INTRODUCTION
Prolotherapy is an injection-based complementary and alternative medical (CAM) therapy for chronic musculoskeletal pain. It has been used for for approximately 100 years, however, its modern applications can be traced to the 1950s when the prolotherapy injection protocols were formalized by George Hackett,1 a general surgeon in the U.S., based on his clinical experience of over 30 years. While prolotherapy techniques and injected solutions vary by condition, clinical severity, and practitioner preferences, a core principle is that a relatively small volume of an irritant or sclerosing solution is injected at sites on painful ligament and tendon insertions, and in adjacent joint space over the course of several treatment sessions.1, 2 Interest in prolotherapy among physicians and patients is high. It is becoming increasingly popular in the U.S. and internationally, and is actively used in clinical practice.3, 4 A 1993 survey sent to osteopathic physicians estimated that 95 practitioners in the US were estimated to have performed prolotherapy on approximately 450,000 patients. However, only 27% of surveys were returned, likely dramatically underestimated true number of practitioners. 5 No formal survey has been done since 1993. The current number of practitioners actively practicing prolotherapy is not known but is likely several thousand in the US based on attendance at CME conferences and physician listings on relevant websites. Prolotherapy has been assessed as a treatment for a wide variety of painful chronic musculoskelatal conditions which are refractory to “standard of care” therapies. While anecdotal clinical success guides the use of prolotherapy for many conditions, clinical trial literature supporting evidence-based decision-making for the use of prolotherapy exists for low back pain, several tendinopathies and osteoarthritis.
The name of prolotherapy has changed over time. Consistent with existing hypotheses and understanding of possible mechanisms of action, the name of this therapy has evolved. Nomenclature has reflected practitioners’ perceptions of prolotherapy’s therapeutic effects on tissue. Historically, this injection therapy was called “sclerotherapy” because early solutions were thought to be scar-forming. “Prolotherapy” is currently the most commonly used name, and is based on the presumed “proliferative” effects on chronically injured tissue. It has also been called “regenerative injection therapy” (“RIT”), 2, 6 and some contemporary authors name the therapy according to the injected solution.7 The precise mechanism of action is not known.
The National Institute of Health identifies prolotherapy as a CAM therapy and has funded two ongoing clinical prolotherapy trials. The Centers for Medicare and Medicaid Services and the Veteran’s Administration have reviewed the prolotherapy literature for low back pain and all musculoskeletal indications, respectively, and determined existing evidence to be inconclusive. Neither recommends third party compensation for prolotherapy. However, neither included the most recent clinically positive studies or reviews in their review.7–9 Private insurers are beginning to cover prolotherapy for selected indications and clinical circumstances; however, most patients pay “out-of-pocket”.
PROLOTHERAPY TECHNIQUE
While no formal practice guidelines have been published, prolotherapy treatment commonly consists of several injection sessions delivered every 2 to 6 weeks over the course of several months. During an individual prolotherapy session, therapeutic solutions are injected at sites of painful and tender ligament and tendon insertions, and in adjacent joint spaces. Injected solutions (“proliferants”) have historically been hypothesized to cause local irritation, with subsequent inflammation and tissue healing, resulting in enlargement and strengthening of damaged ligamentous, tendon and intra-articular structures.10, 11 These processes were thought to improve joint stability, biomechanics, function and ultimately, to decrease pain.1, 2
MECHANISM OF ACTION
The mechanism of action for prolotherapy has not been clearly established and, until recently, received little attention. Supported by pilot-level evidence, the three most commonly used prolotherapy solutions have been hypothesized to act via different pathways: hypertonic dextrose by osmotic rupture of local cells, phenol-glycerine-glucose (P2G) by local cellular irritation, and morrhuate sodium by chemotactic attraction of inflammatory mediators12 and sclerosing of pathologic neovascularity associated with tendinopathy.13, 14 The potential of prolotherapy to stimulate release of growth factors favoring soft tissue healing has also been suggested as a possible mechanism.15, 16
In vitro and animal model data have not fully corroborated these hypotheses. An inflammatory response in a rat knee ligament model has been reported for each solution, though it was not significantly different from that caused by needle stick alone or saline injections.17 However, animal model data do suggest a significant biological effect of morrhuate sodium and dextrose solutions compared to controls. Rabbit medial collateral ligaments injected with morrhuate sodium were significantly stronger (31%), larger (47%), and thicker (28%), and had a larger collagen fiber diameter (56%) than saline-injected controls;10 increase in cell number, water content, ground substance amount and a variety of inflammatory cell types were hypothesized to account for these changes.11 Rat patellar tendons injected with morrhuate sodium were able to withstand a mean maximal load of 136% (± 28%) – significantly more than the uninjected control tendon.18 Interestingly, in the same study, tendons injected with saline control solution were significantly weaker than uninjected controls.18 Dextrose has been minimally assessed in animal models. Recent studies showed that injured medial collateral rat ligaments injected with 15% dextrose had a significantly larger cross-sectional area compared to both non-injured and injured saline-injected controls.19 P2G solution has received the least research attention; although it is in active clinical use, no animal or in vitro study has assessed P2G effect using an injury model. Clinically, most clinicians report using these solutions as single agents, though concentration varies. In clinical practice, physicians sometimes mix prolotherapy solutions, or use solutions serially in a single injection session depending on experience and local practice patterns. Neither effect of varied concentration nor mixtures have been assessed in basic science nor clinical studies and no clinical trial has compared different solutions against one another.
CLINICAL EVIDENCE
Early Research
Since its inception, prolotherapy has been primarily utilized outside of academic centers. This has lead to a pragmatic orientation of existing prolotherapy studies, and a relative paucity of major rigorous clinical trials in spite of significant clinical activity. While the first randomized controlled trial (RCT) did not appear until 1987, clinicians have enthusiastically reported the results of more modest, pilot-level clinical trials.
A 2005 systematic review of prolotherapy for all indications found 42 published reports of clincal prolotherapy trials since 1937.20 Thirty-six of the studies were case reports and case series that included 3928 patients aged from 12 to 88 years. These uncontrolled studies provide the earliest and most clinically-oriented evidence for prolotherapy. Each study reported positive findings for patients with chronic, painful, refractory conditions. Report quality of the included studies varied widely; their internal methodological strength was generally consistent with publication date. The older case studies documented injectants and methods that are no longer in use. Contemporary solutions were noted to start with P2G in the 1960s, dextrose in the 1980s, and morrhuate sodium in the early 1990s. The case reports and case series highlighted the fact that, over time, prolotherapy has been used and studied for a continually growing set of clinical indications. These case studies have also been used as pilots to develop new assessment techniques that could help elucidate pathophysiology of the condition in question 7, and test methodology for future, more robust randomized trials.21 In general, while lacking control groups and randomization, these pragmatic studies22 had the advantage of assessing effectiveness of prolotherapy in “real life settings” that patients encounter, including the prolotherapist’s ability to select the patient and to individually tailor the injection protocol. Most of the subjects (72%, 2691/3741) assessed in the early literature were treated for low back pain. However, other indications assessed by these early studies included knee osteoarthritis, shoulder dislocation, neck strain, costochondritis, lateral epicondylosis and fibromyalgia.
Contemporary Research
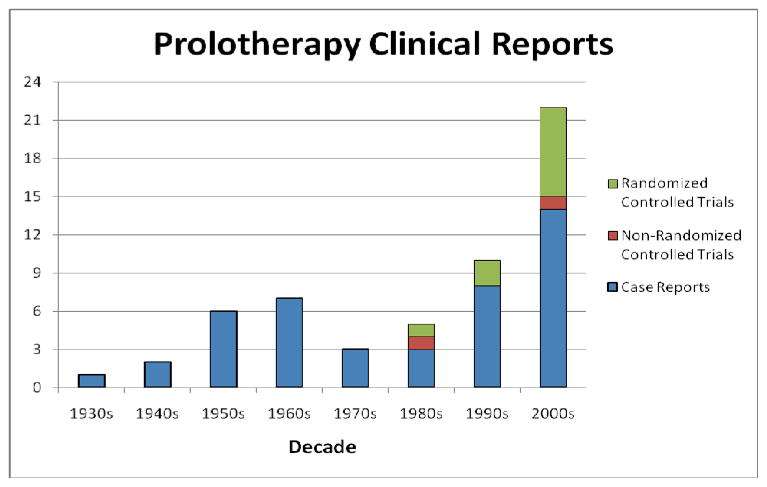
Since the mid 1980s, research on prolotherapy effects has accelerated and the number and methodological quality of studies assessing prolotherapy have increased dramatically (Figure 1).
Figure 1.
Number of published clinical studies on prolotherapy since 1937.
To date, prolotherapy has been best assessed as a treatment for low back pain, osteoarthritis and tendinopathy, each of which is a significant cause of pain and disability, and is often refractory to best standard-of-care therapies. The severity and prevalence of each condition is age-related. Because the U.S. population is aging, finding new effective therapies for these conditions can have an impact on both individual patient care and overall public health. In addition, prolotherapy has been assessed as a treatment for non-specific, non-surgical low back pain, osteoarthritis of the knee and hand, and for several tendinopathies, including lateral epicondylosis, Achilles, adductor and plantar fasciitis. The following section gives a brief description of studies assessing prolotherapy for these clinical indications, and level of evidence associated with each condition; this information is additionally summarized in Table 1.
Table 1.
Strength of evidence for prolotherapy as a treatment for chronic musculoskeletal conditions: Low Back Pain (LBP), Osteoarthritis (OA) and Tendinopathy.
| Key Clinical Recommendation on Prolotherapy | Evidence Rating | Reference/s |
|---|---|---|
| Non-specific LBP: may be effective; conflicting results in several RCTs | B | 25–28 |
| Sacroiliac Joint Dysfunction: may be effective in patients with documented failure of load transfer (disability) at the sacroiliac joint | B | 34 |
| Coccygodynia: may be effective based on prospective case series | B | 35 |
| Lateral epicondylosis: likely effective based on strong positive data in these small RCTs | A | 6, 7 |
| Achilles tendinopathy: may be effective based on high quality prospective case series | B | 9 |
| Plantar fasciitis: may be effective, based on high- quality prospective case series | B | 60 |
| Osteoarthritis: may be effective for knee and ? finger OA, based RCTs of moderately strong methodological quality | B | 17, 61 |
Low Back Pain (LBP)
LBP is among the most common reason patients see a primary care provider. Approximately 80% of Americans experience LBP during their lifetime. An estimated 15–20% of patients develop protracted pain, and approximately 2–8% experience chronic pain. LBP is second only to the common cold as a cause of lost work time. Productivity losses from chronic LBP approach $28 billion annually in the U.S.23
Non-specific LBP
Four RCTs evaluated prolotherapy for musculoskeletal LBP; three used P2G as the injectant24–26 and the fourth used Dextrose.27 Each study used a protocol involving injections to the ligamentous insertions of the L4-S1 spinous processes, sacrum and ilium. While outcome measures varied, a common measure was the percentage number of participants reporting greater than 50% improvement in pain/disability scores at six months.
Two of these four RCTs reported positive findings compared to control injections. Ongley et al.24 and Klein et al.25 compared the treatment effects of prolotherapy combined with an adjacent treatment with injected steroids, spinal manipulation and exercise. In the Ongley study,24 the intervention and control groups differed markedly on the make-up of initial injections and type of spinal manipulation associated with the injections. Significantly more subjects in the prolotherapy (88%) group reported at least 50% reduction in pain severity compared to controls (39%). Also, prolotherapy subjects, compared to controls, reported significantly decreased pain and disability levels.24 Klein et al.25 used more similar treatment protocols in the two assessed groups, with subjects in both groups receiving steroid injections and spinal manipulation prior to prolotherapy. Again, significantly more prolotherapy subjects improved by 50% or more on pain or disability scores (77%) than controls (53%). Pain grid scores were also significantly lower in the prolotherapy group, with individual pain (p=0.06) and disability (p=0.07) scores trending toward significance, compared to the control group.
Two of the four RCTs reported negative outcomes compared to control injections.26 27 Dechow et al.26 implemented a refined study protocol; subjects in both groups underwent three injection therapy sessions without adjacent spinal manipulation or physical therapy. While both groups showed a trend toward improved severity scores on pain questionnaire, pain grid, and somatic perception measures, these changes did not reach statistical significance over time, within or between groups. At 6 months, improvements in both groups were smaller than those of the other RCTs. The largest and most methodologically rigorous prolotherapy study published to date has been conducted by Yelland et al.27 Study subjects (N=110), with an average of 14 years of LBP, were randomized to one of four intervention groups: dextrose and physical therapy, dextrose and “normal activity”, saline injections (“control” injection) and physical therapy, or saline injections and “normal activity”. By 12 months, subjects in all groups reported improved pain (26%–44%) and disability (30%–44%) scores, without significant differences between groups. The majority of subjects (55%) stated that their improvement in regards to both pain and disability had been worth the effort of undergoing the intervention. The percentage of subjects who reached at least 50% pain reduction varied between 36% and 46% though these differences were not statistically significant.
Overall, interpretation of findings from these 4 RCTs is challenging. Both experimental and control groups received different treatment protocols, and none of the trials was designed to elicit a possible mechanism of prolotherapy action. Therefore, it is impossible to attribute effects to prolotherapy or any other specific intervention. A recent Cochrane Collaboration systematic review28 did not find sufficient evidence to recommend prolotherapy for non-specific LBP. However, these four RCTs present overall promising results, calling for well-designed, sufficiently-powered research. All RCTs report improvements for pain and disability in all treatment groups consisting of subjects with chronic, moderate-to-severe LBP. In particular, Yelland et al.27 reported clinical improvement in excess of minimal clinical important difference,29–31 and in excess of subjects’ own perception of the minimum improvement necessary for prolotherapy to be worthwhile (25% for pain and 35% for disability).21, 27
LBP due to Specific Causes
Prolotherapy research methods for LBP have been evolving amid much debate surrounding effectiveness, indications, treatment protocols and solution types.32, 33 Given the promising aspects of the above RCTs for non-specific LBP, combined with anecdotal clinical success, recent clinical researchers have begun to assess prolotherapy in patients with more specific forms LBP and loss of function in an effort to determine specific causes of LBP for which prolotherapy may be most effective.
Cusi et al. assessed 25 subjects with sacroiliac joint dysfunction and pain, refractory to 6 months or more of physical therapy, and with documented failure of load transfer (disability) at the sacroiliac joint.34 They used a strong prolotherapy solution of 18% dextrose, delivered in 3 sets of injections over 12 weeks. Compared to baseline, pain and disability scores on 3 multidimensional outcome measures significantly improved at 26 month follow-up in excess of minimal clinically important difference.
Khan et al. assessed 37 subjects with refractory coccygodynia.35 Using 25% dextrose in up to 3 prolotherapy injection sessions over 2 months, average pain scores, evaluated using a 0–10 visual analog scale (VAS), significantly decreased from a baseline score of 8.5 to 2.5 points at 2 months, far in excess of reported minimal clinical important difference for chronic pain.36 The authors reported “good” pain relief for 30 of 37 subjects, and “no improvement” for the remaining 7.
In an especially novel study, Miller et al. assessed prolotherapy for leg pain due to moderate-to-severe degenerative disc disease as determined by CT spell out discography.37 Subjects (N=76) who failed physical therapy and had substantial but temporary pain relief with two fluoroscopically-guided epidural steroid injections were included. After an average of 3.5 sessions of biweekly, fluoroscopically-guided injections to the relevant disc space with 25% dextrose with bupivacaine, 43% of responders showed a significant, sustained treatment response of 71% improvement in pain score, with VAS score for responders at 8.9 (± 1.4), 2.5 (±2.0), and 2.6 (± 2.2) at baseline, 2 and 18 months, respectively. While these three recent studies of prolotherapy for “specific” LBP were uncontrolled, they suggest the need for future RCTs with more focused clinical indications of axial pain and disability.
Tendinopathies
The strongest data supporting the efficacy of prolotherapy for any musculoskeletal condition, compared to control injections, is for chronic, painful overuse tendon conditions that were formerly called “tendonitis” and are now more correctly termed “tendinosis” or “tendinopathy” to reflect existing, underlying pathophysiology.38 Tendinopathies are common reasons why patients present to primary care providers and various medical specialists.39, 40 Tendinopathies are sometimes discussed as a group because the current understanding of over-use tendinopathies identifies them as sharing underlying non-inflammatory pathology, resulting from a repetitive motion or overuse injury, and associated with painful degenerative tissue. Histopathology of tendon biopsies in patients undergoing surgery for painful tendinopathy reveals collagen separation,41 thin, frayed, and fragiletendon fibrils, separated from each other lengthwise and disruptedin cross-section, increase in tenocytes with my of ibroblastic differentiation (tendon repair cells), proteoglycan ground substance and neovascularization. Classic inflammatory cells are usually absent.41 Although this aspect of tendinosis was first described 25 years ago42 and content experts have advocated a change in nomenclature (from “tendonitis” to tendinosis),38 the misnomer and use of the term “tendonitis” continues.43 Prolotherapy has been assessed as a treatment for four tendon disorders: lateral epicondylosis, hip adductor, Achilles tendinopathies and plantar fasciitis.
Lateral epicondylosis (LE, “tennis elbow”) is an important common condition of the upper extremity with an incidence of 4–7/1000 patients per year in primary care settings.44–46 Its greatest impact is on workers with repetitive and high-load upper extremity tasks and on athletes. Its most common cause may be low-load, high-repetition activities such as keyboarding, though formal data is lacking.47 Cost and time away from job or activity are significant.48, 49 While many non-surgical therapies have been tested for LE refractory to conservative measures, none have shown to be uniformly effective in the long term.50–52 Scarpone et al. conducted an RCT to determine whether prolotherapy improves self-reported elbow pain, and objectively measured grip strength and extension strength in patients with chronic LE. Twenty adults with at least 6 months of moderately-to-severely painful LE refractory to rest, non-steroidal anti-inflammatory medications (NSAIDs) and corticosteroid injections, were randomized to prolotherapy with dextrose and morrhuate sodium (1 part 5% sodium morrhuate, 1.5 parts 50% dextrose, 0.5 parts 4% lidocaine, 0.5 parts 0.5% sensorcaine and 3.5 parts normal saline)or control injections with normal saline. Three prolotherapy sessions were administered, with injection at the supracondylar ridge, lateral epicondyle and annular ligament. Compared to controls, prolotherapy subjects reported significantly decreased pain scores at 8 and 16 weeks. These between-group differences in pain scores were associated with a significant improvement in prolotherapy subjects (from 5.1±0.8 at baseline, down to 0.5±0.4 at 16 weeks), while the controls did not report significant change (4.5±1.7 to 3.5±1.5). In addition to pain reduction, prolotherapy subjects also showed significantly improved isometric strength compared to controls, and grip strength compared to baseline. These clinical improvements seen in prolotherapy subjects were maintained at 52 weeks.
Achilles tendinopathy is a common overuse injury seen both in athletes and in the general population. This painful condition is a cause of considerable distress and disability.53 Maxwell et al. conducted a well-designed case series to assess whether prolotherapy, administered during a mean of 4 injection sessions, at 6 week intervals, would decrease pain in 36 adults with painful Achilles tendinopathy. In this study, 25% dextrose solution was injected into hypoechoic regions of the Achilles tendon under ultrasound guidance. In addition to self-reported measures, the authors also assessed ultrasound-based tendon thickness, and the degree of hypoechogenicity and neovascularity - ultrasound findings recently reported to correlate to tendinopathy severity.54, 55 At 52 weeks, prolotherapy-treated subjects reported decrease in VAS-assessed pain severity by 88%, 84% and 78% during rest, “usual” activity or sport, respectively. In addition, tendon thickness decreased significantly. The overall grade of tendon pathology, hypoechoic and anechoic tendon regions, and neovascularity were all improved in some, but not all subjects who reported clinical improvement. Therefore, the relationship between ultrasound-assessed characteristics and the degree of clinical improvement remains unclear.
Hip adductor tendinopathy, associated with groin pain, is a common problem among those who engage in kicking sports.56 Topol et al. conducted a case series assessing prolotherapy for chronic groin pain, a condition involving pain and tenderness at tendon and ligament insertions at the groin area.57 Male athletes (N=24), with an average duration of 15.5 months of groin pain in spite of standard therapy, were injected with 12.5% dextrose at the thigh and suprapubic abdominal insertions of the adductor tendon, and at the symphysis pubis at four-week intervals until pain resolved or subjects had no improvement for two consecutive sessions. On average, subjects received 3 prolotherapy sessions. At a mean of 17 months, subjects reported dramatic significant improvements on two pain scales (VAS and the Nirschl Pain Phase Scale). Of 24 subjects, 20 had no pain and 22 returned to sports without restrictions after therapy.
Plantar fasciitis is a common injury among athletes engaged in sports requiring running and among general primary care patients. It is reported to account for 15% of all adult foot complaints requiring professional consultation, and, in a 2002 survey of running-related injuries, plantar fasciitis was the third most prevalent injury.58, 59 Among “standard of care” approaches, there is limited evidence for the effectiveness of any one treatment for plantar fasciitis, including steroid injections.60 Ryan et al. assessed prolotherapy for chronic plantar fasciitis refractory to conservative care.61 Twenty adults with an average of 21 month duration of heal pain underwent ultrasound-guided 25% dextrose injections for an average of 3 treatment sessions delivered at 6 week intervals. Pain scores were assessed, using a 100-point VAS, at baseline and at 11.8 months. Pain severity significantly improved at rest, during activities of daily living and sport activities by 26.5, 49.7 and 56.5 points, respectively, compared to baseline, and 16 of 20 subjects reported good or excellent treatment effects.
Osteoarthritis (OA)
Prolotherapy has been assessed as a treatment for knee and finger osteoarthritis16, 62 and is the subject of ongoing studies.63 Arthritis is a leading cause of disability in the world and in the US, where it affects 43 million persons.64–66 OA is the most common form of arthritis and the most common joint disorder.67 In the US, symptomatic knee OA is present in up to 6% of the population over 30 years old,67 and has an overall incidence of 360,000 cases per year.68 Incidence increases up to 10-fold from ages 30 to 65 and more thereafter.69 OA results in a high burden of disease and substantial economic impact through its high prevalence, time lost off work, and frequent utilization of health care resources.64, 70
Allopathic and CAM treatment recommendations for OA, aimed at correcting modifiable risk factors, symptom control and disease modification, have been published.71, 72 While these modalities may help some patients, none has proven to provide definitive pain control or disease modification for patients with knee OA. The Agency for Research Health and Quality (AHRC) has recently evaluated the most common standard treatment options including glucosamine, chondroitin, visco supplementation and arthroscopic debridement.73 These have not shown to be effective compared to placebo. The high burden of knee OA and the absence of cure continue to stimulate intense search for new agents to modify disease and control symptoms.
Reeves et al. assessed prolotherapy as a treatment for knee and finger OA.16, 62 Subjects with finger or knee pain and radiological evidence of OA were randomly assigned to receive 3 injection sessions of either prolotherapy with 10% dextrose and lidocaine, or lidocaine and bacteriostatic water (control group). In the finger OA trial, intervention subjects significantly improved in ‘pain with movement’ and ‘flexion range’ scores compared to controls; pain scores at rest and with grip showed a tendency to improvement without reaching statistical significance. In the knee OA trial, subjects in both groups reported significant improvements in pain and swelling scores, number of buckling episodes, and flexion range of motion compared to baseline, but without statistically significant differences between the groups. Surprising and potentially important 12-month follow-up in both studies included improved radiological features of OA on plain x-ray films: authors reported decreased joint space narrowing and osteophyte grade in the finger study, and increased patellofemoral cartilage thickness in the knee study. These radiological findings may suggest disease modification properties of prolotherapy. Whether or not subjects in the knee study had a baseline concomitant meniscal pathology was not reported or included in entry criteria. Furthermore, the ability of plain x-ray to quantify patellofemoral cartilage thickness is questionable, limiting impact of these findings.
CONTRAINDICATIONS, SIDE EFFECTS AND ADVERSE EVENTS
Contraindications
Absolute contraindications to prolotherapy are few and include acute infections such as cellulitis, local abscess or septic arthritis. Relative contraindications include acute gouty arthritis and acute fracture.
Common side-effects
The main risk of prolotherapy is pain and mild bleeding as a result of needle trauma. Patients frequently report pain, a sense of fullness and occasional numbness at the injection site at the time of injections. These side effects are typically self-limited. A post-injection pain flare during the first 72 hours after the injections is common clinically but its incidence has not been well documented. An ongoing study of prolotherapy for knee OA pain has noted that 10–20% of subjects experience such flares.74 Pain flares are likewise typically self-limited, and usually respond well to acetaminophen (500–650 mg every four hours as needed). On rare occasions, the occurrence of strong, post-injection pain may require treatment with narcotic medication. Non-steroidal anti-inflammatory agents are not routinely used after the procedure, but may be indicated if the pain does not resolve with other measures. Most patients with pain flares experience diminution of pain in 5–7 days after injections; regular activities can be resumed at this time.
Adverse events
While prolotherapy performed by an experienced injector appears safe, the injection of ligaments, tendons and joints with irritant solutions raises safety concerns. Theoretical risks of prolotherapy injections include lightheadedness, allergic reaction, infection or neurological (nerve) damage. Injections should be performed using universal precautions and the patient should be prone if possible. Dextrose is extremely safe; it is FDA approved for intravenous treatment of hypogylcemia and for caloric supplementation.75 As of 1998, FDA records for intravenous 25% dextrose solution reported no adverse events to Abbott Labs in 60 years.76 Morrhuate sodium is a vascular sclerosant, used in gastrointestinal procedures and vein sclerosing. Allergic reactions to morrhuate sodium are rare. Although P2G is not FDA approved for any indication, it has not been reported in clinical trials to cause significant side effects or adverse events.
Historically, a small number of significant, prolotherapy-related complications have been reported. They were associated with perispinal injections for back or neck pain, using very concentrated solutions, and included 5 cases of neurological impairment from spinal cord irritation77–79 and 1 death in 1959 following prolotherapy with zinc sulfate for low back pain.77 Neither zinc sulfate nor concentrated prolotherapy solutions are currently in general use. In a survey of 95 clinicians using prolotherapy, there were 29 reports of pneumothoraces after prolotherapy for back and neck pain, two of which required hospitalization for a chest tube, and 14 cases of allergic reactions, although none classified as serious.5 A more recent survey of practicing prolotherapists yielded similar results for spinal prolotherapy: spinal headache, pneumothoraces, nerve damage and non-severe spinal cord insult and disc injury were reported.80 The authors concluded these events were no more common in prolotherapy than for other spinal injection procedures. No serious side effects or adverse events were reported for prolotherapy when used for peripheral joint indications.
Practical Prolotherapy
Incorporating Prolotherapy Into Practice
Similar to corticosteroid injections, prolotherapy is an unregulated procedure without certification by any governing body. Formal training is not provided by most medical schools, residencies and fellowships. However, prolotherapy, to be performed appropriately and safely, requires specialized training. In the U.S., it is taught to physicians and other health care providers (authorized to deliver joint-type injections) in semi-formal workshops and formal continuing medical education (CME) by several organizations, including university settings (Table 2).
Table 2.
Educational and Informational Prolotherapy Resources
| Name/URL | Comments |
|---|---|
| “The Anatomy, Diagnosis, and Treatment of Chronic Myofascial Pain with Prolotherapy” http://www.ocpd.wisc.edu/Course_Catalog/ |
Continuing medical education (CME) on the basics of prolotherapy. This 3.5 day conference is offered through the University of Wisconsin School of Medicine and Public Health. All aspects of clinical and research aspects of prolotherapy are covered. |
| Hackett-Hemwall Foundation List of Prolotherapists http://www.hacketthemwall.org/HHF/List_of_Prolotherapists.html |
The Hackett-Hemwall Foundation is a non-profit medical foundation whose mission is to provide high-quality treatment of musculoskeletal problems to underserved people around the world. Physicians listed on the site have completed the Foundation’s high-volume continuing medical education experience in prolotherapy. |
| Commercial Prolotherapy Physician Listing http://www.getprolo.com |
This site lists physicians by state who perform prolotherapy. It includes contact information and a short biography and prolotherapy credentials. Physicians pay to list themselves on this site. |
| American Association of Orthopaedic Medicine http://www.aaomed.org |
The American Association of Orthopaedic Medicine is a non-profit organization which provides information and educational programs on comprehensive nonsurgical musculoskeletal treatment including prolotherapy. This searchable site lists AAOM members who perform prolotherapy. |
Patients and physicians who desire consultation for prolotherapy may have difficulty finding an appropriate consulting prolotherapist. Online resources (Table 2) are available that can help locate a prolotherapist, though information is limited by lack of a credentialing structure and governing body for prolotherapy.
Despite limited institutional support, interest in prolotherapy is increasing, and it is performed in increasing numbers, primarily in two settings. For several decades, prolotherapy has been mostly performed outside of mainstream medicine by independent physicians. More recently, multi-specialty groups that include family or sports medicine physicians, physiatrists, orthopedic surgeons, neurologists or anesthesiologists have been incorporating prolotherapy as a result of positive clinical experience and research reports. Prolotherapy is one of several injection therapies that may promote healing of chronically injured soft tissue. Other therapies receiving active clinical and research attention for chronic musculoskeletal pain include whole blood, platelet rich plasma and polidocanol injections.9 In both settings, prolotherapy is viewed as a valued procedure, primarily reserved for patients who have failed other treatments or in patients who are not surgical candidates.
The authors’ clinic
The authors practice in a community in which several primary care physicians and specialists perform prolotherapy; receptivity to prolotherapy in our setting is growing. Some health insurance plans in our area cover prolotherapy for the indications discussed. Referrals can be made similar to those for more conventional procedures. An initial consultation, including a complete history and physical, is performed by the prolotherapist to determine if the patient is a candidate for prolotherapy. If so, side effects, adverse events and expected course of injections are explained, and the patient is asked to sign a procedure consent form. Information is also provided to patients in written form. (Table 3) The patient is then scheduled for up to three outpatient prolotherapy sessions, typically four- six weeks apart. At each subsequent visit, an interval history is obtained and physical exam is performed. If the patient does not report improvement after three prolotherapy sessions, alternative interventions are pursued.
Table 3.
Prolotherapy at a glance
| What is prolotherapy? | Prolotherapy is an injection-based complementary and alternative medical (CAM) therapy for chronic musculoskeletal pain. This treatment aims to stimulate a natural healing response at the site of painful soft tissue and joints. |
| What is involved? | Prolotherapy treatment typically involves getting a series of 2–5 monthly injections of a topical anesthetic and a solution of other medicines directly on sore tendon or ligaments, or into painful joints. |
| What conditions is it used for, and is it effective? | Prolotherapy is generally used for musculoskeletal pain of greater than 3 months. Conditions that have responded well to prolotherapy in published studies include tennis elbow, Achilles tendinopathy, and other overuse injuries involving tendons. Prolotherapy is also likely effective for knee osteoarthritis and low back pain, though studies assessing these conditions are less conclusive. |
| Is it safe? | Studies indicate that prolotherapy is safe when performed by an experienced practitioner. It does not appear to have a greater risk than other injection techniques, such as steroid injections. |
| Does it hurt? | No one loves getting a shot, though prolotherapy injections typically hurt less than most immunizations. Most patients tolerate prolotherapy injections related pain quite well with only topical and conservative measures. Physicians can pre- treat with a pain reliever if necessary. |
Clinical Recommendations
Present data suggest that prolotherapy is likely an effective therapy for painful overuse tendinopathy. Specifically, Scarpone et al.8 provides level A evidence for prolotherapy as an effective therapy for lateral epicondylosis. Subjects with refractory lateral epicondylosis and treated with prolotherapy reported significant reduction in pain and improved isometric strength compared to those who received control injections. These findings are supported by the Maxwell,7 Topol57 and Ryan61 studies that report strong case series results for Achilles, hip adductor and plantar fasciitis, respectively and provide level B evidence for these conditions. Given that the underlying mechanism of injury and pathophysiologic effects are similar for tendinopathies, prolotherapy is a reasonable option for these conditions as well. Randomized controlled trials for all three tendinopathies and for other tendinopathies are indicated.
Recommendations are more difficult to make for osteoarthritis and low back pain, both of which are associated with more complex anatomy and less clear pathophysiology than that seen in tendinopathies. Side effect and potential adverse events of prolotherapy are likely to be more serious when performed for spinal or intra-articular indications and must be weighed against the potential for improvement. Existing studies provide level B evidence that prolotherapy is effective for non-specific low back pain compared to a patient’s baseline condition. Given that subjects with refractory, disabling low back pain significantly improved compared to their own baseline status in the Yelland study,27 patients may reasonably try prolotherapy when performed by an experienced injector. Future studies with more focused inclusion criteria may help determine which specific low back pathologies respond to prolotherapy. Existing studies provide level B evidence that prolotherapy is effective for knee and finger osteoarthritis compared to control injections.16, 62 Prolotherapy by an experienced physician is a treatment modality worth of consideration by primary care physicians for these conditions, especially when they are refractory to more conventional therapy.
Acknowledgments
Jeffrey Patterson, DO
Grant Support: None
Contributor Information
David Rabago, Email: David.rabago@fammed.wisc.edu, University of Wisconsin School of Medicine and Public Health, Department of Family Medicine, 777 S. Mills St., Madison WI, 53715, Ph 608-265-2487, Fax 608-263-5813.
Andrew Slattengren, University of Wisconsin School of Medicine and Public Health, Department of Family Medicine, 777 S. Mills St., Madison WI, 53715.
Aleksandra Zgierska, University of Wisconsin-Madison, Department of Family Medicine, 777 S. Mills St., Madison WI, 53715.
References
- 1.Hackett GS, Hemwall GA, Montgomery GA. Ligament and tendon relaxation treated by prolotherapy. 5. Oak Park: Gustav A. Hemwall; 1993. [Google Scholar]
- 2.Linetsky FS, FRafael M, Saberski L. Pain management with regenerative injection therapy (RIT) In: Weiner RS, editor. Pain Management. Boca Raton: CRC Press; 2002. pp. 381–402. [Google Scholar]
- 3.Matthews JH. Nonsurgical treatment of pain in lumbar spinal stenosis: Letter to the editor. Am Fam Physician. 1999;59(2):280–284. [PubMed] [Google Scholar]
- 4.Schnirring L. Are your patients asking about prolotherapy? Physician Sportsmed. 2000 August;28(8):15–17. [Google Scholar]
- 5.Dorman TA. Prolotherapy: A survey. The Journal of Orthopaedic Medicine. 1993;15(2):49–50. [Google Scholar]
- 6.Linetsky FS, Botwin K, Gorfin L, Jay GW. Regeneration injection therapy (RIT): Effectiveness and appropriate usage. Florida Academy of Pain Medicine. 2001 http://www.gracermedicalgroup.com/resources/articles/rf_file_0025.pdf.
- 7.Maxwell NJ, Ryan MB, Taunton JE, Gillies JH, Wong AD. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roent. 2007;189(4):W215–220. doi: 10.2214/AJR.06.1158. [DOI] [PubMed] [Google Scholar]
- 8.Scarpone M, Rabago D, Zgierska A, Arbogest J, Snell ED. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clinical J Sports Med. 2008;18:248–254. doi: 10.1097/JSM.0b013e318170fc87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabago D, Best TM, Zgierska A, Zeisig E, Ryan M, Crane D. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet rich plasma. BJSM. 2009 doi: 10.1136/bjsm.2008.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu YK, Tipton CM, Matthes RD, Bedford TG, Maynard JA, Walmer HC. An in-situ study of the influence of a sclerosing solution in rabbit medial collateral ligaments and its junction strength. Connective Tissue Research. 1983;11:95–102. doi: 10.3109/03008208309004846. [DOI] [PubMed] [Google Scholar]
- 11.Maynard JA, Pedrini VA, Pedrini-Mille A, Romanus B, Ohlerking F. Morphological and biochemical effects of sodium morrhuate on tendons. Journal of Orthopaedic Research. 1985;3:236–248. doi: 10.1002/jor.1100030214. [DOI] [PubMed] [Google Scholar]
- 12.Banks A. A rationale for prolotherapy. Journal of Orthopaedic Medicine. 1991;13(3):54–59. [Google Scholar]
- 13.Hoksrud A, Ohberg L, Alfredson H, Bahr R. Ultrasound-guided sclerosis of neovessels in painful chronic patellar tendinopathy. Am J Sports Med. 2006;34:1738–1746. doi: 10.1177/0363546506289168. [DOI] [PubMed] [Google Scholar]
- 14.Zeisig E, Fahlstrom M, Ohberg LHA. A 2-year sonographic follow-up after intratendinous injection therapy in patients with tennis elbow. Br J Sports Med. 2008 doi: 10.1136/bjsm.2008.049874. [DOI] [PubMed] [Google Scholar]
- 15.Kim SR, Stitik TP, Foye PM. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: a physiatric perspective. Journal of Physical Medicine and Rehabilitation. 2004;83(5):379–389. doi: 10.1097/01.phm.0000124443.31707.74. [DOI] [PubMed] [Google Scholar]
- 16.Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health M. 2000;6(2):68–80. [PubMed] [Google Scholar]
- 17.Jensen K, Rabago D, Best TM, Patterson JJ, Vanderby R. Early inflammatory response of knee ligaments to prolotherapy in a rat model. J Orthop Res. 2008;26:816–823. doi: 10.1002/jor.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aneja A, Spero G, Weinhold P, et al. Suture plication, thermal shrinkage and sclerosing agents. American Journal of Sports Medicine. 2005;33:1729–1734. doi: 10.1177/0363546505275492. [DOI] [PubMed] [Google Scholar]
- 19.Jensen KT. PhD Dissertation: Healing response of knee ligaments to prolotherapy in a rat model. Madison: Biomedical Engineering, University of Wisconsin; 2006. [Google Scholar]
- 20.Rabago D, Best T, Beamsly M, Patterson J. A systematic review of prolotherapy for chronic musculoskeletal pain. Clinical J Sports Med. 2005;15(5):376–380. doi: 10.1097/01.jsm.0000173268.05318.a4. [DOI] [PubMed] [Google Scholar]
- 21.Yelland M, Yeo M, Schluter P. Prolotherapy injections for chronic low back pain: results of a pilot comparative study. Austrailian Musculoskeletal Medicine. 2000;5(2):20–30. [Google Scholar]
- 22.Ernst E, Pittler MH, Stevinson C, White A. Randomised clinical trials: pragmatic or fastidious? Focus on Alternative and Complementary Therapies. 2001;63(3):179–180. [Google Scholar]
- 23.Wheeler AH. Pathophysiology of Chronic Back Pain. 2007 http://www.emedicine.com/neuro/topic516.htm.
- 24.Ongley MJ, Klein RG, Dorman TA, Eek BC, Hubert LJ. A new approach to the treatment of chronic low back pain. Lancet. 1987 July 18;2:143–146. doi: 10.1016/s0140-6736(87)92340-3. [DOI] [PubMed] [Google Scholar]
- 25.Klein RG, Eek BC, DeLong WB, Mooney V. A randomized double-blind trial of dextrose-glycerine-phenol injections for chronic, low back pain. J Spinal Disord. 1993;6(1):23–33. [PubMed] [Google Scholar]
- 26.Dechow E, Davies RK, Carr AJ, Thompson PW. A randomized, double-blind, placebo-controlled trial of sclerosing injections in patients with chronic low back pain. Rheumatology. 1999;38:1255–1259. doi: 10.1093/rheumatology/38.12.1255. [DOI] [PubMed] [Google Scholar]
- 27.Yelland M, Glasziou P, Bogduk N, Schluter P, McKernon M. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized trial. Spine. 2004;29(1):9–16. doi: 10.1097/01.BRS.0000105529.07222.5B. [DOI] [PubMed] [Google Scholar]
- 28.Yelland MJ, Del Mar C, Pirozo S, Schoene ML. Prolotherapy injections for chronic low back pain: A systematic review. Spine. 2004;29:2126–2133. doi: 10.1097/01.brs.0000141188.83178.b3. [DOI] [PubMed] [Google Scholar]
- 29.Bellamy N, Carr A, Dougados M, Shea B, Wells G. Towards a definition of “difference” in osteoarthritis. J Rheumatology. 2001;28(2):427–430. [PubMed] [Google Scholar]
- 30.Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: A comparison of two techniques. J Clin Epidemiol. 1996;49:1215–1219. doi: 10.1016/s0895-4356(96)00206-5. [DOI] [PubMed] [Google Scholar]
- 31.Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. Journal of Rheumatology. 1993;20:557–560. [PubMed] [Google Scholar]
- 32.Loeser JD. Point of view. Spine. 2004;29(1):16. [Google Scholar]
- 33.Reeves KD, Klein RG, DeLong WB. Letter to the editor. Spine. 2004;29(16):1839–1840. doi: 10.1097/01.brs.0000134587.24738.68. [DOI] [PubMed] [Google Scholar]
- 34.Cusi M, Saunders J, Hungerford B, Wisbey-Roth B, Lucas P, Wilson S. The use of prolotherapy in the sacro-iliac joint. Br J Sports Med. 2008 doi: 10.1136/bjsm.2007.042044. [DOI] [PubMed] [Google Scholar]
- 35.Khan SA, Kumar A, Varshney MK, Trikha V, Yadav CS. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. 2008;16:27–29. doi: 10.1177/230949900801600107. [DOI] [PubMed] [Google Scholar]
- 36.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 37.Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121. [PubMed] [Google Scholar]
- 38.Khan KM, Cook JL, Kannus P, Maffuli N, Bonar SF. Time to abandon the ‘tendinitis’ myth. BMJ. 2002;324:626–627. doi: 10.1136/bmj.324.7338.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bongers PM. The cost of shoulder pain at work. Variation in work tasks and good job opportunities are essential for prevention. BMJ. 2001;322:64–65. doi: 10.1136/bmj.322.7278.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson JJ, Best TM. Common overuse tendon problems: a review and recommendations for treatment. Am Fam Physician. 2005;72:811–818. [PubMed] [Google Scholar]
- 41.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 42.Puddu G, Ippolito E, Postacchini FA. classification of Achilles tendon disease. Am J Sports Med. 1976;4:145–150. doi: 10.1177/036354657600400404. [DOI] [PubMed] [Google Scholar]
- 43.Johnson GW, Cadwallader K, Scheffel SB, Epperly TD. Treatment of lateral epicondylitis. Am Fam Physician. 2007;76:843–848. 849–850, 853. [PubMed] [Google Scholar]
- 44.Verhar J. Tennis elbow: anatomical, epidemiological and therapeutic aspects. Int Orthop. 1994;18:263–267. doi: 10.1007/BF00180221. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton P. The prevalence of humeral epicondylitis: a survey in general practice. J R Coll Gen Pract. 1986;36:464–465. [PMC free article] [PubMed] [Google Scholar]
- 46.Kivi P. The etiology and conservative treatment of lateral epicondylitis. Scand J Rehabil Med. 1983;15:37–41. [PubMed] [Google Scholar]
- 47.Gabel GT. Acute and chronic tendinopathies at the elbow. Curr Opin Rheumatol. 1999;11:138–148. doi: 10.1097/00002281-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Ono Y, Nakamura R, Shimaoka M, Hattori Y, Ichihara G. Epicondylitis among cooks in nursery schools. Occup Environ Med. 1998;55:172–179. doi: 10.1136/oem.55.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritz BR. Humeral epicondylitis among gas and waterworks employees. Scand J Work Environ Health. 1995;21:478–486. doi: 10.5271/sjweh.64. [DOI] [PubMed] [Google Scholar]
- 50.Buchbinder R, Green S, White M, Barnsley L, Smidt N, Assendelft WJ. Shock wave therapy for lateral elbow pain. The Cochrane Collaboration. 2005;3 doi: 10.1002/14651858.CD003524. [DOI] [PubMed] [Google Scholar]
- 51.Smidt N, van der Windt DA, Assendelft WJ, Deville WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359:657–662. doi: 10.1016/S0140-6736(02)07811-X. [DOI] [PubMed] [Google Scholar]
- 52.Struijs PA, Smidt N, Arola H, Dijk CNv, Buchbinder R, Assendelft WJ. Orthotic devices for the treatment of tennis elbow. The Cochrane Collaboration. 2005;3 doi: 10.1002/14651858.CD001821. [DOI] [PubMed] [Google Scholar]
- 53.Kvist M. Achilles tendon injuries in athletes. Sports Med. 1994;18:173–2001. doi: 10.2165/00007256-199418030-00004. [DOI] [PubMed] [Google Scholar]
- 54.Zeisig E, Ohberg L, Alfredson H. Extensor origin vascularity related to pain in patients with tennis elbow. Knee Surg Sports Traumatol Arthrosc. 2006;14:659–663. doi: 10.1007/s00167-006-0060-7. [DOI] [PubMed] [Google Scholar]
- 55.Alfredson H, Ohberg L. Sclerosing injections to areas of neovascularization reduce pain in chronic Achilles tendinopathy: a double-blind randomised trial. Knee Surg Sports Traumatol Arthrosc. 2005;13:338–344. doi: 10.1007/s00167-004-0585-6. [DOI] [PubMed] [Google Scholar]
- 56.Holmich P, Uhrskou P, Ulnits L. Effectiveness of active physical training as treatment of long-standing adductor-related groin pain in athletes: a randomized controlled trial. Lancet. 1999;353:439–443. doi: 10.1016/S0140-6736(98)03340-6. [DOI] [PubMed] [Google Scholar]
- 57.Topol GA, Reeves KD, Hassanein KM. Efficacy of dextrose prolotherapy in elite male kicking-sport athletes with groin pain. Arch Phys Rehabil. 2005;86:697–702. doi: 10.1016/j.apmr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Buchbinder R. Plantar Fasciitis. N Engl J Med. 2004;350:2159–2166. doi: 10.1056/NEJMcp032745. [DOI] [PubMed] [Google Scholar]
- 59.Taunton J, Ryan M, Clement D, McKenzie D, Lloyd-Smith D, Zumbo B. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36:95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crawford F, Thomson C. Interventions for treating plantar heel pain. Cochrane Database. 2003;3(CD000416) doi: 10.1002/14651858.CD000416. [DOI] [PubMed] [Google Scholar]
- 61.Ryan MB, Wong AD, Gillies JH, Wong J, Taunton JE. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:303–306. doi: 10.1136/bjsm.2008.050021. [DOI] [PubMed] [Google Scholar]
- 62.Reeves KD, Hassanein K. Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP,PIP, and Trapeziometacarpal) joints: evidence of clinical efficacy. J Altern Complem Med. 2000;6(4):311–320. doi: 10.1089/10755530050120673. [DOI] [PubMed] [Google Scholar]
- 63.Rabago D. The efficacy of prolotherapy in osteoarthritic knee pain. NIH-NCCAM Grant, 1K23 AT001879–01; In Progress. [Google Scholar]
- 64.Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3–6. [PubMed] [Google Scholar]
- 65.CDC. Prevalence and impact of chronic joint symptoms-seven states, 1996. MMWR. 1998;47:345–351. [PubMed] [Google Scholar]
- 66.CDC. Prevalence of disabilities and associated health conditions-United States, 1991–1992. MMWR. 1994;43:730–739. [PubMed] [Google Scholar]
- 67.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis & Rheumatism. 1998;41(8):1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 68.Wilson MG, Michet CJ, Ilstrup DM, Melton LJ. Ideopathic symptomatic osteoarthritis of the hip and knee: A population-based incidence study. Mayo Clic Proc. 1990;65:1214–1221. doi: 10.1016/s0025-6196(12)62745-1. [DOI] [PubMed] [Google Scholar]
- 69.Oliveria SA, Felson DT, Klein RA, Reed JI, Walker AM. Estrogen replacement therapy and the development of osteoarthritis. Epidemiology. 1996;7:415–419. doi: 10.1097/00001648-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Levy E, Ferme A, Perocheau D, et al. Socioeconomic costs of osteoarthritis in France. Rev Rhum. 1993;60:63S–67S. [PubMed] [Google Scholar]
- 71.Felson DT, Lawarence RC, Hochberg MC, et al. Osteoarthritis: new insights, part 2: treatment approaches. Annals of Internal Medicine. 2000;133:726–737. doi: 10.7326/0003-4819-133-9-200011070-00015. [DOI] [PubMed] [Google Scholar]
- 72.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 73.Samson DJ, Grant MD, Ratko TA, Bonnell CJ, Ziegler KM, Aronson N. Treatment of primary and secondary osteoarthritis of the knee. Agency for Healthcare Research and Quality: Evidence Report/Technology Assessment. 2007:151. [PMC free article] [PubMed] [Google Scholar]
- 74.Rabago D, Zgierska A, Mundt M, Kijowski R, DeLucia R, Longlais B. Efficacy of prolotherapy for knee osteoarthritis: Results of a prospective case series (poster presentation). North American Research Conference on Complementary and Integrative Medicine; 2009. [Google Scholar]
- 75.AbbottLabs. FDA indications for 50% dextrose. 2004 http://www.fda.gov/cder/foi/nda/98/19445-s4-s6.htm.
- 76.AbbottLabs. Approval Documentation for 25% Dextrose submitted to FDA. Abbott Laboratories; Online documentation] [Google Scholar]
- 77.Schneider RC, Williams JJ, Liss L. Fatality after injection of sclerosing agent to precipitate fibro-osseous proliferation. Journal of the American Medical Association. 1959 August 8;170(15):1768–1772. doi: 10.1001/jama.1959.03010150012003. [DOI] [PubMed] [Google Scholar]
- 78.Keplinger JE, Bucy PC. Paraplegia from treatment with sclerosing agents - report of a case. Journal of the American Medical Association. 1960;173(12):1333–1336. doi: 10.1001/jama.1960.03020300045014. [DOI] [PubMed] [Google Scholar]
- 79.Hunt WE, Baird WC. Complications following injection of sclerosing agent to precipitate fibro-osseous proliferation. Journal of Neurosurgery. 1961;18:461–465. doi: 10.3171/jns.1961.18.4.0461. [DOI] [PubMed] [Google Scholar]
- 80.Dagenais S, Ogunseitan O, Haldeman S, Wooley JR, Newcomb RL. Side effects and adverse events related to intraligamentous injection of sclerosing solutions (prolotherapy) for back and neck pain: a survey of practitioners. Arch Phys Med Rehabil. 2006;87:909–913. doi: 10.1016/j.apmr.2006.03.017. [DOI] [PubMed] [Google Scholar]