Abstract
Background
Lung cancer screening could present a “teachable moment” for promoting smoking cessation and relapse prevention. Understanding the risk perceptions of older individuals who undergo screening will guide these efforts.
Purpose
This paper examines National Lung Screening Trial (NLST) participants’ perceptions of risk for lung cancer and other smoking-related diseases. We investigated (1) whether risk perceptions of lung cancer screening participants differed between current and former smokers and (2) which factors (sociodemographic, smoking and medical history, cognitive, emotional, and knowledge) were associated with these risk perceptions.
Methods
We analyzed baseline data collected from 630 NLST participants prior to their initial screen. Participants were older (55–74 years), heavy (minimum 30 pack years) current or former smokers. A ten-item risk perception measure was developed to assess perceived lifetime risk of lung cancer and other smoking-related diseases.
Results
The risk perception measure had excellent internal consistency (alpha=0.93). Former smokers had lower risk perceptions compared to current smokers. Factors independently associated with high risk perceptions among current smokers included having a personal history of a smoking-related disease, higher lifetime maximum number of cigarettes smoked daily, having lived with a smoker, high worry, high perceived severity of lung cancer and smoking-related diseases, and accurate knowledge of tenfold increased risk of lung cancer for a one pack per day smoker. Factors independently associated with high risk perceptions among former smokers included being White, past history of smoking within 30 min of waking, high worry, and accurate knowledge of tenfold increased risk of lung cancer for a one pack per day smoker.
Conclusions
Using a comprehensive risk perception measurement, we found that current and former smokers held different risk perceptions. Former and current smokers’ smoking and medical history, race, emotional concerns, behavior change cognitions, and knowledge should be considered during a prescreening risk communication session. We highlight the theoretical and risk communication implications for former and current smokers undergoing lung cancer screening.
Keywords: Risk perceptions, Lung cancer, Cigarette smoking
Introduction
Cigarette smoking is responsible for 87% of lung cancers [1, 2]. Compared to a never smoker, the relative risk of lung cancer is 10 for a one pack per day male smoker; it increases to 25 for a two pack per day smoker [3]. Smoking cessation has health benefits that increase with duration of abstinence, but even after 10 years of abstinence, former smokers are at increased risk of lung cancer and other smoking-related diseases (SRDs) compared with never smokers [4]. Approximately half of all continuing smokers die prematurely, losing on average 13–15 years of life [5].
Patients diagnosed with lung cancer have a poor prognosis; fewer than 20% survive 5 years after diagnosis [6, 7], but Henschke and others have proposed that detecting lung cancers at earlier, more treatable stages using spiral computed tomography (CT) screening will reduce lung cancer mortality [8, 9]. “Teachable moments” for health promotion activities are hypothesized to occur during cancer care [10]. Although much of the research in this area has focused on smoking cessation following a cancer diagnosis [11–16], lung cancer screening may also be a teachable moment in which to promote smoking cessation among current smokers and reinforce relapse prevention among former smokers [17]. Undergoing lung cancer screening may change cancer-related risk perceptions, an intermediate step that facilitates smokers’ intentions and motivation to quit [18–21]. However, to date, no one has comprehensively measured or examined the risk perceptions of older individuals who seek lung cancer screening.
Smokers’ risk perceptions are not well understood. There is no agreement on how smokers view their risks; this lack of agreement is likely due to the variability in how risk perceptions and outcomes are measured. Although some researchers have reported that smokers overestimate the health risks of smoking [22–24], others have found that smokers have an accurate or underestimated perception of their risk [25–30]. Smokers may display an optimistic distortion when they are asked to rate their own risk of acquiring a SRD compared to the “average smoker” or “other smokers” [31–35]. One study examined risk perceptions of older (50–72 years), heavy smokers and found that they underestimated their risks of premature mortality, cancer, and heart disease [25].
There are many benefits to smoking cessation for older patients. At age 60 and over, cessation can decrease rates of smoking-induced disease [36]. Within 2 years of quitting, smokers who quit after 65 years of age reduce their risk of death compared to current smokers. Smoking cessation in older adults decreases the risk of coronary events (within 1 year of quitting) and smoking-related cancers (within 5 to 10 years of quitting) [37]. However, few smoking cessation and relapse prevention studies have been conducted with older smokers.
The research that has been done with older smokers shows promise for cessation interventions with this population. Studies of smoking patterns and attitudes among older, heavy smokers found that most intend to quit in the next year [38], and 42% make an annual 24-h quit attempt [39]. Compared to younger smokers, older smokers are more likely to be successful if they attempt to quit [36] and are more likely to utilize smoking cessation assistance [40, 41]. A recent study demonstrated that 19% of smokers 65 years and older who were provided with a telephone quitline and low-cost pharmacotherapy were able to quit smoking and maintain their quit at 12-month follow-up [42]. An observational study of smokers’ behaviors following CT screening found that older age was associated with smoking abstinence at 12-month follow-up; there was a 25% abstinence rate in the oldest age group (70 years and over), compared to a 12% abstinence rate in the youngest age group (50–54 years) [20].
We examined prescreening lung cancer risk perceptions among older current and former heavy smokers participating in the National Lung Screening Trial (NLST) to inform future work using lung cancer screening as a teachable moment for promoting cessation and preventing relapse. We developed a questionnaire based on theoretical cognitive and emotional components from the Health Belief Model (HBM) [43], the Precaution Adoption Process Model [44], and the Self-Regulation Model [45]. The HBM [46, 47] states that individuals are more likely to make a preventive behavior change such as quitting smoking if they feel the threat is serious, they are at risk, they are confident that they could make a change, and they believe that making a change would be beneficial. The theory further posits that a “cue to action,” such as screening for lung cancer, can stimulate the behavior change process. Previous research has documented that intentions to quit are directly influenced by risk perceptions [48, 49]. Weinstein’s Precaution Adoption Process Model (1988) builds on the HBM by outlining the stages that individuals pass through before making a preventive behavior change; an individual must become aware of the health threat, believe that it poses a threat to others, believe that it poses a threat to himself or herself (in other words, overcome their optimistic bias), and then decide whether to take a precaution (e.g., change behavior). The Self-Regulation Model extends beyond the HBM to integrate emotional, as well as cognitive, responses to a threat. It posits that when a health threat is presented, two processes in health behavior change occur: (1) cognitive processes that construct beliefs about the illness and develop a plan of how to cope with it (e.g., risk perceptions of lung cancer and perceived benefits of coping with it) and (2) emotional processes involving fear and arousal (e.g., worry).
Our main objectives were to investigate (1) whether risk perceptions of lung cancer screening participants differed between current and former smokers and (2) which factors (sociodemographic, smoking and medical history, cognitive, emotional, and risk knowledge) were associated with these risk perceptions. Study findings will facilitate a better understanding of the components of risk perceptions, and this information can be used to enhance risk communication cessation interventions for current and former smokers who are undergoing lung cancer screening. This study is novel in that we (1) developed a comprehensive, multi-item measure of risk perceptions that included personal and comparative risk questions for lung cancer as well as SRD outcomes, (2) assessed behavior change constructs and knowledge among an older, high risk population, and (3) conducted assessments that captured risk perceptions prior to lung cancer screening.
Methods
Design
This cross-sectional study examined lung cancer and other SRD risk perceptions among a subset of participants of the NLST. The NLST is a collaboration between the American College of Radiology Imaging Network (ACRIN) and the National Cancer Institute Lung Screening Study. The NLST randomly assigned more than 54,000 participants at 33 sites to be screened with either spiral CT or chest X-ray. These participants are being followed for up to 8 years to determine whether there is a difference in lung cancer mortality between the two screening arms. Should lung cancer mortality be reduced in the CT arm of the trial, CT screening might become the standard of care.
Participants and Data Collection
The National Cancer Institute launched a national campaign when the ACRIN/NLST study began. The Cancer Information Services, an NCI in-house resource, developed a study eligibility protocol to screen potential participants who called in response to the media campaign. Recruitment efforts included public service announcements on local radio stations, advertisements in local newspapers, and communication with individual physicians and physician practices. All interested individuals were screened, over the telephone, for eligibility. At the time of recruitment into the trial, participants were 55–74 years of age, current or former (quit within the past 15 years) smokers with a history of 30 pack years or more, had no history of lung cancer, had not been treated for any cancer within the past 5 years other than a non-melanoma skin cancer, and were not participating in any other screening or cancer prevention trial.
Permission was obtained from the ACRIN/NLST executive committee to administer this questionnaire as a sub-study within the ACRIN arm of the trial. Twenty-three ACRIN study sites participated in the ACRIN arm of the NLST; eight sites opted to participate in this sub-study (Appendix). From December 2003 to March 2004, all trial enrollees at the eight sub-study participating sites completed our study questionnaire as part of the trial prescreening baseline questionnaire battery.
Pilot Work
Prior to trial administration, the study questionnaire was cognitively tested with 15 participants from the Brown University ACRIN site. Participants were asked if questions were (1) confusing, (2) difficult to answer (because of response categories), (3) repetitive, and (4) upsetting. The questionnaire was revised, based on these pilot results, and included changes to item wording (e.g., “such as emphysema, stroke, and heart disease” was added after “other than lung cancer” to clarify the term “smoking-related diseases”), item placement, item additions, response category options, and formatting (e.g., removal of unnecessary section headings and form formatting changed to match other trial forms). Institutional Review Board approval to administer the questionnaire was obtained from Partners Healthcare and all eight participating study sites.
Measures
ACRIN/NLST Baseline Sociodemographic, Medical, and Smoking History Variables
The trial baseline questionnaires [50] include measures on sociodemographics (age, education, gender, household income, marital status, and race/ethnicity), smoking history (smoking status, number of years quit, number of years smoked, highest number of cigarettes smoked per day, smoked within 30 min of waking, quit attempt in the past year, lived with a smoker, worked with a smoker, occupation with hazardous exposure, and smoking intentions), and medical history (personal history of cancer or SRD, family history of lung cancer, and experiencing cough or shortness of breath). Intentions to quit/stay quit were assessed on a 10-point scale with items ranging from, “I enjoy smoking so much I will never consider quitting no matter what happens” to “I have quit and I am 100% confident that I will never smoke again” [51].
Risk Perception Sub-study Questionnaire
Using constructs from behavior change theories, we developed a 25-item questionnaire. The questionnaire included risk perception items and theoretically based cognitive and emotional behavior change constructs. We also included knowledge of smoking risks questions; Kreuter and Strecher posited that in order to understand the risk perception biases of an individual, it is necessary to also assess and incorporate an individual’s knowledge about smoking risks [52]. We built upon previously published measures of these cognitive and emotional constructs. Questions appeared in the following order: risk perceptions [53–55], knowledge of smoking risks [23, 24, 27, 52], worry [53, 56], self-efficacy to quit smoking/stay quit [57], perceived benefits of quitting smoking [53, 58], and perceived severity [59].
Risk Perceptions
An individual’s risk perception profile is constructed based on his/her views of his/her personal and comparative risk. Since each informs and influences the other, we captured individuals’ personal risk reports (own risk) as well as their comparative risk reports (risk in relation to others). Since SRDs are also likely to be detected from screening, we asked about risk of SRDs, as well as risk of lung cancer. Risk perceptions were assessed from ten questions. To assess perceived personal risk, we posed four questions on a 5-point Likert scale about the likelihood (very unlikely to very likely) and danger (strongly disagree to strongly agree) of developing lung cancer and a SRD. Perceived comparative risk was assessed from six questions presented on a 5-point Likert scale. Since risk perceptions differ based on how individuals are asked and who is in the comparison group [27, 29, 53–55, 58], comparative risk was assessed with three different referent groups. Participants were asked if they were in danger of developing lung cancer and other SRDs, compared to the average person (strongly disagree to strongly agree). Participants were also asked about their chances of developing lung cancer and a SRD, compared to others of the same age and sex and other former/current smokers (much lower to much higher).
Knowledge, Cognitive, and Emotional Constructs Knowledge of smoking risks was assessed by three questions asking participants to quantify a smoker’s risk of developing lung cancer (“A smoker who smokes one pack of cigarettes a day is at how many times risk of developing lung cancer, compared to a nonsmoker?”) and the number of years of life lost due to smoking (“On average, smokers die nearly _____ years earlier than nonsmokers.”). In addition, participants were asked about the percentage of smokers who would get lung cancer (“Among 100 smokers, how many will get lung cancer because they smoke?”). Worry about lung cancer and other SRDs was assessed by four questions about intensity (4-point Likert scale, not at all to extremely) and frequency of worry (4-point Likert scale, not at all to all of the time); items were combined to create a single score (range=4–16; alpha=.89). Self-efficacy was measured by one question about level of confidence to quit smoking/remain quit (5-point Likert scale, not at all to extremely). Perceived benefits of quitting smoking was measured by three questions about the benefits of quitting in terms of decreasing risk for lung cancer and other SRDs and increasing life expectancy (4-point Likert scale, not at all to very much); these items were combined to create a single score (range=3–12; alpha=0.89). Perceived severity was assessed by four questions about the health consequences and severity of lung cancer and other SRDs (5-point Likert scale, not at all to extremely); these items were combined to create a single score (range 4–20; alpha=0.85).
Analysis
Statistical analyses were conducted using SPSS 16.0 statistical software. Current and former smokers were compared with respect to sociodemographics, smoking history, medical history, knowledge of smoking risks, risk perceptions, and cognitive and emotional behavior change constructs; chi-square and Fisher’s exact tests were used for categorical variables, analysis of variance for continuous variables, and Kruskal–Wallis for ordinal variables. The internal consistency of the risk perception outcome variable was assessed by calculating Cronbach’s alpha. Univariate analyses were run (analysis of variance for categorical variables, Pearson correlation for continuous variables, and Spearman rank for ordinal variables) to test for associations between the risk perception outcome variable and sociodemographics, smoking history, medical history, risk knowledge, and cognitive and emotional behavior change constructs. Factors associated with the risk perception outcome variable at p≤0.10 level in the univariate analyses are included in the table; two linear regression models were run, one for current smokers and one for former smokers. Spearman rank tests were run to examine the association between smoking behavior intentions and the risk perception outcome variable.
Results
Participant Characteristics
The study sample consisted of 630 ACRIN/NLST participants; 55% were current smokers (Table 1). Former smokers were older, more likely to be male and White, less likely to be among the lower income brackets, and more likely to be married. On average, former smokers had stopped smoking 7.1 years prior (SD=4.9), and 42.6% of current smokers had made an intentional 24-h quit attempt in the past year (Table 2). Former smokers had smoked for an average of 45 years (SD=5.7), and current smokers had smoked for an average of 43 years (SD=5.4; p<0.001). Former and current smokers did not differ on medical history variables, with the exception that 70.4% of current smokers, compared to only 51.6% of former smokers, experienced smoking-related symptoms such as a cough or shortness of breath (p<0.001).
Table 1.
Sociodemographic characteristics of participants
| Total ever smokers (n=630) | Current smoker (n=345) | Former smoker (n=285) | p value | |
|---|---|---|---|---|
| Age, mean (SD) | 60.9 (4.9) | 60.3 (4.6) | 61.6 (5.2) | 0.001 |
| Female gender, N (%) | 292 (46.3) | 181 (52.5) | 111 (38.9) | 0.001 |
| Education, N (%) | 0.06 | |||
| <HS graduate | 57 (9.0) | 35 (10.1) | 22 (7.7) | |
| HS graduate/GED | 189 (30.0) | 115 (33.3) | 74 (26.0) | |
| Post HS | 207 (32.9) | 110 (31.9) | 97 (34.0) | |
| College and beyond | 154 (24.4) | 73 (21.2) | 81 (28.4) | |
| Household income, N (%) | 0.001 | |||
| <$15,000 | 79 (12.5) | 55 (15.9) | 24 (8.4) | |
| $15,000–34,999 | 148 (23.5) | 93 (27.0) | 55 (19.3) | |
| $35,000–64,999 | 178 (28.3) | 82 (23.8) | 96 (33.7) | |
| $65,000 and above | 140 (22.2) | 80 (23.2) | 60 (21.1) | |
| Marital status, N (%) | 0.001 | |||
| Married/living as married | 398 (63.2) | 197 (57.1) | 201 (70.5) | |
| Widowed/divorced/separated | 204 (32.4) | 133 (38.6) | 71 (24.9) | |
| Never married | 25 (4.0) | 15 (4.3) | 10 (3.5) | |
| Race/ethnicity, N (%) | 0.03 | |||
| White | 573 (91.0) | 306 (88.7) | 267 (93.7) | |
| Black | 50 (7.9) | 36 (10.4) | 14 (4.9) | |
| Other | 7 (1.1) | 3 (0.9) | 4 (1.4) |
Note that column “%” may not add up to 100% if missing data.
Table 2.
Smoking and medical history
| Total ever smokers (n=630) | Current smoker (n=345) | Former smoker (n=285) | p value | |
|---|---|---|---|---|
| Smoking history | ||||
| Smoked within 30 min of waking, N (%) | 445 (70.7) | 260 (75.4) | 185 (65.1) | 0.003 |
| Quit attempt in the past year, N (%) | – | 147 (42.6) | – | – |
| Highest number of cigarettes/day, mean (SD) | 36.4 (16.2) | 35.6 (15.4) | 37.2 (17.0) | 0.22 |
| Number of years quit, mean (SD) | – | – | 7.1 (4.9) | – |
| Number of years smoked, mean (SD) | 44.1 (5.6) | 43.4 (5.4) | 45.0 (5.7) | <0.001 |
| Ever lived with a smoker, N (%) | 557 (88.4) | 313 (90.7) | 244 (85.6) | <0.05 |
| Ever worked with a smoker, N (%) | 575 (91.3) | 310 (89.9) | 265 (93.0) | 0.17 |
| Has occupational exposure, N (%) | 222 (35.2) | 122 (35.4) | 100 (35.1) | 0.94 |
| Medical history | ||||
| Has family history of lung cancer, N (%) | 138 (22.2) | 84 (24.6) | 54 (19.3) | 0.12 |
| Has personal history of cancer, N (%) | 65 (10.3) | 35 (10.1) | 30 (10.5) | 0.88 |
| Has personal history of SRD, N (%) | 168 (26.7) | 96 (27.8) | 72 (25.3) | 0.47 |
| Has cough or shortness of breath, N (%) | 390 (61.9) | 243 (70.4) | 147 (51.6) | <0.001 |
Note that SRD indicates smoking-related disease.
Risk Perceptions and Behavior Change Constructs
The ten-item smoking risk perceptions measure possessed excellent internal consistency (alpha=0.93). The risk perception total scores were summed; the mean total score was 35.2 [SD=7.5; range=10 (low) to 50 (high)] (Table 3). Compared to former smokers, current smokers had higher risk perceptions [means=36.9 (CS) and 33.1 (FS) respectively, p<0.001] and greater worry about lung cancer and SRDs [range=4–16; means=9.8 (CS) and 8.9 (FS) respectively, p<0.001]. Compared to current smokers, former smokers were more confident that they could remain quit [range, 1–5; means=4.6 (FS) and 2.7 (CS) respectively, p< 0.001] and believed in greater perceived benefits from quitting [range, 3–12; means=9.8 (FS) and 9.0 (CS) respectively, p<0.001]. Former smokers endorsed greater perceived severity of lung cancer and SRDs, compared to current smokers [range, 4–20; means 18.6 (FS) and 18.1 (CS) respectively, p<0.01].
Table 3.
Behavior change constructs and knowledge of smoking risks
| Total ever smokers (n=630) | Current smoker (n=345) | Former smoker (n=285) | p value | |
|---|---|---|---|---|
| Risk perceptions, mean (SD) | ||||
| Risk perceptions for lung cancer and SRDs | 35.2 (7.5) | 36.9 (7.3) | 33.1 (7.1) | <0.001 |
| Cognitive and emotional constructs, mean (SD) | ||||
| Perceived benefits of quitting smoking | 9.4 (2.2) | 9.0 (2.2) | 9.8 (2.0) | <0.001 |
| Confidence to quit smoking/remain quit | 3.6 (1.4) | 2.7 (1.1) | 4.6 (0.8) | <0.001 |
| Perceived severity of lung cancer and SRDs | 18.3 (2.1) | 18.1 (2.3) | 18.6 (1.7) | 0.01 |
| Worry about lung cancer and SRDs | 9.4 (2.9) | 9.8 (2.9) | 8.9 (2.7) | <0.001 |
| Knowledge of smoking risks | ||||
| Percent smokers who will get lung cancer, mean (SD) | 40.5 (23.3) | 39.2 (23.6) | 42.0 (23.0) | 0.16 |
| Average years decreased life for smokers, N (%) | 0.08 | |||
| 0–5 | 142 (30.6) | 75 (31.9) | 67 (29.3) | |
| 6–10 | 258 (55.6) | 136 (57.9) | 122 (53.3) | |
| 11 and higher | 64 (13.8) | 24 (10.2) | 40 (17.5) | |
| One pack/day smoker’s risk of developing lung cancer, N (%) | 0.01 | |||
| 0–2× risk | 208 (34.4) | 130 (39.5) | 78 (28.4) | |
| 5× risk | 183 (30.3) | 87 (26.4) | 96 (34.9) | |
| 10–20× risk | 213 (35.3) | 112 (34.0) | 101 (36.7) | |
Note that column “%” may not add up to 100% if missing data.
Note that SRD indicates smoking-related disease.
Factors associated with the risk perception outcome variable at p≤0.10 level in the univariate analyses were included in the multivariable analyses.
Knowledge of Smoking Risks
Both current and former smokers overestimated lung cancer prevalence among smokers; they estimated that approximately 40% of smokers would get lung cancer (Table 3). However, both current and former smokers underestimated other risks of smoking; current smokers were even more likely to do so. The majority of former and current smokers underestimated average years of life lost for smokers; only 18% of former smokers and 10% of current smokers accurately responded that smokers die greater than 10 years earlier than nonsmokers. Forty percent of current smokers and 28% of former smokers believed that a pack a day smoker had minimal (0–2×) elevated risk of lung cancer attributable to smoking; only 34% of current smokers and 37% of former smokers accurately identified the tenfold or greater risk for lung cancer.
Multivariable Analyses of Factors Associated with Risk Perceptions
In the multivariable analyses, we built separate models for current and former smokers (Table 4). The model for current smokers yielded six significant correlates of risk perceptions. Factors that were independently associated with high risk perceptions among current smokers were having a personal history of a SRD (β=0.16, SE=0.78, p= 0.001), higher lifetime maximum number of cigarettes smoked daily (β=0.18, SE=0.02, p<0.001), having lived with a smoker (β=0.10, SE=1.17, p=0.04), high worry (β=0.38, SE=0.15, p<0.001), high perceived severity of lung cancer and SRDs, (β=0.11, SE=0.17, p=0.04), and accurate knowledge of tenfold increased risk of lung cancer for a one pack per day smoker (0–2× risk compared to 10× risk; β=−0.19, SE=0.95, p=0.003). The model for former smokers yielded four significant correlates of risk perceptions. Factors that were independently associated with high risk perceptions among former smokers were being White (β=−0.20, SE=0.99, p=<0.001), smoked within 30 min of waking (β=0.18, SE=0.80, p=0.001), high worry (β=0.48, SE=0.16, p<0.001), and accurate knowledge of tenfold increased risk of lung cancer for a one pack per day smoker (0–2× risk compared to 10× risk; β=−0.18, SE=1.02, p=0.006).
Table 4.
Linear regression: factors associated with risk perceptions for lung cancer and smoking-related diseases (SRDs)
| Variables | β | SE | p value |
|---|---|---|---|
| Current smokers | |||
| Age | −0.04 | 0.08 | 0.37 |
| Experience cough or shortness of breath | −0.02 | 0.78 | 0.75 |
| Has personal history of SRD | 0.16 | 0.78 | 0.001 |
| Smoked within 30 min of waking | 0.04 | 0.80 | 0.39 |
| Highest number of cigarettes/day | 0.18 | 0.02 | <0.001 |
| Has family history of lung cancer | 0.08 | 0.84 | 0.11 |
| Ever worked with a smoker | 0.09 | 1.17 | 0.06 |
| Ever lived with a smoker | 0.10 | 1.17 | 0.04 |
| Worry about lung cancer and SRD | 0.38 | 0.15 | <0.001 |
| Perceived severity of lung cancer and SRD | 0.11 | 0.17 | 0.04 |
| Perceived benefits of quitting smoking | 0.07 | 0.17 | 0.19 |
| One pack/day smoker’s risk of developing lung cancer (0–2× risk)a | −0.19 | 0.95 | 0.003 |
| One pack/day smoker’s risk of developing lung cancer (5× risk)a | −0.02 | 0.45 | 0.68 |
| Former smokers | |||
| Black raceb | −0.20 | 0.99 | <0.001 |
| “Other” raceb | −0.02 | 3.31 | 0.66 |
| Experience cough or shortness of breath | 0.01 | 0.80 | 0.82 |
| Has personal history of SRD | 0.07 | 0.92 | 0.22 |
| Smoked within 30 min of waking | 0.18 | 0.80 | 0.001 |
| Number of years quit | −0.09 | 0.08 | 0.13 |
| Has family history of lung cancer | 0.08 | 1.01 | 0.15 |
| Ever lived with a smoker | 0.01 | 1.08 | 0.95 |
| Worry about lung cancer and SRD | 0.48 | 0.16 | <0.001 |
| Perceived severity of lung cancer and SRD | −0.04 | 0.24 | 0.45 |
| Perceived benefits of quitting smoking | −0.08 | 0.22 | 0.18 |
| Knowledge of increased risk of lung cancer (0–2× risk)a | −0.18 | 1.02 | 0.006 |
| Knowledge of increased risk from 1 pack/day (5× risk)a | −0.11 | 0.46 | 0.08 |
Note that SRD indicates smoking-related disease
Referent group=10–20× risk
Referent group=White participants
Smoking Risk Perceptions and Behavioral Intentions
Risk perceptions were associated with intentions to quit/stay quit (p<0.001 overall; within current smokers, p< 0.002; within former smokers, p<.01; Fig. 1). The relationship appeared curvilinear; the lowest risk perceptions were among current smokers who would never consider quitting (mean=29.1), and risk perceptions were the highest among current smokers who were planning on quitting in the next 30 days (38.3). Among former smokers, those who were confident that they would not relapse had lower risk perceptions (mean=32.6) compared to those who were concerned about relapse (35.9).
Fig. 1.
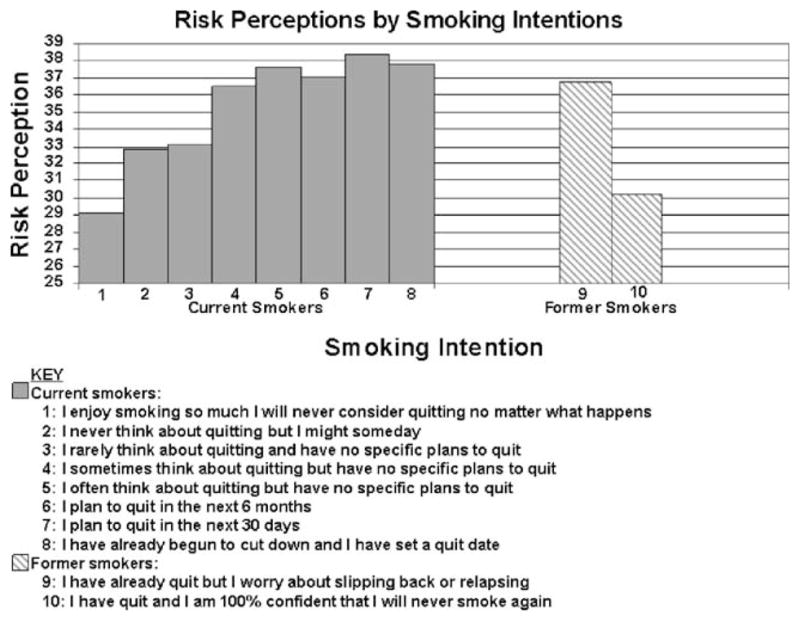
Risk perceptions by smoking intentions
Discussion
This study was designed to understand how older current and former heavy smokers who undergo lung cancer screening perceive their risk of lung cancer and other SRDs. We developed a comprehensive risk perceptions measure that examined whether risk perceptions of lung cancer differed among participants who were current versus former smokers and examined factors associated with risk perceptions.
We developed a ten-item risk perception measure that queried both personal and comparative risk for lung cancer and SRDs, had high internal consistency, and was associated with smoking intentions. Similar to work conducted by Weinstein and colleagues in 2005 [30], current smokers with quitting intentions had higher perceived risk than smokers who did not plan on quitting. In the next phase of our work, through longitudinal analyses of these data, we will ascertain whether these intentions translate into actual behaviors.
Most research examining smokers’ risk perceptions has used nonsmokers as a comparison group and deemed that smokers underestimate their lung cancer risk relative to nonsmokers [30, 55]. However, some studies have compared risk perceptions of current versus former smokers and have yielded mixed results as to whether current smokers have higher or lower risk perceptions compared to former smokers [28, 32, 58, 60, 61]. Using our risk perception measure, we found that current smokers’ risk perceptions were higher than those of former smokers; this was true when group comparisons were made using the total risk perception measure and for each of the ten personal and comparative risk questions. Moreover, only approximately 10% of current smokers responded that their risk of getting lung cancer or a SRD was lower than that of other smokers. Therefore, this group of current, heavy smokers who undergo lung screening did not display an optimistic bias, as might have been expected based on previous research. These findings are also surprising in light of key work that suggests that heavy smoking and older age is associated with being more likely to underestimate risk [25, 60, 62]. Our results indicate that older individuals with a significant smoking history who seek out lung cancer screening might hold different perceptions than would be otherwise expected.
Although it is believed that perceived risk can drive behavior change [52], our finding that former, heavy smokers had lower risk perceptions than current, heavy smokers suggests that changing behavior may drive perceived risk; quitting smoking likely lowered former smokers’ risk beliefs. This finding is consistent with the risk reappraisal hypothesis [63] that posits that if a change is made to decrease disease risk, in turn, risk perceptions decrease subsequent to this behavior change. This raises the question whether formerly heavy smokers who are undergoing lung screening overestimate the risk reduction achieved from quitting, which is potentially indicative of optimistic bias.
According to the HBM, in order to make a behavior change, individuals must believe that smoking would lead to serious health consequences and that quitting smoking will reduce the likelihood of getting lung cancer or a SRD. Accordingly, former smokers, compared to current smokers, endorsed greater perceived severity for lung cancer and SRDs and believed in greater perceived benefits from quitting. Former smokers recognized the threat, took action, and believed that this change would be of benefit. This is consistent with research that has shown that current smokers, compared to former smokers, report fewer benefits of quitting [32, 53, 59] and that older, continuing smokers in particular are pessimistic about the benefits of quitting smoking [53, 64].
In examining factors associated with risk perceptions, our findings support the Self-Regulation Model in that they underscore the important role of emotional factors in driving risk perceptions. Although many behavior change models include only cognitive constructs, in our study, worry was a salient factor associated with risk perceptions of both current and former smokers. This finding is consistent with findings from breast and colon screening studies in which cancer-related worry was associated with perceived risk [65–67]. In the cancer screening literature, it is unclear if worry leads to or deters from seeking cancer screening [56, 67–74] or if worry and risk, together, influence behavior [75]. Similarly, for patients undergoing lung cancer screening, it is unclear at which level worry would positively influence risk perceptions and post-screening health promotion behaviors.
Our results also indicated that knowledge of smoking risks was an important factor associated with risk perceptions for both former and current smokers. Current and former smokers who underestimated a 1 pack per day smoker’s increased risk of lung cancer due to smoking had lower risk perceptions for lung cancer and SRDs. These findings are similar to previous research that demonstrated that smokers underestimate the impact on quality of life and disability from smoking [76, 77].
Among former smokers, past history of smoking within 30 min of waking, a proxy measure indicating greater nicotine dependence, was associated with elevated risk perception. It is possible that, within former smokers, those who had a higher level of nicotine dependence feel less of a sense of control over their future health and thus feel at greater risk for diseases.
Among former smokers, being Black was associated with lower risk perceptions for lung cancer and SRDs. Although there is a dearth of research conducted on racial differences and smoking-related risk perceptions, one study conducted among 144 smokers found that Black smokers were more likely to underestimate their lung cancer risk, compared to nonsmokers, and were less likely to believe that quitting smoking would reduce their lung cancer risk [53]. A population-based study also reported that Blacks were less likely than Whites to perceive cancer risk [62]. Racial differences have been shown to influence lung cancer treatment decision making in that Black patients have lower rates of surgical treatment and are more likely to refuse surgery [78], even after access to care has been demonstrated [79]. Our results suggest that race could influence lung cancer risk perceptions, particularly for previously heavy smokers who quit smoking; this is an area for future research.
Some study limitations must be noted. The participants in this NLST sub-study are not representative of all older current or former heavy smokers. In this self-selected population of trial participants, one would assume that worry and risk perceptions would be relatively high. This is a high-risk group of individuals with smoking histories of at least 30 pack years who elected to participate in a screening trial; this may have restricted the range of responses for the risk perception outcomes since, at high levels of smoking consumption, there are decreasing increments of risk [80]. The majority of NLST participants was White and had a fairly high education level. Lastly, about 25% of participants did not answer the question about smokers’ years of life lost; perhaps it was a potentially uncomfortable question to quantify. Because of the missing data, and since this question was associated with the knowledge of a pack per day smoker’s increased risk of lung cancer, it was not included in the multivariable model.
Our findings have important implications for prescreening risk assessments. We found that risk perceptions and risk knowledge among current and former heavy smokers is a complex issue, particularly when addressing risks for both lung cancer and SRDs; this implies that a prescreening assessment must include an assessment of both risk perception and risk knowledge. Our results also suggest that current, heavy smokers undergoing lung screening may not have an optimistic bias and, in fact, may be pessimistic about the value of quitting. Likewise, former, heavy smokers undergoing lung screening may benefit from reinforcement of the importance of staying quit. Therefore, prescreening comprehensive risk assessments would maximize the utility of lung screening as a teachable moment to promote smoking cessation and relapse prevention.
Once risk is assessed, prescreening risk communication sessions can be guided by the contextualized approach to risk communication [81]. The main features of this approach are that (1) information is provided on antecedents, (2) information is provided on consequences, and (3) information is given on how to minimize risk. Our findings indicate that, for lung cancer screening patients, antecedents to focus upon might be increasing recognition of risk due to an individual’s smoking and medical history; care must be taken to do so in a way that would appropriately elevate worry. Our study showed that adverse consequences, such as smoking morbidity, might be an important area in which to focus and educate upon. For current smokers, minimizing risk could be done by emphasizing benefits to be gained by quitting and offering encouragement by sharing research on how older smokers successfully quit. This could be particularly salient for current smokers, given previous research that indicates their proclivity to diminish the benefits of quitting. For former smokers, minimizing risk could be done by supporting their quit status while including a cautionary note about the importance of maintaining quit and decreasing exposure.
In closing, this study demonstrated differences in risk perceptions and risk knowledge between current versus former smokers who were participating in NLST. Our results indicate that older, current heavy smokers who are undergoing lung cancer screening have higher risk perceptions compared to older, former heavy smokers. These findings suggest that lung cancer screening presents a unique opportunity to intervene with risk perceptions to potentially enhance smoking cessation and relapse prevention efforts in this group. As it is important to understand the determinants of risk perceptions in order to develop effective risk communication messages [81], this work elucidated the prescreening determinants of risk and emphasized the particular importance of assessing and discussing worry, risk knowledge, and perception of addiction prior to screening.
Acknowledgments
This project was funded by a grant from the American Cancer Society’s Mentored Research Scholar Award (MRSG-005-05-CPPB), the ACRIN/NLST Trial (U01 CA79778 S2), and a NHLBI Midcareer Investigator Award in Patient-Oriented Research (#K24-HL04440). The authors wish to thank Dr. Deni Aberle, without whose support the study would not have been possible. We are also appreciative of the efforts of ACRIN staff members Irene Mahon and Maria Oh, Brown University staff JoRean Sicks and Amanda Adams, and Dr. Park’s research assistant Jennifer Pandiscio. Lastly, we are very grateful for the assistance and approval of the eight ACRIN/NLST participating Principal Investigators, site coordinators, and staff at the Brown University Center for Statistical Sciences.
Appendix
PARTICIPATING STUDY SITES
Brown University, Providence, Rhode Island
The Cancer Institute of New Jersey, New Brunswick, New Jersey
Mayo Clinic, Rochester, Minnesota
Dartmouth Hitchcock Medical Center, Lebanon, New Hampshire
St. Elizabeth Health Center, Youngstown, Ohio
Jewish Hospital Heart and Lung Institute, Louisville, Kentucky
Moffitt Cancer Center, Tampa, Florida
Johns Hopkins University, Baltimore, Maryland
References
- 1.Cancer facts & figures. Atlanta: American Cancer Society; 2006. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309(6959):901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dresler CM, Leon ME, Straif K, Baan R, Secretan B. Reversal of risk upon quitting smoking. Lancet. 2006;368(9533):348–349. doi: 10.1016/S0140-6736(06)69086-7. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995–1999. MMWR Morb Mortal Wkly Rep. 2002;51(14):300–303. [PubMed] [Google Scholar]
- 6.Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91 (8):675–690. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 7.Screening for lung cancer. The Medical Letter. 2001;43(1109):61–64. [PubMed] [Google Scholar]
- 8.Henschke CI, Yankelevitz DF, Miettinen OS International Early Lung Cancer Action Program Investigators. Computed tomographic screening for lung cancer: The relationship of disease stage to tumor size. Arch Intern Med. 2006;166(3):321–325. doi: 10.1001/archinte.166.3.321. [DOI] [PubMed] [Google Scholar]
- 9.International Early Lung Cancer Action Program Investigators. Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 10.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: The context of cancer care and survivorship. Cancer Control. 2003;10(4):325–333. doi: 10.1177/107327480301000407. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen N, Schulman S, van den Hoek J, Fowler R. Insights on how to quit smoking: A survey of patients with lung cancer. Cancer Nurs. 1985;8(3):145–150. [PubMed] [Google Scholar]
- 13.Sarna L. Smoking behaviors of women after diagnosis with lung cancer. Image J Nurs Sch. 1995;27(1):35–41. doi: 10.1111/j.1547-5069.1995.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 14.Gritz ER, Nisenbaum R, Elashoff RE, Holmes EC. Smoking behavior following diagnosis in patients with stage I non-small cell lung cancer. Cancer Causes Control. 1991;2(2):105–112. doi: 10.1007/BF00053129. [DOI] [PubMed] [Google Scholar]
- 15.Dresler CM, Bailey M, Roper CR, Patterson GA, Cooper JD. Smoking cessation and lung cancer resection. Chest. 1996;110 (5):1199–1202. doi: 10.1378/chest.110.5.1199. [DOI] [PubMed] [Google Scholar]
- 16.Cox LS, Patten CA, Ebbert JO, et al. Tobacco use outcomes among patients with lung cancer treated for nicotine dependence. J Clin Oncol. 2002;20(16):3461–3469. doi: 10.1200/JCO.2002.10.085. [DOI] [PubMed] [Google Scholar]
- 17.Taylor KL, Cox LS, Zincke N, et al. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56 (1):125–134. doi: 10.1016/j.lungcan.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early lung cancer action project: Overall design and findings from baseline screening. Lancet. 1999;354(9173):99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 19.Townsend CO, Clark MM, Jett JR, et al. Relation between smoking cessation and receiving results from three annual spiral chest computed tomography scans for lung carcinoma screening. Cancer. 2005;103(10):2154–2162. doi: 10.1002/cncr.21045. [DOI] [PubMed] [Google Scholar]
- 20.Cox LS, Clark MM, Jett JR, et al. Change in smoking status after spiral chest computed tomography scan screening. Cancer. 2003;98:2495–2501. doi: 10.1002/cncr.11813. [DOI] [PubMed] [Google Scholar]
- 21.Ostroff JS, Buckshee N, Mancuso CA, Yankelevitz DF, Henschke CI. Smoking cessation following CT screening for early detection of lung cancer. Prev Med. 2001;33(6):613–621. doi: 10.1006/pmed.2001.0935. [DOI] [PubMed] [Google Scholar]
- 22.Kristiansen CM, Harding CM, Eiser JR. Beliefs about the relationship between smoking and causes of death. Basic Appl Soc Psychol. 1983;4:254–261. [Google Scholar]
- 23.Viscusi W. Do smokers underestimate risks? J Polit Econ. 1990;98(6):1253–1269. [Google Scholar]
- 24.Viscusi WK. Smoking: Making the Risky Decision. Oxford: Oxford University Press; 1992. [Google Scholar]
- 25.Schoenbaum M. Do smokers understand the mortality effects of smoking? Evidence from the health and retirement survey. Am J Public Health. 1997;87:755–759. doi: 10.2105/ajph.87.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutton S. How ordinary people in Great Britain perceive the health risks of smoking. J Epidemiol Community Health. 1998;52(5):338–339. doi: 10.1136/jech.52.5.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borland R. What do people’s estimates of smoking related risk mean? Psychol Health. 1997;12:513–521. [Google Scholar]
- 28.Strecher VJ, Kreuter MW, Kobrin SC. Do cigarette smokers have unrealistic perceptions of their heart attack, cancer, and stroke risks? J Behav Med. 1995;18(1):45–53. doi: 10.1007/BF01857704. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein ND. Accuracy of smokers’ risk perceptions. Ann Behav Med. 1998;20(2):135–140. doi: 10.1007/BF02884459. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein ND, Marcus SE, Moser RP. Smokers’ unrealistic optimism about their risk. Tob Control. 2005;14(1):55–59. doi: 10.1136/tc.2004.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strecher VJ, DeVellis BM, Becker MH, Rosenstock IM. The role of self-efficacy in achieving health behavior change. Health Educ Q. 1986;13(1):73–92. doi: 10.1177/109019818601300108. [DOI] [PubMed] [Google Scholar]
- 32.McCoy SB, Gibbons FX, Reis TJ, Gerrard M, Luus CA, Sufka AV. Perceptions of smoking risk as a function of smoking status. J Behav Med. 1992;15(5):469–488. doi: 10.1007/BF00844942. [DOI] [PubMed] [Google Scholar]
- 33.Lee C. Perceptions of immunity to disease in adult smokers. J Behav Med. 1989;12(3):267–277. doi: 10.1007/BF00844871. [DOI] [PubMed] [Google Scholar]
- 34.Leventhal H, Glynn K, Fleming R. Is the smoking decision an ‘informed choice’? Effect of smoking risk factors on smoking beliefs. JAMA. 1987;257(24):3373–3376. [PubMed] [Google Scholar]
- 35.Weinstein ND. Unrealistic optimism about susceptibility to health problems: Conclusions from a community-wide sample. J Behav Med. 1987;10(5):481–500. doi: 10.1007/BF00846146. [DOI] [PubMed] [Google Scholar]
- 36.Burns DM. Cigarette smoking among the elderly: Disease consequences and the benefits of cessation. Am J Health Promot. 2000;14(6):357–361. doi: 10.4278/0890-1171-14.6.357. [DOI] [PubMed] [Google Scholar]
- 37.LaCroix AZ, Omenn GS. Older adults and smoking. Clin Geriatr Med. 1992;8(1):69–87. [PubMed] [Google Scholar]
- 38.Brogan & Partners. Focus group research report. Ann Arbor, MI: The Center for Social Gerontology Tobacco Cessation Program Research; 2001. [Google Scholar]
- 39.Arday DR, Lapin P, Chin J, Preston JA. Smoking patterns among seniors and the Medicare stop smoking program. J Am Geriatr Soc. 2002;50(10):1689–1697. doi: 10.1046/j.1532-5415.2002.50461.x. [DOI] [PubMed] [Google Scholar]
- 40.Ossip-Klein DJ, Carosella AM, Krusch DA. Self-help interventions for older smokers. Tob Control. 1997;6(3):188–193. doi: 10.1136/tc.6.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan GD, Noll EL, Orleans CT, Rimer BK, Amfoh K, Bonney G. Reaching midlife and older smokers: Tailored interventions for routine medical care. Prev Med. 1996;25:346–354. doi: 10.1006/pmed.1996.0065. [DOI] [PubMed] [Google Scholar]
- 42.Joyce GF, Niaura R, Maglione M, et al. The effectiveness of covering smoking cessation services for Medicare beneficiaries. Health Serv Res. 2008;43:2106–21023. doi: 10.1111/j.1475-6773.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenstock I. Historical origins of the health belief model. Health Educ Monogr. 1974;2:328–335. [Google Scholar]
- 44.Weinstein ND. The precaution adoption process. Health Psychol. 1988;7(4):355–386. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- 45.Leventhal H, Safer MA, Panagis DM. The impact of communications on the self-regulation of health beliefs, decisions, and behavior. Health Educ Q. 1983;10(1):3–29. doi: 10.1177/109019818301000101. [DOI] [PubMed] [Google Scholar]
- 46.Rosenstock IM. Adoption and maintenance of lifestyle modifications. Am J Prev Med. 1988;4(6):349–352. [PubMed] [Google Scholar]
- 47.Rosenstock IM. The health belief model. In: Glanz K, Lewis F, Rimer B, editors. Health Behavior and Health Education: Theory, Research, and Practice. San Francisco: Jossey-Bass; 1990. pp. 39–62. [Google Scholar]
- 48.Romer D, Jamieson P. The role of perceived risk in starting and stopping smoking. In: Slovic P, editor. Smoking: Risk, Perception & Policy. Thousand Oaks: Sage; 2001. pp. 64–80. [Google Scholar]
- 49.Mullens AB, McCaul KD, Erickson SC, Sandgren AK. Coping after cancer: Risk perceptions, worry, and health behaviors among colorectal cancer survivors. Psycho-oncology. 2004;13(6):367–376. doi: 10.1002/pon.751. [DOI] [PubMed] [Google Scholar]
- 50.American College of Radiology Imaging Network. [Accessed 9 June 2009];Protocol 6654 data forms. Available at: http://www.acrin.org/Default.aspx?tabid=282.
- 51.Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 52.Kreuter MW, Strecher VJ. Changing inaccurate perceptions of health risk: Results from a randomized trial. Health Psychol. 1995;14(1):56–63. doi: 10.1037//0278-6133.14.1.56. [DOI] [PubMed] [Google Scholar]
- 53.Lyna P, McBride C, Samsa G, Pollak KI. Exploring the association between perceived risks of smoking and benefits to quitting: Who does not see the link? Addict Behav. 2002;27(2):293–307. doi: 10.1016/s0306-4603(01)00175-7. [DOI] [PubMed] [Google Scholar]
- 54.Diefenbach MA, Weinstein ND, O’Reilly J. Scales for assessing perceptions of health hazard susceptibility. Health Educ Res. 1993;8(2):181–192. doi: 10.1093/her/8.2.181. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein N. Smokers’ recognition of their vulnerability to harm. In: Slovic P, editor. Smoking: Risk, Perception, and Policy. Thousand Oaks: Sage; 2001. pp. 81–96. [Google Scholar]
- 56.Lerman C, Schwartz M. Adherence and psychological adjustment among women at high risk for breast cancer. Breast Cancer Res Treat. 1993;28(2):145–155. doi: 10.1007/BF00666427. [DOI] [PubMed] [Google Scholar]
- 57.Rigotti NA, Park ER, Regan S, et al. Efficacy of telephone counseling for pregnant smokers: A randomized controlled trial. Obstet Gynecol. 2006;108(1):83–92. doi: 10.1097/01.AOG.0000218100.05601.f8. [DOI] [PubMed] [Google Scholar]
- 58.Price JH, Everett SA. Perceptions of lung cancer and smoking in an economically disadvantaged population. J Commun Health. 1994;19(5):361–375. doi: 10.1007/BF02260405. [DOI] [PubMed] [Google Scholar]
- 59.Aiken LS, West SG, Woodward CK, Reno RR, Reynolds KD. Increasing screening mammography in asymptomatic women: Evaluation of a second-generation, theory-based program. Health Psychol. 1994;13(6):526–538. doi: 10.1037//0278-6133.13.6.526. [DOI] [PubMed] [Google Scholar]
- 60.Ayanian JZ, Cleary PD. Perceived risks of heart disease and cancer among cigarette smokers. JAMA. 1999;281(11):1019–1021. doi: 10.1001/jama.281.11.1019. [DOI] [PubMed] [Google Scholar]
- 61.Hahn EJ, Rayens MK, Hopenhayn C, Christian WJ. Perceived risk and interest in screening for lung cancer among current and former smokers. Res Nurs Health. 2006;29:359–370. doi: 10.1002/nur.20132. [DOI] [PubMed] [Google Scholar]
- 62.Honda K, Neugut AI. Associations between perceived cancer risk and established risk factors in a national community sample. Cancer Detect Prev. 2004;28:1–7. doi: 10.1016/j.cdp.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Brewer NT, Weinstein ND, Cuite CL, Herrington JE. Risk perceptions and their relation to risk behavior. Ann Behav Med. 2004;27(2):125–130. doi: 10.1207/s15324796abm2702_7. [DOI] [PubMed] [Google Scholar]
- 64.Orleans CT, Jepson C, Resch N, Rimer BK. Quitting motives and barriers among older smokers. The 1986 adult use of tobacco survey revisited. Cancer. 1994;74(7 Suppl):2055–2061. doi: 10.1002/1097-0142(19941001)74:7+<2055::aid-cncr2820741712>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 65.Collins V, Halliday J, Warren R, Williamson R. Cancer worries, risk perceptions and associations with interest in DNA testing and clinic satisfaction in a familial colorectal cancer clinic. Clin Genet. 2000;58(6):460–468. doi: 10.1034/j.1399-0004.2000.580606.x. [DOI] [PubMed] [Google Scholar]
- 66.Watson M, Lloyd S, Davidson J, et al. The impact of genetic counselling on risk perception and mental health in women with a family history of breast cancer. Br J Cancer. 1999;79:868–874. doi: 10.1038/sj.bjc.6690139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Absetz P, Aro AR, Sutton SR. Experience with breast cancer, pre-screening perceived susceptibility and the psychological impact of screening. Psycho-oncology. 2003;12(4):305–318. doi: 10.1002/pon.644. [DOI] [PubMed] [Google Scholar]
- 68.Kash KM, Holland JC, Halper MS, Miller DG. Psychological distress and surveillance behaviors of women with a family history of breast cancer. J Natl Cancer Inst. 1992;84(1):24–30. doi: 10.1093/jnci/84.1.24. [DOI] [PubMed] [Google Scholar]
- 69.Kash KM, Dabney MK. Psychological aspects of cancer screening in high-risk populations. Med Pediatr Oncol. 2001;36(5):519–524. doi: 10.1002/mpo.1124. [DOI] [PubMed] [Google Scholar]
- 70.Lerman C, Trock B, Rimer BK, et al. Psychological side effects of breast cancer screening. Health Psychol. 1991;10(4):259–267. doi: 10.1037//0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- 71.Lerman C, Trock B, Rimer BK, et al. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med. 1991;114(8):258–267. doi: 10.7326/0003-4819-114-8-657. [DOI] [PubMed] [Google Scholar]
- 72.Diefenbach MA, Schnoll RA, Miller SM, Brower L. Genetic testing prostate cancer: Willingness and predictors of interest. Cancer Pract. 2000;8(2):82–86. doi: 10.1046/j.1523-5394.2000.82006.x. [DOI] [PubMed] [Google Scholar]
- 73.McCaul KD, Branstetter AD, Schroeder DM, Glasgow RE. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychol. 1996;15(6):423–429. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- 74.Hay JL, McCaul KD, Magnan RE. Does worry about breast cancer predict screening behaviors? A meta-analysis of the prospective evidence. Prev Med. 2006;42(6):401–408. doi: 10.1016/j.ypmed.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Diefenbach MA, Miller SM, Daly MB. Specific worry about breast cancer predicts mammography use in women at risk for breast and ovarian cancer. Health Psychol. 1999;18(5):532–536. doi: 10.1037//0278-6133.18.5.532. [DOI] [PubMed] [Google Scholar]
- 76.Oncken C, McKee S, Krishnan-Sarin S, O’Malley S, Mazure CM. Knowledge and perceived risk of smoking-related conditions: A survey of cigarette smokers. Prev Med. 2005;40(6):779–784. doi: 10.1016/j.ypmed.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 77.Sloan FA, Smith VK, Taylor DH. The Smoking Puzzle. Cambridge: Harvard University Press; 2003. [Google Scholar]
- 78.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341 (16):1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 79.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24(3):413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 80.Sastre MT, Mullet E, Sorum PC. Relationship between cigarette dose and perceived risk of lung cancer. Prev Med. 1999;28(6):566–571. doi: 10.1006/pmed.1999.0482. [DOI] [PubMed] [Google Scholar]
- 81.Vernon SW. Risk perception and risk communication for cancer screening behaviors: A review. J Natl Cancer Inst Monogr. 1999;25:101–119. doi: 10.1093/oxfordjournals.jncimonographs.a024184. [DOI] [PubMed] [Google Scholar]


