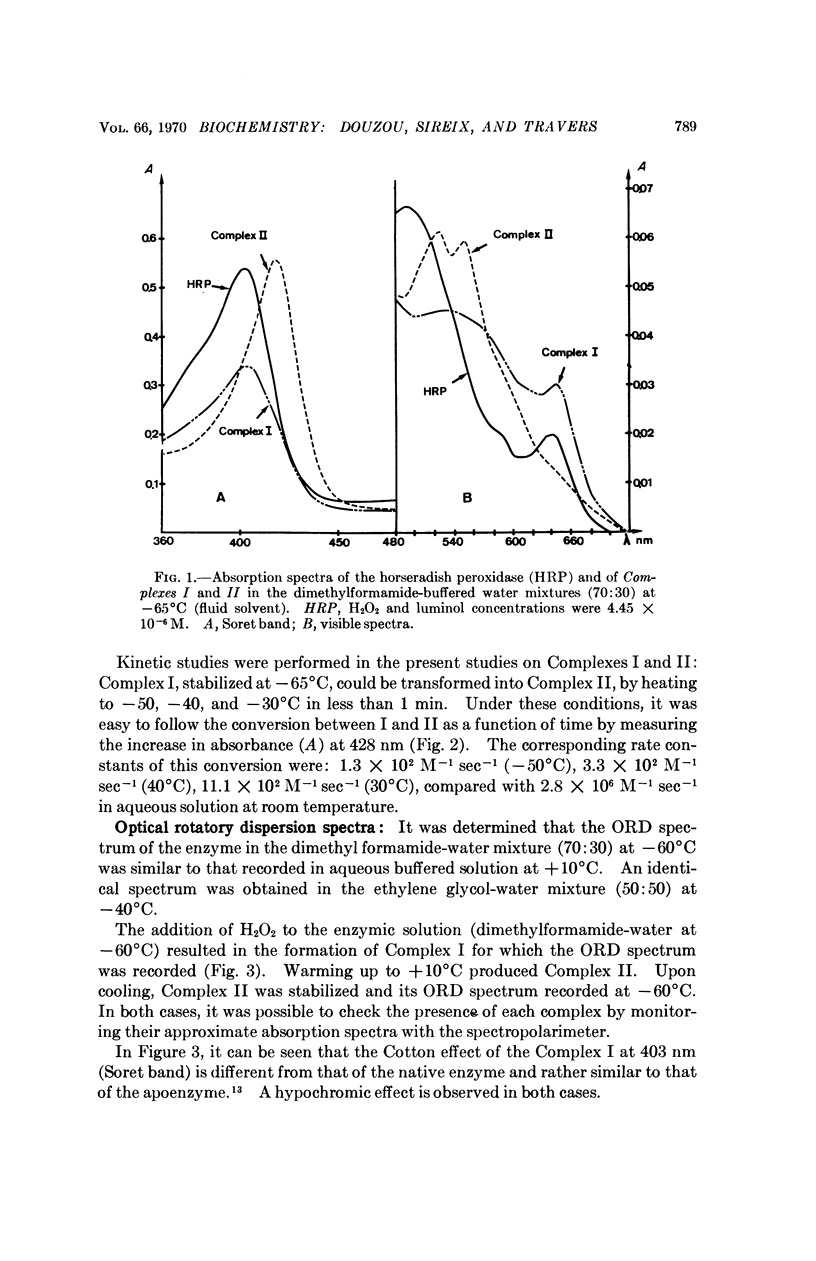
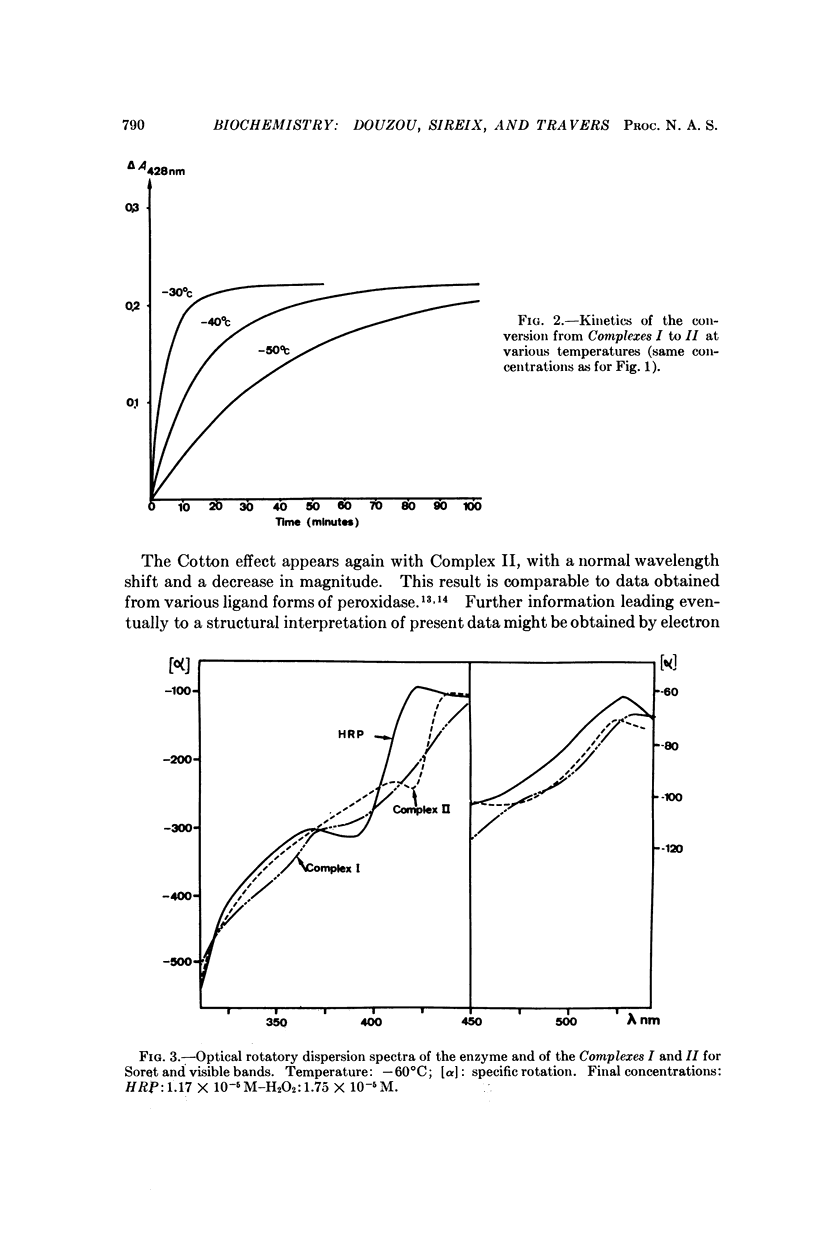
Abstract
Media are described which make it possible to study mechanisms of enzyme action at low temperature. The applicability of techniques developed are illustrated by results obtained on formation kinetics of complexes produced by the interaction of horseradish peroxidase with hydrogen peroxide. It is shown that the various steps in the time course of the reaction can be readily resolved with isolation of each intermediate in concentrations sufficient to permit rate studies between consecutive steps. The potential of the method for elaboration of enzyme mechanisms is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIELSKI B. H., FREED S. ENZYMATIC REACTIONS BELOW 0-DEGREES OF ALPHA-CHYMOTRYPSIN IN METHANOL-WATER SOLVENTS. Biochim Biophys Acta. 1964 Aug 26;89:314–323. doi: 10.1016/0926-6569(64)90220-2. [DOI] [PubMed] [Google Scholar]
- Banerjee R., Douzou P., Lombard A. Kinetics of association of carbon monoxide with haemoglobin at low temperature. Nature. 1968 Jan 6;217(5123):23–25. doi: 10.1038/217023a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B. The spectra of the enzyme-substrate complexes of catalase and peroxidase. Arch Biochem Biophys. 1952 Dec;41(2):404–415. doi: 10.1016/0003-9861(52)90469-4. [DOI] [PubMed] [Google Scholar]
- Douzou P., Hoa G. H., Michelson A. M. Synthetic polynucleotides in fluid supercooled aqueous alcohol solutions. Biochim Biophys Acta. 1969 Jun 17;182(2):334–341. doi: 10.1016/0005-2787(69)90184-1. [DOI] [PubMed] [Google Scholar]
- Ellis W. D., Dunford H. B. The effect of ligand binding and acid splitting on the optical rotatory dispersion of ferric horseradish peroxidase. Can J Biochem. 1968 Oct;46(10):1231–1235. doi: 10.1139/o68-184. [DOI] [PubMed] [Google Scholar]
- Freed S. Chemical-biochemical signal and noise. Science. 1965 Oct 29;150(3696):576–584. doi: 10.1126/science.150.3696.576. [DOI] [PubMed] [Google Scholar]
- Shiga T., Layani M., Douzou P. Etude spectroscopique de la D-amino acide oxydase a basse température. Bull Soc Chim Biol (Paris) 1967;49(5):507–520. [PubMed] [Google Scholar]
- Travers F., Debey M. P., Douzou P., Michelson A. M. Optical rotatory dispersion of polyglutamic and polyuridylic acids at low temperatures in fluid hydro-alcoholic solvents. Biochim Biophys Acta. 1969 Nov 11;194(1):265–271. doi: 10.1016/0005-2795(69)90202-5. [DOI] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L. OPTICALLY ACTIVE METALLOPROTEIN CHROMOPHORES. III. HEME AND NONHEME IRON PROTEINS. Biochemistry. 1963 Nov-Dec;2:1335–1340. doi: 10.1021/bi00906a027. [DOI] [PubMed] [Google Scholar]


