Abstract
Unaffordable prices still bar the end-users in China from accessing ARV drugs. Patent protection of ARV drugs has dramatically limited the availability of these lifesaving drugs to AIDS patients in China.
The way Chinese government can go to breakthrough the ARV drug patents are suggested as:
Make more generic drugs available through compulsory licensing, impartment from other countries or building ARVs plants by partnerships between governments or generic drug companies.
Do a thorough and detailed research on the patent application of ARV drugs to find out the loophole.
Try patent pool to make AIDS medicines more affordable and appropriate for patients.
INTRODUCTION
China, a vast country of 1.3 billion people, faces tremendous logistical challenges in combating the HIV/AIDS epidemic. The most recent statistics released by the Chinese government in December 2008 showed that the number of HIV-infected individuals reported was about 260,000 and the number of full-blown AIDS patients was more than 77,000 at the end of September 2008. In China, the government has recognized the imminent threat that HIV/AIDS poses to its population and responded with a national antiretroviral (ARV) treatment program providing ARV drugs free to those most in need [1]. It has been five years since the national free ARV therapy was put into effect in 2003. Generally speaking, this urgent and helpful action was quite meaningful to the promotion and extension of the China’s AIDS prevention and control project [2]. Nearly RMB25,000 (about US$3571) for one patient’s yearly treatment, the improved first-line treatment (Zidovudine (AZT) or Stavudine (D4T) + lamivudine(3TC) + efavirenz (EFV)) in China costs five to eight times as much as the previously recommended regimen. With increasing numbers of AIDS patients failing on their first-line therapy, there is also an urgent need to find affordable second-line treatments. But only twelve of the more than 30 kinds of ARV drugs are available in the country and seven of them need to be imported because of protection of drug patents. And now the prices of AIDS medicines are still on the rise. As a result, unaffordable prices still bar the end-users in China from accessing these lifesaving drugs. Under this backcloth, prospects from production generic ARV drugs, compulsory licensing and patent pool show that China still has a long way to go in ensuring access of necessary ARV drugs for AIDS patient.
HIV EPIDEMIC AND ARV PATENT IN CHINA
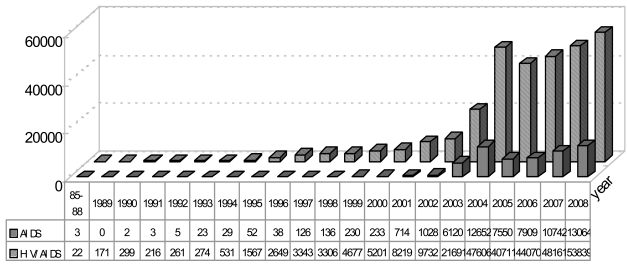
Since 1985, the HIV epidemic has become more visible in China. The cases reported are increasing every year and have spread throughout the country [3]. Despite new HIV infections are increasing at a much slower pace (Fig. 1), WHO warned that, if there were no effective preventive measures adopted, that the number of HIV/AIDS cases would reach 10 million in China by 2010 [4], and the number of AIDS patient would be about 1,097,000 to 2,155,000. According to the report released by the National Center for AIDS and Sexually Transmitted Disease Control and Prevention, HIV/AIDS has begun to spread from specific groups into the general population and many risk factors of HIV transmission remain uncontrolled. In the recent years, unprotected sex is gradually becoming a major route of transmission for HIV/AIDS epidemic in China, and with increases in the number of female HIV patients infected through sex, mother-to-child transmission is bound to increase. The percentage of unprotected sex between men and women reached 40.4% and that of unprotected sex between men was about 5.1% in 2008. At the present time, the HIV epidemic still presents a huge challenge for developing China in areas of awareness, financial support, staffing, prevention, treatment and care.
Fig. (1).
HIV/AIDS individual reported in China from 1985 to September 2008 (Divisions of Treatment and Care, National Center for STD/AIDS Prevention and Control, Chinese Center for Disease Prevention and Control, Beijing).
The treatment of HIV/AIDS that has advanced tremendously over the past two decades decreased the AIDS mortality rate and many patients may now be able to live a normal life. Thirty-two drugs have been authorized by FDA for the HIV/AIDS therapy and can be categorized into six kinds, namely nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors, Fusion inhibitors, entry inhibitors - CCR5 co-receptor antagonist, HIV integrase strand transfer inhibitors and Multi-class Combination Products [5] (Table 1). Most of these drugs are expensive because of patent protection and it’s a big problem for poor and resource-limited countries such as China. Over the past decade activist pressure, the emergence of competition from generic manufacturers, and direct negotiation with pharmaceutical companies have all contributed to a dramatic drop in the price of certain drugs in developing countries. The availability of cheap antiretroviral drugs has been instrumental in treatment scale-up for resource-poor settings hard hit by the AIDS epidemic. Despite significant advances, a number of problems related to the price of anti-AIDS drugs remain. Not all drugs to treat AIDS are available at a suitably cheap price for poor countries, meaning that many of the newer, more effective drugs are only available in the West.
Table 1.
Drugs Used in the Treatment of HIV Infection [5]
| Brand Name | Generic Name | Manufacturer Name | Approval Date |
|---|---|---|---|
| A | |||
| Combivir* | lamivudine and zidovudine | GlaxoSmithKline | 27-Sep-97 |
| Emtriva | emtricitabine, FTC | Gilead Sciences | 02-Jul-03 |
| Epivir*# | lamivudine, 3TC | GlaxoSmithKline | 17-Nov-95 |
| Epzicom | abacavir and lamivudine | GlaxoSmithKline | 02-Aug-04 |
| Hivid | zalcitabine, dideoxycytidine, ddC | Hoffmann-La Roche | 19-Jun-92 |
| Retrovir*# | zidovudine, azidothymidine, AZT, ZDV | GlaxoSmithKline | 19-Mar-87 |
| Trizivir | abacavir, zidovudine, and lamivudine | GlaxoSmithKline | 14-Nov-00 |
| Truvada | tenofovir disoproxil fumarate and emtricitabine | Gilead Sciences, Inc. | 02-Aug-04 |
| Videx EC | enteric coated didanosine, ddI EC | Bristol Myers-Squibb | 31-Oct-00 |
| Videx*# | didanosine, dideoxyinosine, ddI | Bristol Myers-Squibb | 9-Oct-91 |
| Viread* | tenofovir disoproxil fumarate, TDF | Gilead | 26-Oct-01 |
| Zerit* | stavudine, d4T | Bristol Myers-Squibb | 24-Jun-94 |
| Ziagen | abacavir sulfate, ABC | GlaxoSmithKline | 17-Dec-98 |
| (generic version) | Didanosine (ddI)Delayed Release capsules | Barr Lab. | 3-Dec-04 |
| B | |||
| Intelence | etravirine | Tibotec Therapeutics | 18-Jan-08 |
| Rescriptor | delavirdine, DLV | Pfizer | 4-Apr-97 |
| Sustiva*# | efavirenz, EFV | Bristol Myers-Squibb | 17-Sep-98 |
| Viramune*# | nevirapine, NVP | Boehringer Ingelheim | 21-Jun-96 |
| C | |||
| Agenerase | amprenavir, APV | GlaxoSmithKline | 15-Apr-99 |
| Aptivus | tipranavir, TPV | Boehringer Ingelheim | 22-Jun-05 |
| Crixivan*# | indinavir, IDV, | Merck | 13-Mar-96 |
| Fortovase | saquinavir (no longer marketed) | Hoffmann-La Roche | 7-Nov-97 |
| Invirase | saquinavir mesylate, SQV | Hoffmann-La Roche | 6-Dec-95 |
| Kaletra*# | lopinavir and ritonavir, LPV/RTV | Abbott Laboratories | 15-Sep-00 |
| Lexiva | Fosamprenavir Calcium, FOS-APV | GlaxoSmithKline | 20-Oct-03 |
| Norvir | ritonavir, RTV | Abbott Laboratories | 1-Mar-96 |
| Prezista | darunavir | Tibotec, Inc. | 23-Jun-06 |
| Reyataz* | atazanavir sulfate, ATV | Bristol-Myers Squibb | 20-Jun-03 |
| Viracept* | nelfinavir mesylate, NFV | Agouron Pharmaceuticals | 14-Mar-97 |
| D | |||
| Fuzeon | enfuvirtide, T-20 | Hoffmann-La Roche & Trimeris | 13-Mar-03 |
| E | |||
| Selzentry | maraviroc | Pfizer | 06-August-07 |
| F | |||
| Isentress | raltegravir | Merck & Co., Inc. | 12--Oct-07 |
| G | |||
| Atripla | efavirenz, emtricitabine and tenofovir disoproxil fumarate | Bristol-Myers Squibb and Gilead Sciences | 12-July-06 |
Nucleoside reverse transcriptase inhibitors (NRTIs);
Nonnucleoside reverse transcriptase inhibitors (NNRTIs);
Protease inhibitors (PIs);
Fusion inhibitors;
Entry Inhibitors - CCR5 co-receptor antagonist;
HIV integrase strand transfer inhibitors;
Multi-class Combination Products.
Available in Chinese market
free drugs provided by the government.
Although the challenges facing HIV/AIDS control in China are many, the Chinese government is making a strong commitment to implement effective AIDS control measures across the whole country [6,7]. Since early 21st century, the Chinese government has vigorously responded to the HIV/AIDS epidemic and announced the “China Care Project”, a new policy for comprehensive HIV/AIDS prevention and treatment. A major accomplishment in prevention and treatment came in 2003, with the establishment of the “Four Frees and One Care” policy [8]. The government has provided free ARV treatment to 58,000 AIDS patients since then. Thanks to the treatment, the mortality rate among AIDS patients dropped from 28.7 percent in 2002 to 5.8 percent in 2007, according to a survey of the National Center for AIDS and Sexually Transmitted Disease Control and Prevention. In recent years the Central Government financial input to AIDS prevention and care has been significantly increased. Central government resources devoted to AIDS was RMB 15 million (about $ 2.1 million) in the middle of 90's of 20th century, which increased to 944 million (about $135 million)in 2007. In accordance with the incomplete data from local AIDS Working Committee Office, local financial commitments showed a corresponding increase. China is in urgent need of low-cost, less-side effects, and eutherapeutic ARV drugs. With the coordination and joint efforts by State Council AIDS Working Committee Office (SCAWCO), the Ministry of Finance, the State Administration of Taxation, the National Development & Reform Commission and the General Administration of Customs, the State Council was maintaining customs exemption for the import of ARV drugs and approving tax exemptions for the local production of ARV drugs in 2007. The exemption helped stabilize the price of ARV drugs and the price of some drugs decreased. As the number of pediatric cases increased, some pediatric drugs have been included in the plan for domestic production [9]. The government has been urging the patent owners or pharmaceutical companies under the administrative protection to localized their drug production and thereby to lower the price of ARV drugs. The government also has been persuading brand companies to give up their patent rights in China and help the Chinese government to provide effective treatment for AIDS patients [10].
Being a large developing country, China has provided patent protection for new drugs since 1993. Most ARV drugs have patent protection in China. The price of most ARV drugs is beyond the affordability of AIDS patients. Nevertheless, China's current ARV choices are severely limited due to patent restrictions and many large pharmaceutical companies (known as ‘Big Pharma’) already registered their products in China but have not sale of these drugs in China such as lamivudine (3TC), Trizivir(Zidovudine, AZT +ABC+3TC), abacavir (ABC) and saquinavir (SQV). Only twelve of thirty-two ARV drugs are available in China including five kinds of generic drugs (didanosine DDI, stavudin D4T, AZT, nevirapine NVP, and indinavir IDV). The free ARV drugs listed in “For Frees and One Care” policy include five generic drugs and 3TC, efavirenz (EFV), Kaletra (lopinavir and ritonavir, LPV/RTV) and tenofovir (TDF). Kaletra and TDF are second-line drugs and only patients with confirmed resistance to first-line drugs can switch to them. The Ministry of Health imported other drugs(3TC, EFV, Kaletra and TDF) from Brand companies. The cost of ARV drugs per patient per year was as high as RMB 30,000(about $4,225) to 50,000(about $7,143) before August 2002 when first generic drugs came into Chinese market. There are four pharmaceutical manufacturers involved in the production of the national free ARV drugs, including five kinds of generic drugs (DDI, D4T, AZT, NVP, and IDV) in China. The price of these drugs is only one-tenth of that imported drugs and some drugs are even cheaper. The price of D4T capsule (20mg) capsule produced by a pharmaceutical manufacturer in Shanghai is only RMB 0.9 (about $0.13), but that of imported D4T (20mg, Zerit, Bristol Myers-Squibb) is as high as RMB 22.7 (about $3.24). These cheap generic ARV drugs make up a large number of first line drugs and have dramatically reduced the cost of antiretroviral therapy (ART) in China. The cost of ART has reduced dramatically after generic drugs came into Chinese market. This cost may be cut down to RMB3,000 (about $422.5) per year for one patient after localization of all the ARV drugs and the market space will more than RMB 30 billion(about $4 billion). There are still difficulties in obtaining supplies of second-line ART drugs because the high price of ARV drugs. So the physicians still have not so much choice when they determine the ART regimen.
GENERIC DRUGS AND COMPULSORY LICENSING IN CHINA
In order for the proprietary drug makers to recoup the money they spent on drug creation, brand company are granted a ‘patent’ (an intellectual property right), which is an exclusive right that prevents others from making, using, selling, offering to sell, or importing their drug. The patent typically lasts for twenty years. 3TC (300mg, Epivir, GlaxoSmithKline) is the most important drug in the first line and second line regimen in China. GlaxoSmithKline has the patent of 3TC and Combivir (3TC+AZT), and there is only Combivir in Chinese market and price is as high as RMB 1300(about $186). Negotiation with GlaxoSmithKline has reduced the price of 3TC dramatically but this dosage form completely depends on government procurement because the dosage form in Chinese market is Heptode (100mg) treating hepatitis B. The company recently announced that the production of Combivir would be localization in China. It meant that the application of production of generic 3TC from Chinese government had been rejected. The government still has to spend a large amount of money in importing this drug. Generic 3TC is available in many developing country such as India. But no one knows when generic 3TC will appear in China.
Legislation in favor of the pharmaceutical industries’ right to patent their drugs - TRIPS - was introduced in 1995. TRIPS - The Agreement on Trade Related Aspects of Intellectual Property Rights –introduced intellectual property law into the international trading system for the first time and applies to all members of the World Trade Organization (WTO). The implementation of TRIPS was to have a huge impact on generic drug production. As a result, newer drugs are under patent and unaffordable prices still prevent in the resource-poor countries such as China from accessing these lifesaving drugs. Legally a country can get around TRIPS patent enforcement by issuing a compulsory license. A compulsory license is a government license that enables someone other than the patent holder to copy patented products and processes without fear of prosecution. Governments can issue them if a patent owner abuses their rights by, for example, failing to offer their product in the market, or offering it at a price that is too high for potential buyers to afford. Normally, to copy drugs for this reason, the generic company has to negotiate with the original manufacturer to agree royalties (money paid to the patent holder to make up for the loss of profit exclusivity). However, following the 2001 Doha agreement a country can issue a compulsory licence for a drug that treats a disease causing a severe health emergency in that country without royalties being paid [11]. Despite endorsement by the WTO, because of its complicated nature, compulsory licensing has been used very little by low- and middle-income countries. In fact, to date only a few country such as Thailand and Brazil have issued a compulsory licence for an antiretroviral drug and provides an excellent example of why other countries have been reluctant to follow suit [12]. It was once said that Chinese government would use compulsory licence, but it was proved to be a hearsay at last. Some people believe that it is can not be regarded as a public health emergency with the number of HIV-infected individuals reported in China. In fact, it is a misunderstanding of TRIPS. AIDS is regarded as a public health emergency in Doha agreement and there is no definition of the number or proportion of AIDS patients. Chinese government can consider compulsory licence just in order to provide necessary drug for AIDS patiens. Or it can serve as a bargaining chip when the government negotiates with brand pharmaceutical manufacturers.
China currently produces first-line ARV drugs along with the active pharmaceutical ingredients (APIs) for second lines too: really, China could become the ARV factory for the poor world because of its cheapest APIs and industrial scaleup very interested in the under-served markets. Unequivocal choices are lacking, however, between pursuance of TRIPS flexibilities of WTO (which China belongs to) and huge business with the multinational giants (including direct price cuttings for brand ARV drugs) [13]. The government should do a thorough and detailed research on the patent application of ARV drugs to find out the breakthrough. Although the production method of 3TC is still in the patent protection, the new drug protection and administrative protection of 3TC already expired. It is invalid that GlaxoSmithKline assert their patent of production method of 3TC have the faction of product patent of this drug, because the company abused patent rights and expanded monopoly. Or the government can use compulsory licensing and authorize production of generic 3TC.
The World Trade Organization (WTO) issued the so-called ‘paragraph 6 waiver’ which allows members who are unable to produce pharmaceuticals at home and are suffering a serious health crisis to import generics from other nations under compulsory licences (providing exported drugs are not part of a commercial or industrial policy of the exporting country) [14]. Many developing country have been trying their best to provide cheap pharmaceuticals for people suffering from AIDS. India is the largest supplier of generic ARV drugs to low- and middle- income countries, exporting two thirds of the drugs it manufactures [15]. Brazil, Thailand and South Africa also produce a significant amount of generic drugs and have used their expertise to help a number of African nations - such as Zambia, Ghana, Tanzania, Uganda, Ethiopia and Zimbabwe - develop local AIDS drug manufacturing facilities [16]. The manufacture and export of generic drugs was not only a turning point in terms of the price of ARV drugs, but also helped to revolutionize treatment for resource-poor settings by simplifying HIV/AIDS treatment. In 2001, Indian generic drug manufacturer, Cipla, announced that it would sell a generic copy of a triple-therapy antiretroviral for US$350 per patient per year. This had an incredible impact as the competition this generated dramatically drove down the price of anti-AIDS drugs for developing countries, thereby increasing the range of affordable options for national treatment programmers. To date, the Chinese government has snobbed the Indian ARV drugs even though they are cheapest through the Clinton Foundation, a consortium which China is a member of. How much longer will it be worthwhile to Chinese government ignoring the saving money opportunities by Indian generics? Not for long, if China-India trade agreements of November 2006 and January 2008 are supposed to give rise to a mutually profitable partnership for ARV drugs manufacturing and marketing [13].
PATENT POOL
In 2008, the international drug purchasing agency UNITAID took the groundbreaking decision to pursue the idea of establishing a patent pool that could hold the key to future access to affordable newer medicines. A patent pool is a mechanism whereby patent owners agree to pull their patents and license them to one entity called a Patent Pool. As a result, any company who would like to use an invention within the patent pool can do so provided that it pays a royalty fee to the Pool. Patent pools as a tool for the collective management of intellectual property rights are not a new concept. The potential use of patent pools in the pharmaceutical industry has been raised by the World Health Organisation (WHO) as a possible solution to promoting better access to medicines in developing countries. Specifically for AIDS, the pool would hold licences on various patented medicines, which generic companies could then produce at a lower cost for poor countries [12] Although the creation of a patent pool for HIV/AIDS drugs is at an early stage, there is hope that it will make the production of generic versions of antiretrovirals easier to negotiate and therefore faster and more efficient. A patent pool could help make AIDS medicines more affordable and appropriate for patients. For example, it could facilitate the development of fixed-dose combinations of first- and second-line regimens for adults and children. By combining multiple drugs into one pill, treatment is easier to take for patients, which can enhance patient adherence, improve health outcomes, and reduce the risk of resistance. The next steps undertaken by UNITAID will be to set up a task force to develop an operational plan for the creation of a patent pool. It is a good news for most developing countries including China. The pool will be voluntary. So the plan stands or falls on the willingness of the patent holders to put their intellectual property in the pool and for others to make use of the patents, in exchange for royalties [17]. For the large under-served market in China, a patent pool could become a win-win strategy for the government and brand companies.
THE WAY FORWARD FOR CHINA
Any such radical changes to the current system are unlikely any time soon, leaving it up to governments, drugs manufacturers, campaigners and non-governmental organizations to force the price of AIDS drugs down, enabling health authorities to secure the drugs their people so desperately need.
The way Chinese government can try to breakthrough the ARV drug patents are suggested as:
Make more generic drugs available through compul-sory licensing, impartment from other countries or building ARVs plants by partnerships between gover-nments or generic drug companies.
Do a thorough and detailed research on the patent application of ARV drugs to find out the loophole.
Try patent pool to make AIDS medicines more affordable and appropriate for patients.
REFERENCES
- 1.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China’s free ART program. Cell Res. 2005;15:877–82. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 2.Cao YZ, Lu HZ. Care of HIV-infected patients in China. Cell Res. 2005;15:883–890. doi: 10.1038/sj.cr.7290363. [DOI] [PubMed] [Google Scholar]
- 3.Zhang KL, Ma SJ. Epidemiology of HIV in China. Br Med J. 2002;324:803–804. doi: 10.1136/bmj.324.7341.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin L, Yoda T, Suzuki C, et al. Combating HIV/AIDS in mainland China: an epidemiological review of prevention and control measures. South Asian J Trop Med Public Health. 2005;36:1479–86. [PubMed] [Google Scholar]
- 5.Drugs Used in the Treatment of HIV infection 2008. Available from: http://www.fda.gov/oashi/aids/virals.html .
- 6.Sheng L, Cao WK. HIV/AIDS epidemiology and prevention in China. Chin Med J (Engl) 008 ;121:1230–6. [PubMed] [Google Scholar]
- 7.Wu Z, Rou K, Cui H. The HIV/AIDS epidemic in China:history, current strategies and future challenges. AIDS Educ Prev. 2004;16:7–17. doi: 10.1521/aeap.16.3.5.7.35521. [DOI] [PubMed] [Google Scholar]
- 8.WEN Jia-bao. Regulations on AIDS prevention and treatment. Decree of the State Council of the People’s Republic of China. 2006. p. 457.
- 9.A joint assessment of HIV/AIDS prevention, treatment and care in China 2007.
- 10.Chen QF. The retrospect and prospect of ART in China. China Rural Health Serv Adm. 2007;27:450–2. [Google Scholar]
- 11.World Trade Organization. Preparation Guide for Hammun, Trips and Generic Drugs 2008.
- 12.Medecins Sans Frontier ‘Affordability, Availability and Adaptability of AIDS Drugs in Developing Countries: An Ongoing Challenge’ 2008.
- 13.Dionisio D, Messeri D. Impending flop for brand antiretrovirals in the emerging markets? Open AIDS J. 2008;2:68–71. doi: 10.2174/1874613600802010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WTO "Implementation of paragraph 6 of the Doha Declaration on the TRIPS Agreement and public health" . 2003.
- 15.UNAIDS. Report on the global AIDS epidemic. 2008.
- 16.IRIN PlusNews "ZAMBIA: Manufacture of anti-AIDS drugs set to begin", IRIN (2005) "GHANA: Government ploughs ahead with plans to produce AIDS drugs locally", Guardian (Tanzania) (2005) "Tanzania Firm to Start Manufacturing AIDS Drugs", BBC (2007) "Uganda opens first HIV drug plant", IOL (2004) "Ethiopia begins Aids drug production", IOL (8 June 2004) "Zim starts producing anti-Aids drugs" 2004.
- 17.http://www.accessmed-msf.org/main/medical-innovation/unitaid-gives-green-light-to-patent-pool/