Abstract
Background
Together with smoking, the level of lung function attained in early adulthood is among the strongest predictors of chronic obstructive pulmonary disease. Whether airway function measured shortly after birth is a determinant of this level is currently unknown.
Methods
Non-selected infants were enrolled at birth in the Tucson Children's Respiratory Study in 1980-84. Infant maximal expiratory flows at functional residual capacity (V'maxFRC) were measured by the chest compression technique at 2 months (mean±SD: 2.3±1.9m); values were logarithmically transformed and adjusted for length. Forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and forced expiratory flow between 25% and 75% of FVC (FEF25-75) were measured at ages 11, 16 and 22 years before and after 180μg of albuterol. Participant characteristics were determined at enrollment and at each time of testing.
Findings
Airway function was available for 123 participants in infancy and at least once at ages 11, 16 or 22 years. Using a random effects model, participants in the lowest quartile for infant V'maxFRC had persistently lower values for FEV1/FVC ratio (-5.2%, p<0.001), FEF25-75 (-663ml/s, p<0.001) and FEV1 (-233ml, p=0.001) through age 22 compared to the upper three quartiles, after adjusting for height, weight, age and sex. The magnitude and significance of the effect did not change appreciably after additionally adjusting for current wheeze, smoking, atopy and parental asthma.
Interpretation
Diminished airway function present shortly after birth is a risk factor for airflow obstruction in early adult life.
Introduction
Thirty years ago, Burrows and coworkers made the seminal observation that adults with a history of pediatric respiratory illness had lower levels of lung function and were more likely to develop obstructive lung disease than those without such a history (1). Although one plausible interpretation of this finding is that respiratory infections can damage the lung and predispose to obstructive lung disease, it is also possible that events occurring before any respiratory illness can predispose to both these early illnesses and to subsequent chronic impairment of lung function (2). In support of this latter contention, we and others demonstrated that children who presented with lower respiratory illnesses during the first years of life had lower maximal expiratory flows shortly after birth and before any such illnesses developed (3-6). These results suggested the hypothesis that the origins of chronic obstructive pulmonary disease could be found in fetal life, and specifically, in the factors that determine intrauterine growth of lungs and airways.
Longitudinal studies have suggested that a significant proportion of lung function deficits present during the third decade of life, and especially among subjects who have a diagnosis of asthma, persist into late adulthood and predispose for the development of chronic obstructive pulmonary disease (7). In this study we assessed to what degree these deficits are already present in the early post-neonatal period.
Methods
Participants
Healthy infants were enrolled at birth in the Tucson Children's Respiratory Study, a longitudinal non-selected birth cohort study of the early origins of respiratory disease; 1246 infants were enrolled between 1980 and 1984. The chest compression technique for assessing pulmonary function in infancy was developed as the last 376 infants were enrolled in the study. Only these infants were eligible for pulmonary function testing and 169 were tested shortly after birth (mean±SD: 2.3±1.9 months). Details of the selection and exclusion criteria and the have been reported (3). Informed consent was obtained from participants or their parents, and the Institutional Review Board of the University of Arizona approved the study.
Pulmonary Function Tests
In infancy, partial expiratory flow-volume curves were obtained by the chest-compression technique and the maximal expiratory flow at functional residual capacity (V'maxFRC, in milliliters per second) was recorded; technique details have been previously published (4, 8). Among the 169 participants with infant pulmonary function data, follow-up spirometry was obtained according to American Thoracic Society standards for 123 individuals at ages 11 (mean±SD 10.9±0.4yr, n=109), 16 (16.8±0.5yr, n=87) and/or 22 years (21.7±0.6yr, n=83) using a custom built, pneumotachometer-based system running software on a portable computer at age 11(9) and a portable Schiller Spirovit SP-1 (Schiller AG, Baar, Switzerland) at ages 16 and 22 (10). Systems were calibrated with a Jones flow-volume calibrator (Model FVC-3000; Jones Medical Instrumentation Company, Oakbrook, IL). None of the children had used a bronchodilator within 6 hours of testing. Subsequent to baseline measurements, a fixed dose of 2 puffs of albuterol (180 μg) was administered from a metered-dose inhaler and aerochamber holding device (Monaghan Medical Corp, Plattsburgh, NY) and the post bronchodilator spirometry obtained after 15 minutes. Spirometry indices included the forced vital capacity (FVC, milliliters), forced expiratory volume in one second (FEV1, milliliters), and the forced expiratory flow between 25 and 75% of the FVC (FEF25-75, milliliters per second). Response to bronchodilator (BD) was calculated for all spirometric indices as (post-BD - pre-BD) / (Post-BD + pre-BD)*100 at ages 11, 16 and 22. Methacholine challenges were performed at age 11 as previously reported (11) and bronchial hyperresponsiveness was defined as methacholine-dose response slope values below the 10th percentile for a healthy reference subgroup (skin test negative, had never wheeze, nor had been diagnosed as having asthma). The study nurses recorded height, weight, and age at the time of testing.
Phenotypic Information
At enrollment, parents completed a questionnaire describing their ethnicity, history of physician-diagnosed asthma, years of education, current age and current smoking. Additional information concerning pregnancy and the delivery of the infant was also collected. Physician confirmed lower respiratory illnesses were ascertained for each child from birth through age 3 years and concurrent symptoms recorded by the physician (12). Any occurrence of wheeze during the previous year was determined by a questionnaire completed at the time of follow-up spirometry (11, 16 and 22 years). Skin prick tests to 6 local aeroallergens (Hollister-Stier Laboratories, Everett, WA) were performed at ages 11, 16 and 22 as previously described (13). Tests were read at 20 min and wheal size recorded as the sum of the 2 diameters at right angles to each other (in mm); wheal sizes of ≥ 3mm were considered positive. Participants with at least one positive test were considered atopic. Active smoking by the subjects was determined from salivary cotinine levels (>10ng/ml) at age 16 years (competitive radioimmunoassay; Foundation for Blood Research, Scarborough, ME) and by self-completed questionnaire at age 22.
Statistical Analysis
Airway function measured in infancy (V'maxFRC, ml/sec) was logarithmically transformed (base e), adjusted for infant length and standardized to the average length of the infants at 2 months of age (57.4 cm) as previously reported (4). In the current study, length adjusted infant V'maxFRC was used as either a continuous (natural logarithm of ml/sec) or as a categorical predictive variable grouped into quartiles. Follow-up spirometry indices at ages 11, 16 and 22 years (FVC, FEV1, FEF25-75, and the FEV1/FVC ratio) were approximately normally distributed. For separate analyses at each survey, the follow-up spirometry indices were adjusted for concurrent height, weight, and sex, and the standardized residuals used as the outcome measure. Pearson correlation was used to evaluate the relation between normally distributed continuous variables. R2, calculated as the square of the Pearson correlation coefficient, was used to estimate the proportion of variability explained by infant airway function. A random effects model was used to assess follow-up spirometry longitudinally (14)(15). A more detailed description of these models is provided in the Web Appendix. The basic random effects models included age, height and weight as time-dependent covariates, gender and infant V'maxFRC as time-independent covariates. Potential confounders and other important covariates (parental variables: history of physician-diagnosed asthma, years of education, current age and smoking history; subject variables: ethnicity, the occurrence of early lower respiratory illnesses, current wheeze, active smoking and current atopy) were each entered separately in the basic random effects model. Covariates related to any of the follow-up spirometry indices with p-values <0.1 as well as covariates with biological significance, were retained for a final multifactorial model. Two-sided p-values of less than 0.05 were considered significant. STATA version 9.0 and SPSS version 14.0 were used for analyses.
Results
Participants included in the present study (n=123) had more educated mothers (p=0.02) and more paternal asthma (p=0.01) compared to all other CRS enrollees (n=1123) (Table 1). There were no significant differences between the two groups in sex and ethnic group distribution, in the occurrence of lower respiratory illnesses, allergy skin test reactivity, wheeze, or in parental smoking or age. The frequency of maternal asthma and paternal level of education was similar for the two groups.
Table 1.
Characteristics of participants included in the present study compared to all others enrolled in the study
| Participant Characteristics |
Included Participants |
All Others |
|||
|---|---|---|---|---|---|
| N | % | N | % | P* | |
| Male | 123 | 52.9 | 1123 | 48.8 | 0.4 |
| Non-Hispanic White | 123 | 61.8 | 1123 | 58.6 | 0.5 |
| LRI by age 3yr | 102 | 52.9 | 786 | 59.2 | 0.2 |
| RSV LRI by age 3yr | 102 | 22.6 | 764 | 24.1 | 0.7 |
| Atopic at age 6yr† | 115 | 39.1 | 646 | 37.9 | 0.8 |
| Active Wheeze at Age: | |||||
| 11yr | 122 | 21.3 | 827 | 27.6 | 0.2 |
| 16yr | 107 | 21.5 | 632 | 26.6 | 0.3 |
| 22yr | 101 | 46.5 | 623 | 37.7 | 0.09 |
| Maternal: | |||||
| Asthma | 122 | 10.7 | 1033 | 11.0 | 0.9 |
| Smoking | 123 | 19.5 | 1120 | 17.5 | 0.6 |
| Education >12yr | 123 | 77.2 | 1118 | 67.3 | 0.02 |
| Age >28yr | 123 | 41.5 | 1122 | 38.2 | 0.5 |
| Paternal: | |||||
| Asthma | 114 | 19.3 | 980 | 11.2 | 0.01 |
| Smoking | 121 | 28.1 | 1105 | 31.8 | 0.4 |
| Education >12yr | 121 | 73.6 | 1096 | 70.0 | 0.4 |
| Age >28yr | 121 | 58.7 | 1109 | 53.1 | 0.2 |
P-values based on Pearson Chi-square statistic
At least one positive allergy skin prick test
The association of length-adjusted infant V'maxFRC with potential confounders was assessed (Web Appendix ETable 1). Males had lower infant V'maxFRC compared to females (p=0.003). There were no significant differences in infant V'maxFRC related to ethnicity or parental history of asthma, smoking, age or education level. In addition, birth weight, type of delivery, and number of previous pregnancies were unrelated to infant V'maxFRC. The lack of relation of these covariates to infant lung function must be interpreted with caution, however, given the current sample size. These results did not change when the analyses were stratified by gender (data not shown).
Unadjusted baseline spirometry indices for males and females at ages 11, 16 and 22 are shown in ETable 2. There was a highly significant correlation between length-adjusted infant V'maxFRC and height-, weight- and sex-adjusted FEV1/FVC ratio and FEF25-75 at ages 11, 16 and 22 (Table 2 and EFigure 1). Infant airway function accounted for between 9% and 14% of the variability in subsequent adjusted FEV1/FVC ratio and FEF25-75 at ages 11, 16 and 22. No significant association was found between length-adjusted infant V'maxFRC and adjusted FVC and FEV1 at any separate survey. Correlation coefficients between infant V'maxFRC and post-bronchodilator FEV1/FVC ratio and FEF25-75 were slightly lower than those for pre-bronchodilator values, but remained significant for ages 11, 16 and 22 years (Table 2). The relation between length-adjusted V'maxFRC and subsequent FEV1/FVC ratio and FEF25-75 was present for males and females at ages 11, 16 and 22, with small fluctuations in statistical significance at different ages (ETable 3).
Table 2.
Age specific Pearson correlation coefficients between length-adjus V'maxFRC* (natural logarithm of ml/sec) and adjusted pre- and post-bronch function at ages 11, 16 and 22. ted infant odilator lung
| Height, weight and sex adjusted lung function† at | ||||||
|---|---|---|---|---|---|---|
| Age 11yr (n=109) | Age 16yr (n=87) | Age 22yr (n=83) | ||||
| r‡ | p | r‡ | p | r‡ | p | |
| Pre-bronchodilator | ||||||
| FEV1/FVC Ratio | 0.38 | <0.001 | 0.32 | 0.003 | 0.34 | 0.002 |
| FEF25-75 | 0.34 | <0.001 | 0.33 | 0.002 | 0.30 | 0.006 |
| FEV1 | 0.18 | 0.06 | 0.18 | 0.09 | 0.18 | 0.1 |
| FVC | -0.03 | 0.7 | -0.03 | 0.8 | -0.07 | 0.5 |
| Post-bronchodilator | ||||||
| FEV1/FVC Ratio | 0.26 | 0.007 | 0.28 | 0.01 | 0.30 | 0.006 |
| FEF25-75 | 0.31 | 0.001 | 0.28 | 0.01 | 0.27 | 0.01 |
| FEV1 | 0.09 | 0.4 | 0.14 | 0.2 | 0.13 | 0.2 |
| FVC | -0.06 | 0.6 | -0.02 | 0.9 | -0.04 | 0.7 |
V'maxFRC measured in infancy was logarithmically transformed, adjusted for length and standardized to the average length of the infants at 2 months of age (57.4 cm) as previously reported (4)
Spirometry indices were regressed with height, weight and sex and the standardized residuals saved and used as the outcome measure
Pearson correlation coefficient
A longitudinal random effects model was used to assess the relation between length-adjusted infant V'maxFRC and subsequent lung function. Infant V'maxFRC was directly associated with subsequent pre-bronchodilator FEV1/FVC ratio (p<0.001) and FEF25-75 (p<0.001) through age 22 years after adjusting for age, height, weight and sex (Table 3). In the longitudinal model, infant V'maxFRC was directly associated with FEV1 (p=0.01), but there was no association with FVC. Only current wheeze and current atopy, tested in the models as time-dependent covariates, and active smoking by the participant at ages 16 or 22 or both, met the cutoff for inclusion in the random effects models (p≤0.1); maternal smoking at enrollment was additionally retained. When the models shown in Table 3 were repeated including current wheeze, current atopy and smoking, there were essentially no changes in the magnitude or significance of the relation between infant V'maxFRC and subsequent lung function (Web Appendix ETable 4a.) The associations between infant lung function and post-bronchodilator FEV1/FVC ratio and FEF25-75 were similar to those observed using pre-bronchodilator values, although the association between infant lung function and post-bronchodilator FEV1 did not reach statistical significance (Table 3 and Web Appendix ETable 4b).
Table 3.
Random effects models* for the association between length-adjusted infant V'maxFRC (natural logarithm of ml/sec as a continuous predictive variable) and pre- and post-bronchodilator lung function at ages 11, 16 and 22 years
| Pre-bronchodilator | ||||||||
|---|---|---|---|---|---|---|---|---|
| FEV1/FVC Ratio (%) | FEF25-75 (ml/s) | FEV1 (ml) | FVC (ml) | |||||
| β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | |
| Infant V'maxFRC | ||||||||
| (ln of ml/s) | 4.7 (1.0) | <0.001 | 566 (128) | <0.001 | 168 (64) | 0.009 | -2.8 (72) | 0.9 |
| Age (yr) | -0.4 (0.1) | <0.001 | -8.5 (12.7) | 0.5 | 18.2 (6.8) | 0.007 | 41.0 (8.0) | <0.001 |
| Height (cm) | 0.1 (0.03) | 0.007 | 51.6 (4.4) | <0.001 | 45.7 (2.4) | <0.001 | 49.5 (2.8 | <0.001 |
| Weight (kg) | -0.06 (0.02) | 0.009 | -2.4 (3.0) | 0.4 | 2.5 (1.6) | 0.1 | 6.8 (1.8) | <0.001 |
| Male | -1.9 (1.0) | 0.052 | 140 (129) | 0.3 | 305 (65) | <0.001 | 464 (72.7) | <0.001 |
| Model Constant | 56 (6) | <0.001 | -7617 (787) | <0.001 | -5653 (408) | <0.001 | -5480 (464) | <0.001 |
| Post-bronchodilator | ||||||||
|---|---|---|---|---|---|---|---|---|
| Infant V'maxFRC | ||||||||
| (ln of ml/s) | 3.1 (0.8) | <0.001 | 489 (124) | <0.001 | 113 (60.1) | 0.061 | -2.4 (68) | 0.9 |
| Age (yr) | -0.2 (0.1) | 0.031 | 17.0 (14.8) | 0.2 | 26.6 (7.0) | <0.001 | 40.6 (8.0) | <0.001 |
| Height (cm) | 0.06 (0.03) | 0.048 | 49.8 (5.1) | <0.001 | 44.7 (2.4) | <0.001 | 48.5 (2.8) | <0.001 |
| Weight (kg) | -0.06 (0.02) | 0.003 | -1.5 (3.2) | 0.6 | 2.5 (1.6) | 0.10 | 6.6 (1.8) | <0.001 |
| Male | -2.1 (0.8) | 0.010 | 42.6 (126) | 0.7 | 312 (61.0) | <0.001 | 460 (68.9) | <0.001 |
| Model Constant | 68 (5) | <0.001 | -6943 (821) | <0.001 | -5274 (394) | <0.001 | -5305 (447) | <0.001 |
Models include 123 individuals with 279 total follow-up spirometry data points. Age, height and weight were entered as time-dependent covariates; infant V'maxFRC and sex as time-independent covariates
The relation between infant V'maxFRC and subsequent lung function was assessed separately in males and females by stratifying the longitudinal models by gender. Length-adjusted V'maxFRC was directly associated with subsequent pre-bronchodilator FEV1/FVC ratio and FEF25-75 (p<0.001) in both males and females (ETable 3) and there was no significant relation with FEV1 or FVC after adjusting for height, weight and age. The associations between infant lung function and post-bronchodilator FEV1/FVC ratio and FEF25-75 were similar for males and females (ETable 3).
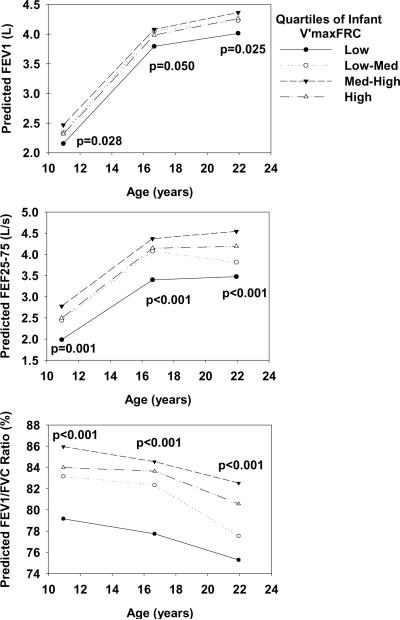
The relation between infant V'maxFRC and subsequent lung function was further assessed by grouping infant V'maxFRC into quartiles. Using random effects models, the predicted values for FEV1, FEV1/FVC ratio and FEF25-75 for males were plotted by quartiles for length-adjusted infant V'maxFRC in Figure 1. Participants in the lowest quartile for infant V'maxFRC had persistently diminished values for FEV1/FVC ratio (-5.2% [95%CI: -7.4, -3.0], p<0.001), FEF25-75 (-666ml/s [-955,-378], p<0.001) and FEV1 (-234ml [-377,-91], p=0.001) through age 22 compared to the upper three quartiles combined, after adjusting for height, weight, age and sex. Additionally, there was no significant age by infant V'maxFRC interaction. Similar results were obtained for the lowest quartile for infant V'maxFRC and adjusted post-bronchodilator FEV1/FVC ratio (-3.6% [-5.4, -1.9], p<0.001), FEF25-75 (-602ml/s [-881,-322], p<0.001) and FEV1 (-176ml [-311,-42], p=0.1) through age 22 years compared to the upper three quartiles.
Figure 1.
Predicted FEV1, FEF25-75 and FEV1/FVC ratio mean values for males at ages 11, 16 and 22 years by length-adjusted infant V'maxFRC quartile groups using random effects models. Predicted values were standardized to the mean height and weight for males at ages 11, 16 and 22 years. An interaction term between survey (age 11, 16 and 22) and quartiles for infant V'maxFRC was included in the models. P-values were estimated at each survey from the models.
The response to bronchodilator was calculated for all four spirometric indices. Infant V'maxFRC was inversely related to bronchodilator response for FEV1/FVC ratio at ages 11 and 22 and FEV1 at age 22, though trends at the other ages were consistent albeit not significant (ETable 5). When evaluated using the random effects model, infants in the lowest quartiles of infant V'maxFRC had significantly higher responses to bronchodilator for FEV1/FVC ratio (+1.18% [0.4, 1.9], p=0.002), FEF25-75 (+3.37% [0.9, 5.8], p=0.006) and FEV1 (+1.38% [0.4, 2.4], p=0.006) through age 22 years compared to the upper three quartiles. In contrast, the proportion of children with bronchial hyperresponsiveness to methacholine at age 11 was unrelated to infant V'maxFRC (28.6%, 27.8%, 25.0% and 31.3% for the lowest to highest quartiles respectively, trend chi2 p=0.9).
Concordant with previous reports from this same cohort (3), infants in the lowest quartile for V'maxFRC were characterized by an increased risk for the development of lower respiratory illnesses in the first three years of life (77%) compared to children in the upper three quartiles combined (45%, p=0.005, Table 4). Infants in the lowest quartile were not significantly different from those in the upper three quartiles regarding ethnicity, sex, maternal asthma or smoking, and they were not more likely to have current wheeze at ages 11, 16 and 22 years (Table 4).
Table 4.
Characteristics of infants by quartiles of infant V'maxFRC
| Infant V'maxFRC (ml/s) Quartiles | % | % | % LRI by 3 | % Atopic at | % Maternal | % Wheeze at | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Male | NHW* | Yr | 6yr | Asthma | Smoking | 11yr | 16yr | 22yr | |
| 1 (23.1 - 78.3) | 27 | 66.7 | 59.3 | 76.9 | 40.7 | 15.4 | 18.5 | 25.9 | 21.7 | 52.2 |
| 2 (79.0 - 119.1) | 33 | 66.7 | 57.6 | 52.0 | 46.7 | 12.1 | 30.3 | 21.2 | 26.9 | 46.2 |
| 3 (120.3 - 165.7) | 31 | 45.2 | 64.5 | 44.0 | 24.1 | 3.2 | 19.4 | 20.0 | 14.3 | 37.5 |
| 4 (167.3 - 242.3) | 32 | 34.4 | 65.6 | 38.5 | 44.8 | 12.5 | 9.4 | 18.8 | 23.3 | 50.0 |
| Trend Chi2 | 0.003 | 0.5 | 0.005 | 0.8 | 0.5 | 0.2 | 0.5 | 0.8 | 0.8 | |
| Lower Quartile compared to upper three quartiles | ||||||||||
| 1 (23.1 - 78.3) | 27 | 66.7 | 59.3 | 76.9 | 40.7 | 15.4 | 18.5 | 25.9 | 21.7 | 52.2 |
| 2-4 (79.0 - 242.3) | 96 | 49.0 | 62.5 | 44.7 | 38.6 | 9.4 | 19.8 | 20.0 | 21.4 | 44.9 |
| Pearson Chi2 | 0.1 | 0.8 | 0.005 | 0.8 | 0.4 | 0.9 | 0.5 | 0.9 | 0.5 | |
Non-Hispanic White
Discussion
We found that up to 14% of the variance in measurements of airway function (FEV1, FEV1/FVC ratio, and FEF25-75) in young adults was explained by the maximal flows at functional residual capacity (V'maxFRC), an index of airway function obtained from partial expiratory flow-volume curves, measured in the same subjects at a mean age of 2 months. Correlations with infant lung function were statistically significant for both pre- and post-bronchodilator indices of airway function at ages 11-22 years, but results were stronger for the former than for the latter. Moreover, subjects who had diminished airway function as infants had significantly increased responses to bronchodilators. We did not measure post-bronchodilator infant lung function, and thus we do not know if the weaker association between infant lung function and bronchodilator responsiveness was due to inter-individual variability in congenital responses to bronchodilators. It is likely, however, that both reversible and irreversible determinants of maximal flows during forced expirations may explain the correlations observed prior to bronchodilator administration. We found no association between infant lung function and subsequent bronchial responses to methacholine suggesting that structural characteristics of the lung, and not intrinsic airway hyperresponsivess, are responsible for the observed tracking of airway function from birth to early adult life.
A more detailed analysis of the data suggested that a significant proportion of the correlation between infant airway function and lung function up to adult life was attributable to subjects who had already diminished airway function shortly after birth. These subjects, who were so classified because they were in the lowest quartile for length-adjusted infant V'maxFRC, had, for example, mean (95%CI) predicted FEV1/FVC ratios of 75.1% (73%-77%) at age 22 years, that is, a level that was 5.2% lower than mean values for subjects in the other 3 quartiles. This could indicate that subjects with airflow obstruction at birth are those more likely to remain in the lowest end of the distribution until early adult life, whereas tracking of airway function may be less evident in children with normal airways. However, we cannot exclude the possibility that, when using the passive chest compression technique to obtain partial flow-volume curves (8), maximal flows could have been more readily obtained in infants with congenitally narrower airways than in those with larger airway. If this were the case, however, our results would underestimate the correlation between infant airway function and lung function obtained with full flow-volume curves in older children and adults.
Previous studies had demonstrated that spirometric parameters show marked tracking during school age, that is, individuals within a cohort remain at a relatively constant deviation from the mean over time (16, 17). Our results suggest that tracking at the lowest levels of airway function is already established shortly after birth, and that subjects who are born with low levels of V'maxFRC have persistently low levels of lung function up to age 22 years. Similarly, Turner et al (18) reported positive correlation between airway function in infancy and lung function measured at ages 6 and 11 years in children with different wheezing phenotypes. Filippone et al (19) observed that in children with bronchopulmonary dysplasia, airway function tracks from age 2 to age 9 years. Hoo et al (20) found strong tracking of airway function between birth and 9 months of age in infants of both normal and low birth weight. Taken together, these findings support the hypothesis that the factors that control airway development in-utero play an important role in determining the level of airway function that a subject will attain by early adult life. This hypothesis is compatible with the observation that branching of the bronchial tree is complete by 16 weeks of gestation (21), and that the number of terminal bronchiolar duct endings does not increase postnatally (22). It should also be noted that elastic recoil plays a key role in determining maximal expiratory flow (23, 24), not only because it is the motive force behind flow, but also because of its role in maintaining airway patency. Unfortunately, we did not measure static recoil in this epidemiologic study because of the invasive nature of its measurement. It is, however, not inconceivable that life-long alterations in the static recoil of the lung either alone or in combination with altered airway conductance could have led to our findings.
Using data obtained from these same study subjects, we previously showed that children who wheezed during viral infection in the first years of life, and especially those whose symptoms had remitted by age 6 (“transient wheezers”), had lower premorbid V'maxFRC levels than children who never wheezed during the pre-school years (4). Other long-term birth cohort studies had previously shown that adults who had lower respiratory illnesses in early life had lower levels of lung function than those with no such history (25). Our current results suggest that alterations in airway development in-utero predispose both to lower respiratory illnesses and to subsequent deficits in lung function during adult life. This does not exclude the possibility that lower respiratory illnesses in early life, especially among children whose symptoms persist beyond the pre-school years, may themselves cause additional deficits in lung function growth that become apparent after birth (10). In addition, other studies have shown that passive smoking (26), and air pollution (27), among other factors (28), may affect airway function.
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death in the U.S. (29) and is projected to become the third leading cause of death worldwide by 2020 (30). Its defining characteristic is the presence of irreversible airflow obstruction, which is conventionally defined as the presence of a post-bronchodilator FEV1/FVC ratio of <70% (31). Although cigarette smoking is known to be the major risk factor for this disease (32-34), 5-10% of non-smoking young adults and up to 30% of non-smoking adults aged 65 or more have evidence of COPD (35). Among non-smokers, impaired lung function is also a significant predictor of mortality due to ischemic heart disease and stroke (36). Individuals who enter adult life with lung function deficits are more likely to develop chronic obstructive pulmonary disease during the later adult years, especially if they had lower respiratory illnesses in early life (37). In our cohort, individuals in the lowest quartile of infant lung function start adulthood with significant deficits in FEF25-75, FEV1 and the FEV1/FVC ratio. We postulate that, even if these individuals were to show lung function decline during adults life at rates similar to those observed in non-smokers (38), they would reach the threshold of FEV1 and FEV1/FVC ratio that define COPD (31) at an earlier age than their peers. It is thus possible that fetal determinants of airway function may predispose not only to airflow obstruction and chronic obstructive pulmonary disease, but also to non-respiratory morbidity and mortality during adult life.
The factors that determine normal and impaired pulmonary development in-utero are not well understood. Lung morphogenesis is a highly regulated process that may be disturbed by both genetic and environmental factors in-utero (39). Among these factors, maternal smoking during pregnancy has been consistently associated with lower levels of lung function in both infants (40) (41) and older children (42). Children with chronic lung disease of prematurity have significantly impaired lung function growth (43), as do, to a lesser extent, premature children who did not have chronic lung disease of prematurity (44). Our result suggest that a better understanding of the mechanisms that control normal lung growth in-utero may contribute significantly to the development of strategies for the prevention of chronic obstructive pulmonary disease in adult life.
Supplementary Material
Acknowledgements
We thank Bruce Saul for data management and our study nurses, Marilyn Lindell and Lydia de la Ossa, for data collection and participant follow-up. This work was supported by National Heart Lung and Blood Institute grants HL-14136 and HL-56177.
Supported by NIH grant: HL-56177
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of the funding source The funding source had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of Interest Statement We declare that we have no conflict of interest.
References
- 1.Burrows B, Knudson RJ, Lebowitz MD. The relationship of childhood respiratory illness to adult obstructive airway disease. Am Rev Respir Dis. 1977 May;115(5):751–60. doi: 10.1164/arrd.1977.115.5.751. [DOI] [PubMed] [Google Scholar]
- 2.Samet JM, Tager IB, Speizer FE. The relationship between respiratory illness in childhood and chronic air-flow obstruction in adulthood. Am Rev Respir Dis. 1983 Apr;127(4):508–23. doi: 10.1164/arrd.1983.127.4.508. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988 Oct 27;319(17):1112–7. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 4.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ, The Group Health Medical Associates Asthma and wheezing in the first six years of life. N Engl J Med. 1995 Jan 19;332(3):133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 5.Tager IB, Hanrahan JP, Tosteson TD, Castile RG, Brown RW, Weiss ST, et al. Lung function, pre- and post-natal smoke exposure, and wheezing in the first year of life. Am Rev Respir Dis. 1993 Apr;147(4):811–7. doi: 10.1164/ajrccm/147.4.811. [DOI] [PubMed] [Google Scholar]
- 6.Dezateux C, Stocks J, Dundas I, Fletcher ME. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. American journal of respiratory and critical care medicine. 1999 Feb;159(2):403–10. doi: 10.1164/ajrccm.159.2.9712029. [DOI] [PubMed] [Google Scholar]
- 7.James AL, Palmer LJ, Kicic E, Maxwell PS, Lagan SE, Ryan GF, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. American journal of respiratory and critical care medicine. 2005 Jan 15;171(2):109–14. doi: 10.1164/rccm.200402-230OC. [DOI] [PubMed] [Google Scholar]
- 8.Tepper RS, Morgan WJ, Cota K, Wright A, Taussig LM. Physiologic growth and development of the lung during the first year of life. Am Rev Respir Dis. 1986 Sep;134(3):513–9. doi: 10.1164/arrd.1986.134.3.513. [DOI] [PubMed] [Google Scholar]
- 9.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999 Aug 14;354(9178):541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 10.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. American journal of respiratory and critical care medicine. 2005 Nov 15;172(10):1253–8. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997 Nov;52(11):946–52. doi: 10.1136/thx.52.11.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Children's Respiratory Study. II. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989 Jun;129(6):1232–46. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 13.Stern DA, Lohman IC, Wright AL, Taussig LM, Martinez FD, Halonen M. Dynamic changes in sensitization to specific aeroallergens in children raised in a desert environment. Clin Exp Allergy. 2004 Oct;34(10):1563–669. doi: 10.1111/j.1365-2222.2004.02088.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown H, Prescott R. Applied Mixed Models in Medicine. John Wiley & Sons, LTD; 1999. [Google Scholar]
- 15.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997 Oct 30;16(20):2349–80. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Hibbert ME, Lannigan A, Landau LI, Phelan PD. Lung function values from a longitudinal study of healthy children and adolescents. Pediatr Pulmonol. 1989;7(2):101–9. doi: 10.1002/ppul.1950070209. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993 Feb;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 18.Turner SW, Palmer LJ, Rye PJ, Gibson NA, Judge PK, Cox M, et al. The relationship between infant airway function, childhood airway responsiveness, and asthma. American journal of respiratory and critical care medicine. 2004 Apr 15;169(8):921–7. doi: 10.1164/rccm.200307-891OC. [DOI] [PubMed] [Google Scholar]
- 19.Filippone M, Sartor M, Zacchello F, Baraldi E. Flow limitation in infants with bronchopulmonary dysplasia and respiratory function at school age. Lancet. 2003 Mar 1;361(9359):753–4. doi: 10.1016/S0140-6736(03)12633-5. [DOI] [PubMed] [Google Scholar]
- 20.Hoo AF, Stocks J, Lum S, Wade AM, Castle RA, Costeloe KL, et al. Development of lung function in early life: influence of birth weight in infants of nonsmokers. American journal of respiratory and critical care medicine. 2004 Sep 1;170(5):527–33. doi: 10.1164/rccm.200311-1552OC. [DOI] [PubMed] [Google Scholar]
- 21.Bucher U, Reid L. Development of the intrasegmental bronchial tree: the pattern of branching and development of cartilage at various stages of intra-uterine life. Thorax. 1961 Sep;16:207–18. doi: 10.1136/thx.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beech DJ, Sibbons PD, Howard CV, van Velzen D. Terminal bronchiolar duct ending number does not increase post-natally in normal infants. Early Hum Dev. 2000 Sep;59(3):193–200. doi: 10.1016/s0378-3782(00)00095-5. [DOI] [PubMed] [Google Scholar]
- 23.Dawson SV, Elliott EA. Wave-speed limitation on expiratory flow-a unifying concept. Journal of applied physiology. 1977 Sep;43(3):498–515. doi: 10.1152/jappl.1977.43.3.498. [DOI] [PubMed] [Google Scholar]
- 24.Pride NB, Permutt S, Riley RL, Bromberger-Barnea B. Determinants of maximal expiratory flow from the lungs. Journal of applied physiology. 1967 Nov;23(5):646–62. doi: 10.1152/jappl.1967.23.5.646. [DOI] [PubMed] [Google Scholar]
- 25.Johnston ID, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med. 1998 Feb 26;338(9):581–7. doi: 10.1056/NEJM199802263380904. [DOI] [PubMed] [Google Scholar]
- 26.Cook DG, Strachan DP, Carey IM. Health effects of passive smoking. 9. Parental smoking and spirometric indices in children. Thorax. 1998 Oct;53(10):884–93. doi: 10.1136/thx.53.10.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Islam T, Gauderman WJ, Berhane K, McConnell R, Avol E, Peters JM, et al. The Relationship between Air Pollution, Lung Function and Asthma in Adolescents. Thorax. 2007 May 21; doi: 10.1136/thx.2007.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajekar R. Environmental factors and developmental outcomes in the lung. Pharmacology & therapeutics. 2007 May;114(2):129–45. doi: 10.1016/j.pharmthera.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance--United States, 1971-2000. Respir Care. 2002 Oct;47(10):1184–99. [PubMed] [Google Scholar]
- 30.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997 May 24;349(9064):1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 31.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. American journal of respiratory and critical care medicine. 2001 Apr;163(5):1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 32.Bosse R, Sparrow D, Garvey AJ, Costa PT, Jr., Weiss ST, Rowe JW. Cigarette smoking, aging, and decline in pulmonary function: A longitudinal study. Arch Environ Health. 1980 Jul-Aug;35(4):247–52. doi: 10.1080/00039896.1980.10667500. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977 Jun 25;1(6077):1645–8. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tager IB, Segal MR, Speizer FE, Weiss ST. The natural history of forced expiratory volumes. Effect of cigarette smoking and respiratory symptoms. Am Rev Respir Dis. 1988 Oct;138(4):837–49. doi: 10.1164/ajrccm/138.4.837. [DOI] [PubMed] [Google Scholar]
- 35.Mannino DM, Watt G, Hole D, Gillis C, Hart C, McConnachie A, et al. The natural history of chronic obstructive pulmonary disease. Eur Respir J. 2006 Mar;27(3):627–43. doi: 10.1183/09031936.06.00024605. [DOI] [PubMed] [Google Scholar]
- 36.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. Bmj. 1996 Sep 21;313(7059):711–5. doi: 10.1136/bmj.313.7059.711. discussion 5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss ST, Ware JH. Overview of issues in the longitudinal analysis of respiratory data. American journal of respiratory and critical care medicine. 1996 Dec;154(6 Pt 2):S208–11. doi: 10.1164/ajrccm/154.6_Pt_2.S208. [DOI] [PubMed] [Google Scholar]
- 38.Burrows B, Knudson RJ, Camilli AE, Lyle SK, Lebowitz MD. The “horse-racing effect” and predicting decline in forced expiratory volume in one second from screening spirometry. Am Rev Respir Dis. 1987 Apr;135(4):788–93. doi: 10.1164/arrd.1987.135.4.788. [DOI] [PubMed] [Google Scholar]
- 39.Whitsett JA. Disorders of lung morphogenesis. Paediatr Respir Rev. 2006;7(Suppl 1):S248. doi: 10.1016/j.prrv.2006.04.188. [DOI] [PubMed] [Google Scholar]
- 40.Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992 May;145(5):1129–35. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- 41.Stocks J, Dezateux C. The effect of parental smoking on lung function and development during infancy. Respirology. 2003 Sep;8(3):266–85. doi: 10.1046/j.1440-1843.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 42.Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Vora H, Rappaport EB, et al. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000;55(4):271–6. doi: 10.1136/thorax.55.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Northway WH, Jr., Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, et al. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990 Dec 27;323(26):1793–9. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- 44.Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ. Lung function and exercise capacity in young adults born prematurely. American journal of respiratory and critical care medicine. 2006 Apr 15;173(8):890–6. doi: 10.1164/rccm.200507-1140OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.