Abstract
This article examines the effect of AIDS-related mortality of the prime-age adult population on marriage behavior among women in Malawi. A rise in prime-age adult mortality increases risks associated with the search for a marriage partner in the marriage market. A possible behavioral change in the marriage market in response to an increase in prime-age adult mortality is to marry earlier to avoid exposure to HIV/AIDS risks. We test this hypothesis by using micro data from Malawi, where prime-age adult mortality has drastically increased. In the analysis, we estimate the probability of prime-age adult mortality that sample women have observed during their adolescent period by utilizing retrospective information on deaths of their siblings. Empirical analysis shows that excess prime-age adult mortality in the local marriage market lowers the marriage age for females and shortens the interval between the first sex and first marriage.
It has been increasingly recognized that HIV/AIDS has drastically raised mortality rates among prime-age adults. Excessive mortality of prime-age adults can influence various aspects of household behavior, since the loss of prime-age adults decreases the household income and thus human capital investments of the next generation (e.g., Ainsworth, Beegle, and Koda 2005; Ueyama 2007; Yamauchi, Buthelezi, and Velia 2008). The problem is not confined only to household behavior, but influences perceptions on potential risks in family formation, such as finding a marriage partner (e.g., Caldwell et al. 1999). For example, in a society where the AIDS epidemic is prevalent, agents may try to secure a safe partner by making decisions at an early stage of the marriage-partner search. Since the decision to marry is key to the way family is structured, current HIV/AIDS mortality risks can potentially have long-term impacts that propagate to the next generation.
Earlier studies showed that even with HIV/AIDS epidemics in the society, agents hardly change their sexual behavior despite widespread knowledge and awareness of HIV/AIDS (Bloom et al. 2000; Caldwell et al. 1999; Lagarde, Emmanual, and Enel 1996).1 These studies highlighted traditional marriage institutions and rituals to explain why Africans do not respond to risks and dangers caused by HIV/AIDS.
However, the above proposition has been questioned recently, given the fact that awareness and knowledge of HIV/AIDS seems to be common in many of the sub-Saharan African populations, especially in high-HIV-prevalence countries (de Walque 2006, using Demographic and Health Surveys [DHS] from various countries). In particular, those living in countries with a high prevalence of HIV have an opportunity to discuss the risks of infection and gather information on precautionary measures. For example, according to the 2004 Malawi DHS that we use in our analysis, 98.7% of respondents have heard about HIV/AIDS, 83.3% know where to go to take an HIV test, 70% have discussed with their spouses strategies to avoid AIDS, and 65% know someone who has HIV/AIDS or died of AIDS.
At the same time, a growing corpus of descriptive research has examined changing sexual behavior in response to the HIV/AIDS epidemic. Through a focus group discussion in Uganda, Mukiza-Gapere and Ntozi (1995) found that younger generations are more fearful of marriage because they are not sure of the HIV status of their potential partners and witnessed many newly married people dying of AIDS. Ng’weshemi et al. (1996) examined men’s sexual behavior in urban Tanzania and found a significant decline in men’s extramarital sexual activities in recent years. Using longitudinal data from Malawi, Smith and Watkins (2005) and Helleringer and Kohler (2005) found that Malawians have begun changing their sexual and marriage behaviors in recent years.
Clark (2004) investigated the link between early marriage and risks of HIV infection among adolescent women by comparing sexually active married women aged 15–24 and sexually active but unmarried women of the same age group. She claimed that married women face a higher risk of infection than unmarried women of the same age group due to (1) a high frequency of sexual activity, (2) a decrease in condom use, and (3) a larger age difference between partners (spouses). Gregson et al. (2002) found that women whose sexual partners are older (large age difference) are more likely to be HIV-infected than those whose partners are of a similar age. Bongaarts (2007) pointed out that rising marriage age is associated with a longer period of premarital sex, which increases infection risk.
In this article, we attempt to identify causal effects of AIDS mortality risks on marriage behavior among women by using the 2004 DHS data from Malawi.2 We hypothesize that an increase in prime-age adult mortality in the marriage market (correlated with the epidemic of HIV/AIDS) leads to safer behavior among young women in the marriage market; specifically, they have a shorter period of premarital sexual activity before marriage, marry at a younger age, and secure a younger partner.3 These behavioral changes reduce the probability of HIV infection during both singlehood and marriage.
The above changes have further implications for demographics and human capital investment of the next generation. First, early marriage means a longer span of marriage, particularly during women’s reproductive ages. Therefore, the fertility rate is expected to increase. Ceteris paribus, this leads to a reduction in human capital investments in children due to the quality-quantity trade-off (Becker and Lewis 1973).
Second, early marriage can potentially lower educational attainment among women, negatively affecting women’s bargaining power within the household, which affects intrahousehold resource allocation and welfare outcomes of children (e.g., Thomas 1990, 1994). Therefore, early marriage among women also adversely affects human capital formation of the next generation.
Verifying our hypothesis is not an easy task since, in many countries, mortality rate and marriage age are negatively correlated over time. Due to improved nutrition and medical science, the mortality rate has decreased until recently, which partly contributed to rapid population growth in developing countries. Marriage age, on the other hand, has had an upward trend in many countries (Harwood-Lejeune 2000; Manda and Meyer 2005), probably due to the increase in opportunity cost for marriage as women became more educated, along with economic development. Until recently, we therefore observed a negative correlation between marriage age and mortality rate. Therefore, when looking at correlation, we observe a negative correlation between mortality rate and marriage age. However, the question at hand is different. In this case, the mortality rate is increasing. What we try to untangle is the relationship between an increase in mortality and the effect on marriage age.
We use the following empirical strategy. Using the retrospective death records of respondents’ siblings in the 2004 Malawi Demographic and Health Survey, we calculate district-wide prime-age adult mortality probabilities that each birth cohort has faced, and relate it to marriage behavior for each birth cohort. We take a recent reversal of the mortality trend among prime-age adults as an exogenous change. We use the district-wide age-specific mortality probability in the population aged 26–30 as the reference for a prime-age adult mortality. Then we assume that women aged 11–15 observe the reference mortality (in the age 26–30 group) to form perceptions on HIV/AIDS risks. The perception formed at this age will, in turn, influence the marriage decision later in their lives.
However, caution is needed when using respondents’ siblings to infer the reference prime-age mortality rate in the region because the death of older siblings directly impacts the marriage behavior of younger siblings. The death of a sibling changes intrafamily resource allocation, which affects marriage decisions among young women. For example, if an income earner dies, the drop in household income may encourage young women in the household to get married earlier to join another family.4 The death of older siblings may also affect knowledge flow to younger siblings regarding marriage. This effect needs to be controlled in the estimation.
The article is organized as follows. The next section describes our data set and key demographic characteristics of the sample population, such as the trends in prime-age adult mortality and first-marriage age in Malawi. We show that the probability of prime-age adult mortality estimated from the DHS siblings data differs across districts and is drastically larger in high-HIV-prevalence districts. We also observe an upward trend of marriage age among different cohorts, which has only recently been reversed among young women, especially in regions severely affected by HIV/AIDS.
The following section sets up a dynamic model that describes marriage decisions in the presence of excess mortality during singlehood. Excess mortality lowers the average marriage age because early marriage secures a safe partner, which increases survival rate among married agents. Following that is a discussion of our empirical strategy. Since we observe marriage age only if the respondent is married, we use tobit and duration models to account for the right-censoring issue—distinguishing between complete and incomplete singlehood samples.
We found that the excess mortality due to AIDS that has been observed in recent years has led to a decrease in women’s age for their first marriage. This finding goes against a stylized fact that in many countries, marriage age for women has an upward trend over time. Our results suggest that the recent emergence of the HIV/AIDS epidemic is the main cause. We also found that young women became more conservative in their sexual activity, shortening the sexually active premarital period and hence reducing infection risk. Concluding remarks are offered in the final section.
EMPIRICAL MOTIVATION
Data
This study uses the 2004 Malawi Demographic and Health Survey (DHS).5 The DHS surveys, conducted in various developing countries since the mid-1980s, are nationally representative and are designed to collect information on marriage, fertility, family planning, reproductive health, child health, and HIV/AIDS. The sample size (respondent women) of the 2004 Malawi DHS is 11,245. Reproductive-age women aged 15–49 are the focus of the survey, although the most recent DHS surveys also included husbands’ and household questionnaires. The questionnaire also includes a list of female respondents’ siblings and asks whether each of these siblings is still alive at the time of the survey. It has additional information on siblings’ current age and, if deceased, year of death and age at death.
The DHS surveys are large databases suitable for researchers who analyze socioeconomic impacts of HIV/AIDS. It includes rich information on marital status, current and past sexual behavior (including premarital and extramarital sexual activities), knowledge and attitude toward HIV/AIDS, and the result of HIV testing for subsample respondents.6
For the purpose of our study, the birth-death records of respondents’ siblings are particularly important. To examine the changes of marriage behavior arising from the AIDS epidemic, ideally the information on the regional HIV prevalence in different periods should be used. However, since there are not many surveillance sites collecting HIV test results in the country, it is difficult to investigate the regional differences and dynamic changes in the prevalence rate from aggregated statistics. The DHS survey can provide only the current estimates of HIV prevalence rate. Therefore, our analysis uses prime-age adult mortality as a proxy for HIV prevalence (for a similar approach, see Ainsworth et al. 2005; Chapoto and Janye 2006; Mather et al. 2004; and Yamano and Jayne 2005).
Our methodology is as follows. Using birth-death records of respondents’ siblings, we compute the probability of age-specific mortality, which is defined as the probability of dying between ages X and Y (X < Y); that is, the conditional probability of dying at age Y given that he/she lived until age X.7 We group people by five-year birth cohorts to track their age-specific mortality8: 1984–1988, 1979–1983, 1974–1978, 1969–1973, 1964–1968, 1959–1963, 1954–1958, and 1949–1953.
Trends in Adult Mortality
Table 1 reports estimates of the age-specific adult mortality probabilities by birth cohort in Malawi, calculated from the death information of all respondents’ siblings. To delineate the trend in mortality rates by birth cohort, the following age-specific probabilities of prime-age adult mortality are calculated: mortality probability in ages 16–20, 21–25, 26–30, 31–35, 36–40, 41–45, and 46–50.
Table 1.
Age-Specific Adult Mortality Probabilitiesa, by Birth Cohort
| Birth Cohort | Age 16–20 | Age 21–25 | Age 26–30 | Age 31–35 | Age 36–40 | Age 41–45 | Age 46–50 |
|---|---|---|---|---|---|---|---|
| 1979–1983 | 24.55 (0.04) |
||||||
| 1974–1978 | 16.16 (0.02) |
34.41 (0.07) |
|||||
| 1969–1973 | 13.55 (0.02) |
29.76 (0.07) |
61.85 (0.21) |
||||
| 1964–1968 | 10.98 (0.02) |
15.01 (0.03) |
37.15 (0.11) |
68.92 (0.28) |
|||
| 1959–1963 | 11.62 (0.02) |
12.07 (0.02) |
20.58 (0.05) |
41.37 (0.16) |
99.32 (0.61) |
||
| 1954–1958 | 14.98 (0.04) |
9.98 (0.02) |
15.36 (0.04) |
21.94 (0.07) |
66.30 (0.39) |
93.43 (0.69) |
|
| 1949–1953 | 17.21 (0.07) |
11.38 (0.04) |
17.71 (0.07) |
19.84 (0.08) |
31.28 (0.17) |
51.28 (0.37) |
112.11 (1.26) |
Notes: Standard errors are in parentheses. See Ueyama and Yamauchi (2008) for the data of age-specific mortality probabilities by gender.
Number of deaths per 1,000.
The data show that the probabilities of death are drastically higher for the younger cohorts. This tendency is especially pronounced in the mortality of prime-age adults: those aged 26–30, 31–35, and 36–40. For example, the probability of death at ages 26–30 for the 1969–1973 birth cohort (ages 31–35 in 2004) is 61.85 per 1,000, which is higher than that of other birth cohorts. The level is nearly four times greater than that for the 1949–1953 cohort.
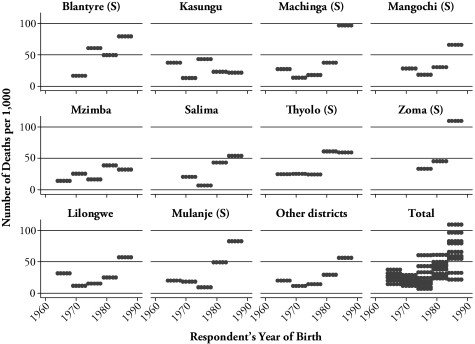
Figure 1 shows the probability of mortality for respondents aged 26–30 by both birth year and district to illustrate regional differences in the rising trend in prime-age adult mortality.9 In general, we find a recent upward trend in the mortality for all regions. However, changes vary by district. Districts in the southern region, such as Blantyre, Machinga, Zomba, and Mulanje, saw a more drastic increase in the mortality than districts in the northern and central regions. In contrast, changes in the mortality are gradual in the northern or central regions. Also, for the whole of Malawi, the variance of adult mortality across districts increased recently.
Figure 1.
Trends in Adult Mortality for Respondents Aged 26–30, by Birth Year and District
Notes: (S) denotes that the district is in the Southern region. The graph plots the number of deaths per 1,000. See Ueyama and Yamauchi (2008) for data on mortality probabilities at ages 26–30 by district.
This finding of more drastic increases in prime-age adult mortality in the southern region than in the northern and central regions is consistent with the regional trend in the HIV/AIDS epidemic. The southern region has the highest HIV prevalence rate of all regions. According to the DHS, the HIV prevalence rates are 17.5%, 8.1%, and 6.4% in the southern, northern, and central regions, respectively.
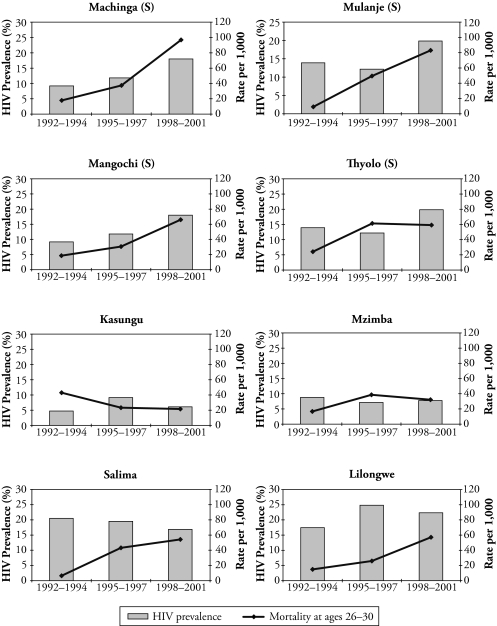
To highlight the relationship between the HIV prevalence rate and prime-age adult mortality, Figure 2 shows the trends in both indicators by district. We use the HIV prevalence rates from surveillance data within the district. The surveillance HIV prevalence rates come from the HIV database at the U.S. Census Bureau.10 While survey period varies across the surveillance sites, we have the HIV prevalence rate estimates between 1992 and 2001 for most sites. To accommodate missing values and to smooth the trend, we take the three-year average prevalence rates in the following three periods: 1992–1994, 1995–1997, and 1998–2001. Note that we do not have surveillance sites in the Blantyre and Zomba districts to infer their HIV prevalence rates. We use surveillance sites in rural areas except for in the Lilongwe district.
Figure 2.
HIV Prevalence and Prime-Age Adult Mortality, by District and Year
Notes: (S) denotes that the district is in the Southern region. Except for Lilongwe, the HIV prevalence rates come from rural surveillance sites. The HIV prevalence rates of Lilongwe are from urban sites.
Source: HIV prevalence rates of surveillance sites are based on HIV/AIDS Surveillance Data Base (available online at http://hivaidssurveillancedb.org/hivdb/) maintained by the U.S. Census Bureau. (For more details, see USAIDS/WHO [2006].) Mortality rates at ages 26–30 are authors’ estimates.
We use our estimates of mortality at ages 26–30 in the three periods to compare with the HIV prevalence rates. That is, for 1992–1994, we use the mortality from cohorts born between 1959 and 1963.
We find rising trends both in the HIV prevalence and in the prime-age adult mortality rates in most districts.11 In particular, the correlation seems to be stronger in the southern region. In this region, both the prevalence rates and the mortality rise sharply. The Pearson correlation coefficient between the prevalence and the probability of mortality at ages 26–30 is .70 in the southern region (number of observations = 15), while it is .44 in the whole of Malawi (number of observations = 27).
Age at First Marriage and Premarital Sexual Activities
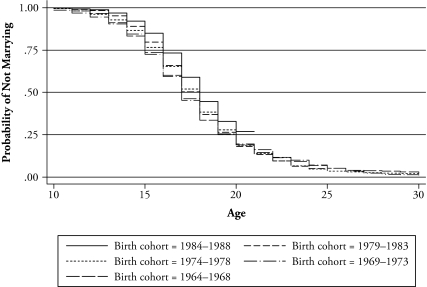
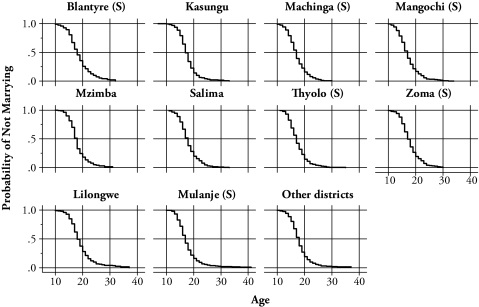
Figures 3 and 4 present the Kaplan-Meier estimates of the single proportion by birth cohort and district. While there do not seem to be drastic structural changes in timing of marriage among birth cohorts, in general, there is a gradual shift toward marriage at later ages. However, for the younger cohorts, marriage age seems to become younger (Figure 3). This result is consistent with the findings in Manda and Meyer (2005), who analyzed the timing of marriage by using the data from the 2000 Malawi DHS. Though the distributions are slightly different by district, there do not seem to be any differences in timing of marriage across districts (Figure 4).
Figure 3.
Kaplan-Meier Plot of the Probability of Not Marrying, by Birth Cohort
Figure 4.
Kaplan-Meier Estimates of the Probability of Not Marrying, by District
Notes: (S) denotes that the district is in the Southern region.
Table 2 shows regional differences in the marriage timing and premarital sexual behaviors by birth cohort. Panel A shows the proportions of those who were ever married by age 17 and age 19.12 The data suggest that the proportion married by both age 17 and age 19 is higher for younger cohorts. This tendency seems to be remarkable in the southern region, especially in districts where the HIV epidemic has spread, such as Blantyre, Machinga, Thyolo, and Mulamje.
Table 2.
Trends in the Timing of Marriage and Premarital Activities, by Birth Cohort and District
| A. Age at First Marriage |
||||||||
|---|---|---|---|---|---|---|---|---|
| Proportion Married by Age 17 |
Proportion Married by Age 19 |
|||||||
| 1979–1983 Cohort | 1974–1978 Cohort | 1969–1973 Cohort | 1964–1968 Cohort | 1979–1983 Cohort | 1974–1978 Cohort | 1969–1973 Cohort | 1964–1968 Cohort | |
| Southern Region | ||||||||
| Blantyre | 0.47 | 0.41 | 0.45 | 0.54 | 0.66 | 0.65 | 0.65 | 0.73 |
| Machinga | 0.59 | 0.64 | 0.55 | 0.69 | 0.81 | 0.75 | 0.72 | 0.82 |
| Mangochi | 0.63 | 0.64 | 0.62 | 0.52 | 0.77 | 0.83 | 0.76 | 0.74 |
| Thyolo | 0.66 | 0.51 | 0.56 | 0.68 | 0.81 | 0.74 | 0.77 | 0.84 |
| Zomba | 0.46 | 0.47 | 0.55 | 0.66 | 0.74 | 0.72 | 0.78 | 0.76 |
| Mulanje | 0.61 | 0.58 | 0.58 | 0.61 | 0.81 | 0.77 | 0.80 | 0.72 |
| North/Central Region | ||||||||
| Kasungu | 0.56 | 0.54 | 0.53 | 0.55 | 0.81 | 0.79 | 0.79 | 0.80 |
| Mzimba | 0.53 | 0.51 | 0.59 | 0.53 | 0.76 | 0.79 | 0.82 | 0.81 |
| Salima | 0.53 | 0.47 | 0.61 | 0.46 | 0.72 | 0.69 | 0.84 | 0.69 |
| Lilongwe | 0.34 | 0.40 | 0.54 | 0.51 | 0.60 | 0.62 | 0.75 | 0.74 |
| Other Districts | 0.50 | 0.46 | 0.54 | 0.49 | 0.77 | 0.73 | 0.73 | 0.73 |
| All of Malawi | 0.50 | 0.48 | 0.55 | 0.54 | 0.73 | 0.72 | 0.74 | 0.75 |
| B. Premarital Sexual Activities |
||||||||
| Proportion of Women Who Had First Premarital Sex |
Years Between First Sex and Marriage |
|||||||
| 1979–1983 Cohort | 1974–1978 Cohort | 1969–1973 Cohort | 1964–1968 Cohort | 1979–1983 Cohort | 1974–1978 Cohort | 1969–1973 Cohort | 1964–1968 Cohort | |
| Southern Region | ||||||||
| Blantyre | 0.51 | 0.45 | 0.43 | 0.35 | 1.74 | 2.07 | 2.21 | 1.69 |
| Machinga | 0.30 | 0.38 | 0.31 | 0.33 | 0.83 | 1.58 | 1.72 | 1.09 |
| Mangochi | 0.31 | 0.27 | 0.28 | 0.27 | 0.79 | 0.76 | 0.98 | 2.12 |
| Thyolo | 0.56 | 0.61 | 0.57 | 0.58 | 1.63 | 2.12 | 2.19 | 2.21 |
| Zomba | 0.58 | 0.60 | 0.42 | 0.42 | 1.98 | 1.78 | 1.60 | 1.86 |
| Mulanje | 0.45 | 0.42 | 0.44 | 0.35 | 1.19 | 1.38 | 1.77 | 1.81 |
| North/Central Region | ||||||||
| Kasungu | 0.28 | 0.25 | 0.26 | 0.20 | 0.47 | 0.43 | 0.79 | 0.58 |
| Mzimba | 0.26 | 0.21 | 0.11 | 0.19 | 0.90 | 0.78 | 0.41 | 0.44 |
| Salima | 0.27 | 0.37 | 0.24 | 0.24 | 0.59 | 1.23 | 0.91 | 1.07 |
| Lilongwe | 0.31 | 0.34 | 0.31 | 0.29 | 0.95 | 1.71 | 1.16 | 1.57 |
| Other Districts | 0.36 | 0.35 | 0.30 | 0.31 | 0.98 | 1.22 | 1.27 | 1.51 |
| All of Malawi | 0.37 | 0.37 | 0.33 | 0.32 | 1.07 | 1.32 | 1.34 | 1.43 |
Panel B of Table 2 describes premarital sexual activity. The left side shows the proportion of women who experienced premarital sex, and the right side shows the average years between the first sex and marriage by birth cohort and by district. The data show regional differences in premarital sexual activity. More unmarried women in the southern region are sexually active than in the northern and central regions. Also, the interval between first sex and marriage is longer in the southern region. The trends in premarital sexual activity by birth cohort seem to be an inverse U shape in many districts. Until recently, before the HIV/AIDS epidemic emerged, the share of women who had premarital sex tended to increase. However, as the epidemic has spread in the general population, people have become more cautious about sexual activity. This trend is pronounced in the interval years between the first sex and marriage. Especially in the southern region, the interval becomes shorter for younger cohorts than older ones in all districts except Zomba.
THEORETICAL HYPOTHESES
A Simple Model
Our model is based on a search framework in which agents who are single look for their marriage partners. As discussed in Yamauchi (2007), if AIDS-related mortality shock has decreased survival probability (i.e., discount factor) for single agents without equally affecting that for married agents, this gap in survival probability (defined as 1 minus the death rate, or discount factor in dynamic optimization) increases the incentive to marry.
If the search for a marriage partner involves some risk of HIV infection, an increase in the AIDS-related mortality rate increases the search cost (e.g., using condoms and acquiring more information on partners). Since the incentive to continue the search decreases, agents may delay the starting age for sexual activity and may decide to marry earlier. Together with a decrease in survival probability, this factor decreases the expected value of continuing the search, which increases the probability of marriage.
However, if the partner search does not involve sexual contact, an increase in prime-age adult mortality does not affect marriage behavior. Moreover, as a common form of risk aversion, women may decrease the use of sexual contact as the prime way to secure a marriage partner in the face of increased adult mortality due to the high HIV/AIDS prevalence. If so, prime-age adult mortality does not necessarily change marriage behavior among young women. Our main hypothesis critically depends on whether adult mortality increases the cost of partner search.
We assume that agents know how AIDS affects mortality risks and how marriage can function to reduce the risk. First, it is controversial to assume perfect information on the AIDS effect on mortality risks in the context of sub-Saharan Africa because some communities think the cause of death is more spiritual. In this case, we will not find any effect of increased mortality on marriage behavior in our empirical analysis. Our intention in this section is to lay out behavioral foundations for our empirical hypotheses to test in the next sections.
We introduce a simple model to describe the effects of the AIDS epidemic on the marriage decision. The stationary dynamic problem is summarized in the Bellman equation,
where VM(v) and VS are the values of marriage and singlehood, respectively, given marriage value v ~ F(v). Given that marriage is assumed to be a permanent decision,13 the value of marriage is written as
where W is wage income. After marriage, agents get only (1 – α) of wage income. The value of singlehood does not depend on v;
where c is search cost, which can increase due to the AIDS epidemic (e.g., due to HIV infection risks, agents use condoms and need better information on partners). Under the condition that β0 > β* > β where β0 is the non-AIDS survival probability, the gap in the survival probability between marriage and singlehood gives an incentive to get married earlier to protect one’s human capital.
The marriage decision is given as the following optimization,
The threshold point for spouse value v* (v above, which implies marriage) is
where m* = 1 – β*. The agent chooses to marry with a partner whose value v is greater than v*. Determinants of v* will affect marriage age.
Hypotheses
In the above model, whether the agent had an incentive to marry earlier or later depends on (changes in) survival probabilities in marriage and singlehood. If an increase in adult mortality decreases survival rate β (among singles) and increases search cost c (through sexual activities during singlehood), these factors will decrease v*, which increases the probability of getting married. On the other hand, an increase in the survival probability in marriage, β*, increases the incentive to marry. If β* is smaller than β (i.e., singlehood is safer than marriage), then agents have an incentive to delay marriage.
Though we have not explicitly incorporated sexual activity (e.g, delaying first sex) during singlehood in the model, it is feasible that the agent makes an action to control exposure to HIV/AIDS risks. In singlehood, the agent can make a risk-avoiding investment z (delaying first sex) with per-period cost p, which increases β(z). We interpret z as an action to limit exposure to HIV/AIDS risks. It is easy to show that z* increases as EV(v′) increases and decreases as p increases. Thus, searches without sexual activity increases the survival probability in singlehood.
Combining implications on marriage age and sexual activities during singlehood, we cannot make predictions about the total effect of increased adult mortality on first sex—the effect is ambiguous. Marriage age is expected to decrease, which lowers age for first sex, ceteris paribus. However, we also predict less sexual activity during singlehood, which increases the age at first sex, ceteris paribus. We test these hypotheses in the next sections using individual-level data from Malawi.
EMPIRICAL STRATEGY
To construct reference mortality rates for a respondent woman, we use district-level mortality probabilities for ages 26–30 when the respondent was aged 11–15. It is assumed that women aged 11–15 decide the timing of their marriage based on the observed mortality in adults aged 26–30 in the same district. For example, the mortality probability of women aged 26–30 in the birth cohort 1939–1943 is used in the analysis of marriage behavior of the 1949–1953 birth cohort.14
The second assumption is that the district is taken as the marriage market. According to our data, most postmarriage mobility is within a district. More than half of married women remain within the same village. This observation implies that the search for a spouse is also likely to be bounded within the district.
The recent increase in adult mortality is most pronounced in the southern region. Though cross-sectional variations across districts may reflect regional characteristics, dynamic changes observed in recent years are mainly attributed to the HIV/AIDS shocks which recently hit prime-age adults in the country (see the “Trends in Adult Mortality” section).
Siblings’ deaths also affect intrahousehold resource allocation, which influences marriage behavior among young women. When using the mortality rate estimates based on retrospective sibling records, young women living in districts with high prime-age adult mortality rates are more likely to face their siblings’ deaths. If we do not take into account direct effects of siblings’ deaths, we may overestimate the impact of HIV/AIDS epidemics in a marriage market on marriage behavior. Therefore, we have to control for this effect when estimating the effect of district-level aggregate mortality on marriage behavior. To do so, we include two additional variables on deceased siblings: (1) the proportion of siblings who died before reaching age 15, and (2) the proportion of adult siblings who died before the respondent reached the age of 15. Because infant and childhood mortality makes up a substantial portion of siblings’ deaths and child mortality is strongly correlated with income level, the former indicates living standards of the respondent’s family. The latter indicator is more related to HIV/AIDS within the household because the age range of siblings is in the prime ages.
We estimate the following equation
where is the marriage decision (e.g., marriage age) for woman i of cohort c in cluster j and district k, mkc is the district-level mortality at ages 26–30 at the time the respondent woman i was aged 11–15, xijkc is a set of variables that includes the proportion of deceased siblings (aged 26–30 when the respondent was aged 11–15), μi is the individual fixed effect, ϕc is the cohort (birth year) fixed effect, ηjk is the location fixed effect (district or cluster), and εijkc is an error term.
In the analyses, includes (1) first-marriage age, (2) age at first sex, and (3) the interval between first sex and first marriage, which is defined as the age at first marriage minus the age at first sex, meaning a period of exposure to premarital sexual activities. As for characteristics of the household during childhood, we include information on the type of place of residence during childhood (such as city, town, and countryside), number of siblings, and birth order in all analyses. We also control for ethnicity and religion and include age fixed effects (dummy variables) in all regressions.
We hypothesize that an increase in prime-age adult mortality due to AIDS motivates younger generations to abstain from risky sexual behaviors. In societies with a severe epidemic, people hasten the timing of marriage, delay their first intercourse, and reduce the period of premarital sexual activities. Thus, we expect the coefficients on district-level mortality to be negative for the marriage age and the period of premarital sexual activities but positive for the age of first intercourse. However, the relationship between the age at first sex and prime-age adult mortality is ambiguous. If one assumes that all girls engage in premarital sex, then the relationship is positive. However, if they become more conservative and abstain from premarital sex altogether, then the age at first sex is the same as the age at first marriage. In this case, the relationship would be negative. Therefore, the interval is a better measure to examine whether younger generations are changing their premarital sexual behavior in response to the HIV/AIDS epidemic because this incorporates the possibility of abstinence.
In the analysis of marriage age, we observe marriage age only if the respondent is married. For single women, we still do not know at what age they will marry. This is the same in the analysis of age at first sex. This problem is similar to the distinction between complete and incomplete tenure, in which we know only years of tenure when a worker has already quit his or her job. To solve this right-censoring problem in our estimation, we use a tobit model to separate samples of complete and incomplete singlehood, taking into account that the single may get married when they are older.
The married (complete singlehood) and the single (incomplete singlehood) form the following likelihood:
where is the probability that the potential marriage age is higher than the current age (i.e., he or she is currently single), and is the probability of observing marriage age y*. The same framework applies to the analysis of the interval between first sex and marriage age, since we can compute this variable only when the respondent is married. Likewise, we apply a tobit model to the analysis of age at first sex to account for virgin females.
We assume that mkc is uncorrelated with μi because mkc is an aggregate factor at the district level that is differentiated by cohort group and is unlikely to be correlated with individual unobserved characteristics under the assumptions that μi is distributed similarly in each district or cluster and uncorrelated with cohort given birth-year fixed effects.15 To check the assumption that mkc is uncorrelated with ui, we also include educational level to proxy for the woman’s (and her family’s) income level. It is plausible that the mortality shock affects poor (and uneducated) households more adversely than well-off (and educated) households. Put more simply, poverty may influence marriage behavior. Because we are controlling for birth-year fixed effects and village fixed effects in our framework, the inclusion of educational level means that we compare educated and uneducated women (and families) within the same cohort-village group.
This perspective also provides an interesting insight into the difference in the speed of adjustment in response to the local mortality change. We hypothesize that the more-educated can identify changes in the mortality rate more quickly and be in a position to promptly modify their marriage behavior than the uneducated. This conjecture has been verified in other studies that have examined social learning and learning by doing in different contexts (e.g., Foster and Rosenzweig 1995; Yamauchi 2004).
EMPIRICAL RESULTS
Table 3 shows estimation results, correcting for the right-censoring problem. We analyze age at first marriage, age at first sex, and years between first intercourse and first marriage. For single women in our sample, we use the current age for the lower bounds to correct for potential bias due to the right-censoring problem.
Table 3.
Tobit Analysis of the Impact of Adult Mortality on Marriage
| Variable | Age at First Marriage |
Interval Between First Sex and Marriage |
Age at First Sex |
|||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | |
| District Adult Mortality in Childhood | –0.008* (2.53) |
–0.007* (2.23) |
–0.036** (6.90) |
–0.033** (6.58) |
–0.004 (1.48) |
–0.003 (1.37) |
| Number of Siblings | 0.033* (1.99) |
0.023 (1.41) |
0.001 (0.04) |
–0.003 (0.11) |
0.036** (2.76) |
0.025† (1.92) |
| Birth Order | –0.014 (0.77) |
–0.017 (0.96) |
–0.017 (0.55) |
–0.023 (0.77) |
–0.029* (2.00) |
–0.029* (2.06) |
| Proportion of Deceased Infant/Child Siblings | –0.927** (5.39) |
–0.689** (3.99) |
–1.310** (4.50) |
–1.027** (3.53) |
–0.625** (4.54) |
–0.586** (4.25) |
| Proportion of Deceased Adult Siblings | –1.095** (2.92) |
–0.861* (2.34) |
–1.396* (2.18) |
–1.119† (1.79) |
–0.723* (2.41) |
–0.476 (1.61) |
| Childhood Place of Residence Is a Town | –0.463* (2.27) |
–0.234 (1.14) |
–0.703* (2.09) |
–0.361 (1.07) |
0.008 (0.05) |
0.015 (0.09) |
| Childhood Place of Residence Is a Countryside | –1.113** (5.98) |
–0.504** (2.66) |
–1.514** (4.95) |
–0.602† (1.93) |
–0.460** (3.15) |
–0.287† (1.93) |
| District Fixed Effects | yes | yes | yes | |||
| Village (cluster) Fixed Effects | yes | yes | yes | |||
| Number of Observations | 9,161 | 9,161 | 8,527 | 8,527 | 9,141 | 9,141 |
| Uncensored Observations | 7,723 | 7,723 | 7,106 | 7,106 | 8,294 | 8,294 |
| Censored Observations | 1,438 | 1,438 | 1,421 | 1,421 | 847 | 847 |
Notes: Absolute t statistics are in parentheses. The models also control for a constant term and women’s age (birth year), ethnicity, and religion.
p < .10;
p < .05;
p < .01
The results of marriage age are shown in columns 1 and 2, those of the interval between first sex and marriage are shown in columns 3 and 4, and those of age at first sex are presented in columns 5–6. Columns 1, 3, and 5 show the results with controls for district fixed effects. We find that the district-level mortality probability of a prime-age adult significantly reduces age at first marriage (column 1). These findings indicate that women who saw higher adult mortality in the neighborhood during their adolescence are more likely to marry at a younger age. Marriage is still customary in Malawi; therefore, it is not common to refuse marriage. One possible interpretation is that women tend to marry younger in an area experiencing high HIV prevalence to find a safe spouse. At the same time, the negative correlation between women’s marriage age and prime-age adult mortality may be interpreted as men choosing younger women to marry because younger women, especially virgins, have a lower probability of being HIV-positive.
Column 3 shows that the HIV epidemic has had a significant effect on reducing premarital sexual behavior. Women who faced higher adult mortality during their adolescence are likely to shorten the interval between first sexual intercourse and first marriage. However, we do not find any significant correlation between the age of first intercourse and mortality during their adolescence (column 5). This is because first marriage is frequently interdependent with the first intercourse. On the one hand, the HIV/AIDS epidemic motivates unmarried women to delay or avoid premarital sexual activities; on the other hand, early marriage as a risk-mitigating strategy causes them to be sexually active at an earlier age. Because these effects offset each other, the empirical result for age at first sex becomes ambiguous. Combined with our previous result, this finding means that women take a two-pronged strategy: reducing premarital sexual activities and marrying at a younger age.
In addition to the regional mortality effect, the proportion of deceased siblings is an important factor affecting the timing of marriage. The death of siblings significantly decreases first-marriage age and shortens the time span between first intercourse and first marriage. There are two reasons for this negative effect. First, the death of siblings may cause a reduction in household income, which encourages young women to get married to secure their livelihood elsewhere. Second, young women can learn from siblings’ deaths within the household that the HIV/AIDS epidemic is a significant problem among the population from which they choose their spouses. Both factors decrease first-marriage age.
The right-censoring problem causes an upward bias in the parameter estimates of interest. Ignoring incomplete singlehood samples (especially young women), we lose information from a group of women who may marry later. Because mortality rate estimates are cohort-specific in our analysis, a substantial proportion of the recent cohort would be dropped from our sample. Therefore, the negative impact of mortality on marriage behavior may be underestimated without the sample of younger women (the recent cohort).
The results in columns 1, 3, and 5 in Table 3, though corrected for the right-censoring problem, may be biased due to a correlation between village-specific and individual-specific fixed unobservable variables and explanatory variables in the equation. However, since our mortality measure is at the district level, potential bias will be minimal.
To check the robustness, columns 2, 4, and 6 show results with village fixed effects. The results are quite similar to those with district fixed effects. The coefficient estimates on adult mortality are significant and negative in the analyses of marriage age and the interval between first sex and marriage but are insignificant in the analysis of age at first sex. The magnitude of the effects of adult mortality on age at first marriage and the period of premarital sexual activity is large. For example, if the probability of prime-age adult mortality increases from 0 to 60 per 1,000, the timing of marriage comes earlier by half a year, and the period of premarital sexual activity is shortened by about 2 years. (The recent probability of mortality at ages 26–30 is about 60 per 1,000 in many southern districts, where HIV prevalence is high.)
Table 4 includes interaction terms between the district-level adult mortality rates and the birth cohorts of women. The base category of birth cohort is women who were born between 1984 and 1988 (the youngest cohort). In this group, district-level adult mortality significantly decreases first-marriage age. The interactions are insignificant in most cases, implying that there are no significant differences with the base (youngest) cohort.
Table 4.
Tobit Analysis of the Impact of Adult Mortality on Marriage, With Controls for Village Fixed Effects (with interaction between district adult mortality and birth cohort)
| Variable | Age at First Marriage (1) |
Interval Between First Sex and Marriage (2) |
|---|---|---|
| District Adult Mortality in Childhood | –0.010** (2.70) |
–0.046** (7.88) |
| 1979–1983 cohort × Adult mortality | 0.001 (0.13) |
0.041** (3.91) |
| 1974–1978 cohort × Adult mortality | 0.010 (1.41) |
0.034** (2.96) |
| 1969–1973 cohort × Adult mortality | 0.020 (1.33) |
0.063* (2.44) |
| 1964–1968 cohort × Adult mortality | 0.011 (0.66) |
0.052† (1.78) |
| Number of Siblings | 0.023 (1.40) |
–0.001 (0.05) |
| Birth Order | –0.016 (0.91) |
–0.021 (0.71) |
| Proportion of Deceased Infant/Child Siblings | –0.686** (3.98) |
–1.036** (3.57) |
| Proportion of Deceased Adult Siblings | –0.854* (2.32) |
–1.141† (1.82) |
| Childhood Place of Residence Is a Town | –0.228 (1.11) |
–0.332 (0.98) |
| Childhood Place of Residence Is a Countryside | –0.503** (2.65) |
–0.601† (1.93) |
| Number of Observations | 9,161 | 8,527 |
| Uncensored Observations | 7,723 | 7,106 |
| Censored Observations | 1,438 | 1,421 |
Notes: Absolute t statistics are in parentheses. The models include a constant term and dummy variables for village and women’s age (birth year), ethnicity, and religion.
p < .10;
p < .05;
p < .01
We observe some cohort-specific heterogeneity in the age difference between first sex and first marriage. The negative effect of adult mortality is significant only in the base group of the most recent cohort. The effect seems to be much smaller, not different from zero, among older cohorts.
As discussed, we check the robustness of the results in Table 4 by including highest level of education attained. Because in most cases there is only one respondent in the sample household, individual characteristics also represent household condition. Though, ideally, we would like to include income measures for each respondent as of her childhood or adolescence, the data do not have such retrospective information. We think that educational attainment can capture income level if we control the cohort effect (or trend) in educational attainment. Table 5 includes dummy variables representing primary, secondary, and higher education attained in the right-censoring-controlled tobit model.16
Table 5.
Tobit Analysis of the Impact of Adult Mortality on Marriage, With Controls for Respondents’ Education and Village Fixed Effects
| Variable | Age at First Marriage |
Interval Between First Sex and Marriage |
||
|---|---|---|---|---|
| (1) | (2) | (3) | (4) | |
| District Adult Mortality in Childhood | –0.0053† (1.79) |
–0.015** (2.87) |
–0.0293** (5.89) |
–0.0668** (7.41) |
| Highest Education Attained (ref. = no education) | ||||
| Primary education | 0.5821** (6.50) |
0.2112 (1.28) |
–0.1587 (1.02) |
–0.9118** (3.17) |
| Secondary education | 3.5993** (26.63) |
3.6652** (14.82) |
3.2789** (14.22) |
–0.0199 (0.05) |
| Higher education | 6.9723** (14.06) |
6.778** (6.41) |
6.2455** (7.64) |
–2.2777 (1.28) |
| Primary education × Adult mortality | 0.0128** (2.60) |
0.0303** (3.55) |
||
| Secondary education × Adult mortality | 0.001 (0.17) |
0.0955** (8.79) |
||
| Higher education × Adult mortality | 0.0075 (0.24) |
0.2669** (5.33) |
||
| Number of Siblings | 0.0074 (0.47) |
0.0068 (0.43) |
–0.0129 (0.47) |
–0.0064 (0.24) |
| Birth Order | –0.0196 (1.16) |
–0.019 (1.12) |
–0.0266 (0.90) |
–0.0273 (0.93) |
| Proportion of Deceased Infant/Child Siblings | –0.3614* (2.20) |
–0.3556* (2.17) |
–0.6674* (2.34) |
–0.6588* (2.32) |
| Proportion of Deceased Adult Siblings | –0.5779 (1.64) |
–0.5889† (1.67) |
–0.7957 (1.30) |
–0.6712 (1.10) |
| Childhood Place of Residence Is a Town | –0.2635 (1.34) |
–0.2578 (1.32) |
–0.3865 (1.17) |
–0.3046 (0.92) |
| Childhood Place of Residence Is a Countryside | –0.0523 (0.29) |
–0.0524 (0.29) |
–0.1332 (0.43) |
–0.0617 (0.20) |
| Number of Observations | 9,161 | 8,527 | 8,527 | 8,527 |
| Uncensored Observations | 7,723 | 7,106 | 7,106 | 7,106 |
| Censored Observations | 1,438 | 1,421 | 1,421 | 1,421 |
Notes: Absolute t statistics are in parentheses. Models include a constant term and dummy variables for village and women’s age (birth year), ethnicity, and religion.
p < .10;
p < .05;
p < .01
The first two columns of Table 5 show the results for age at first marriage. District-level mortality has a significant impact on the timing of marriage, even after we control for educational level. The coefficients on dummy variables for primary, secondary, and higher education are significant and positive, meaning that educated women tend to marry later than uneducated women. The magnitude of the coefficient on higher education is larger than that on primary education. To test whether the impact of adult mortality on the timing of marriage differs by educational levels, column 2 adds the interaction terms between district adult mortality and dummy variables for educational levels. The coefficient on adult mortality is still significant and negative. The coefficients on dummy variables for secondary and higher education are very similar to those in column 1, though the coefficient on primary education becomes insignificant. The coefficient on the interaction term between adult mortality and primary education is significant and positive, suggesting that for women who have completed only primary school, the mortality effect is weakened. For others, the mortality effect remains robust.
Similar results are obtained for the interval between first sex and marriage. The coefficients on adult mortality are significant and negative. Women who attained primary school have a shorter interval between first intercourse and marriage than do uneducated women. In column 4, we find that the mortality effect is weak among educated women. They have a longer period of singlehood after first sex.
To check the robustness of the above tobit results, we employ a duration (survival) analysis of the marriage decision. Table 6 shows the results of the proportional hazard model with time-variant explanatory variables. The measure of survival duration is number of years until marriage. We use the district-level mortality probability at ages 26–30 in t – 5 (years) for potential decision making at t (t = 1,2,3,….,T). This is a time-variant, district-level, prime-age adult mortality probability. Other explanatory variables are the same as in the tobit model. Since in the general setup we treat “marriage” as an exit state, a hazard ratio greater than 1 means the woman is more likely to enter marriage—that is, she has a shorter duration until marriage.
Table 6.
A Duration Analysis of the Impact of Adult Mortality on Marriage Timing (odds ratios)
| Variable | Conditional Hazard: Marriage |
|||
|---|---|---|---|---|
| (1) | (2) | (3) | (4) | |
| District Adult Mortality in Childhood | 1.049** (59.32) |
1.062** (27.48) |
1.05** (59.77) |
1.049** (39.60) |
| 1979–1983 cohort × Adult mortality | 0.995* (2.31) |
|||
| 1974–1978 cohort × Adult mortality | 0.993** (2.98) |
|||
| 1969–1973 cohort × Adult mortality | 0.979** (8.35) |
|||
| 1964–1968 cohort × Adult mortality | 0.944** (11.27) |
|||
| Highest Education Attained (ref. = no education) | ||||
| Primary education | 0.94* (2.45) |
0.86** (3.22) |
||
| Secondary education | 0.38** (17.99) |
0.37** (9.59) |
||
| Higher education | 0.24** (6.38) |
0.54 (1.35) |
||
| Primary education × Adult mortality | 1.00† (1.72) |
|||
| Secondary education × Adult mortality | 1.00 (0.40) |
|||
| Higher education × Adult mortality | 0.98† (1.83) |
|||
| Number of Siblings | 0.996 (0.72) |
0.996 (0.90) |
1.004 (0.71) |
1.004 (0.76) |
| Birth Order | 1.002 (0.34) |
1.002 (0.42) |
1.002 (0.42) |
1.002 (0.40) |
| Proportion of Deceased Infant/Child Siblings | 1.219** (3.89) |
1.203** (3.76) |
1.085 (1.61) |
1.085 (1.61) |
| Proportion of Deceased Adult Siblings | 1.146 (1.06) |
1.105 (0.77) |
1.095 (0.76) |
1.097 (0.77) |
| Childhood Place of Residence Is a Town | 1.218* (2.31) |
1.147† (1.73) |
1.162† (1.89) |
1.162† (1.89) |
| Childhood Place of Residence Is a Countryside | 1.369** (3.94) |
1.321** (3.84) |
1.075 (0.97) |
1.073 (0.95) |
| Number of Observations | 134,477 | 134,477 | 134,477 | 134,477 |
Notes: Robust z statistics are in parentheses. All regressions include dummy variables for district and women’s age (birth year), ethnicity, and religion.
p < .10;
p < .05;
p < .01
All the estimates are shown as hazard ratios, and absolute z values are reported in parentheses. The mortality rate at age 26–30 is significant, and the hazard ratio is greater than unity, implying that women who faced higher district-level prime-age mortality during childhood married earlier in their lives (column 1). In column 2, we interacted birth cohort indicators with the district-level adult mortality. The results are similar to those obtained in the tobit model in Table 4. The youngest cohort (baseline cohort) changes their marriage timing in response to an increase in adult mortality in the region.
Column 3 includes women’s educational levels, and column 4 adds its interaction with the district-level adult mortality. The results are very similar to those in Table 5 (tobit model). Even after we control for women’s education, the impact of adult mortality is still significant. The probability of marriage decreases with women’s educational level. The interactions between adult mortality and women’s education are significant for primary and higher education, but their odds ratios are approximately equal to 1. This suggests that the impact of adult mortality on marriage age is not different largely by educational level. Women who face higher district-level adult mortality tend to hasten their marriage regardless of their educational levels. In Table 6, therefore, we confirm our key empirical results in the survival analysis also.
CONCLUSION
This article shows that excess mortality arising from AIDS observed in recent years decreased women’s age for their first marriage in Malawi. This finding goes against a stylized fact that in many countries, marriage age for women has been rising over time. The recent emergence of an HIV/AIDS epidemic reversed this trend in Malawi and likely in other countries in sub-Saharan Africa. Young women also became more conservative in their sexual activity, shortening the premarital sexually active period.
The findings have some implications for human capital formation among women and for the next generations. First, early marriage means less schooling among young women, which may weaken their bargaining power in the household and consequently have negative effects on children. Second, a longer period of marriage may also imply an increase in fertility, which also has a negative effect on child schooling through the so-called quantity-quality trade-off. Therefore, it is possible that AIDS-related excess mortality has negative effects on human capital formation among women and the next generations through changes in women’s marriage behavior. The detailed investigation of the impact of women’s early marriage on human capital formation is an issue to be tackled in the future.
Acknowledgments
The authors thank Scott Drimie, Kathleen Beegle, and anonymous referees for useful comments. We are grateful to the Japan Society for the Promotion of Sciences; Japan’s Ministry of Education, Culture, Sport, Science and Technology’s the 21st Century Center of Excellence Project (National Graduate Institute for Policy Studies); and the Regional Network on AIDS, Livelihoods and Food Security (RENEWAL) for financial support.
Footnotes
In addition, a number of epidemiological and anthropological studies documented recent trends in the rising age at marriage and increased instances in premarital sex for young girls in sub-Saharan Africa without focusing on recent HIV/AIDS epidemics (Manda and Meyer 2005; Mensch, Grant, and Blanc 2006; Zaba et al. 2004).
Unlike many previous studies, ours uses a nationally representative household survey from Malawi, where the HIV prevalence is high and the excess prime-age adult mortality has increased in recent years.
Due to data limitations, we examine women’s behavioral responses. However, our empirical results are applicable to an interpretation of men’s behavioral responses. Men as well as women seek younger partners (who have lower probability of being infected) to marry and decide whom to marry at a younger age to reduce the risk of HIV infection. Men’s preference for younger wives accelerates women’s early marriage. We predict a negative correlation between marriage age and HIV/AIDS epidemic among both men and women.
For instance, Beegle and Krutikova (2007) found that orphaned girls tend to marry at significantly younger ages than non-orphan girls.
The official Web site of the DHS is http://www.measuredhs.com/start.cfm.
The result of HIV test question allows for an in-depth analysis of sociodemographic and behavioral factors of HIV infection (e.g., de Walque 2006; Gersovitz 2005). In addition, although HIV prevalence rates are usually estimated from surveillance data taken from pregnant women attending antenatal clinics and high-risk populations, the DHS can provide nationally representative estimates of HIV infection, which is more accurate than the surveillance method.
Since respondents are, by definition, alive during the survey period, mortality estimates could be downwardly biased if survival probabilities among siblings are positively correlated.
See Ueyama and Yamauchi (2008) for data on the district-wide age-specific mortality probabilities.
See the Web site of the U.S. Census Bureau, HIV/AIDS Surveillance Database: http://www.census.gov/ipc/www/hivaidsd.html. See UNAIDS/WHO (2006) for time-series infection estimates of each surveillance site.
Another reason for the difficulty in comparing the correlation between HIV prevalence and adult mortality is a long time lag between infection and death due to AIDS. Current excess mortality due to AIDS may reflect past HIV prevalence.
The median age at the time of first marriage is 17, and the 75th percentile of women ever married in our sample is age 19.
It is not hard to include separation (divorce) probability, though we simplify this aspect. We also ignore polygamy in this section as we focus on women’s decisions.
The above assumption may be arbitrary. However, if we expand the age range of adult mortality rates, then more data are required for the calculation of mortality rates in the past. Since the data set has only eight cohorts, expanding the age range for adult mortality will result in a smaller number of cohorts of women that we can study.
The individual fixed effect is subsumed in the residual in our estimation.
Changes in labor-market conditions (such as returns to schooling) affect the incentive to invest in schooling, which influences women’s marriage behavior. Given the possibility that schooling and marriage decisions are interrelated, it is important to control completed schooling levels in the analysis. However, we assume that schooling is exogenously determined.
Contributor Information
MIKA UEYAMA, Japan International Cooperation Agency Research Institute 10-5, Ichigaya Honmura-cho, Shinjuku-ku, Tokyo, 162-8433, JAPAN; e-mail:Ueyama.Mika@jica.go.jp..
FUTOSHI YAMAUCHI, International Food Policy Research Institute, Washington DC; e-mail:f.yamauchi@cgiar.org..
REFERENCES
- Ainsworth M, Beegle K, Koda G. “The Impact of Adult Mortality and Parental Deaths on Primary Schooling in North-Western Tanzania”. Journal of Development Studies. 2005;41:412–39. [Google Scholar]
- Becker GS, Lewis HG. “On the Interaction Between the Quantity and Quality of Children”. Journal of Political Economy. 1973;81:279–88. [Google Scholar]
- Beegle K, Krutikova S.2007“Adult Mortality and Children’s Transition Into Marriage”World Bank Policy Research Working Paper 4139. World Bank; Washington, DC [Google Scholar]
- Bloom SS, Banda C, Songolo G, Mulendema S, Cunningham AE, Boerma JT. “Looking for Change in Response to the AIDS Epidemic: Trends in AIDS Knowledge and Sexual Behavior in Zambia, 1990 Through 1998”. Journal of Acquired Immune Deficiency Syndrome. 2000;25:77–85. doi: 10.1097/00042560-200009010-00011. [DOI] [PubMed] [Google Scholar]
- Bongaarts J. “Late Marriage and the HIV Epidemic in Sub-Saharan Africa”. Population Studies. 2007;61:73–83. doi: 10.1080/00324720601048343. [DOI] [PubMed] [Google Scholar]
- Caldwell JC, Caldwell P, Anarfi J, Awusabo-Asare K, Ntozi J, Orubuloye IO, Marck J, Cosford W, Colombo R, Hollings E. Resistances to Behavioural Change to Reduce HIV/AIDS Infection in Predominantly Heterosexual Epidemics in Third World Countries. Canberra: Health Transition Centre, National Centre for Epidemiology and Population Health, The Australian National University; 1999. [Google Scholar]
- Chapoto A, Jayne TS. “Socio-economic Characteristics of Individuals Affected by AIDS-Related Prime Age Mortality in Zambia.”. In: Gillespie S, editor. AIDS, Poverty, and Hunger: Challenges and Responses. Washington, DC: International Food Policy Research Institute; 2006. pp. 33–55. [Google Scholar]
- Clark S. “Early Marriage and HIV Risks in Sub-Saharan Africa”. Studies in Family Planning. 2004;35:149–60. doi: 10.1111/j.1728-4465.2004.00019.x. [DOI] [PubMed] [Google Scholar]
- de Walque D.2006“Who Gets AIDS and How? The Determinants of HIV Infection and Sexual Behaviors in Burkina Faso, Cameroon, Ghana, Kenya, and Tanzania”World Bank Policy Research Working Paper No. 3844World Bank; Washington, DC [Google Scholar]
- Foster AD, Rosenzweig MR. “Learning by Doing and Learning From Others: Human Capital and Technical Change in Agriculture”. Journal of Political Economy. 1995;103:1176–209. [Google Scholar]
- Gersovitz M. “The HIV Epidemic in Four African Countries Seen Through the Demographic and Health Surveys”. Journal of African Economies. 2005;14:191–246. [Google Scholar]
- Gregson S, Nyamukapa CA, Garnett GP, Mason PR, Zhuwau T, Caraël M, Chandiwana SK, Anderson RM. “Sexual Mixing Patterns and Sex Differentials in Teenage Exposure to HIV Infection in Rural Zimbabwe”. Lancet. 2002;359:1896–903. doi: 10.1016/S0140-6736(02)08780-9. [DOI] [PubMed] [Google Scholar]
- Harwood-Lejeune AL. “Rising Age at Marriage and Fertility in Southern and Eastern Africa”. European Journal of Population. 2000;17:261–80. [Google Scholar]
- Helleringer S, Kohler H-P. “Social Networks, Perceptions of Risk, and Changing Attitudes Towards HIV/AIDS: New Evidence From a Longitudinal Study Using Fixed-Effects Analysis”. Population Studies. 2005;59:265–82. doi: 10.1080/00324720500212230. [DOI] [PubMed] [Google Scholar]
- Lagarde G, Emmanual P, Enel C. “Knowledge, Attitudes and Perception of AIDS in Rural Senegal: Relationship to Sexual Behavior and Behavior Change”. AIDS. 1996;10:327–34. doi: 10.1097/00002030-199603000-00012. [DOI] [PubMed] [Google Scholar]
- Manda S, Meyer R. “Age at First Marriage in Malawi: Bayesian Multilevel Analysis Using a Discrete Time-to-Event Model”. Journal of the Royal Statistical Society. 2005;168:439–55. [Google Scholar]
- Mather D, Donovan C, Jayne TS, Weber M, Mazhangara E, Bailey L, Yoo K, Yamano T, Mghenyi E.2004“A Cross-Country Analysis of Household Responses to Adult Mortality in Rural Sub-Saharan Africa: Implications for HIV/AIDS Mitigation and Rural Development Policies.”MSU International Development Working Paper No. 82. Department of Agricultural Economics, Michigan State University.
- Mensch BS, Grant MJ, Blanc AK. “The Changing Context of Sexual Initiation in Sub-Saharan Africa”. Population and Development Review. 2006;32:699–727. [Google Scholar]
- Mukiza-Gapere J, Ntozi JPM. “Impact of AIDS on Marriage Patterns, Customs and Practices in Uganda”. Health Transition Review. 1995;5:201–208. [PubMed] [Google Scholar]
- Ng’weshemi JZ, Boerma JT, Pool R, Barongo L, Senkoro K, Maswe M, Insingo R, Schapink D, Nnko S, Borgdorff MW. “Changes in Male Sexual Behavior in Response to the AIDS Epidemic: Evidence From a Cohort Study in Urban Tanzania”. AIDS. 1996;10:1415–20. doi: 10.1097/00002030-199610000-00015. [DOI] [PubMed] [Google Scholar]
- Smith K, Watkins S. “Perceptions of Risk and Strategies for Prevention: Responses to HIV/AIDS in Rural Malawi”. Social Science and Medicine. 2005;60:649–60. doi: 10.1016/j.socscimed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Thomas D. “Intra-household Resource Allocation: An Inferential Approach”. Journal of Human Resources. 1990;25:635–64. [Google Scholar]
- Thomas D. “Like Father, Like Son; Like Mother, Like Daughter”. Journal of Human Resources. 1994;29:950–88. [Google Scholar]
- Ueyama M.2007“Mortality, Mobility and Schooling Outcomes Among Orphans: Evidence From Malawi”IFPRI Discussion Paper No 710International Food Policy Research Institute; Washington, DC [Google Scholar]
- Ueyama M, Yamauchi F.2008“Marriage Behavior Response to Prime-Age Adult Mortality: Evidence From Malawi”IFPRI Discussion Paper No 764. International Food Policy Research InstituteWashington, DC: [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS/WHO 2006Epidemiological Fact Sheets on HIV/AIDS and Sexually Transmitted Infections: MalawiUSAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance, Geneva. Available online at http://www.who.int/GlobalAtlas/predefinedReports/EFS2006/EFS_PDFs/EFS2006_MW.pdf
- Yamano T, Jayne TS. “Working-Age Adult Mortality and Primary School Attendance in Rural Kenya”. Economic Development and Cultural Change. 2005;53:619–54. [Google Scholar]
- Yamauchi F. “Are Experience and Schooling Complementary? Evidence From Migrants’ Assimilation Process in the Bangkok Labor Market”. Journal of Development Economics. 2004;74:489–513. [Google Scholar]
- Yamauchi F. International Food Policy Research Institute; Washington, DC: 2007. “Marriage, Schooling and Excess Mortality in Prime-Age Adults: Evidence From South Africa” IFPRI Discussion Paper No 691. [Google Scholar]
- Yamauchi F, Buthelezi T, Velia M. “Impacts of Prime-Age Adult Mortality on Labour Supply: Evidence From Adolescents and Women in South Africa”. Oxford Bulletin of Economics and Statistics. 2008;70:375–98. [Google Scholar]
- Zaba B, Pisani E, Slaymaker E, Boerma JT. “Age at First Sex: Understanding Recent Trends in African Demographic Surveys”. Sexually Transmitted Infections. 2004;80(Suppl. 2):28–35. doi: 10.1136/sti.2004.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]