sulfonylhydrazone-substituted imidazo 1,2-a pyridines as novel PI3 kinase p110 alpha inhibitors. Bioorg Med Chem. 2007;15:5837–5844. doi: 10.1016/j.bmc.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 50.Hayakawa M, Kaizawa H, Moritomo H, Koizumi T, Ohishi T, Yamano M, Okada M, Ohta M, Tsukamoto S, Raynaud FI, Workman P, Waterfield MD, Parker P. Synthesis and biological evaluation of pyrido 3′,2′: 4,5 furo 3,2-d pyrimidine derivatives as novel PI3 kinase p110 alpha inhibitors. Bioorg Med Chem Lett. 2007;17:2438–2442. doi: 10.1016/j.bmcl.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 51.Hayakawa M, Kaizawa H, Kawaguchi K, Ishikawa N, Koizumi T, Ohishi T, Yamano M, Okada M, Ohta M, Tsukamoto S, Raynaud FI, Waterfield MD, Parker P, Workman P. Synthesis and biological evaluation of imidazo 1,2-a pyridine derivatives as novel PI3 kinase p110 alpha inhibitors. Bioorg Med Chem. 2007;15:403–412. doi: 10.1016/j.bmc.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 52.Hayakawa M, Kaizawa H, Moritomo H, Koizumi T, Ohishi T, Okada M, Ohta M, Tsukamoto S, Parker P, Workman P, Waterfield M. Synthesis and biological evaluation of 4-morpholino-2-phenylquinazolines and related derivatives as novel PI3 kinase p110 alpha inhibitors. Bioorg Med Chem. 2006;14:6847–6858. doi: 10.1016/j.bmc.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 53.Marion F, Williams DE, Patrick BO, Hollander I, Mallon R, Kim SC, Roll DM, Feldberg L, Van Soest R, Andersen RJ. Liphagal, a selective inhibitor of PI3 kinase alpha isolated from the sponge Aka coralliphaga: Structure elucidation and biomimetic synthesis. Org Lett. 2006;8:321–324. doi: 10.1021/ol052744t. [DOI] [PubMed] [Google Scholar]
- 54.Ihle NT, Paine-Murrieta G, Berggren MI, Baker A, Tate WR, Wipf P, Abraham RT, Kirkpatrick DL, Powis G. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol Cancer Ther. 2005;4:1349–1357. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110 alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ihle NT, Williams R, Chow S, Chew W, Berggren MI, Paine-Murrieta G, Minion DJ, Halter RJ, Wipf P, Abraham R, Kirkpatrick L, Powis G. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004;3:763–772. [PubMed] [Google Scholar]
- 57.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 58.McMullen JR, Jay PY. PI3K(p110 alpha) inhibitors as anti-cancer agents - Minding the heart. Cell Cycle. 2007;6:910–913. doi: 10.4161/cc.6.8.4124. [DOI] [PubMed] [Google Scholar]
- 59.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 60.Staal SP. Molecular-Cloning of the Akt Oncogene and Its Human Homologs Akt1 and Akt2 - Amplification of Akt1 in a Primary Human Gastric Adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin XD, Murray JM, Rico AC, Wang MX, Chu DT, Zhou YS, Del Rosario M, Kaufman S, Ma S, Fang E, Crawford K, Jefferson AB. Discovery of 2-pyrimidyl-5-amidothiophenes as potent inhibitors for AKT: Synthesis and SAR studies. Bioorg Med Chem Lett. 2006;16:4163–4168. doi: 10.1016/j.bmcl.2006.05.092. [DOI] [PubMed] [Google Scholar]
- 62.Zhu GD, Gong JC, Claiborne A, Woods KW, Gandhi VB, Thomas S, Luo Y, Liu XS, Shi Y, Guan R, Magnone SR, Klinghofer V, Johnson EF, Bouska J, Shoemaker A, Oleksijew A, Stoll VS, De Jong R, Oltersdorf T, Li Q, Rosenberg SH, Giranda VL. Isoquinoline-pyridine-based protein kinase B/Akt antagonists: SAR and in vivo antitumor activity. Bioorg Med Chem Lett. 2006;16:3150–3155. doi: 10.1016/j.bmcl.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 63.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson PG, Anderson KC. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandal M, Younes M, Swan EA, Jasser SA, Doan D, Yigitbasi O, McMurphey A, Ludwick J, El-Naggar AK, Bucana C, Mills GB, Myers JN. The Akt inhibitor KP372-1 inhibits proliferation and induces apoptosis and anoikis in squamous cell carcinoma of the head and neck. Oral Oncol. 2006;42:430–439. doi: 10.1016/j.oraloncology.2005.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng ZH, Samudio IJ, Zhang WG, Estrov Z, Pelicano H, Harris D, Frolova O, Hail N, Chen WJ, Kornblau SM, Huang P, Lu YL, Mills GB, Andreeff M, Konopleva M. Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase 3 signaling by a small-molecule KP372-1 induces mitochondrial dysfunction and apoptosis in acute myelogenous leukemia. Cancer Res. 2006;66:3737–3746. doi: 10.1158/0008-5472.CAN-05-1278. [DOI] [PubMed] [Google Scholar]
- 66.Koul D, Shen RJ, Bergh S, Sheng XY, Shishodia S, Lafortune TA, Lu YL, de Groot JF, Mills GB, Yung WKA. Inhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastoma. Mol Cancer Ther. 2006;5:637–644. doi: 10.1158/1535-7163.MCT-05-0453. [DOI] [PubMed] [Google Scholar]
- 67.Gills JJ, Holbeck S, Hollingshead M, Hewitt SM, Kozikowski AP, Dennis PA. Spectrum of activity and molecular correlates of response to phosphatidylinositol ether lipid analogues, novel lipid-based inhibitors of Akt. Mol Cancer Ther. 2006;5:713–722. doi: 10.1158/1535-7163.MCT-05-0484. [DOI] [PubMed] [Google Scholar]
- 68.Li Q, Woods KW, Thomas S, Zhu GD, Packard G, Fisher J, Li TM, Gong JC, Dinges J, Song XH, Abrams J, Luo Y, Johnson EF, Shi Y, Liu XS, Klinghofer V, Jong RD, Oltersdorf T, Stoll VS, Jakob CG, Rosenberg SH, Giranda VL. Synthesis and structure-activity relationship of 3,4′-bispyridinylethylenes: Discovery of a potent 3-isoquinolinylpyridine inhibitor of protein kinase B (PKB/Akt) for the treatment of cancer. Bioorg Med Chem Lett. 2006;16:2000–2007. doi: 10.1016/j.bmcl.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 69.Li Q, Li TM, Zhu GD, Gong JC, Claibone A, Dalton C, Luo Y, Johnson EF, Shi Y, Liu XS, Klinghofer V, Bauch JL, Marsh KC, Bouska JJ, Arries S, De Jong R, Oltersdorf T, Stoll VS, Jakob CG, Rosenberg SH, Giranda VL. Discovery of trans-3,4′-bispyridinylethylenes as potent and novel inhibitors of protein kinase B (PKB/Akt) for the treatment of cancer: Synthesis and biological evaluation. Bioorg Med Chem Lett. 2006;16:1679–1685. doi: 10.1016/j.bmcl.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 70.Tang HJ, Jin XH, Wang SM, Yang DJ, Cao YY, Chen JY, Gossett DR, Lin JY. A small molecule compound inhibits AKT pathway in ovarian cancer cell lines. Gynecol Oncol. 2006;100:308–317. doi: 10.1016/j.ygyno.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 71.Georgakis GV, Li Y, Rassidakis GZ, Medeiros LJ, Mills GB, Younes A. Inhibition of the phosphatidylinositol-3 kinase/Akt promotes G1 cell cycle arrest and apoptosis in Hodgkin lymphoma. Br J Haematol. 2006;132:503–511. doi: 10.1111/j.1365-2141.2005.05881.x. [DOI] [PubMed] [Google Scholar]
- 72.Shi Y, Liu XS, Han EK, Guan R, Shoemaker AR, Oleksijew A, Woods KW, Fisher JP, Klinghofer V, Lasko L, McGonigal T, Li Q, Rosenberg SH, Giranda VL, Luo Y. Optimal classes of chemotherapeutic agents sensitized by specific small-molecule inhibitors of Akt in vitro and in vivo. Neoplasia. 2005;7:992–1000. doi: 10.1593/neo.05355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luo Y, Shoemaker AR, Liu XS, Woods KW, Thomas SA, de Jong R, Han EK, Li TM, Stoll VS, Powlas JA, Oleksijew A, Mitten MJ, Shi Y, Guan R, McGonigal TP, Klinghofer V, Johnson EF, Leverson JD, Bouska JJ, Mamo M, Smith RA, Gramling-Evans EE, Zinker BA, Mika AK, Nguyen PT, Oltersdorf T, Rosenberg SH, Li Q, Giranda VL. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005;4:977–986. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 74.Mandal M, Kim S, Younes MN, Jasser SA, El-Naggar AK, Mills GB, Myers JN. The Akt inhibitor KP372-1 suppresses Akt activity and cell proliferation and induces apoptosis in thyroid cancer cells. Br J Cancer. 2005;92:1899–1905. doi: 10.1038/sj.bjc.6602595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Shin I, Edl J, Biswas S, Lin PC, Mernaugh R, Arteaga CL. Proapoptotic activity of cell-permeable anti-Akt single-chain antibodies. Cancer Res. 2005;65:2815–2824. doi: 10.1158/0008-5472.CAN-04-2898. [DOI] [PubMed] [Google Scholar]
- 76.Lindsley CW, Zhao ZJ, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 77.Barnett SF, Defeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, Kahana JA, Kral AM, Leander K, Lee LL, Malinowski J, McAvoy EM, Nahas DD, Robinson RG, Huber HE. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem J. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hiromura M, Okada F, Obata T, Auguin D, Shibata T, Roumestand C, Noguchi M. Inhibition of Akt kinase activity by a peptide spanning the beta A strand of the proto-oncogene TCL1. J Biol Chem. 2004;279:53407–53418. doi: 10.1074/jbc.M403775200. [DOI] [PubMed] [Google Scholar]
- 79.Meuillet EJ, Ihle N, Baker AF, Gard JM, Stamper C, Williams R, Coon A, Mahadevan D, George BL, Kirkpatrick L, Powis G. In vivo molecular pharmacology and antitumor activity of the targeted Akt inhibitor PX-316. Oncol Res. 2004;14:513–527. doi: 10.3727/0965040042380487. [DOI] [PubMed] [Google Scholar]
- 80.Jin X, Gossett DR, Wang S, Yang D, Cao Y, Chen J, Guo R, Reynolds RK, Lin J. Inhibition of AKT survival pathway by a small molecule inhibitor in human endometrial cancer cells. Br J Cancer. 2004;91:1808–1812. doi: 10.1038/sj.bjc.6602214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaw YJ, Yang YT, Garrison JB, Kyprianou N, Chen CS. Pharmacological exploitation of the alpha 1-adrenoreceptor antagonist doxazosin to develop a novel class of antitumor agents that block intracellular protein kinase B/Akt activation. J Med Chem. 2004;47:4453–4462. doi: 10.1021/jm049752k. [DOI] [PubMed] [Google Scholar]
- 82.Yang L, Dan HC, Sun M, Liu QY, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, Sebti SM, Cheng JQ. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 83.Castillo SS, Brognard J, Petukhov PA, Zhang CY, Tsurutani J, Granville CA, Li M, Jung M, West KA, Gills JG, Kozikowski AP, Dennis PA. Preferential inhibition of Akt and killing of Akt-dependent cancer cells by rationally designed phosphatidylinositol ether lipid analogues. Cancer Res. 2004;64:2782–2792. doi: 10.1158/0008-5472.can-03-1530. [DOI] [PubMed] [Google Scholar]
- 84.Luo Y, Smith RA, Guan R, Liu XS, Klinghofer V, Shen JW, Hutchins C, Richardson P, Holzman T, Rosenberg SH, Giranda VL. Pseudosubstrate peptides inhibit Akt and induce cell growth inhibition. Biochemistry. 2004;43:1254–1263. doi: 10.1021/bi034515p. [DOI] [PubMed] [Google Scholar]
- 85.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 86.Meuillet EJ, Mahadevan D, Vankayalapati H, Berggren M, Williams R, Coon A, Kozikowski AP, Powis G. Specific inhibition of the Akt1 pleckstrin homology domain by D-3-deoxy-phosphatidyl-myo-inositol analogues. Mol Cancer Ther. 2003;2:389–399. [PubMed] [Google Scholar]
- 87.Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Anti-cancer alkyl-lysophospholipids inhibit the phosphatidylinositol 3-kinase-Akt/PKB survival pathway. Anti-Cancer Drugs. 2003;14:167–173. doi: 10.1097/00001813-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 88.Reuveni H, Livnah N, Geiger T, Kleid S, Ohne O, Cohen I, Benhar M, Gellerman G, Levitzki A. Toward a PKB inhibitor: Modification of a selective PKA inhibitor by rational design. Biochemistry. 2002;41:10304–10314. doi: 10.1021/bi0202530. [DOI] [PubMed] [Google Scholar]
- 89.Vink SR, Lagerwerf S, Mesman E, Schellens JHM, Begg AC, van Blitterswijk WJ, Verheij M. Radiosensitization of squamous cell carcinoma by the alkylphospholipid perifosine in cell culture and xenografts. Clin Cancer Res. 2006;12:1615–1622. doi: 10.1158/1078-0432.CCR-05-2033. [DOI] [PubMed] [Google Scholar]
- 90.Li X, Luwor R, Lu Y, Liang K, Fan Z. Enhancement of antitumor activity of the anti-EGF receptor monoclonal antibody cetuximab/C225 by perifosine in PTEN-deficient cancer cells. Oncogene. 2006;25:525–535. doi: 10.1038/sj.onc.1209075. [DOI] [PubMed] [Google Scholar]
- 91.Rahmani M, Reese E, Dai Y, Bauer C, Payne SG, Dent P, Spiegel S, Grant S. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–2432. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- 92.Smorenburg CH, Seynaeve C, Bontenbal M, Planting A, Sindermann H, Verweij J. Phase II study of miltefosine 6% solution as topical treatment of skin metastases in breast cancer patients. Anti-Cancer Drugs. 2000;11:825–828. doi: 10.1097/00001813-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 93.Clive S, Gardiner J, Leonard RCF. Miltefosine as a topical treatment for cutaneous metastases in breast carcinoma. Cancer Chemother Pharmacol. 1999;44:S29–S30. doi: 10.1007/s002800051114. [DOI] [PubMed] [Google Scholar]
- 94.Terwogt JMM, Mandjes IAM, Sindermann H, Beijnen JH, Huinink W. Phase II trial of topically applied miltefosine solution in patients with skin-metastasized breast cancer. Br J Cancer. 1999;79:1158–1161. doi: 10.1038/sj.bjc.6690184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dummer R, Krasovec M, Roger J, Sindermann H, Burg G. Topical Administration of Hexadecylphosphocholine in Patients with Cutaneous Lymphomas - Results of a Phase-I/Ii Study. J Am Acad Dermatol. 1993;29:963–970. doi: 10.1016/0190-9622(93)70275-x. [DOI] [PubMed] [Google Scholar]
- 96.Feun LG, Blessing JA, Barrett RJ, Hanjani P. A Phase-Ii Trial of Tricyclic Nucleoside Phosphate in Patients with Advanced Squamous-Cell Carcinoma of the Cervix - a Gynecologic-Oncology-Group Study. Am J Clin Oncol -Cancer Clin Trials. 1993;16:506–508. doi: 10.1097/00000421-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 97.Feun LG, Savaraj N, Bodey GP, Lu K, Yap BS, Ajani JA, Burgess MA, Benjamin RS, McKelvey E, Krakoff I. Phase-1 Study of Tricyclic Nucleoside Phosphate Using a 5-Day Continuous Infusion Schedule. Cancer Res. 1984;44:3608–3612. [PubMed] [Google Scholar]
- 98.Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Molecular Cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 99.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu QW, Crenshaw EB, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 101.Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 102.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 103.Bernardi R, Guernah I, Jin D, Grisendi S, Alimonti A, Teruya-Feldstein J, Cordon-Cardo C, Simon MC, Rafii S, Pandolfi PP. PML inhibits HIF-1 alpha translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779–785. doi: 10.1038/nature05029. [DOI] [PubMed] [Google Scholar]
- 104.Sehgal SN, Baker H, Vezina C. Rapamycin (Ay-22,989), a New Antifungal Antibiotic.2. Fermentation, Isolation and Characterization. J Antibiot. 1975;28:727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 105.Neuhaus P, Klupp J, Langrehr JM. mTOR inhibitors: An overview. Liver Transplant. 2001;7:473–484. doi: 10.1053/jlts.2001.24645. [DOI] [PubMed] [Google Scholar]
- 106.Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, Dinopoulos A, Thomas G, Crone KR. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 107.Galanis E, Buckner JC, Maurer MJ, Kreisberg JL, Ballman K, Boni J, Peralba JM, Jenkins RB, Dakhil SR, Morton RF, Jaeckle KA, Scheithauer BW, Dancey J, Hidalgo M, Walsh DJ. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A north central cancer treatment group study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 108.Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, Semiglazov V, Borner M, Salzberg M, Ostapenko V, Illiger HJ, Behringer D, Bardy-Bouxin N, Boni J, Kong S, Cincotta M, Moore L. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 109.Witzig TE, Geyer SM, Ghobrial I, Inwards DJ, Fonseca R, Kurtin P, Ansell SM, Luyun R, Flynn PJ, Morton RF, Dakhil SR, Gross H, Kaufmann SH. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 110.Margolin K, Longmate J, Baratta T, Synold T, Christensen S, Weber J, Gajewski T, Quirt I, Doroshow JH. CCI-779 in metastatic melanoma - A phase II trial of the California Cancer Consortium. Cancer. 2005;104:1045–1048. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 111.Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, Fink K, Robins HI, De Angelis L, Raizer J, Hess K, Aldape K, Lamborn KR, Kuhn J, Dancey J, Prados MD. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 112.Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 113.Raymond E, Alexandre J, Faivre S, Vera K, Materman E, Boni J, Leister C, Korth-Bradley J, Hanauske A, Armand JP. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22:2336–2347. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 114.Campistol JM, Gutierrez-Dalmau A, Torregrosa JV. Conversion to sirolimus: A successful treatment for posttransplantation Kaposi’s sarcoma. Transplantation. 2004;77:760–762. doi: 10.1097/01.tp.0000115344.18025.0b. [DOI] [PubMed] [Google Scholar]
- 115.Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, Park Y, Lion SH, Marshall B, Boni JP, Dukart G, Sherman ML. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 116.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 117.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 118.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 120.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 121.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, Henley A, Di-Stefano F, Ahmad Z, Guillard S, Bjerke LM, Kelland L, Valenti M, Patterson L, Gowan S, Brandon AD, Hayakawa M, Kaizawa H, Koizumi T, Ohishi T, Patel S, Saghir N, Parker P, Waterfield M, Workman P. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 123.Whyte DB, Holbeck SL. Correlation of PIK3Ca mutations with gene expression and drug sensitivity in NCI-60 cell lines. Biochem Biophys Res Commun. 2006;340:469–475. doi: 10.1016/j.bbrc.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 124.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang XM, Yu JS, Malmstrom PO, Mansukhani M, Enoksson J, Hibshoosh H, Borg A, Parsons R. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 125.Chan CT, Metz MZ, Kane SE. Differential sensitivities of trastuzumab (Herceptin (R))-resistant human breast cancer cells to phosphoinositide-3 kinase (PI-3K) and epidermal growth factor receptor (EGFR) kinase inhibitors. Breast Cancer Res Treat. 2005;91:187–201. doi: 10.1007/s10549-004-7715-1. [DOI] [PubMed] [Google Scholar]
- 126.Jordan NJ, Gee JMW, Barrow D, Wakeling AE, Nicholson RI. Increased constitutive activity of PKB/Akt in tamoxifen resistant breast cancer MCF-7 cells. Breast Cancer Res Treat. 2004;87:167–180. doi: 10.1023/B:BREA.0000041623.21338.47. [DOI] [PubMed] [Google Scholar]
- 127.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 128.Perez-Tenorio G, Stal O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86:540–545. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]


