Abstract
Establishment of the maternal-fetal interface is characterized by the influx of maternal NK cells, macrophages, and T cells into the decidua. Although a great deal has been learned about the function of NK cells in the decidua, comparatively little is known of decidual T cell function. NKT cells are an unusual T cell subset capable of producing both Th1-like and Th2-like cytokines. Unlike conventional αβ T cells that recognize peptides in the context of MHC molecules, NKT cells recognize glycolipids presented by the MHC class I-like molecule, CD1d. Recent reports have demonstrated that NKT cells and CD1d are present at the maternal-fetal interface. Moreover, activation of NKT cells can have dramatic effects on pregnancy. In this article, we will review basic aspects of NKT cell biology and summarize the recent literature on NKT cells at the maternal-fetal interface.
Keywords: NKT, CD1d, pregnancy, decidua, placenta, abortion
INTRODUCTION
Successful placentation is a dynamic process that, in humans and mice, is dependent on highly invasive fetal trophoblasts migrating through the decidualized endometrium where they degrade tissue and extracellular matrix through the production of a wide variety of proteinases and collagenases. Throughout their migration through the decidua, trophoblasts are in intimate contact with maternal decidual leukocytes, which comprise up to 40% of all cells in the decidua (Trundley and Moffett 2004). The local maternal immune response is characterized largely by cells of the innate arm, such as decidual NK cells and macrophages. Because they constitute the largest proportion of decidual lymphocytes (up to 70%), decidual NK cells have been the focus of the majority of research into decidual leukocyte function. In contrast, much less is known about the functions of decidual macrophages and T cells, which constitute up to 20% and 10% of the uterine leukocyte pool, respectively.
Recently, we and others reported the presence of NKT cells in the human and mouse decidua (Boyson et al. 2002; Ito et al. 2000; Tsuda et al. 2001). This small T cell subset has been demonstrated to modulate both innate and adaptive immune responses (Kronenberg and Gapin 2002) and, although the role of these cells in the decidua is unknown, experiments in the mouse have demonstrated that NKT stimulation dramatically impacts pregnancy, resulting in rapid pregnancy loss (Ito et al. 2000). Here, we review some basic aspects of NKT cell biology, summarize the literature to date regarding NKT cells at the maternal-fetal interface and finally, propose a model of possible decidual NKT cell function.
NKT cells
NKT cells comprise a heterogeneous subset of αβ T cells characterized by the expression of both T cell and NK cell markers. Although the subset was originally identified in mice as NK1.1+ T cells, truncation of the term to NKT has resulted in a somewhat ambiguous term that encompasses a number of T cell subsets. As investigation into NKT cells has expanded across disciplines, this terminology has led to considerable confusion and has spurred attempts to develop a more standardized nomenclature (Godfrey et al. 2004)(Table I).
Table I.
Classification of NKT cells
| Type I (classical) |
Type II (non-classical) |
NKT-like | References | |||
|---|---|---|---|---|---|---|
| Mouse | Human | Mouse | Mouse | Human | ||
| TCR α | Vα14-Jα18 invariant |
Vα24-Jα18 invariant |
Diverse | Diverse | Diverse | (Cardell et al. 1995;Lantz and Bendelac 1994) |
| TCR β | Vβ8.2, Vβ7,Vβ2 |
Vβ11 | Diverse | Diverse | Diverse | (Cardell et al. 1995;Lantz and Bendelac 1994; Porcelli et al. 1996) |
|
CD1d- restricted |
Yes | Yes | Yes | No | No | (Bendelac et al. 1995;Cardell et al. 1995;Exley et al. 1997) |
|
Known Antigensa |
αGalCer iGb3, GD3 αGlcUCer αGalUCer BbGL-II |
αGalCer iGb3, GD3 αGlcUCer αGalUCer BbGL-II |
Sulfatide | ND | ND | (Jahng et al. 2004;Kawano et al. 1997;Kinjo et al. 2006; Kinjo et al. 2005; Mattner etal. 2005; Spada et al 1998; Sriram et al 2005; Wu et al. 2003;Zajonc et al. 2005;Zhou et al. 2004) |
|
Surface Markers |
NK1.1 +/−b CD4, DN |
CD161 +/− CD4, DN, CD8 |
NK1.1+/− CD4, DN |
NK1.1+ DX5+ |
CD56+ CD161+ |
(Cardell et al. 1995;Hammond et al. 2001;Jahng et al. 2004;Spada et al. 1998) |
αGalCer, αGalactosylceramide; iGb3, isoglobotrihexosylceramide; GD3, disialoganglioside GD3; GSL; glycosphingolipid; αGlcUCer, α-glucuronosylceramide; αGalUCer, α-galacturonosylceramide; DN, double negative; ND, not determined
the NK1.1 allele is expressed in only a few commonly used inbred strains such as C57BL/6, SJL, FVB/N, NZB, and NZW.
The most widely studied NKT cells are the classical NKT cells, also known as Type I, or iNKT cells. These NKT cells recognize glycolipids presented by the monomorphic class I MHC-like glycoprotein CD1d (Bendelac 1995a; Bendelac et al. 1995; Kawano et al. 1997). More specifically, classical NKT cells are characterized by their recognition of the prototypical CD1d ligand, the marine sponge glycosphingolipid alpha-galactosylceramide (αGalCer), which induces the rapid production of a wide variety of cytokines by the entire classical NKT cell subset (Kawano et al. 1997). Recognition is mediated through a semi-invariant T cell receptor (TCR), consisting of an invariant TCR α chain (Vα14-Jα18 in mouse or the homologous Vα24-Jα18 in human) paired preferentially with a variable (primarily Vβ8+, Vβ7+, or Vβ2+ in the mouse, or Vβ11 in human) TCR β chain (Bendelac 1995b; Dellabona et al. 1994; Lantz and Bendelac 1994; Porcelli et al. 1996). Classical NKT cells are either CD4+CD8- or CD4-CD8- (DN), although CD8αα expression can be induced in human NKT cells after activation. Humans CD4+ and DN NKT cells are functionally distinct in that the DN population is characterized by a predominant Th1-like pattern of cytokine secretion, high levels of perforin expression, and high levels of NKG2D expression, while the CD4+ population secretes both Th1-like and Th2-like cytokines (Gumperz et al. 2002). In mice, however, these distinct differences are not as clear.
Most classical NKT cells are CD161 (NK1.1)+. Identification of classical NKT cells using this marker alone, however, has proven problematic. First, only a few commonly used inbred mouse strains, such as C57BL/6, possess the CD161 alleles that encode the NK1.1 alloantigen recognized by the PK136 mAb (Carlyle et al. 2006). Second, accumulating data suggest the existence of NK1.1− classical NKT cells (McNab et al. 2007; Michel et al. 2007). Finally, it is becoming clear that non-classical NKT and CD1d–independent T cell subsets can also express NK1.1 (Behar and Cardell 2000b; Slifka et al. 2000). Likewise, in humans, most classical NKT cells express CD161, yet only a very small fraction of CD161+ T cells are classical NKT (Lanier et al. 1994). It is not uncommon to find in the human literature the name NKT in reference to the CD3+CD56+ T cell population, though most data suggests that only a small fraction of this subset are classical NKT (Kim et al. 2002). Currently, the most accurate means with which to identify classical NKT cells is through the use of αGalCer-loaded CD1d multimers, some of which are available commercially (Proimmune, BD Biosciences) or through reagent resource programs (NIH tetramer facility).
The remainder of the CD1d–restricted T cell population is made up of non-classical (type II) NKT cells. These T cells differ from classical NKT cells in that they possess a diverse TCR repertoire and do not recognize αGalCer (Behar and Cardell 2000a; Cardell et al. 1995). Less is known about this T cell subset due to the lack of reagents with which to study them. Recent reports identifying sulfatide as a CD1d ligand that can stimulate non-classical NKT cells should pave the way for more thorough characterization of this subset (Zajonc et al. 2005). Interestingly, non-classical NKT cells appear to be quite different functionally from classical NKT cells, in that they appear to function as suppressor cells (Ambrosino et al. 2007; Halder et al. 2007; Terabe et al. 2005) and have been reported to suppress classical NKT cell function (Ambrosino et al. 2007). Thus, classical and non-classical NKT cells may each comprise part of an immunoregulatory network.
The remainder of the NKT cell population that co-express NK cell markers (e.g., CD3+CD56+ T cells in humans), and thus are “NKT-like,” are not CD1d–restricted. This is a heterogeneous group of T cells which may include a number of diverse T cell subsets and they will therefore not be discussed here.
CD1d and glycolipids
Both type I and type II NKT cells recognize glycolipids presented by CD1d (Brigl and Brenner 2004). This is a monomorphic MHC class I-like molecule that is normally expressed on a variety of cells including B cells, dendritic cells, monocyte/macrophages, and epithelial cells (Kronenberg 2005). Although it is similar to classical class I MHC in that it is paired with β2m, CD1d differs in that it has a large, hydrophobic groove that accommodates the hydrophobic tails of its glycolipid ligands. The most widely used CD1d ligand is the marine sponge-derived glycosphingolipid αGalCer which activates all classical NKT cells (Kawano et al. 1997). However, although indispensable in its use as a tool with which to study classical NKT and CD1d biology, αGalCer is considered a non-physiological ligand since it cannot be found in vertebrates or microorganisms (Kinjo et al. 2005; Mattner et al. 2005; Sriram et al. 2005). Efforts by a number of laboratories to identify physiological exogenous ligands has led to the identification of bacterial cell wall glycolipids that stimulate classical NKT cells when presented by CD1d (Kinjo et al. 2005; Mattner et al. 2005; Sriram et al. 2005). In addition, one group has demonstrated that a melanoma-derived ligand, ganglioside GD3, is capable of being presented by CD1d to NKT cells (Wu et al. 2003).
NKT cells are unusual in that they show a propensity to recognize CD1d on cells in the absence of exogenous ligands. Thus, a number of laboratories have sought to identify endogenous CD1d ligands which has led to the identification of isoglobotrihexosylceramide (iGb3) as a mammalian endogenous ligand that stimulates both human and mouse NKT cells (Zhou et al. 2004), although there is controversy over its role in thymic selection (Porubsky et al. 2007). Therefore, NKT cells may recognize endogenous ligands produced in certain tissue microenvironments or during certain pathological states, or they may act in a more classical sense by recognizing pathogen glycolipids presented by CD1d.
NKT cells in the immune response
NKT cells influence a broad spectrum of immunological responses, including autoimmunity (Singh et al. 2001; Wilson et al. 1998), tolerance induction (Seino et al. 2001; Sonoda et al. 1999; Sonoda et al. 2001; Sonoda and Stein-Streilein 2002), tumor immunology (Cui et al. 1997; Kawano et al. 1998; Kawano et al. 1999; Smyth et al. 2000), and infectious disease (Hansen et al. 2003; Kinjo et al. 2005; Kumar et al. 2000; Mattner et al. 2005; Sriram et al. 2005). This diversity of function is due to the ability of NKT cells to rapidly produce a wide variety of cytokines such as IL-2, IL-4, IL-5, IL-10, IL-13, IL-17, IL-21, IFN-γ, and TNF-α, and GM-CSF (Kronenberg 2005). Production of both Th1-like as well as Th2-like cytokines may explain the paradoxical proinflammatory or tolerogenic ability of NKT cells exhibited in different contexts (Wilson and Delovitch 2003). Interestingly, the nature of the CD1d ligands themselves can bias NKT cells toward a Th1 or Th2 cytokine profile (Oki et al. 2004). OCH, a truncated version of αGalCer in which the sphingosine chain is shortened, elicits primarily a Th2 cytokine profile from classical NKT cells (Miyamoto et al. 2001). More recent work examining panels of synthetic glycolipids have underscored the ability of ligands to modulate the cytokine profiles of NKT cells. In addition to the obvious implications for therapeutic applications, these observations raise the possibility that different endogenous glycolipids may be associated with altered NKT cell function.
Direct and Indirect pathways for NKT cell activation
Exogenous CD1d glycolipid ligands, such as αGalCer, stimulate NKT cells after being endocytosed and loaded onto CD1d in APCs, a process that has been termed the direct pathway of NKT stimulation. Recently, it has been demonstrated that stimulation of APCs with TLR ligands such as LPS and CpG oligonucleotides induce the loading of endogenous mammalian glycolipid ligands on CD1d, that induce the activation of NKT cells. Since neither LPS nor CpG oligonucleotide is capable of stimulating NKT cells directly, this process has been termed the indirect pathway of NKT stimulation (Brigl et al. 2003; Paget et al. 2007). These intriguing observations suggest that NKT cells may be able to play a role in a broad spectrum of immunological responses to pathogens and that their response may not be limited only to pathogens that possess CD1d agonist glycolipid ligands. Indeed, evidence for this indirect pathway of NKT cell activation provides an explanation for the demonstration that NKT cells are critical in LPS-induced endotoxic shock (Dieli et al. 2000). Moreover, Nagarajan et al., demonstrated recently that NKT cells are involved in regulating TLR-induced macrophage TNF production (Nagarajan and Kronenberg 2007).
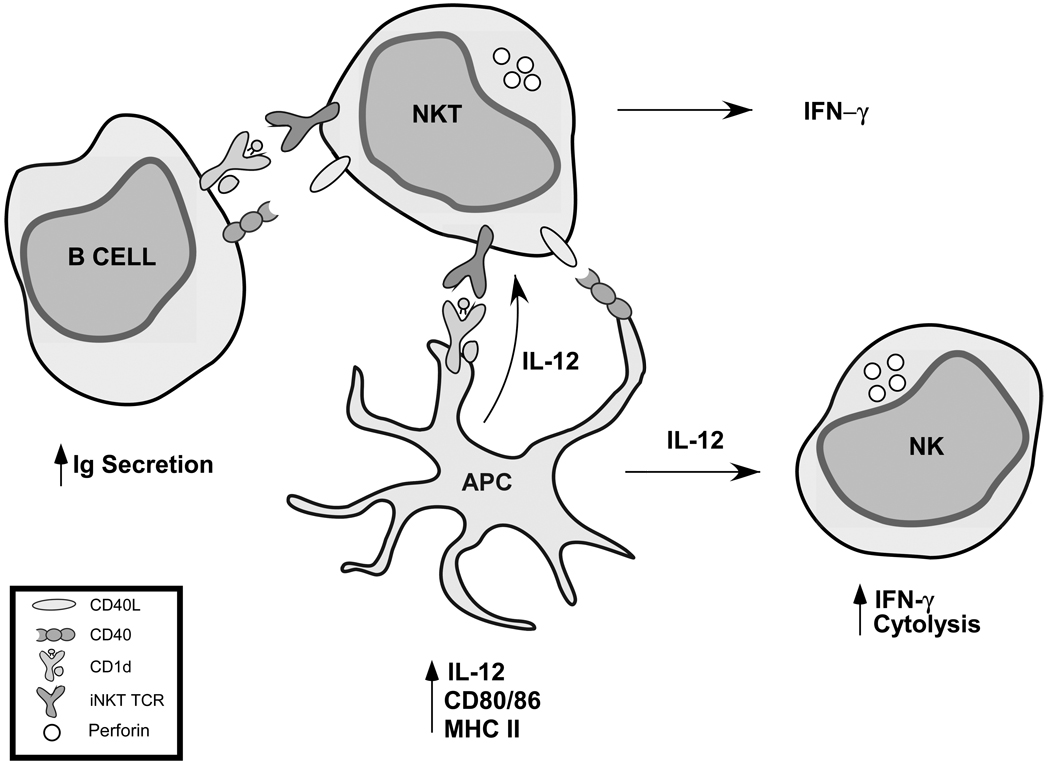
NKT cells, therefore, may play an early role in the development of the immune response and thus they are often referred to as a “bridge” between the innate and adaptive arms of the immune system. Apart from their ability to rapidly produce a wide variety of cytokines, activation of NKT cells results in the activation of a number of immune cell subsets (Fig. 1). NKT cell recognition of αGalCer on dendritic cells (DCs) leads to upregulation of CD40L on the NKT cell, which can then bind to CD40 on DCs and induce DC maturation and IL-12 secretion (Kitamura et al. 1999). In addition to DC activation, NKT cell activation leads to activation of B cells (Carnaud et al. 1999) as well as NK cells (Carnaud et al. 1999; Eberl and MacDonald 2000). The precise mechanism through which NK cell transactivation occurs is not known, although it is dependent on IL-12 and IFN-γ, and is mediated through DCs and/or macrophages (Bezbradica et al. 2005; Wesley et al. 2005a). Thus, NKT cells are emerging as potentially powerful modulators of both the innate and adaptive arms of the immune system.
Figure 1.
Modulation of innate and adaptive immune responses by NKT cells. Activation of NKT cells leads to activation of a number of leukocyte subsets including B cells, dendritic cells (DCs), and NK cells.
NKT cells and CD1d at the maternal-fetal interface
NKT cells have been described in both the mouse and human decidua (Boyson et al. 2002; Ito et al. 2000; Tsuda et al. 2001). For the reasons discussed above, however, a variety of surrogate markers have been used to identify this population. Studies examining human decidual NKT cells are in general agreement that the classical NKT cell population is present at a frequency of approximately 0.5%, which is similar to what has been reported in the mouse (Boyson et al. 2002; Ito et al. 2000; Tsuda et al. 2001). Similarly, in both humans and mice, classical NKT cells appear to be highly activated and exhibit a Th1-like bias towards production of IFN-γ (Boyson et al. 2002; Ito et al. 2000). Although one report did not observe this Th1-like bias (Tsuda et al. 2001), the analysis was done on decidual CD3+Vα24+ cells, only a fraction of which are classical NKT cells (Boyson et al. 2002). As discussed earlier, there are few markers with which one can unambiguously identify the non-classical (type II) NKT population in either mice or humans. However, a recently published study describes that, in addition to classical NKT cells, the CD3+CD161+ decidual T cell population contains CD1d–restricted T cells that had diverse TCRs and exhibited a Th2-like cytokine profile, secreting IL-4 and IL-10 (Trapani et al. 1989). These characteristics are consistent with these cells being non-classical (Type II) NKT cells and suggest the presence of both types of NKT cells in the human decidua.
In contrast to the data in humans, there are somewhat conflicting reports regarding the composition of the murine decidual NK1.1+ T cell population. One report suggested that NK1.1+ T cells in the decidua expressed invariant TCR α chains and recognized αGalCer, and thus were classical NKT cells. It was noted, however, that decidual NKT cells preferentially expressed a Vβ7 TCR instead of Vβ8.2 that is more commonly observed in the spleen and liver (Ito et al. 2000). These observations were supported by two other groups that detected invariant Vα14-Jα18 transcripts (Dang et al. 2000; Wang et al. 2002). However, it was also reported that while nearly all the decidual NK1.1+ T cells are Vα14+, there was preferential pairing with Vβ3 (Dang et al. 2000). In light of these data, it is intriguing that decidual NK1.1+ T cells are dependent on β2m, but not CD1d (Dang and Heyborne 2001), indicating that they are restricted by an unknown β2m–associated protein. Even more interesting is that the restricting MHC class I molecule does not need to be expressed on maternal tissue for decidual NK1.1+ T cell development. Instead, expression of β2m on paternally-derived tissue is sufficient for development of the decidual NK1.1+ T cell population, suggesting that this T cell subset develops extrathymically (Dang and Heyborne 2001). Clearly, a more thorough characterization of this T cell population is needed. Resolution of these issues will require characterization of the NK1.1+ T cell population using αGalCer-loaded CD1d tetramers and functional assays.
Finally, a number of studies have documented in humans numerical and functional differences in the CD3+CD56+ NKT cell population. As indicated earlier, the identity of these cells is unknown and they have no obvious equivalent in the mouse. These cells are unlikely to be CD1d–restricted T cells since these markers have been demonstrated to be a poor predictor of CD1d–reactivity (Kim et al. 2002).
In addition to the presence of NKT cells at the human maternal-fetal interface, expression of CD1d has also been documented in early and in late gestation. Jenkinsson et al., first reported CD1d transcription in both trophoblast and choriocarcinoma cell lines (Jenkinson et al. 1999), which was followed by the demonstration that CD1d is expressed on both the villous and extravillous trophoblasts and that its expression increases as gestation progresses (Boyson et al. 2002; Shao et al. 2005). In addition to trophoblasts in anchoring villi, CD1d is expressed on trophoblasts that have invaded well into the maternal decidua (Boyson et al. 2002). Although it has never been formally demonstrated, this pattern of expression is consistent with the pattern observed for HLA-G and suggests that CD1d and HLA-G could be co-expressed on the same cells. In mice, the situation is again less clear than in humans. Although CD1d transcripts are clearly present in early gestation (day 6–8) decidual tissue (data not shown) and CD1d protein expression on decidual cell preparations has been reported (Dang et al. 2000), the cells which express CD1d have yet to be defined.
In addition to CD1d expression, the placenta possesses other features conducive to productive CD1d–NKT cell interactions. For example, it was recently demonstrated that a number of genes involved in sphingolipid metabolism were highly upregulated in the mouse decidua, and that disruption of sphingolipid metabolism caused pregnancy loss in mice (Mizugishi et al. 2007). In addition, prosaposin, an accessory molecule essential for glycosphingolipid loading onto CD1d (Kang and Cresswell 2004; Zhou et al. 2004) is known to be expressed at high levels in the decidua (Sun et al. 1994). Finally, although the profile of glycolipids in the placenta is still unclear, it is intriguing that ganglioside GD3, a recently described CD1d agonist ligand (Wu et al. 2003), has been demonstrated to be a predominant species in the rat placenta (Itonori et al. 1995).
NKT cells and immune-mediated pregnancy loss
There is a large body of literature in humans linking pregnancy loss with the presence of infection and pro-inflammatory cytokines (Buhimschi et al. 2005; Engel et al. 2005; Goepfert et al. 2001; Goldenberg et al. 2000; Lockwood and Kuczynski 1999; Romero et al. 2004). In light of the fact that recognition of bacterial cell wall glycolipids by NKT cells results in the production of large amounts of IFN-γ and TNF (Kinjo et al. 2005; Mattner et al. 2005; Sriram et al. 2005), it is interesting that activation of NKT cells with the agonist CD1d ligand αGalCer rapidly induces pregnancy loss in C57BL/6J mice at all stages of gestation (Boyson et al. 2006; Ito et al. 2000).
The mechanisms through which αGalCer induces pregnancy loss are still unclear. It is ineffective in NKT- or CD1d–deficient mice, indicating that NKT cells are required and is dependent on TNF-α, IFN-γ, and perforin (Boyson et al. 2006; Ito et al. 2000). Recently, it was demonstrated that NKT cell activation induced preterm birth as well as early to mid-gestation pregnancy loss (Boyson et al. 2006). Surprisingly, key differences were observed in the mechanisms between early and mid-late gestation pregnancy loss, with perforin being required for early gestation pregnancy loss (up to day 8), but not during mid-late gestation (day 9 and after). Conversely, mid-late, but not early, gestation pregnancy loss was associated with strain-dependent variability in serum cytokine production. Significantly greater levels of a number of cytokines, including TNF, are produced in response to αGalCer in highly susceptible C57BL/6J mice, whereas these levels are lower in resistant strains such as BALB/cJ and C3H/HeJ (Boyson et al. 2006)(data not shown). Interestingly, these observations were similar to those seen in a model of pregnancy loss induced by CD40 cross-linking, which efficiently induced pregnancy loss early in gestation (up to day 8) but was ineffective at later stages of gestation (Erlebacher et al. 2004). Collectively, these observations suggest that there are distinct requirements for immune-mediated pregnancy loss at different stages of gestation.
Since NKT cells are known to activate a number of other leukocyte subsets, it is entirely possible that pregnancy loss due to their activation is only the first step in a cascade of events. Although Ito et al., speculated that NKT cells were directly involved in trophoblast cell death (Ito et al. 2000), NKT cells are present in relatively low numbers compared to uNK cells which share many effector functions with NKT cells. It is possible, therefore, that uNK cells contribute to NKT cell-mediated pregnancy loss. Mouse uNK cells, which are analogous to decidual CD16−CD56bright NK cells, have recently been demonstrated to be critical in maternal decidual artery remodeling via their production of IFN-γ (Bilinski et al. 2008). Conversely, uNK cells have also been implicated in mouse models of pregnancy loss (Chaouat 1994; de Fougerolles and Baines 1987; Erlebacher et al. 2004; Murphy et al. 2005). With this in mind, it is interesting that NKT cell activation via αGalCer is known to result in spleen NK cell activation (Carnaud et al. 1999; Eberl and MacDonald 2000), a process known as “transactivation.” Through a mechanism that is still unclear, but which probably involves IFN-γ secretion by NKT cells and IL-12 secretion by DCs and/or macrophages (Bezbradica et al. 2005; Wesley et al. 2005b), NK cells are activated within hours of NKT cell activation, and begin to produce IFN-γ and increase their cytotoxic activity.
Thus, it is possible that NKT-mediated pregnancy loss may involve uNK cell transactivation. To test the hypothesis that an NKT-NK axis could function in the decidua, αGalCer was administered to pregnant mice on day 7.5 of gestation. Splenocytes and decidual leukocytes were isolated 5 hours later and IFN-γ production was assessed by intracellular staining. This preliminary analysis revealed that, similar to spleen NK cells, decidual uNK cells became activated and produced IFN-γ within hours of NKT cell activation (data not shown). Whether this mechanism is critical in mediating NKT-induced pregnancy loss, however, is still unknown. Also unclear is whether functional NKT-NK interactions take place in the absence of an agonist ligand such as αGalCer. However, the demonstration that NKT cell function is capable of modulating decidual uNK cell function, especially uNK cell IFN-γ production, argues that further examination of the NKT-NK axis at the maternal-fetal interface is warranted.
A role for NKT cells in LPS-induced pregnancy loss??
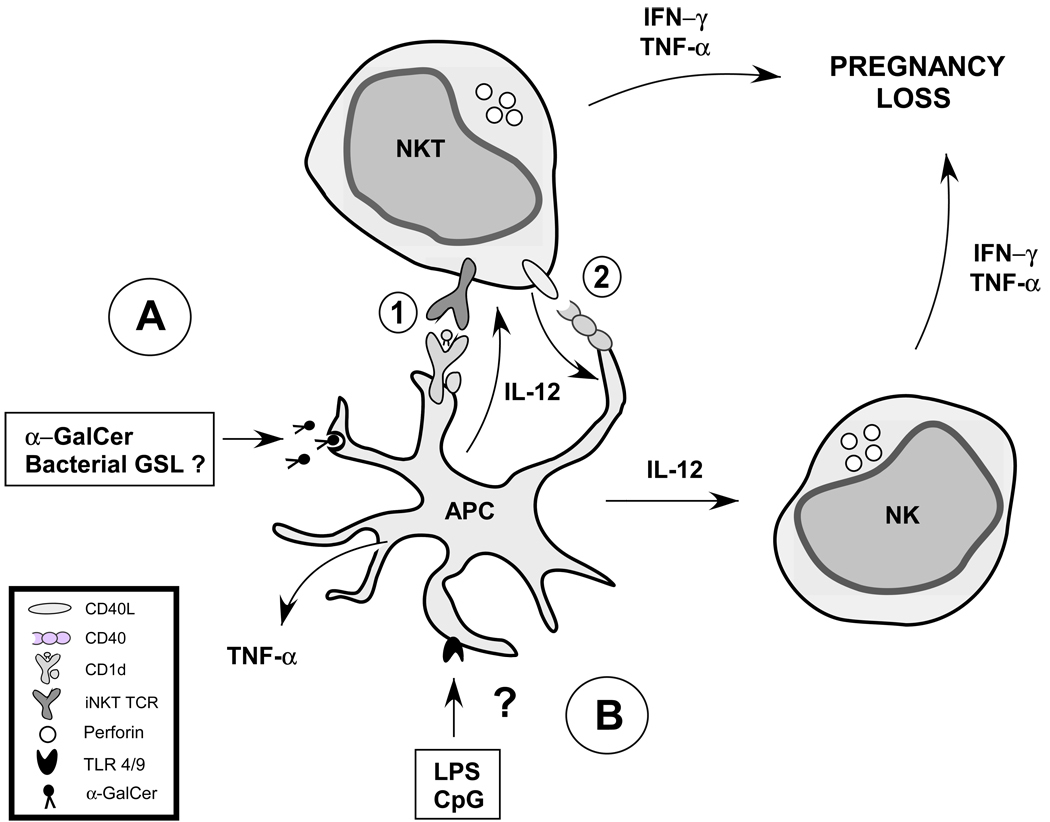
The induction of pregnancy loss by endotoxin (LPS) has been studied for at least sixty years (Zahl and Bjerknes 1943). It is a widely used model to study the effects of inflammation on pregnancy loss at all stages of gestation. As discussed above, there is a growing body of evidence to suggest that NKT cells may play a critical role in many of the classic immune responses that occur after TLR cross-linking by LPS or CpG (Brigl et al. 2003; Paget et al. 2007). For example, serum TNF production as well as NK cell IFN-γ production in response to LPS are dramatically reduced in NKT-deficient mice (Nagarajan and Kronenberg 2007). A model has emerged in which NKT cells act to “license” innate immune cells such as macrophages or DCs to reach their full functional capacity. It is possible therefore, to devise a hypothesis (Fig. 2) in which NKT cells could play a role in immune-mediated pregnancy loss through direct recognition of bacterial cell wall glycolipids (direct pathway), or through recognition of endogenous agonist ligands loaded onto CD1d as a result of TLR ligation (indirect pathway).
Figure 2.
Hypothetical model of NKT cells in immune-mediated pregnancy loss. NKT cells are activated directly by bacterial cell wall glycolipids, or they can be activated indirectly via LPS or CpG-stimulated APCs. Therefore, a hypothetical model may be constructed which integrates both of these pathways in immune-mediated pregnancy loss. A) Exogenous pathway. Pathogen-derived cell wall glycolipids are endocytosed by an APC and loaded onto CD1d. B) Endogenous pathway. Pathogen-derived TLR ligands such as LPS trigger TLR4 signaling on an APC (DC and/or macrophage) which induces loading of endogenous CD1d agonist ligands onto CD1d. Activation of NKT cells through TCR recognition of CD1d and agonist ligand leads to cytokine production and upregulation of CD40L (CD154). Cross-linking of CD40 expressed on the APC by CD40L in turn leads to APC activation and results in secretion of IL-12, which induces NK cell. IL-12 production leads to NK cell activation and subsequent production of IFN-γ and TNF.
CONCLUSIONS
Successful pregnancy requires the coordinate regulation of the innate and adaptive arms of the immune system. CD1d–restricted NKT cells are a novel subset of T cells with a demonstrated ability to modulate the function of both the innate and adaptive arms of the immune system. Especially interesting with regard to their possible function at the maternal-fetal interface is their ability to mediate both pro-inflammatory or tolerogenic immune responses in a context-dependent manner. Although we still know very little of their function at the maternal-fetal interface, their ability to dramatically impact pregnancy upon activation with an agonist ligand, together with the demonstration that they may be able to modulate decidual uNK cell function, suggests that NKT cells are important in coordinating functional interactions among decidual leukocytes at the maternal-fetal interface.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants R01AI067897 and P20RR021905. We thank previous members of the laboratory for their contributions and we thank Elizabeth Bonney for helpful discussion.
REFERENCES
- Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, Yamamura T, Kumar V, Berzofsky JA. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J. Immunol. 2007;179:5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- Behar SM, Cardell S. Diverse CD1d-restricted T cells: diverse phenotypes, and diverse functions. Semin. Immunol. 2000a;12:551–560. doi: 10.1006/smim.2000.0273. [DOI] [PubMed] [Google Scholar]
- Behar SM, Cardell S. Diverse CD1d–restricted T cells: diverse phenotypes, and diverse functions. Semin. Immunol. 2000b;12:551–560. doi: 10.1006/smim.2000.0273. [DOI] [PubMed] [Google Scholar]
- Bendelac A. CD1: presenting unusual antigens to unusual T lymphocytes. Science. 1995a;269:185–186. doi: 10.1126/science.7542402. [DOI] [PubMed] [Google Scholar]
- Bendelac A. Mouse NK1+ T cells. Curr. Opin. Immunol. 1995b;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, Unutmaz D, Van Kaer L, Joyce S. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J. Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- Bilinski MJ, Thorne JG, Oh MJ, Leonard S, Murrant C, Tayade C, Croy BA. Uterine NK cells in murine pregnancy. Reproductive biomedicine online. 2008;16:218–226. doi: 10.1016/s1472-6483(10)60577-9. [DOI] [PubMed] [Google Scholar]
- Boyson JE, Nagarkatti N, Nizam L, Exley MA, Strominger JL. Gestation stage-dependent mechanisms of invariant natural killer T cell-mediated pregnancy loss. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4580–4585. doi: 10.1073/pnas.0511025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson JE, Rybalov B, Koopman LA, Exley M, Balk SP, Racke FK, Schatz F, Masch R, Wilson SB, Strominger JL. CD1d and invariant NKT cells at the human maternal-fetal interface. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13741–13746. doi: 10.1073/pnas.162491699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d–restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Christner R, Buhimschi CS. Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. Bjog. 2005;112:173–181. doi: 10.1111/j.1471-0528.2004.00340.x. [DOI] [PubMed] [Google Scholar]
- Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J. Exp. Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle JR, Mesci A, Ljutic B, Belanger S, Tai LH, Rousselle E, Troke AD, Proteau MF, Makrigiannis AP. Molecular and genetic basis for strain-dependent NK1.1 alloreactivity of mouse NK cells. J. Immunol. 2006;176:7511–7524. doi: 10.4049/jimmunol.176.12.7511. [DOI] [PubMed] [Google Scholar]
- Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- Chaouat G. Synergy of lipopolysaccharide and inflammatory cytokines in murine pregnancy: alloimmunization prevents abortion but does not affect the induction of preterm delivery. Cell. Immunol. 1994;157:328–340. doi: 10.1006/cimm.1994.1231. [DOI] [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Dang Y, Beckers J, Wang CR, Heyborne KD. Natural killer 1.1(+) alpha beta T cells in the periimplantation uterus. Immunology. 2000;101:484–491. doi: 10.1046/j.1365-2567.2000.t01-1-00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Heyborne KD. Cutting edge: regulation of uterine NKT cells by a fetal class I molecule other than CD1. J. Immunol. 2001;166:3641–3644. doi: 10.4049/jimmunol.166.6.3641. [DOI] [PubMed] [Google Scholar]
- de Fougerolles AR, Baines MG. Modulation of the natural killer cell activity in pregnant mice alters the spontaneous abortion rate. J. Reprod. Immunol. 1987;11:147–153. doi: 10.1016/0165-0378(87)90018-0. [DOI] [PubMed] [Google Scholar]
- Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4–8-T cells. J. Exp. Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieli F, Sireci G, Russo D, Taniguchi M, Ivanyi J, Fernandez C, Troye-Blomberg M, De Leo G, Salerno A. Resistance of natural killer T cell-deficient mice to systemic Shwartzman reaction. J. Exp. Med. 2000;192:1645–1652. doi: 10.1084/jem.192.11.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 2000;30:985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Engel SA, Erichsen HC, Savitz DA, Thorp J, Chanock SJ, Olshan AF. Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology. 2005;16:469–477. doi: 10.1097/01.ede.0000164539.09250.31. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Zhang D, Parlow AF, Glimcher LH. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J. Clin. Invest. 2004;114:39–48. doi: 10.1172/JCI20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J. Exp. Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- Goepfert AR, Goldenberg RL, Andrews WW, Hauth JC, Mercer B, Iams J, Meis P, Moawad A, Thom E, VanDorsten JP, Caritis SN, Thurnau G, Miodovnik M, Dombrowski M, Roberts J, McNellis D. The Preterm Prediction Study: association between cervical interleukin 6 concentration and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am. J. Obstet. Gynecol. 2001;184:483–488. doi: 10.1067/mob.2001.109653. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally Distinct Subsets of CD1d-restricted Natural Killer T Cells Revealed by CD1d Tetramer Staining. J. Exp. Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J. Clin. Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond KJ, Pellicci DG, Poulton LD, Naidenko OV, Scalzo AA, Baxter AG, Godfrey DI. CD1d–restricted NKT cells: an interstrain comparison. J. Immunol. 2001;167:1164–1173. doi: 10.4049/jimmunol.167.3.1164. [DOI] [PubMed] [Google Scholar]
- Hansen DS, Siomos MA, Buckingham L, Scalzo AA, Schofield L. Regulation of murine cerebral malaria pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity. 2003;18:391–402. doi: 10.1016/s1074-7613(03)00052-9. [DOI] [PubMed] [Google Scholar]
- Ito K, Karasawa M, Kawano T, Akasaka T, Koseki H, Akutsu Y, Kondo E, Sekiya S, Sekikawa K, Harada M, Yamashita M, Nakayama T, Taniguchi M. Involvement of decidual Valpha14 NKT cells in abortion. Proc. Natl. Acad. Sci. U. S. A. 2000;97:740–744. doi: 10.1073/pnas.97.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itonori S, Shirai T, Kiso Y, Ohashi Y, Shiota K, Ogawa T. Glycosphingolipid composition of rat placenta: changes associated with stage of pregnancy. Biochem. J. 1995;307(Pt 2):399–405. doi: 10.1042/bj3070399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson HJ, Wainwright SD, Simpson KL, Perry AC, Fotiadou P, Holmes CH. Expression of CD1D mRNA transcripts in human choriocarcinoma cell lines and placentally derived trophoblast cells. Immunology. 1999;96:649–655. doi: 10.1046/j.1365-2567.1999.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5:175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, Tanaka Y, Taniguchi M. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, Akutsu Y, Motohashi S, Iizasa T, Endo H, Fujisawa T, Shinkai H, Taniguchi M. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999;59:5102–5105. [PubMed] [Google Scholar]
- Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11–16. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, Kawano T, Taniguchi M, Nishimura T. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)- 12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- Kumar H, Belperron A, Barthold SW, Bockenstedt LK. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 2000;165:4797–4801. doi: 10.4049/jimmunol.165.9.4797. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Chang C, Phillips JH. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J. Immunol. 1994;153:2417–2428. [PubMed] [Google Scholar]
- Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8- T cells in mice and humans. J. Exp. Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Kuczynski E. Markers of risk for preterm delivery. J. Perinat. Med. 1999;27:5–20. doi: 10.1515/JPM.1999.001. [DOI] [PubMed] [Google Scholar]
- Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- McNab FW, Pellicci DG, Field K, Besra G, Smyth MJ, Godfrey DI, Berzins SP. Peripheral NK1.1 NKT cells are mature and functionally distinct from their thymic counterparts. J. Immunol. 2007;179:6630–6637. doi: 10.4049/jimmunol.179.10.6630. [DOI] [PubMed] [Google Scholar]
- Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Li C, Olivera A, Bielawski J, Bielawska A, Deng CX, Proia RL. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J. Clin. Invest. 2007;117:2993–3006. doi: 10.1172/JCI30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J. Immunol. 2005;175:4084–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- Oki S, Chiba A, Yamamura T, Miyake S. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J. Clin. Invest. 2004;113:1631–1640. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, Trottein F. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Gerdes D, Fertig AM, Balk SP. Human T cells expressing an invariant V alpha 24-J alpha Q TCR alpha are CD4- and heterogeneous with respect to TCR beta expression. Hum. Immunol. 1996;48:63–67. doi: 10.1016/0198-8859(96)00090-0. [DOI] [PubMed] [Google Scholar]
- Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5977–5982. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil. Steril. 2004;82:799–804. doi: 10.1016/j.fertnstert.2004.05.076. [DOI] [PubMed] [Google Scholar]
- Seino KI, Fukao K, Muramoto K, Yanagisawa K, Takada Y, Kakuta S, Iwakura Y, Van Kaer L, Takeda K, Nakayama T, Taniguchi M, Bashuda H, Yagita H, Okumura K. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2577–2581. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Jacobs AR, Johnson VV, Mayer L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J. Immunol. 2005;174:7539–7547. doi: 10.4049/jimmunol.174.12.7539. [DOI] [PubMed] [Google Scholar]
- Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Pagarigan RR, Whitton JL. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J. Immunol. 2000;164:2009–2015. doi: 10.4049/jimmunol.164.4.2009. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J. Exp. Med. 1999;190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda KH, Faunce DE, Taniguchi M, Exley M, Balk S, Stein-Streilein J. NK T cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J. Immunol. 2001;166:42–50. doi: 10.4049/jimmunol.166.1.42. [DOI] [PubMed] [Google Scholar]
- Sonoda KH, Stein-Streilein J. CD1d on antigen-transporting APC and splenic marginal zone B cells promotes NKT cell-dependent tolerance. Eur. J. Immunol. 2002;32:848–857. doi: 10.1002/1521-4141(200203)32:3<848::AID-IMMU848>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d–specific ligands for NKT cells. Eur. J. Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- Sun Y, Witte DP, Grabowski GA. Developmental and tissue-specific expression of prosaposin mRNA in murine tissues. Am. J. Pathol. 1994;145:1390–1398. [PMC free article] [PubMed] [Google Scholar]
- Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J. Exp. Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JA, Mizuno S, Kang SH, Yang SY, Dupont B. Molecular mapping of a new public HLA class I epitope shared by all HLA-B and HLA-C antigens and defined by a monoclonal antibody. Immunogenetics. 1989;29:25–32. doi: 10.1007/BF02341610. [DOI] [PubMed] [Google Scholar]
- Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Sakai M, Michimata T, Tanebe K, Hayakawa S, Saito S. Characterization of NKT cells in human peripheral blood and decidual lymphocytes. Am. J. Reprod. Immunol. 2001;45:295–302. doi: 10.1111/j.8755-8920.2001.450505.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Li C, Kawamura H, Watanabe H, Abo T. Unique sensitivity to alpha-galactosylceramide of NKT cells in the uterus. Cell. Immunol. 2002;215:98–105. doi: 10.1016/s0008-8749(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Wesley JD, Robbins SH, Sidobre S, Kronenberg M, Terrizzi S, Brossay L. Cutting Edge: IFN-{gamma} Signaling to Macrophages Is Required for Optimal V{alpha}14i NK T/NK Cell Cross-Talk. J. Immunol. 2005a;174:3864–3868. doi: 10.4049/jimmunol.174.7.3864. [DOI] [PubMed] [Google Scholar]
- Wesley JD, Robbins SH, Sidobre S, Kronenberg M, Terrizzi S, Brossay L. Cutting edge: IFN-gamma signaling to macrophages is required for optimal Valpha14i NK T/NK cell cross-talk. J. Immunol. 2005b;174:3864–3868. doi: 10.4049/jimmunol.174.7.3864. [DOI] [PubMed] [Google Scholar]
- Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3:211–222. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, Strominger JL, Hafler DA. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature. 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahl PA, Bjerknes C. Induction of decidua-placental hemorrhage in mice by the endotoxins of certain gram-negative bacteria. Proc. Soc. Exp. Biol. Med. 1943;54:329–332. [Google Scholar]
- Zajonc DM, Maricic I, Wu D, Halder R, Roy K, Wong CH, Kumar V, Wilson IA. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J. Exp. Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]