Abstract
Doxorubicin (Dox) is a very potent anti-cancer agent but its usage is limited by its dose-dependent irreversible cardiotoxicity. Despite intensive research efforts, the mechanism of Dox cardiotoxicity remains to be poorly understood and consequently the means available for clinicians to prevent or effectively manage Dox cardiotoxicity are very limited. Recent studies have excitingly revealed that a therapeutic dose of Dox can activate ubiquitin-proteasome system (UPS) mediated proteolysis in cardiomyocytes and that the UPS-mediated degradation of a number of pivotal cardiac transcription factors and/or survival factors is enhanced by Dox treatment. These suggest that the Dox induced UPS activation may represent a new mechanism underlying Dox cardiotoxicity. Notably, recent experimental studies suggest that proteasome activation promotes cardiac remodeling during hypertension. This review surveys the current literature on the impact of Dox on the UPS and the potential mechanisms by which UPS activation may compromise the heart during Dox therapy.
Introduction
The ubiquitin-proteasome system (UPS) mediates the specific degradation of most cellular proteins, in addition to fulfilling important non-proteolytic obligations in the cell [1]. UPS-mediated proteolysis can be broken down into two main steps: ubiquitination and proteasome-mediated degradation. Ubiquitination is an ATP dependent process that attaches ubiquitin molecules to a substrate protein by a series of enzymatic reactions involving the ubiquitin activating enzyme (E1), ubiquitin conjugating enzyme (E2), ubiquitin ligase (E3), and occasionally the ubiquitin chain elongation factor (E4) [2]. The degradation of polyubiquitinated proteins is predominantly done by the 26S proteasome [1, 3]. The 26S proteasome consists of a 20S catalytic core flanked by one or two 19S regulatory caps. In some cases, the proteasome may degrade or cleave proteins in an ubiquitin-independent manner [4, 5].
The functioning of a protein can be altered by its expression level, structural integrity, posttranslational modifications, and/or interaction with other proteins. The UPS can regulate protein function potentially at each of these levels. The expression level of a protein in the cell is determined by the equilibrium between its synthesis and degradation. Although the synthesis side of the equation has been historically investigated and appreciated a lot more, the degradation side plays an as important role in maintaining protein homeostasis in the cell. To fulfill its obligation to the cell a protein must attain and maintain a specific conformation via folding/refolding and removing the terminally misfolded species through a process known as protein quality control in which the UPS is a major player. The UPS can exert posttranslational modifications on a target protein through several ways: first, ubiquitination of a protein molecule can either target the protein for degradation or alter its function without affecting its stability; second, in a few known cases the proteasome can cleave a protein molecule to generate a fragment that is an active form of the protein in a signaling pathway. Hence, it is readily conceivable that alteration of UPS function would have a profound implication in various cellular processes. Indeed, the UPS plays an essential role in not only protein quality control but also the regulation of a number of cellular functions such as transcription, cell cycle, and even cell death [1, 3, 6].
Doxorubicin (Dox) is a potent anti-cancer agent of the anthracycline family. Unfortunately, its clinical chemotherapeutic use is limited by its severe toxicity on the heart when the accumulative dose reaches a threshold. The cardiotoxicity especially subchronic and delayed cardiotoxicity is manifested by dose-dependent cardiomyopathy and refractory congestive heart failure with the unique pathological changes being distention of the endoplasmic reticulum, swelling of mitochondria, cytoplasmic vacuolization, and myofibrillar disarray and loss (sarcopenia) in cardiomyocytes as well as apoptosis [7, 8]. A great deal of research has been carried out to investigate the molecular mechanisms by which Dox selectively impairs the heart. As a result, a number of mechanisms were proposed although most of them are attributable to the basis that Dox increases the production of reactive oxidative species (ROS) in cardiomyocytes. Accordingly, anti-ROS therapy using iron-chelating agents, for example, has been clinically used along with Dox to battle the cardiotoxicity. However, success of the anti-ROS strategy has so far been quite modest [8], indicating that the current understanding of Dox cardiotoxicity is very incomplete. Emerging studies suggest that UPS dysfunction may be involved in Dox cardiotoxicity [9••, 10••]. In this mini-review, we will highlight recent reports that revealed Dox induces UPS dysfunction and discuss the potential molecular mechanisms by which UPS activation contributes to Dox cardiotoxicity.
Dox Increases UPS Activities
Once introduced to the body Dox passively diffuses through the cell membrane into the cytoplasm where Dox interacts with the proteasome. The Dox-proteasome complex will then translocate to the nucleus where Dox will release from the proteasome and bind to DNA due to its higher binding affinity for DNA [11]. The elucidation of the ability of Dox to bind the proteasome in the cell has raised the question of whether Dox alters proteasome function. This question has become more relevant recently in terms of both deciphering Dox pharmacological actions and the mechanisms underlying Dox cardiotoxicity. This is because proteasome inhibition has been clinically employed to treat certain types of cancer and proteasome dysfunction is increasingly associated with cardiac malfunction. So far, quite a few studies have begun addressing this important question and revealed intriguing and likely important findings although the findings are, in some cases, conflicting. It seems that Dox treatment increases the expression of several key ubiquitin E3 ligases while activating the proteasome at a therapeutically relevant dose. Consistently, Dox has been shown to significantly enhance the proteasome-mediated degradation of key regulatory proteins in cardiomyocytes.
Dox increases the expression of ubiquitin E3 ligases
In general, intracellular proteins must be polyubiquitinated in order to be degraded by the 26S proteasome. For a normal cellular protein, ubiquitination is likely the rate-limiting step for its degradation by the UPS. The specificity of the ubiquitination lies primarily with the ubiquitin E3 ligases. Increased degradation of muscle proteins as seen in muscle atrophy is often associated with the upregulation of the expression of muscle-specific E3 ligases, such as artogin-1 and MuRF's (muscle-specific RING finger proteins). Increases in ubiquitin E3 ligase expression and activity have been implicated in cardiac pathological conditions including cardiac hypertrophy and reverse remodeling [12, 13].
The COOH-terminal of heat shock protein cognate 70-interacting protein (CHIP) is a U box-containing ubiquitin E3 ligase as well as a co-chaperone of HSP70 [14]. It was found in cultured cells that Dox triggered posttranscriptional increases in CHIP protein expression while proteasome inhibitors significantly decreased CHIP protein levels in the same cell culture setting [10••]. The CHIP increase was accompanied reciprocally by HSP70 depletion [10••], consisting with HSP70 being a substrate of the CHIP ubiquitin ligase activity [14]. Since CHIP is an essential E3 ligase for the ubiquitination of misfolded/abnormal proteins the increase in CHIP by Dox may represent a compensatory response from the cell to boost the capability to remove abnormal proteins generated by Dox induced oxidative stress. However, the reciprocal decrease of HSP70 likely diminishes the cell's ability to handle stress and may contribute to Dox cytotoxicity. It remains to be determined whether Dox upregulates CHIP in the heart and if so, whether this up-regulation would contribute to Dox cardiotoxicity.
Atrogin-1 is a striated muscle specific E3 ligase that is upregulated during muscle atrophy and promotes the degradation of muscle proteins, thereby playing critical roles in muscle wasting [15, 16]. A recent study by Yamamoto et al. demonstrated that atrogin-1 levels rise in response to Dox treatment as shown by increased transcript and protein levels. The induction of atrogin-1 by Dox was mediated by the activation of p38 mitogen-activated protein kinase (MAPK) but could be antagonized by Akt [17••]. Consistently, it was demonstrated that Akt levels are reduced in response to Dox treatment, allowing the levels of atrogin-1 to increase [18, 19]. Dox was able to induce cardiomyocyte atrophy which was alleviated by the proteasome inhibitor, MG 132, indicating a role for the UPS in Dox induced atrophy [17••]. Atrogin-1 overexpression was sufficient to recapitulate Dox treatment induced cardiomyocyte atrophy [17••], illustrating that increasing atrogin-1 by Dox in cardiomyocytes may contribute to Dox cardiotoxicity.
Dox increases proteasome proteolytic activities
In UPS-mediated protein degradation, peptide cleavage is primarily carried out by the 26S proteasome which is composed of a 20S catalytic core and one or two 19S regulatory caps. The activity of the 20S proteasome can be and often is assessed in vitro by the conventional proteasome peptidase activity assays but a simple in vitro method to evaluate the function of the 19S proteasome is currently lacking. Consequently, we have to rely on surrogate full length protein substrates expressed in cultured cells or intact animals to assess the proteolytic function of the 19S and the 26S proteasomes. An example of such a surrogate substrate is GFPu (or GFPdgn) which is created by fusion of an ubiquitination signal sequence, degron CL1, to the carboxyl terminus of green fluorescence protein (GFP) [9••, 20]. To assess the acute effect of Dox on UPS proteolytic function in vivo we treated the GFPdgn transgenic mice with an intraperitoneal injection of Dox (25 mg/kg). The protein levels but not transcript levels of GFPdgn were significantly decreased at 6 hours after the Dox injection. This demonstrated for the first time in intact animals that Dox can enhance UPS proteolytic function [9••].
Similar results were seen in vitro with cultured mouse cardiomyocytes also experiencing decreased GFPdgn levels in response to Dox treatment in a dose dependent fashion [9••]. These results were later validated using GFPu infected neonatal rat ventricular myocytes and monitoring changes in endogenous proteasome substrates, such as β-catenin and c-Jun [10••]. Experiments using the cyclohexamide chase and proteasome inhibitors further confirmed that GFPu was indeed destabilized by Dox treatment in a proteasome-dependent manner [9••]. This indicates that Dox has the ability to increase UPS proteolytic activity.
Dox treatment increases the production of ROS which may increase the amount of oxidized proteins. Dox treatment induced increases in the degradation of UPS surrogate protein substrates or endogenous proteins could be indirectly through the effect of reactive oxygen species (ROS) generated by Dox indiscreetly on protein substrate. As substrates, oxidized proteins may indirectly activate UPS proteolysis. Alternatively, Dox may directly activate the ubiquitination step as discussed earlier or directly activate the proteasome. A study by Liu et al. has addressed these questions from several perspectives. First, they showed in cultured neonatal rat ventricular myocytes that Dox treatment dose-dependently reduced the steady levels of endogenous ubiquitinated proteins and significantly attenuated the accumulation of ubiquitinated proteins caused by proteasome inhibitors or the overexpression of a misfolded protein [10••]. Second, using isolated 20S proteasomes in test tubes they revealed an increase in proteasome chymotrypsin-like activity in response to Dox treatment at clinically relevant doses of 1 μM to 5 μM, while at a higher and clinically irrelevant dose, of 10 μM proteasome proteolytic activity was inhibited [10••]. Another recent study by Tsimokha et al. showed in cultured neoplastic cells that Dox treatment increases the phosphorylation of proteasome subunits and enhances chymotrypsin-like activity of 26S proteasomes [21]. These experiments demonstrated that Dox is able to exert bidirectional direct effects on the proteasome, depending on the availability of free Dox to the proteasome.
Some earlier in vitro studies had reported an inhibiting effect of Dox on the proteasome [22, 23]. In reviewing these studies, it becomes apparent that nearly all those studies used Dox at a concentration greater than 5 μM, a concentration that is difficult to achieve in conventional clinical regimen. The bidirectional in vitro effect of Dox revealed recently by Liu et al. [10••], therefore, provides important evidence explaining the discrepancy in the previous literature. The mechanism by which Dox activates the proteasome remains to be elucidated.
How would Dox-induced UPS activation contribute to Dox cardiotoxicity?
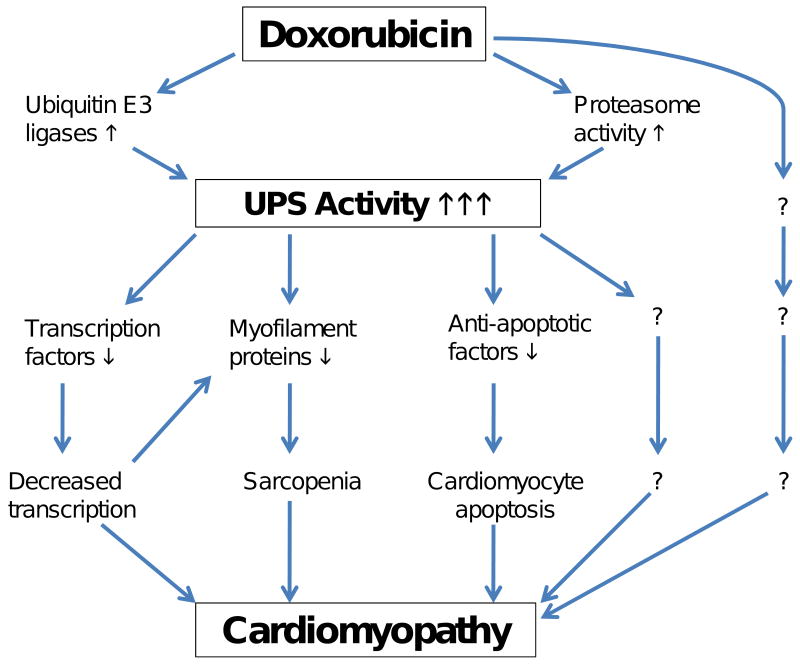
In a general term, this important question has not been directly answered yet. However, a number of studies have revealed that UPS-mediated degradation of key transcription factors, myofibrils, and cell survival factors in cardiomyocytes are enhanced by Dox treatment. These reasonably link Dox-mediated UPS activation to several major known pathogenic factors of Dox cardiotoxicity, namely transcription depression [24••, 25••], sarcopenia [17••, 26], and cardiomyocyte death [27], which are not mutually exclusive (Figure 1).
Figure 1.
An illustration of the potential pathways by which the doxorubicin induced activation of the ubiquitin-proteasome system (UPS) causes cardiomyopathy.
Transcription suppression
Several recent reports show that the UPS-mediated degradation of key transcription factors is enhanced by Dox. This not only corroborates Dox-mediated UPS activation but also serves an important contributing factor to Dox-induced transcription inhibition, a major mechanism underlying Dox cardiotoxicity.
β-Catenin is not only a critical structural protein of the intercalated disk in the heart but also a versatile transcription factor that is critical to cardiac development and remodeling. β-Catenin is part of a large protein complex consisting of glycogen synthase kinase (GSK)-3β, the tumor suppressor gene product adenomatous polyposis coli, and axin/conductin [28]. In absence of Wnt, GSK-3β is constitutively active and phosphorylates β-catenin and promotes its UPS-mediated degradation. During proteasome inhibition with MG 132 the levels of β-catenin in 3T3 cells increased while treatment with Dox was able to attenuate this increase [10••]. More importantly, Dox treatment significantly destabilized β-catenin proteins in cultured neonatal rat ventricular myocytes [10••].
The nuclear factors of activated T-cells (NFATs) are an important family of transcription factors. Through ROS and mitochondrial mediated calcium release, NFAT-4 can be activated by Dox in the typical calcium-calcineurin dependent manner. This activation can cause cardiomyocyte death [29]. NFAT-5 is a relatively new member of the NFAT family, however, its activity is regulated in a calcineurin-independent manner because of its lack of the N-terminal NFAT homology region harboring the calcineurin regulatory motif [30]. NFAT-5 is vital to cardiomyocyte development as NFAT-5 inhibition results in decreased cell viability [24••]. Experiments with siRNA for NFAT-5 and adenoviral dominant negative NFAT-5 treated cardiomyocytes compared to their control counterparts demonstrated that NFAT-5 is necessary for myocyte survival and that the Dox induced decrease of NFAT-5 promotes cell injury [24••]. Dox treatment was shown to decrease significantly the levels of NFAT-5 without changing the transcript levels. Cyclohexamide chase further confirmed that Dox destabilizes NFAT-5 [24••]. Proteasome inhibition was able to alleviate the Dox induced decrease of NFAT-5, suggesting that NFAT-5 experiences increased proteasome-mediated degradation in response to Dox treatment [24••]. Taurine is an amino acid with strong cardioprotective actions during pathological conditions such as ischemia and heart failure [31]. Taurine is absorbed into cardiomyocytes from the plasma through the taurine transporter (TauT). The TauT gene is a target gene of NFAT5. Consistent with the enhanced degradation of NFAT5 by Dox, TauT transcript levels were significantly decreased by Dox treatment [31]. Increased NFAT5 degradation by the proteasome leads to decreased levels of TauT, which reduces the ability of the cell to take up taurine thus diminishing the cardioprotective effects and leaving the cell more prone to pathology.
p300 is a transcriptional coactivator essential to heart development, regulation of cardiac cell specific genes, and cardiac hypertrophy. Poizat et al. showed that exposure of cultured cardiomyocytes to Dox rapidly depleted transcripts for key regulators of cardiac gene expression, including MEF2C, dHAND, and NKX2.5. This depletion was preventable by the delivery of exogenous p300. They further demonstrated that Dox treatment decreased p300 protein levels without changing p300 mRNA [32]. The decrease in p300 protein levels was caused by 26S-proteaosme mediated degradation [32]. Again, Dox induced p38 MAPK activation might have triggered p300 degradation because the p38 inhibitor stabilized p300 levels [25••]. The authors suggest that it is the loss of p300 that decreases the cellular expression of anti-apoptotic proteins, thereby predisposing the cell to apoptosis [25••]. The increased degradation of these protective transcription factors appears to be an underlying mechanism of Dox induced cardiomyopathy.
Sarcopenia
As manifested by myofibril disarray, myofibril loss, and cytoplasmic vacuolization in cardiomyocytes, sarcopenia is a prominent pathological change and is considered an important pathogenic factor in Dox cardiomyopathy. The degradation of nearly all myofibrillar proteins depends on the UPS; therefore, Dox-induced UPS activation very likely contributes to sarcopenia associated with Dox cardiotoxicity.
As discussed earlier, Dox induces the expression of striated muscle-specific E3 ligase atrogin-1 in vivo and in cultured cardiomyocytes through activating p38 MAPK [17••]. Atrogin-1 is upregulated in myocytes during cachexia and other muscle atrophy conditions and is required for muscle wasting [33, 34]. A transgenic study has shown that cardiac overexpression of atrogin-1 blocked pressure overload cardiac hypertrophy but caused ventricular dilatation and malfunction [35]. It appears that upregulation of atrogin-1 can cause sarcopenia through not only directly degrading myofibril proteins but also decreasing protein synthesis by targeting the eukaryotic initiation factor 3 (eIF3) subunit 5 for degradation [36].
Degradation of some of the sarcomeric proteins may require initial cleavage from other proteases, such as calpain and caspase, to loosen the myofibril proteins for subsequent degradation by the UPS [37]. Titin, the large myofibrillar protein that plays an essential role in the Frank-Starling relationship in the heart [38], is a classical example. Dox induces increased calpain activity and calpain dependent degradation of titin leading to myofilament disarray and thus decreased cardiomyocyte function [26]. While the proteasome is unable to degrade myofibrils like titin due to the large size of the protein, complete degradation of titin likely requires the proteasome because calpains are not able to fully degrade titin [39]. Some investigators, including us, believe that an increase in ubiquitin ligases alone may not sufficient to increase the degradation of their substrates if the substrates are normal proteins because ubiquitination of a normal protein molecule usually requires the protein to undergo certain posttranslational modification(s) to expose or constitute the degradation signal [1]. Therefore, Dox induced loss of myofibril may not only be attributed to its upregulation of ubiquitin E3 ligases. The modification that Dox or its derivatives exert on sarcomeric proteins must play an important role as well. To this end, Dox induced oxidative stress leading oxidative modifications of the substrates (e.g., sarcomeric and other proteins) may be as important as its activation on the UPS. On the other hand, some oxidized proteins may be, according to some studies [4, 40], degraded directly by the 20S proteasome in an ubiquitination-independent manner. In that case, increasing oxidative stress and directly activating 20S proteasome activities by Dox treatment might be sufficient to increase the degradation of myofibrils and cause sarcopenia even in absence of its stimulating effect on the ubiquitination step.
Cardiomyocyte death
Because the regenerative capacity of the heart is, if any, very limited a significant loss of cardiomyocytes can cause the heart to fail. Cardiomyocyte death, especially apoptosis, has been considered a major underlying mechanism for Dox cardiomyopathy [7]. Obviously, Dox induced UPS activation can lead to cardiomyocyte apoptosis by destabilizing cell survival related transcription factors as described above. It has also been shown that Dox increases UPS-mediated degradation of several other cellular proteins, such as ARC and Bcl2, thereby tipping the balance between cell death and survival towards cell death.
ARC (apoptosis repressor with caspase recruitment domain) is a critical protein involved in apoptosis repression and thus cardiomyocyte survival. ARC upregulation was demonstrated to have the ability to antagonize Dox induced cardiomyocyte apoptosis [41••], indicating the vital role of ARC preservation. A recent study by An et al. indicated that the mechanism of action for ARC is to preserve mitochondrial membrane potential, decrease cytochrome c release, reduce caspase-9 and caspase-3 cleavage, and inhibit the pro-apoptotic Bax activation [41••]. Dox induced a time and dose dependent decrease in ARC that parallels decreased cardiomyocyte survival [41••]. Proteasome inhibition was able to partially restore ARC levels thus indicating the involvement of the proteasome in ARC down-regulation [41••]. ARC is targeted for degradation by the ubiquitin E3 ligase minute double minute 2 (MDM2) [42]. MDM2 is a RING-finger protein that is commonly associated as the E3 ligase for p53 [43]. The ubiquitination of ARC by MDM2 and subsequent degradation leaves cardiomyocytes more susceptible to apoptosis [44]. These results indicate the role of enhanced UPS activity in the underlying mechanism of cardiomyocyte apoptosis for the perpetuation of Dox induced cardiotoxicity.
Nuclear Transcription Factor κB (NFκB) can be anti-apoptotic as in cancerous cells but as Wang et al. demonstrated, NFκB activation is pro-apoptotic in endothelial cells and cardiomyocytes after Dox treatment [45]. NFκB activation depends on the UPS-mediated degradation of IκB, the inhibitor of NFκB. Dox induces the activation of NFκB in a time and dose dependent fashion in both endothelial cells and cardiomyocytes [45]. This activation is caused by ROS-triggered IκB phosphorylation and degradation [46]. It is very likely but remains to be shown that the Dox induced UPS activation contributes to the increased degradation of IκB.
The MAPKs p38 and JNK are activated by Dox in cardiomyocytes [17••, 47]. It appears that p38 is required for the upregulation of atrogin-1 by Dox and that the increased amount of atrogin-1 increases the activation of JNK [17••, 47]. MAPK phosphatase-1 (MKP-1) inactivates JNK. Atrogin-1 interacts with MKP-1 and stimulates the proteasomal degradation of MKP-1, thereby allowing sustained JNK activation [47]. During ischemia-reperfusion injury there was a documented increase in atrogin-1 and JNK which lead to decreased levels of the anti-apoptotic Bcl-2 and increased levels of the pro-apoptotic Bax, cleaved caspase-9, and cleaved caspase-3, resulting in an increased amount of cardiomyocyte apoptosis [47]. Therefore, there is a potential link between up-regulation of atrogin-1 and cardiomyocyte apoptosis. The involvement of atrogin-1 in both sarcopenia and apoptosis suggests a critical role for atrogin-1 elevation and thus UPS activation in Dox cardiomyopathy.
Conclusions and Future Directions
Direct and indirect experimental evidence have suggested that a therapeutic dose of Dox activates the UPS in cardiomyocytes both in vivo and in vitro but whether this occurs in humans, especially in Dox treated cancer patients, remains to be investigated. A multitude of evidence is consistent with a detrimental role of Dox induced UPS activation in cardiomyocytes to the heart potentially through eliciting transcription suppression, sarcopenia, and cardiomyocyte apoptosis (Figure 1). However, the contribution of UPS activation to the acute and chronic cardiotoxicity of Dox has not been established. No studies reported so far have tested whether correction of the UPS activation through, for instance, co-administration of a proteasome inhibitor, would mitigate Dox cardiotoxicity. Notably, a phase I clinical trial employing doxorubicin in combination with the proteasome inhibitor, bortezomib, to treat advanced malignancies was reported and concluded that bortezomib and Dox can be safely administered [48]. This clinical trial is currently advancing to a phase II clinical trial. It will be interesting to see whether Dox cardiotoxicity would be mitigated by co-administration of a proteasome inhibitor. Given that the sub-chronic and delayed cardiotoxicity may not be discernible until months and years after Dox treatment, it will take a much longer term to assess the potential beneficial effects on delayed cardiotoxicity, compared with assessing the anti-neoplastic benefits.
Acknowledgments
Dr. X. Wang is an Established Investigator of American Heart Association (AHA). Research in Dr. Wang's laboratory is in part supported by grants R01HL072166, R01HL085629, and R01HL068936 from the National Heart, Lung, and Blood Institute/NIH, grant 0740025N from AHA (to X. W.), and the Physician Scientist Program of University of South Dakota.
References and Recommended Reading
Papers of particular interest, published in the last three years, have been highlighted as:
• Of importance
•• Of major importance
- 1.Wang X, Su H, Ranek MJ. Protein quality control and degradation in cardiomyocytes. J Mol Cell Cardiol. 2008;45:11–27. doi: 10.1016/j.yjmcc.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tu D, Li W, Ye Y, Brunger AT. Inaugural Article: Structure and function of the yeast U-box-containing ubiquitin ligase Ufd2p. Proc Natl Acad Sci U S A. 2007;104:15599–15606. doi: 10.1073/pnas.0701369104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–1328. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- 4.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 5.Powell SR. The ubiquitin-proteasome system in cardiac physiology and pathology. Am J Physiol Heart Circ Physiol. 2006;291:H1–H19. doi: 10.1152/ajpheart.00062.2006. [DOI] [PubMed] [Google Scholar]
- 6.Patterson C, Ike C, Willis PWt, et al. The bitter end: the ubiquitin-proteasome system and cardiac dysfunction. Circulation. 2007;115:1456–1463. doi: 10.1161/CIRCULATIONAHA.106.649863. [DOI] [PubMed] [Google Scholar]
- 7.Minotti G, Menna P, Salvatorelli E, et al. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 8.Simunek T, Sterba M, Popelova O, et al. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61:154–171. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 9••.Kumarapeli AR, Horak KM, Glasford JW, et al. A novel transgenic mouse model reveals deregulation of the ubiquitin-proteasome system in the heart by doxorubicin. FASEB J. 2005;19:2051–2053. doi: 10.1096/fj.05-3973fje. [DOI] [PubMed] [Google Scholar]; The first in vivo study demonstrates that doxorubicin treatment increases UPS-mediated degradation of a surrogate full length protein substrate in mouse hearts.
- 10••.Liu J, Zheng H, Tang M, et al. A therapeutic dose of doxorubicin activates ubiquitin-proteasome system-mediated proteolysis by acting on both the ubiquitination apparatus and proteasome. Am J Physiol Heart Circ Physiol. 2008;295:H2541–2550. doi: 10.1152/ajpheart.01052.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study reveals the dose-dependent bidirectional effects of doxorubicin on the peptidase activity of purified 20S proteasomes. It also reports that doxorubicin upregulates, but proteasome inhibitors downregulates, CHIP (the COOH-terminal of heat shock protein cognate 70-interacting protein).
- 11.Kiyomiya K, Matsuo S, Kurebe M. Proteasome is a carrier to translocate doxorubicin from cytoplasm into nucleus. Life Sci. 1998;62:1853–1860. doi: 10.1016/s0024-3205(98)00151-9. [DOI] [PubMed] [Google Scholar]
- 12.Razeghi P, Baskin KK, Sharma S, et al. Atrophy, hypertrophy, and hypoxemia induce transcriptional regulators of the ubiquitin proteasome system in the rat heart. Biochem Biophys Res Commun. 2006;342:361–364. doi: 10.1016/j.bbrc.2006.01.163. [DOI] [PubMed] [Google Scholar]
- 13.Razeghi P, Taegtmeyer H. Hypertrophy and atrophy of the heart: the other side of remodeling. Ann N Y Acad Sci. 2006;1080:110–119. doi: 10.1196/annals.1380.011. [DOI] [PubMed] [Google Scholar]
- 14.Qian SB, McDonough H, Boellmann F, et al. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006;440:551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes MD, Lecker SH, Jagoe RT, et al. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stitt TN, Drujan D, Clarke BA, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 17••.Yamamoto Y, Hoshino Y, Ito T, et al. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res. 2008;79:89–96. doi: 10.1093/cvr/cvn076. [DOI] [PubMed] [Google Scholar]; The first report shows cardiac upregulation of atrogin-1, a bona fide striated muscle-specific ubiquitin E3 ligase in vivo and in vitro by doxorubicin, delineates the essential role of p38 but not JNK MAPK in this upregulation, and demonstrates the sufficiency of atrogin-1 upregulation to cause cardiomyocyte atrophy.
- 18.Fan GC, Zhou X, Wang X, et al. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–1279. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee K, Zhang J, Tao R, et al. Vincristine attenuates doxorubicin cardiotoxicity. Biochem Biophys Res Commun. 2008;373:555–560. doi: 10.1016/j.bbrc.2008.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Chen Q, Huang W, et al. Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J. 2006;20:362–364. doi: 10.1096/fj.05-4869fje. [DOI] [PubMed] [Google Scholar]
- 21.Tsimokha AS, Mittenberg AG, Kulichkova VA, et al. Changes in composition and activities of 26S proteasomes under the action of doxorubicin--apoptosis inductor of erythroleukemic K562 cells. Cell Biol Int. 2007;31:338–348. doi: 10.1016/j.cellbi.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Kiyomiya K, Satoh J, Horie H, et al. Correlation between nuclear action of anthracycline anticancer agents and their binding affinity to the proteasome. Int J Oncol. 2002;21:1081–1085. [PubMed] [Google Scholar]
- 23.Fekete MR, McBride WH, Pajonk F. Anthracyclines, proteasome activity and multi-drug-resistance. BMC Cancer. 2005;5:114. doi: 10.1186/1471-2407-5-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Ito T, Fujio Y, Takahashi K, Azuma J. Degradation of NFAT5, a transcriptional regulator of osmotic stress-related genes, is a critical event for doxorubicin-induced cytotoxicity in cardiac myocytes. J Biol Chem. 2007;282:1152–1160. doi: 10.1074/jbc.M609547200. [DOI] [PubMed] [Google Scholar]; This study demonstrates that doxorubicin enhances proteasome-mediated degradation of NFAT5, a transcription factor crucial to the expression of a series stress genes, including the taurine transporter.
- 25••.Poizat C, Puri PL, Bai Y, Kedes L. Phosphorylation-dependent degradation of p300 by doxorubicin-activated p38 mitogen-activated protein kinase in cardiac cells. Mol Cell Biol. 2005;25:2673–2687. doi: 10.1128/MCB.25.7.2673-2687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report showing that doxorubicin treatment increases the proteasome-mediated degradation of an essential transcription co-activator: p300 through activating p38 MAPK.
- 26.Lim CC, Zuppinger C, Guo X, et al. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem. 2004;279:8290–8299. doi: 10.1074/jbc.M308033200. [DOI] [PubMed] [Google Scholar]
- 27.Arola OJ, Saraste A, Pulkki K, et al. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–1792. [PubMed] [Google Scholar]
- 28.Haq S, Michael A, Andreucci M, et al. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci U S A. 2003;100:4610–4615. doi: 10.1073/pnas.0835895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalivendi SV, Konorev EA, Cunningham S, et al. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–539. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho SN. The role of NFAT5/TonEBP in establishing an optimal intracellular environment. Arch Biochem Biophys. 2003;413:151–157. doi: 10.1016/s0003-9861(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 31.Ito T, Fujio Y, Schaffer SW, Azuma J. Involvement of transcriptional factor TonEBP in the regulation of the taurine transporter in the cardiomyocyte. Adv Exp Med Biol. 2009;643:523–532. doi: 10.1007/978-0-387-75681-3_54. [DOI] [PubMed] [Google Scholar]
- 32.Poizat C, Sartorelli V, Chung G, et al. Proteasome-mediated degradation of the coactivator p300 impairs cardiac transcription. Mol Cell Biol. 2000;20:8643–8654. doi: 10.1128/mcb.20.23.8643-8654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 34.Murton AJ, Constantin D, Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim Biophys Acta. 2008;1782:730–743. doi: 10.1016/j.bbadis.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Li HH, Kedar V, Zhang C, et al. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest. 2004;114:1058–1071. doi: 10.1172/JCI22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagirand-Cantaloube J, Offner N, Csibi A, et al. The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J. 2008;27:1266–1276. doi: 10.1038/emboj.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajan V, Mitch WE. Ubiquitin, proteasomes and proteolytic mechanisms activated by kidney disease. Biochim Biophys Acta. 2008;1782:795–799. doi: 10.1016/j.bbadis.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuda N, Sasaki D, Ishiwata S, Kurihara S. Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the Frank-Starling mechanism of the heart. Circulation. 2001;104:1639–1645. doi: 10.1161/hc3901.095898. [DOI] [PubMed] [Google Scholar]
- 39.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem. 1996;271:26690–26697. doi: 10.1074/jbc.271.43.26690. [DOI] [PubMed] [Google Scholar]
- 40.Shringarpure R, Grune T, Davies KJ. Protein oxidation and 20S proteasome-dependent proteolysis in mammalian cells. Cell Mol Life Sci. 2001;58:1442–1450. doi: 10.1007/PL00000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.An J, Li P, Li J, et al. ARC is a critical cardiomyocyte survival switch in doxorubicin cardiotoxicity. J Mol Med. 2009;87:401–410. doi: 10.1007/s00109-008-0434-z. [DOI] [PubMed] [Google Scholar]; This study shows that doxorubicin enhances the proteasome-mediated degradation of ARC, an important anti-apoptotic protein in cardiomyocytes, in a dose and time dependent manner.
- 42.Li YZ, Lu DY, Tan WQ, et al. p53 initiates apoptosis by transcriptionally targeting the antiapoptotic protein ARC. Mol Cell Biol. 2008;28:564–574. doi: 10.1128/MCB.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haupt Y, Maya R, Kazaz A, Oren M. MDM2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 44.Nam YJ, Mani K, Wu L, et al. The apoptosis inhibitor ARC undergoes ubiquitin-proteasomal-mediated degradation in response to death stimuli: identification of a degradation-resistant mutant. J Biol Chem. 2007;282:5522–5528. doi: 10.1074/jbc.M609186200. [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Kotamraju S, Konorev E, et al. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q, Sanlioglu S, Li S, et al. GPx-1 gene delivery modulates NFkappaB activation following diverse environmental injuries through a specific subunit of the IKK complex. Antioxid Redox Signal. 2001;3:415–432. doi: 10.1089/15230860152409068. [DOI] [PubMed] [Google Scholar]
- 47.Xie P, Guo S, Fan Y, et al. Atrogin-1/MAFbx enhances simulated ischemia/reperfusion-induced apoptosis in cardiomyocytes through degradation of MAPK phosphatase-1 and sustained JNK activation. J Biol Chem. 2009;284:5488–5496. doi: 10.1074/jbc.M806487200. [DOI] [PubMed] [Google Scholar]
- 48.LoConte NK, Thomas JP, Alberti D, et al. A phase I pharmacodynamic trial of bortezomib in combination with doxorubicin in patients with advanced cancer. Cancer Chemother Pharmacol. 2008;63:109–115. doi: 10.1007/s00280-008-0719-5. [DOI] [PubMed] [Google Scholar]