Abstract
We have previously reported that the majority of phospholipase A2 (PLA2) activity in rabbit ventricular myocytes is membrane-associated, calcium-independent (iPLA2), selective for arachidonylated plasmalogen phospholipids and inhibited by the iPLA2-selective inhibitor bromoenol lactone (BEL). Here, we identified the presence of iPLA2 in rabbit ventricular myocytes, determined the full length sequences for rabbit iPLA2β and iPLA2γ and compared their homology to the human isoforms. Rabbit iPLA2β encoded a protein with a predicated MW of 74 kDa that is 91% identical to the human iPLA2β short isoform. Full length iPLA2γ protein has a predicated MW of 88 kDa and is 88% identical to the human isoform. Immunoblot analysis of iPLA2β and γ in membrane and cytosolic fractions from rabbit and human cardiac myocytes demonstrated a similar pattern of distribution with both isoforms present in the membrane fraction, but no detectable protein in the cytosol. Membrane-associated iPLA2 activity was inhibited preferentially by the R enantiomer of bromoenol lactone (R)-BEL, indicating that the majority of activity is due to iPLA2γ.
Keywords: bromoenol lactone, phospholipase A2, myocardium, plasmalogens, arachidonic acid
INTRODUCTION
Phospholipase A2 catalyzes the hydrolysis of the fatty acid at the sn-2 position of membrane phospholipids, resulting in the generation of multiple biologically-active, membrane phospholipid-derived metabolites [for review, see 1]. The family of PLA2 enzymes has been classified into 15 groups and several subgroups based on the catalytic mechanism, functional and structural features, and sequence homology [1].
We reported previously that the majority of phospholipase A2 (PLA2) activity in rabbit ventricular myocytes is membrane-associated, calcium-independent (iPLA2) and selective for arachidonylated plasmalogen phospholipids [2]. Activation of this membrane-associated iPLA2 occurs when ventricular myocytes are incubated with phorbol 12-myristate 13-acetate, suggesting that activation of iPLA2 is regulated by protein kinase C [3]. We have also demonstrated that rabbit ventricular myocyte membrane-associated iPLA2 is activated in response to either thrombin stimulation [4] or the presence of hypoxia [5]. Multiple types of iPLA2 have been identified in cytosolic, microsomal and mitochondrial fractions from mammalian myocardium [6,7], but to date there is no data concerning the specific iPLA2 isoform that may be present and activated in rabbit ventricular myocytes. Our previous data indicate that rabbit ventricular myocyte iPLA2 activity is inhibited by bromoenol lactone (BEL) but not by methyl arachidonyl fluorophosphonate (MAFP) [2], suggesting that it is either iPLA2β or iPLA2γ.
The iPLA2β isoform is predominantly a cytosolic isoform and highly homologous cDNAs have been cloned from the hamster, mouse, rat and human (approximately 95% identity). In addition, several splice variants of human iPLA2β have been identified [8]. However, in COS-7 cells overexpressing iPLA2β, Larsson Forsell et al demonstrated a 5.5-fold increase in membrane-associated iPLA2 activity [8], and membrane-associated iPLA2β has also been found in rat vascular smooth muscle cells [9].
More recently, a novel membrane associated iPLA2 (iPLA2γ) has been identified [7] which possesses several of the properties demonstrated in our previous studies using rabbit ventricular myocytes, including the optimum pH for activity, sensitivity to BEL and a tight association with the membrane fraction. Homology between iPLA2β and iPLA2γ is confined to the ATP binding motif, the serine lipase site and a region of nine amino acids for which, as yet, there is no known functional significance [7]. The human iPLA2γ gene contains 4 possible translation codons that result in proteins of 63, 74, 77 and 88 kDa [10]. When first described, iPLA2γ was proposed to be localized to peroxisomes due to the presence of a C-terminal SKL signal sequence and recent studies have confirmed that the peroxisomal iPLA2γ is the 63 kDa protein [11]. More recent studies have demonstrated higher molecular weight isoforms of iPLA2γ in mitochondria and endoplasmic reticulum [6].
This study was designed to identify the presence of iPLA2β and iPLA2γ in rabbit ventricular myocytes and to determine the similarity of these isoforms to those identified previously.
EXPERIMENTAL PROCEDURES
Rabbit Ventricular Myocyte Isolation and Culture
Adult rabbits of either sex weighing 2 to 3 kg were anesthetized with intravenous pentobarbitone sodium (50 mg/kg) and the heart rapidly removed. The heart was mounted on a Langendorff perfusion apparatus and perfused for 5 minutes with a Tyrode solution containing (mmol/l) NaCl 118, KCl 4.8, CaCl2 1.2, MgCl2 1.2, NaHCO3 24, KH2PO4 1.2 and glucose 11; the Tyrode solution was saturated with 95%O2/5%CO2 to yield a pH of 7.4. This was followed by a 4 minute perfusion with a Ca free Tyrode solution containing EGTA (100 μM) and a final perfusion for 20 minutes with the Tyrode solution containing 100 μM Ca and 0.033% collagenase. The heart was removed from the perfusion apparatus, the atria were removed and the remaining ventricles were cut into small pieces and incubated in fresh 0.033% collagenase solution at 37°C in a shaking water bath for 4 successive harvests of 20 minutes. Individual myocytes were washed with a HEPES buffer containing (mmol/l): NaCl 133.5, KCl 4.8, MgCl2 1.2, CaCl2 0.3, KH2PO4 1.2, glucose 10 and HEPES 10 (pH=7.4). Extracellular Ca was increased to 1.2 mM in three stages at intervals of 20 minutes. Myocytes were incubated overnight in M199 medium with 10% fetal calf serum at 37°C and then washed three times with 1.2 mM Ca HEPES solution.
Culture of human cardiac myocytes
Human adult cardiac myocytes (Sciencell, San Diego, CA) were cultured in cardiac myocyte medium consisting of 500 ml of basal medium, 25 ml of fetal bovine serum, 5 ml of cardiac myocyte growth supplement and 5 ml of penicillin/streptomycin solution (Sciencell, San Diego, CA).
Generation of iPLA2 clones
Total RNA for use in all cloning steps was isolated with the Versagene Cell Kit (Gentra Systems, Minneapolis, MN). 5′ and 3′ end sequences of rabbit ventricular myocyte iPLA2 were obtained by rapid amplification of cDNA ends following the manufacturer’s instructions for the GeneRacer Kit from Invitrogen (Carlsbad, CA). Briefly, total RNA was dephosphorylated, decapped and ligated to the GeneRacer RNA Oligo to form template for first strand RACE-ready cDNA synthesis. Following cDNA amplification with the appropriate GeneRacer Oligo and gene specific Oligo, 5′ and 3′ end PCR fragments were cloned into the pCR4-TOPO vector and sequenced by automated DNA sequencing with BigDye chemistry. This sequence was conceptually translated and Oligos were designed to yield a full-length amplicon corresponding to the longest open reading frame for each isoform. Oligo dT primed first strand cDNA was generated from total RNA using the Thermoscript RT-PCR System (Invitrogen) and used as template for PCR amplification of full-length iPLA2 clones. These products were cloned into the pCR-XL-TOPO vector and five independent clones of each isoform, −β and −γ, were sequenced. Sequence analysis and assembly was performed using the Vector NTI Suite 9.0.0 (Invitrogen) and the BLAST server at NCBI. Sequence assembly was performed using the ContigExpress program (Invitrogen) and the full-length cDNA sequences were deposited into Genbank (iPLA2-β - Accession # AY744674, iPLA2-γ - Accession # AY738591).
Production of iPLA2γ antibody
A custom-made chicken antibody was obtained from Aves Labs, Inc. (Tigard, OR). Hens were injected four times with a KLH conjugate of the peptide CZSKYIERNEHKMKKVAK and immune eggs were collected for 10 to 15 days after the final injection. The IgY fractions were purified and passed over an affinity column, the column washed and antibody eluted. The final concentration of affinity-purified antibody in the eluant was 0.6 mg/ml.
HPLC Separation of (R)- and (S)-enantiomers of BEL
(R)- and (S)-enantiomers of BEL were isolated from racemic BEL (Calbiochem) using a chirex 3,5-dinitrobenzoyl-(R)-phenylglycine chiral HPLC column (Phenomenex Inc., Torrance, CA) and previously published methods [17]. The column was equilibrated with hexane/dichlroroethane/ethanol (150:15:1, by vol) and the optical enantiomers eluted isocratically at 2 ml/min. Elution of (R)- and (S)-BEL was monitored by UV absorbance at 280 nm. Under these conditions the retention times (Rt) for (R)- and (S)-BEL differ by almost 1 min with the Rt for (S)-BEL being 11.1 min and 12.2 min for (R)-BEL [12]. Peaks corresponding to these Rt were collected, dried under N2, and stored at −20°C. The concentration of each enantiomer was determined spectrophotometrically based on its UV absorbance [12].
Immunoblot analysis of iPLA2 isoforms
Myocytes were suspended in lysis buffer containing (mmol/l) HEPES 20 (pH 7.6), sucrose 250, dithiothreitol 2, EDTA 2, EGTA 2, β-glycerophosphate 10, sodium orthovanadate 1, phenylmethylsulfonyl fluoride 2, leupeptin 20 μg/ml, aprotinin 10 μg/ml and pepstatin A 5 μg/ml (buffer 2). Cells were sonicated on ice for 6 bursts of 10 sec and centrifuged at 10,000 × g at 4°C for 20 min to remove cellular debris and nuclei. Cytosolic and membrane fractions were separated by centrifuging the supernatant at 100,000 × g for 60 min. The pellet was resuspended in lysis buffer and the suspension centrifuged at 100,000 × g for 60 min twice to minimize contamination of the membrane fraction with cytosolic protein. The final pellet was resuspended in lysis buffer containing 0.1% Triton X-100. Protein (cytosol or membrane) was mixed with an equal volume of SDS sample buffer and heated at 95°C for 5 mins prior to loading onto a 10% polyacrylamide gel. Protein was separated by SDS/PAGE at 200 V for 35 mins and electrophoretically transferred to PVDF membranes (Bio-Rad, Richmond, CA) at 100 V for 1 hour. Non-specific sites were blocked with Tris buffered solution containing 0.05% (v/v) Tween-20 (TBST) and 5% (w/v) nonfat milk for 1 hour at room temperature. The blocked PVDF membrane was incubated with primary antibodies to iPLA2β (1 in 1,000 dilution, Cayman Chemical Company, Ann Arbor, MI) or iPLA2γ (1 in 1,000 dilution, Aves Labs Inc., Tigard, OR), followed by horseradish peroxidase-conjugated secondary antibodies. Regions of antibody binding were detected using enhanced chemiluminescence (Amersham, Arlington Heights, IL) after exposure to film (Hyperfilm, Amersham). Multiple exposures of film to the blots were developed.
Phospholipase A2 activity
Myocytes were suspended in 1 ml buffer containing (mmol/l): Sucrose 250, KCl 10, imidazole 10, EDTA 5, dithiothreitol (DTT) 2 with 10% glycerol, pH = 7.8 (buffer 1). The suspension was sonicated on ice six times for 10 seconds (using a microtip probe at 20% power output, 500 Sonic Dismembrator, Fisher Scientific) and the sonicate centrifuged at 20,000 × g for 20 minutes to remove cellular debris and nuclei. The supernatant was then centrifuged at 100,000 × g for 60 minutes to separate the membrane fraction (pellet) from the cytosolic fraction (supernatant). The pellet was washed twice to minimize contamination of the membrane fraction with cytosolic protein by resuspending in buffer1, and centrifuging at 100,000 × g for 60 min. The final pellet was resuspended in buffer 1. Phospholipase A2 activity in cytosolic and membrane fractions was assessed by incubating enzyme (8 μg membrane protein) with 100 μM (16:0, [3H]18:1) plasmenylcholine substrate in assay buffer containing (mmol/l): Tris 10, EGTA 4, 10% glycerol, pH = 7.0 at 37°C for 5 minutes in a total volume of 200 μl. The radiolabeled phospholipid substrate was introduced into the incubation mixture by injection in 5 μl ethanol to initiate the assay. Reactions were terminated by the addition of 100 μl butanol and released radiolabeled fatty acid was isolated by application of 25 μl of the butanol phase to channeled Silica Gel G plates, development in petroleum ether/diethyl ether/acetic acid (70/30/1, v/v) and subsequent quantification by liquid scintillation spectrometry. Protein content of each sample was determined by the Lowry method utilizing freeze dried bovine serum albumin as the protein standard. Radiolabeled (16:0, [3H]18:1) plasmenylcholine was synthesized using [9,10-3H] oleic acid and lysoplasmenylcholine as described in detail previously (4).
Statistical analysis
Statistical comparison of values was performed by Student’s t test. All results are expressed as means ± S.E.M. Statistical significance was considered to be p<0.05.
RESULTS
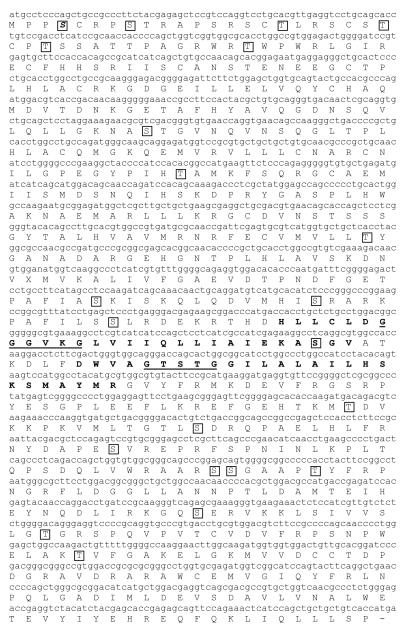
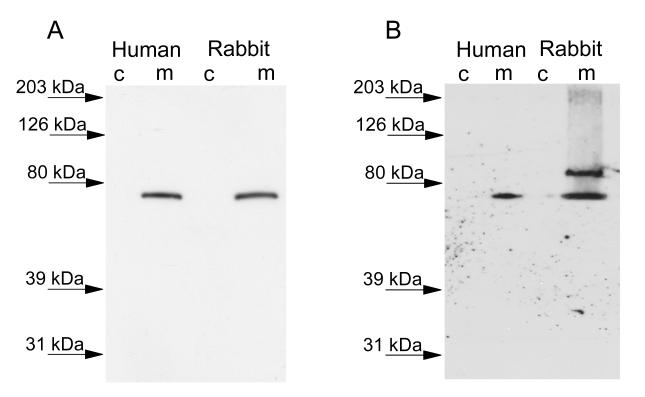
iPLA2-β cDNA was amplified, cloned and sequenced from freshly isolated rabbit ventricular myocytes, (GenBank accession No. 744674), and the amino acid sequence was determined (Figure 1). The longest open reading frame of iPLA2β was found to encode a protein of 665 amino acids in length with a predicted MW of 74 kDa that aBLAST analysis of the non-redundant protein database reveals is 91% identical to the human short isoform. The sequences are highly conserved except for a divergent segment at the N-terminus which is 128 amino acids long in the human and 42 amino acids in the rabbit. Immunoblot analysis of cytosolic and membrane fractions isolated from rabbit and human cardiac myocytes demonstrated an iPLA2β immunoreactive band that ran just below the 80 kDa molecular weight marker (Figure 3A). The human and rabbit immunoreactive bands ran at a similar molecular weight and were present in the membrane fraction only. No immunoreactive band was detected with iPLA2β antibody in the cytosolic fraction of human or rabbit myocytes (Figure 3A).
FIGURE 1.
Full-length rabbit iPLA2β cDNA sequence. Underlines indicate the conserved ATP-binding and lipase active sites. Potential transmembrane domains were predicted using the TMpred server and are indicated with boldface type. Potential PKC-phosphorylation sites were predicted using the NetPhosK 1.0 Server and are boxed.
FIGURE 3.
Immunoblot analysis of the localization of iPLA2β (Panel A) and iPLA2γ (Panel B) in the cytosolic (C) and membrane (M) subcellular fractions isolated from rabbit and human cardiac myocytes. Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Membranes were probed with anti-iPLA2β or anti-iPLA2γ antibodies (1:1,000 dilution) and incubated with horseradish peroxidase-linked secondary antibody (1:50,000 dilution). Immunoblots were detected with enhanced chemiluminescence and exposure to film.
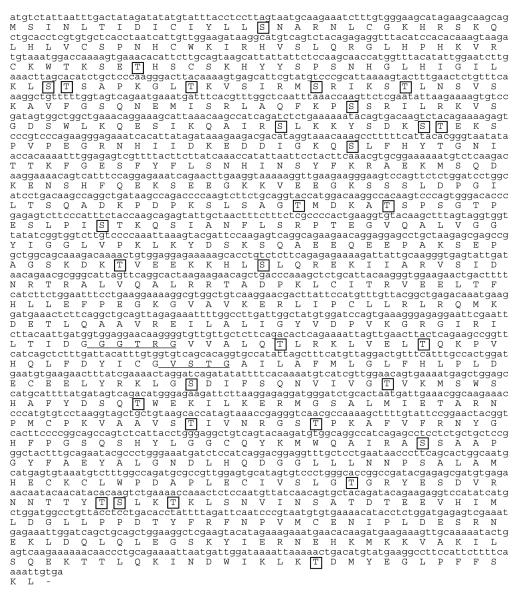
Rabbit ventricular myocyte iPLA2-γ was also cloned and sequenced, and is predicted to encode a full-length protein of 786 amino acids (Figure 2) with a molecular mass of 88 kDa that was found by BLAST analysis to be 88% identical to the human form. It has been reported previously that the human iPLA2-γ sequence contains multiple alternative start sites for translation, which correspond to protein products of 88, 77, 74 and 63 kDa [10]. We analyzed the amino acid sequence for iPLA2γ in rabbit ventricular myocytes and identified 6 methionine residues upstream of the active site which could encode functional PLA2. In addition to the 88, 77, 74, and 63 kDa isoforms found in the human isoform, we also identified two single amino acid substitutions which could encode alternative start sites for 58 and 49 kDa proteins. Immunoblot analysis of rabbit ventricular myocytes detected immunoreactive bands corresponding to the 88 kDa and 73 kDa isoenzymes in the membrane fraction of isolated rabbit ventricular myocytes. Immunoblot analysis of human cardiac myocytes revealed a single band corresponding to the 73 kDa isoform of iPLA2γ in the membrane fraction (Figure 3B). No bands were detected in the cytosolic fractions from either rabbit or human myocytes (Figure 3B).
FIGURE 2.
Full-length rabbit iPLA2γ cDNA sequence. Underlines indicate the conserved ATP-binding and lipase active sites. Potential PKC-phosphorylation sites were predicted using the NetPhosK 1.0 Server and are boxed.
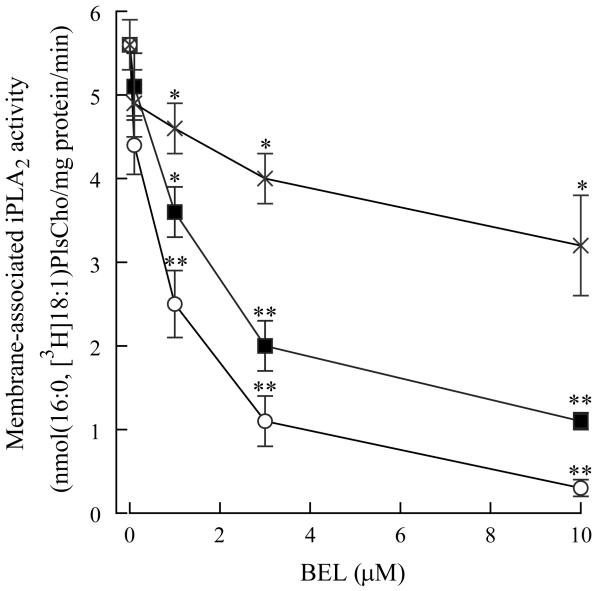
Since we detected both iPLA2β and γ in isolated rabbit ventricular myocytes and the majority of iPLA2 activity is membrane-associated, we incubated isolated membrane fractions with (R)-BEL, (S)-BEL or racemic BEL. Previous studies have indicated that (S)-BEL is approximately an order of magnitude more selective for iPLA2β in comparison to iPLA2γ [12]. Conversely, (R)-BEL is approximately an order of magnitude more selective for iPLA2γ in comparison to iPLA2β [12]. After pretreatment with BEL for 10 mins, membrane-associated iPLA2 activity was significantly decreased with concentrations of (R)-BEL greater than 0.1 μM (Figure 4). At 10 μM, (R)-BEL inhibited iPLA2 activity by 90%, whereas (S)-BEL inhibited iPLA2 activity by approximately 50% (Figure 4)
FIGURE 4.
Membrane-associated iPLA2 activity measured in the absence or presence of bromoenol lactone (BEL). Isolated membrane fractions were incubated with increasing concentrations of R-BEL (open circles), S-BEL (X) or racemic BEL (filled squares) for 10 minutes prior to assay of PLA2 activity. PLA2 activity was measured using 100 μM (16:0, [3H]18:1) plasmenylcholine (PlsCho) in the absence of Ca (4 mM EGTA). Values are means ± SEM for independent results from 6 separate animals. *p<0.05, **p<0.01 when compared to activity in the absence of BEL.
DISCUSSION
Myocardial iPLA2 activity in the rabbit was first described by Ford et al in 1991 [13]. They described a ten-fold increase in microsomal, plasmalogen-selective iPLA2 activity during myocardial ischemia [13]. We have demonstrated increases in membrane-associated, plasmalogen-selective iPLA2 activity in isolated rabbit ventricular myocytes in response to thrombin [14] and hypoxia [5]. The activation of iPLA2 and accelerated plasmalogen phospholipid hydrolysis lead to the production of lysoplasmenylcholine [5, 14], an amphipathic metabolite that can have direct effects on the function of integral membrane proteins. We have shown that lysoplasmenylcholine produces action potential derangements in cardiac myocytes at much lower concentrations than that previously demonstrated for lysoplasmenylcholine [5]. The action potential derangements caused by lysoplasmenylcholine occur as a result of its action on multiple membrane currents [15].
Although several studies have described iPLA2 activation in rabbit ventricular myocytes, this is the first study to sequence iPLA2β and iPLA2γ and to demonstrate that iPLA2γ is the major isoform in the rabbit myocardium. Our immunoblot analysis data suggest that the vast majority of iPLA2 is membrane-associated, and this agrees with our previous data measuring iPLA2 activity [2]. In previously reported studies, iPLA2β has been demonstrated to be primarily cytosolic. It was originally purified from the cytosol of the mouse macrophage cell line P388D1 [16] and its cDNA was later identified in P388D1 and Chinese hamster ovary cells [17] and human B lymphocytes [18]. In humans the difference between the membrane-associated and cytosolic isoforms is the presence of 54 amino acid residues that may lead to a membrane-associated protein [8]. While we did not identify a similar sequence in the rabbit isoform, TMpred analysis suggests that rabbit iPLA2β contains a potential transmembrane domain (indicated by the boldface type in Figure 1).
In a previous study, we have identified the presence of multiple iPLA2 isoforms in several tissues and species [6]. The role of myocardial iPLA2 isoforms has been investigated in several recent studies using mouse models. Calcium-independent PLA2 activity in murine heart is less than 5% of that in human myocardium, suggesting that the mouse could be a species-specific knockdown model of human ischemia-induced arrhythmogenesis [19]. Accordingly, Mancuso et al generated transgenic mice that expressed amounts of iPLA2β activity comparable to that present in human myocardium and demonstrated malignant ventricular arrhythmias, increased fatty acid release in venous effluents and increased lysophospholipid content in the ischemic zone in response to ischemia [19]. These data demonstrate the role of myocardial iPLA2β in the hydrolysis of membrane phospholipids and arryhthmogenesis in response to ischemia. Transgenic mice expressing iPLA2γ in cardiac myocytes demonstrated a marked loss of mitochondrial phospholipids accompanied by mitochondrial dysfunction [20]. In a subsequent study, mice null for iPLA2γ displayed growth retardation, cold intolerance, reduced exercise endurance and abnormal mitochondrial function [21]. In particular, iPLA2γ knockout mice were more susceptible to sudden death following transverse aortic constriction, indicating the importance of iPLA2γ in the ability of the myocardium to respond to stress [21].
We have previously reported that incubation of isolated membrane fractions from rabbit ventricular myocytes demonstrate increased iPLA2 activity when incubated with phorbol 12-myristate 13-acetate in the absence of Ca [3]. These data suggests that a membrane-associated novel PKC isoform modulates iPLA2 activity. Increased iPLA2 activity required the presence of ATP, suggesting that the enzyme is phosphorylated by PKC [3]. Sequence analysis of rabbit ventricular myocyte iPLA2βand γ isoforms reveals the presence of several predicted PKC phosphorylation sites that are indicated by the boxes in Figures 1 and 3.
In conclusion, we have identified and sequenced iPLA2βand iPLA2γ from isolated rabbit ventricular myocytes. These isoforms possess a high degree of sequence homology between rabbit and human and there is a similar protein expression pattern when comparing rabbit and human cardiac myocytes. Both isoforms have several putative PKC phosphorylation sites and our previous studies suggest that membrane-associated iPLA2 is activated directly by a membrane-associated novel PKC isoform [3]. Finally, our data obtained using (R)- and (S)-enantiomers of the iPLA2-selective inhibitor BEL, suggest that the majority of membrane-associated iPLA2 activity is iPLA2γ.
ACKNOWLEDGEMENTS
This research was supported in part by National Heart, Lung and Blood Institute Grant HL 68588 (JM). The authors would like to thank Dr. Brian Cummings for the separation of (R)- and (S)-enantiomers of BEL.
Abbreviations
- BEL
bromoenol lactone
- iPLA2
calcium-independent phospholipase A2
- MAFP
methyl arachidonyl fluorophosphonate
- PKC
protein kinase C
- PLA2
phospholipase A2
REFERENCES
- 1.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 2.McHowat J, Creer MH. Calcium-independent phospholipase A2 in isolated rabbit ventricular myocytes. Lipids. 1998;33:1203–1212. doi: 10.1007/s11745-998-0324-5. [DOI] [PubMed] [Google Scholar]
- 3.Steer SA, Wirsig KC, Creer MH, Ford DA, McHowat J. Regulation of membrane-associated iPLA2 activity by a novel PKC in ventricular myocytes. Am. J. Physiol. 2002;283:C1621–C1626. doi: 10.1152/ajpcell.00109.2002. [DOI] [PubMed] [Google Scholar]
- 4.McHowat J, Creer MH. Thrombin activates a membrane-associated calcium-independent PLA2 in ventricular myocytes. Am. J. Physiol. 1998;274:C447–C454. doi: 10.1152/ajpcell.1998.274.2.C447. [DOI] [PubMed] [Google Scholar]
- 5.McHowat J, Liu SJ, Creer MH. Selective hydrolysis of plasmalogen phospholipids by Ca2+-independent PLA2 in hypoxic ventricular myocytes. Am J Physiol. 1998;274:C1727–C1737. doi: 10.1152/ajpcell.1998.274.6.C1727. [DOI] [PubMed] [Google Scholar]
- 6.Kinsey GR, Cummings BS, Beckett CS, Saavedra G, Zhang W, McHowat J, Schnellmann RG. Identification and distribution of endoplasmic reticulum iPLA2. Biochem Biophys Res Comm. 2005;327:287–293. doi: 10.1016/j.bbrc.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Mancuso DJ, Jenkins CM, Gross RW. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A2. J Biol Chem. 2000;275:9937–9945. doi: 10.1074/jbc.275.14.9937. [DOI] [PubMed] [Google Scholar]
- 8.Forsell PK Larsson, Kennedy BP, Claessen HE. The human calcium-independent phospholipase A2 gene multiple enzymes with distinct properties from a single gene. Eur J Biochem. 1999;262:575–585. doi: 10.1046/j.1432-1327.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 9.Miyake R, Gross RW. Multiple phospholipase A2 activities in canine vascular smooth muscle. Biochim.. BiophysActa. 1992;1165:167–176. doi: 10.1016/0005-2760(92)90183-v. [DOI] [PubMed] [Google Scholar]
- 10.Mancuso DJ, Jenkins CM, Sims HF, Cohen JM, Yang J, Gross RW. Complex transcriptional and translational regulation of iPLA2γ resulting in multiple gene products containing dual competing sites for mitochondrial or peroxisomal localization. Eur J Biochem. 2004;271:4709–4724. doi: 10.1111/j.1432-1033.2004.04435.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Han X, Gross RW. Identification of hepatic peroxisomal phospholipase A2 and characterization of arachidonic acid-containing choline glycerophospholipids in hepatic peroxisomes. FEBS Lett. 2003;546:247–250. doi: 10.1016/s0014-5793(03)00581-7. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins CM, Han X, Mancuso DJ, Gross RW. Identification of calcium-independent phospholipase A2 (iPLA2) β, and not iPLA2γ, as the mediator of arginine vasopressin-induced arachidonic acid release in A-10 smooth muscle cells. Enantioselective mechanism-based discrimination of mammalian iPLA2s. J Biol Chem. 2002;277:32807–32814. doi: 10.1074/jbc.M202568200. [DOI] [PubMed] [Google Scholar]
- 13.Ford DA, Hazen SL, Saffitz JE, Gross RW. The rapid and reversible activation of a calcium-independent plasmalogen-selective phospholipase A2 during myocardial ischemia. J Clin Invest. 1991;88:331–335. doi: 10.1172/JCI115296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHowat J, Creer MH. Selective plasmalogen substrate utilization by thrombin-stimulated Ca2+- independent PLA2 in cardiomyocytes. Am J Physiol. 2000;278:H1933–H1940. doi: 10.1152/ajpheart.2000.278.6.H1933. [DOI] [PubMed] [Google Scholar]
- 15.Liu SJ, Creer MH, Kennedy RH, McHowat J. Alterations in Ca2+ cycling by lysoplasmenylcholine in adult rabbit ventricular myocytes. Am J Physiol. 2003;284:C826–C838. doi: 10.1152/ajpcell.00465.2002. [DOI] [PubMed] [Google Scholar]
- 16.Ackermann EJ, Kempner ES, Dennis EA. Ca2+-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J Biol Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- 17.Balboa MA, Balsinde J, Jones SS, Dennis EA. Identity between the Ca2+-independent phospholipase A2 enzymes from P388D1 macrophages and CHO cells. J Biol Chem. 1997;272:8576–8580. doi: 10.1074/jbc.272.13.8576. [DOI] [PubMed] [Google Scholar]
- 18.Larsson PK, Claesson HE, Kennedy BP. Multiple splice variants of the human calcium-independent phospholipase A2 and their effect on enzyme activity. J Biol Chem. 1998;273:207–214. doi: 10.1074/jbc.273.1.207. [DOI] [PubMed] [Google Scholar]
- 19.Mancuso DJ, Abendschein DR, Jenkins CM, Han X, Saffitz JE, Schuessler RB, Gross RW. Cardiac ischemia activates calcium-independent phospholipase A2β, precipitating ventricular tachyarrhythmias in transgenic mice: Rescue of the lethal electrophysiologic phenotype by mechanism-based inhibition. J Biol Chem. 2003;278:22231–22236. doi: 10.1074/jbc.C300033200. [DOI] [PubMed] [Google Scholar]
- 20.Mancuso DJ, Han X, Jenkins CM, Lehman JJ, Sambandam N, Sims HF, Yang J, Yan W, Yang K, Green K, Abendschein DR, Saffitz JE, Gross RW. Dramatic accumulation of triglycerides and precipitation of cardiac hemodynamic dysfunction during brief caloric restriction in transgenic myocardium expressing human calcium-independent phospholipase A2γ. J Biol Chem. 2007;282:9216–9227. doi: 10.1074/jbc.M607307200. [DOI] [PubMed] [Google Scholar]
- 21.Mancuso DJ, Sims HF, Han X, Jenkins CM, Guan SP, Yang J, Moon SH, Pietka T, Abumrad NA, Schlesinger PH, Gross RW. Genetic ablation of calcium-independent phospholipase A2γ leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J Biol Chem. 2007;282:34611–34622. doi: 10.1074/jbc.M707795200. [DOI] [PMC free article] [PubMed] [Google Scholar]